Abstract
Purpose
Metabolic phenotyping has provided important biomarker findings, which, unfortunately, are rarely replicated across different sample sets due to the variations from different analytical and clinical protocols used in the studies. To date, very few metabolic hallmarks in a given cancer type have been confirmed and validated by use of a metabolomic approach and other clinical modalities. Here, we report a metabolomics study to identify potential metabolite biomarkers of colorectal cancer with potential theranostic value.
Experimental Design
Gas chromatography–time-of-flight mass spectrometry (GC–TOFMS)–based metabolomics was used to analyze 376 surgical specimens, which were collected from four independent cohorts of patients with colorectal cancer at three hospitals located in China and City of Hope Comprehensive Cancer Center in the United States. Differential metabolites were identified and evaluated as potential prognostic markers. A targeted transcriptomic analysis of 29 colorectal cancer and 27 adjacent nontumor tissues was applied to analyze the gene expression levels for key enzymes associated with these shared metabolites.
Results
A panel of 15 significantly altered metabolites was identified, which demonstrates the ability to predict the rate of recurrence and survival for patients after surgery and chemotherapy. The targeted transcriptomic analysis suggests that the differential expression of these metabolites is due to robust metabolic adaptations in cancer cells to increased oxidative stress as well as demand for energy, and macromolecular substrates for cell growth and proliferation.
Conclusions
These patients with colorectal cancer, despite their varied genetic background, mutations, pathologic stages, and geographic locations, shared a metabolic signature that is of great prognostic and therapeutic potential.
Introduction
Cancer cells exhibit distinct metabolic phenotypes that are essential for sustaining high proliferative rates, and resist cell death signals associated with altered flux along key metabolic pathways, such as glycolysis and the tricarboxylic acid cycle (1). As exemplified in the "Warburg effect" (2), an increase in aerobic glycolysis is associated with the characteristic expression, mutation, and posttranslational modification of enzymes involved in a number of key metabolic pathways, presumably due to the adaptation to oxidative stress associated with tumor hypoxia and mitochondrial mutations (3). Therefore, metabolic regulation is closely linked to cancer progression because proliferation is tightly regulated by the availability of nutrients. Moreover, oncogenes that promote proliferation likely both influence and are also conversely influenced by metabolic changes.
The revival of interest in cancer cell metabolism in recent years has prompted the need for metabolomic phenotyping of clinical cancer specimens. However, the identification and validation of a distinct metabolic signature for a specific cancer proves to be challenging, due to the interindividual variability of patients and the differing analytical and clinical protocols used in various studies (4). Although many studies revealed different metabolic profiles in cancerous tissues (5–8), whether a certain cancer tends to maintain a unique metabolic transformation process to sustain uncontrolled proliferation and exhibit a universal "core" metabolome that is consistently identifiable among the tumors of different subjects at different pathologic stages is uncertain.
Colorectal cancer remains one of the most common types of cancers occurring worldwide (9), among which sporadic colorectal cancer represents an estimated 70% of all newly diagnosed cases. It is believed that sporadic colorectal cancer develops slowly through the progressive accumulation of multiple mutations that affect tumor suppressor genes, oncogenes, and downstream metabolic pathways (10). The global metabolic profiling of colon tissue could define metabolic signatures that not only discriminate malignant tissue from nontumor tissue, but also distinguish the clinicopathologic characteristics and treatment outcomes among patients with colorectal cancer. Here, we describe a comprehensive metabolomic analysis of colorectal cancer tissue samples from multiple patient cohorts that consistently detected a panel of differentially expressed metabolites in human colorectal cancer tissues relative to adjacent nontumor tissues.
Materials and Methods
Sample information
Samples were collected as surgical specimens (n = 376) from patients with colorectal cancer treated at four hospitals located in China and the United Stated following the same protocol. All of the samples were collected within 15 minutes after the surgery and immediately frozen at −80°C for metabolomic analysis. The first batch of samples was collected from 85 patients with colorectal cancer from the Fudan University Shanghai Cancer Center (Shanghai, China), in which 55 patients contributed paired samples (i.e., both colorectal cancer tissue and adjacent nontumor tissues located 5 cm from the edge of the tumor), and the other 30 patients contributed colorectal cancer tissue only. In addition, three validation batches of samples were collected from Cancer Hospital affiliated with the Chinese Academy of Medical Sciences (Beijing, China; n = 23, paired tissue samples with two batches of nontumor tissues located 5 and 2–5 cm from the edge of the tumor, respectively), the Second Affiliated Hospital, Zhejiang University School of Medicine (Hangzhou, China; n = 65, paired tissue samples), and the City of Hope Comprehensive Cancer Center (Duarte, CA; n = 20, paired tissue samples). Samples for the gene expression assay were provided by the City of Hope Comprehensive Cancer Center. A total of 56 tissue samples (29 colorectal cancer and 27 adjacent nontumor tissues) from 34 patients were collected, 22 of which were paired samples and 12 were colorectal cancer or control samples only. The age, tumor stage, and tumor location for all of these patients are provided in Table 1. None of the patients was on any neoadjuvant chemotherapy before surgical treatment. The protocol was approved by the Institutional Review Board from each of the four participating hospitals, and all participants in this study signed informed consent before the study.
Table 1.
Demographic and clinical chemistry characteristics of human subjects
| Samples from Shanghai |
Samples from Beijing |
Samples from Hangzhou |
Samples from City of Hope |
Samples for gene analysis |
|
|---|---|---|---|---|---|
| Number of patients | 85 | 23 | 65 | 20 | 34 |
| Age (median, range) | 57, 31–79 | 61, 40–75 | 61, 34–84 | 59, 35–81 | 61, 36–86 |
| Male/female ratio | 49/36 | 10/13 | 43/22 | 8/12 | 10/24 |
| TNM stage | |||||
| Stage 0 | / | / | / | 1 | / |
| Stage I | 7 | 3 | 11 | 2 | 7 |
| Stage II | 35 | 9 | 22 | 2 | 5 |
| Stage III | 37 | 10 | 21 | 14 | 21 |
| Stage IV | 6 | 1 | 11 | 1 | 1 |
Sample processing for metabolomics analysis
The metabolites extraction procedure followed our previous publication with minor modifications (11). Briefly, approximately 50 mg of each tissue sample was weighed and minced using liquid nitrogen. A 250-µL mixture of chloroform, methanol, and water (2:5:2) was added and the samples were vortexed for 1 minute. The samples were then placed at −20°C for 20 minutes to extract metabolites, followed by centrifugation at 12,000 rpm for 10 minutes. The liquid layer was transferred into a new tube. The residue was extracted with 250 µL of methanol using the homogenizer for 10 minutes followed by centrifugation at 12,000 rpm for 10 minutes. The supernatant was combined with the previous extraction. After vortexing, a volume of 150-µL mixture was transferred to a glass vial spiked with internal standards (10-µL heptadecanoic acid at 1 mg/mL and 4-chlorophenylalanine at 0.3 mg/mL), which was then vacuum dried at room temperature. The residue was chemically derivatized with a two-step procedure and then analyzed following the protocols previously published (12) with the Pegasus HT system (Leco Corporation) coupled with an Agilent 6890N gas chromatography. Briefly, A 1-µL derivate was injected with a splitless mode at 270°C. The flow rate for the carrier gas, helium, was 1.0 mL/min. The oven program started at 80°C for 2 minutes, and then ramped to 180°C with 10°C/min, to 230°C with 6°C/min, finally to 295°C with 40 ° C/min and hold for 8 minutes. The transfer interface and ion source was set to 270°C and 220°C, respectively. Data were acquired with m/z range of 30 to 600 at an acquisition rate of 20 spectra per second.
Quantitative real-time PCR analysis
RNA was isolated from tumor tissue or control samples using the Qiagen RNA Easy Kit (Qiagen). Total RNA (1 µg) was converted into cDNA with the RT2 HT First Strand Kit (Cat no. 330411; SABiosciences) according to the manufacturer’s instructions. The synthesized cDNA was used for multiple gene expression analyses using SABiosciences RT2 SYBR Green qPCR Master Mixes in a Standard ABI 7500 system (Life Technologies Corporation). The primer pairs were provided and preloaded in 96-well plates by SABiosciences for a customized gene panel (SABiosciences). β-Actin was used as an internal control.
Statistical analysis
The acquired gas chromatography–time-of-flight mass spectrometry (GC–TOFMS) data were processed (including smoothing, denoising, peak picking, identification, and alignment) using ChromaTOF software (v4.22; Leco Co.) as described in a previous publication (13). Sample information, peak retention time, and peak area (quant mass) were included in the final dataset. Known artificial peaks, such as peaks caused by noise, column bleed, and N,O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA) derivatization agents, were removed from the dataset. The resulting data were normalized to internal standards and the weight of tissue sample before statistical analysis. Principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) were performed with SIMCA-p software (v 12.0; Umetrics). The default 7-fold cross-validation was applied, to guard against overfitting. The variable importance in the projection (VIP) values (VIP > 1.0) are considered to be differentiating variables (14). The Student t test was used for further differentiating variables selection and validation (P < 0.05). Compound identification for GC–TOFMS was performed by comparing the mass fragments with NIST 11 Standard mass spectral databases in ChromaTOF software with a similarity of more than 70% and verified by available reference compounds. Receiver operating characteristic (ROC) analysis, binary logistic regression, and Kaplan–Meier analysis were performed with SPSS software (v20; IBM). The data for gene expression were expressed as means ± SE. Differences were considered statistically significant at P < 0.05 from a Student t test.
Results
Differentially expressed metabolites in colorectal cancer tissues relative to adjacent nontumor tissues
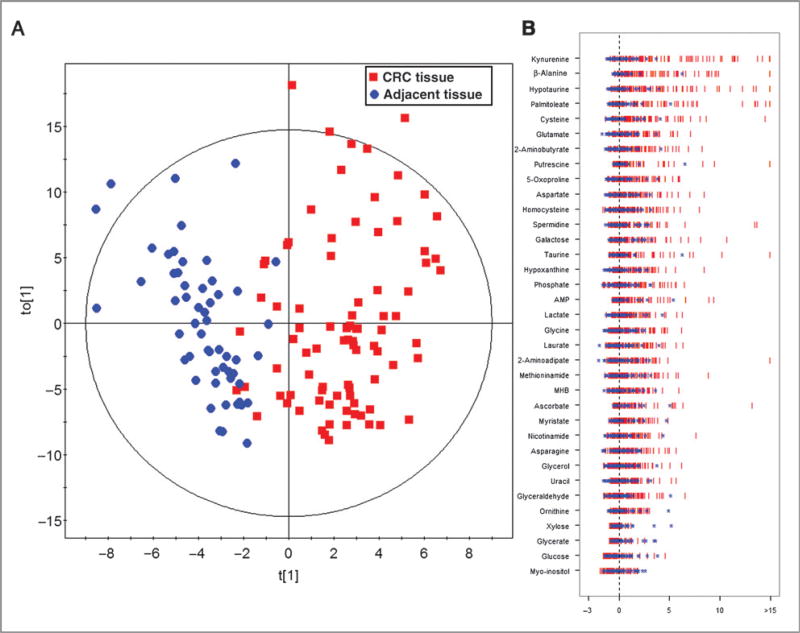
PCA scores plot revealed a trend of separation between 85 tumor tissues and 55 adjacent nontumor tissues collected from Shanghai (Supplementary Fig. S1A). An OPLS-DA model was obtained with one predictive component and two orthogonal components (R2Xcum = 0.374; R2Ycum = 0.706; and Q2cum = 0.532). The scores plot demonstrated a separation between tumor tissues and adjacent nontumor ones with few overlaps (Fig. 1A). A 999-time permutation test was performed to validate the corresponding model. The result showed that the intercept for the Q2 to the γ-axis is below zero [Q2 intercept (0, −0.097); Supplementary Fig. S1B], which indicated the validity of the current model. A number of 35 metabolites were identified with the criteria of VIP > 1 and P value in the Student t test less than 0.05 (Fig. 1B; Table 2).
Figure 1.
OPLS-DA scores plot for the Z-score plot of differentially expressed metabolites in colorectal cancer tissues relative to adjacent nontumor tissues. A, OPLS-DA scores plot for colorectal cancer (CRC) samples collected from Shanghai. B, the Z-score plot of differentiating metabolites between CRC tissues and adjacent nontumor tissues. The values were standardized using the mean values and the SDs of adjacent nontumor tissues in each group. Each vertical line represents one metabolite in one sample, colored by tissue type (blue star, nontumor tissue; red vertical line, CRC tissue). For clarity, Z-score values were cut at 15 SDs. Abbreviations for metabolites: AMP, adenosine-5′-monophosphate; MHB, 3-methyl-3-hydroxybutanoic acid.
Table 2.
Identified differential metabolites between colorectal cancer and adjacent normal controls from each of tissue samples
| Samples from Shanghai | Samples from Hangzhou |
Samples from Beijing |
Samples from City of Hope |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||
| No. | Metabolite name | VIP | FCa,c | Pb,c | FCa,c | Pb,c | FCa,c | Pb,c | FCa,c | Pb,c |
| 1 | Kynurenined | 2.55 | 5.50 | 6.49E–14 | 2.64 | 1.68E–05 | 4.31 | 2.34E–05 | 2.20 | 1.92E–03 |
| 2 | β-Alanined | 2.48 | 5.02 | 3.51E–13 | 4.56 | 9.09E–11 | 6.01 | 3.23E–06 | 2.79 | 1.00E–03 |
| 3 | Glutamated | 2.25 | 1.88 | 3.54E–10 | 1.92 | 8.68E–08 | 1.87 | 4.14E–04 | 1.53 | 8.06E–03 |
| 4 | Cysteined | 2.14 | 2.41 | 8.02E–10 | 1.87 | 3.54E–04 | 1.33 | 4.79E–02 | 1.92 | 1.40E–02 |
| 5 | 2-Aminobutyrated | 2.13 | 1.81 | 3.05E–09 | 1.49 | 7.97E–03 | 1.58 | 4.17E–02 | 1.49 | 4.45E–02 |
| 6 | Palmitoleated | 1.90 | 3.09 | 3.03E–08 | 2.25 | 2.98E–04 | 2.70 | 1.21E–02 | 2.22 | 9.59E–03 |
| 7 | 5-Oxoprolined | 1.96 | 1.76 | 5.89E–08 | 1.45 | 1.88E–03 | 2.13 | 2.16E–02 | 1.47 | 2.35E–02 |
| 8 | Aspartated | 1.92 | 1.80 | 1.17E–07 | 1.58 | 1.95E–03 | 1.49 | 2.17E–02 | 1.70 | 1.65E–02 |
| 9 | Hypoxanthined | 1.84 | 1.71 | 5.16E–07 | 1.36 | 1.28E–03 | 2.12 | 6.12E–06 | 1.38 | 3.80E–02 |
| 10 | Lactated | 1.87 | 1.68 | 5.89E–07 | 1.49 | 4.34E–07 | 1.45 | 1.95E–04 | 1.55 | 2.21E–02 |
| 11 | Myristated | 1.56 | 1.59 | 6.54E–05 | 2.06 | 3.11E–06 | 2.15 | 8.45E–03 | 1.72 | 3.81E–02 |
| 12 | Glycerold | 1.36 | 1.48 | 3.77E–04 | 1.37 | 7.10E–03 | 1.28 | 3.06E–02 | 1.57 | 3.76E–02 |
| 13 | Uracild | 1.36 | 1.39 | 4.59E–04 | 1.47 | 4.59E–04 | 2.37 | 2.77E–03 | 1.58 | 4.29E–02 |
| 14 | Putrescined | 1.21 | 3.57 | 4.61E–04 | 1.48 | 4.84E–02 | 4.46 | 4.57E–03 | 2.78 | 4.94E–02 |
| 15 | Myo-inositold | 1.10 | −1.29 | 8.39E–03 | −1.33 | 3.78E–04 | −1.66 | 4.54E–07 | −1.49 | 1.96E–02 |
| 16 | Hypotaurine | 2.32 | 4.04 | 1.09E–11 | 2.59 | 2.10E–05 | 3.84 | 2.00E–05 | / | / |
| 17 | Spermidine | 1.37 | 1.89 | 1.02E–04 | 1.70 | 1.91E–05 | 2.34 | 2.44E–06 | / | / |
| 18 | Homocysteined | 1.73 | 1.79 | 1.84E–06 | 1.45 | 3.83E–03 | 2.33 | 8.77E–03 | / | / |
| 19 | 4-Aminobutyrate | 1.27 | 2.18 | 2.97E–04 | 1.35 | 5.14E–03 | 2.27 | 6.06E–03 | / | / |
| 20 | Asparagined | 1.33 | 1.47 | 4.40E–04 | 1.36 | 5.14E–03 | 1.96 | 1.86E–02 | / | / |
| 21 | Glycerated | 1.12 | −1.76 | 1.38E–02 | −2.00 | 1.90E–02 | / | / | −1.43 | 1.31E–02 |
| 22 | Nicotinamide | 1.39 | 1.56 | 2.54E–04 | / | / | 1.53 | 2.18E–02 | 1.61 | 5.39E–02 |
| 23 | AMPd | 1.46 | 2.78 | 4.66E–05 | 2.83 | 7.59E–05 | / | / | / | / |
| 24 | Ascorbated | 1.06 | 2.59 | 3.34E–03 | 1.43 | 2.07E–02 | / | / | / | / |
| 25 | Glucosed | 1.09 | −1.51 | 6.50E–03 | −2.37 | 1.01E–06 | / | / | / | / |
| 26 | Xylose | 1.16 | −1.63 | 1.61E–02 | −2.15 | 4.40E–02 | / | / | / | / |
| 27 | Glycined | 1.64 | 1.62 | 7.49E–06 | / | / | 1.53 | 1.22E–02 | / | / |
| 28 | Glyceraldehyded | 1.02 | 1.39 | 7.19E–03 | / | / | 1.37 | 4.15E–02 | / | / |
| 29 | Ornithined | 1.07 | 1.39 | 9.49E–03 | / | / | 2.28 | 1.91E–02 | / | / |
| 30 | Phosphate | 1.64 | 1.53 | 5.84E–06 | / | / | / | / | / | / |
| 31 | Laurated | 1.59 | 1.49 | 1.49E–05 | / | / | / | / | / | / |
| 32 | Galactose | 1.54 | 2.17 | 1.80E–05 | / | / | / | / | / | / |
| 33 | 3-Methy-3-hydrpoxybutyrate | 1.58 | 1.54 | 3.97E–05 | / | / | / | / | / | / |
| 34 | Methioninamide | 1.44 | 1.70 | 7.16E–05 | / | / | / | / | / | / |
| 35 | 2-Aminoadipate | 1.19 | 1.45 | 8.04E–04 | / | / | / | / | / | / |
Abbreviation: AMP, adenosine-50-monophosphate.
FC (fold change) with a positive value indicates a relatively higher level in tumor tissue, whereas a negative value means a relatively lower expression level as compared with the adjacent normal tissues.
P values are calculated from the Student t test.
Metabolites marked with/means these metabolites have a P value higher than 0.05 in the corresponding batch of samples.
The identification of these metabolites were confirmed with our standard compounds.
Replication analysis using colorectal cancer samples collected from independent cohorts
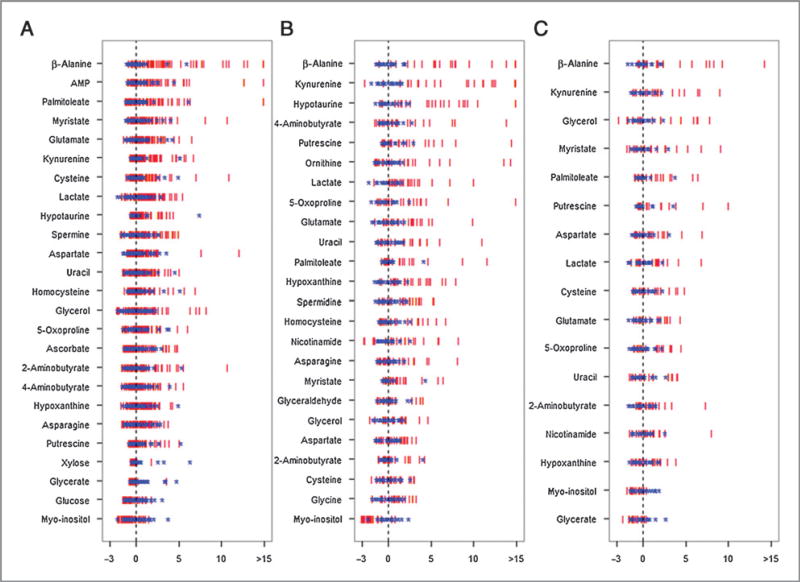
To replicate the metabolomics study of the Shanghai cohort and, thus, to validate the findings of differential metabolites, three independent cohorts of tissue samples were collected from patients with colorectal cancer at three hospitals in Hangzhou and Beijing (China), as well as the City of Hope Comprehensive Cancer Center in the United States, respectively. The samples were analyzed with the same analytical procedures as the samples collected from the Shanghai cohort. Two samples (one in the Hangzhou batch and the other in the City of Hope batch) were excluded from the analysis due to the apparently poor extraction of the metabolites. We focused on these 35 differential metabolites obtained from the Shanghai cohort. A number of 25 metabolites were validated with significantly statistical difference (P < 0.05) between colorectal cancer tissues and adjacent nontumor ones in the Hangzhou cohort of patients with colorectal cancer (n = 65, paired tissue samples). In the Beijing cohort, 24 metabolites were validated in tumor tissues (n = 23, paired tissue samples). In the fourth batch, 17 metabolites were significantly altered (P < 0.05) in tumor tissues from patients treated at the City of Hope (n = 20, paired tissue samples). Z-score plots of each differentially expressed metabolite in colorectal cancer tissue relative to the adjacent nontumor tissue from three validation datasets are shown in Fig. 2. Taken together, 15 metabolites among these 35 differential metabolites were significantly and consistently altered with the same up and down tendency in the three replication cohorts. The panel of 15 metabolites includes significantly elevated β-alanine, palmitoleate, kyrunine, putrescine, cysteine, lactate, glutamate, uracil, hypoxanthine, 5-oxoproline, 2-aminobutyrate, and aspartate, as well as downregulated myo-inositol. In addition, the paired t test was performed on those 15 metabolites with samples in the Hangzhou, Beijing, and City of Hope cohorts. All these 15 metabolites were significantly different between colorectal cancer tissues and adjacent nontumor ones in all these three cohorts (Supplementary Table S1). The chromatogram from two representative samples (one from colorectal cancer tissue and one from adjacent nontumor tissue) was provided in Supplementary Fig. S1C, in which the 15 metabolite markers were marked. In addition, five metabolites were significantly altered in all of samples collected from the three hospitals in China, but not in the samples from City of Hope (details are provided in Table 2). The nontumor tissues were all collected from a section located 5 cm away from the edge of the tumor. We were also able to collect 22 nontumor tissue samples from a section located 2 to 5 cm from the edge of the tumor in the Beijing cohort. Our metabolomic results showed that alterations were less significant in tissue located 2 to 5 cm away from the tumor compared with 5 cm away, indicating a tendency of increased metabolic aberration in nontumor tissue located closer to colorectal cancer tissue (Supplementary Table S1). Multivariate statistics were also performed to the samples in the Hangzhou, Beijing and City of Hope cohorts (Supplementary Text 1 and Supplementary Fig. S2).
Figure 2.
The Z-score plot of differentially expressed metabolites in CRC tissues relative to adjacent nontumor tissues in the validation samples. The values were standardized using the mean values and the SDs of adjacent nontumor tissues in each group. Each vertical line represents one metabolite in one sample, colored by tissue type (blue star, nontumor tissue; red vertical line, CRC tissue). For clarity, Z-score values were cut at 15 SDs. A, samples from Hangzhou; B, samples from Beijing; and C, samples from City of Hope. AMP, adenosine-5′-monophosphate; MHB, 3-methyl-3-hydroxybutanoic acid.
Expression levels of related genes
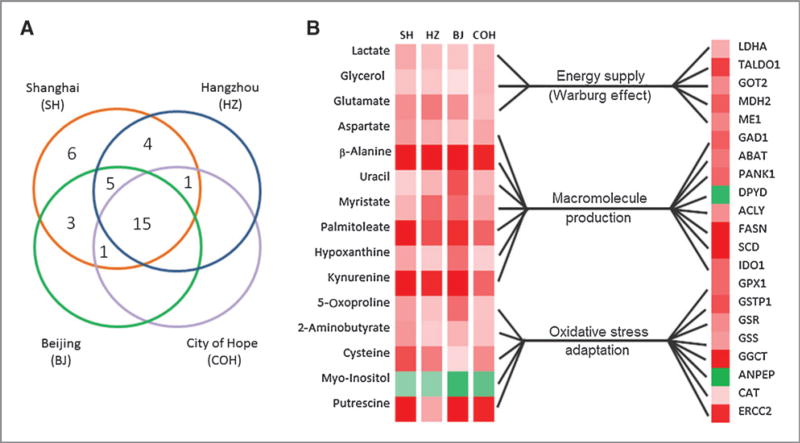
We further analyzed 43 genes in the pathways of glucose metabolism, malate–aspartate shuttle, β-alanine metabolism, tryptophan metabolism, glutathione (GSH) metabolism and oxidative stress, and DNA repair genes. Twenty-one genes were found to be differentially expressed between colorectal cancer and adjacent nontumor tissues with real-time PCR analysis (Supplementary Table S2 and Supplementary Fig. S3A). These differentially expressed genes can be categorized into three types of metabolic transformation to support the needs for increased energy supply, macromolecule production, and the maintenance of redox balance under increased oxidative stress (Fig. 3). The genes with the greatest upregulation were SCD1 (fold change, FC = 6.1) and FASN (FC = 3.48), indicating elevated in situ fatty acid synthesis in cancer tissues (Fig. 3B; Supplementary Table S2).
Figure 3.
Differential expression of metabolites and genes detected in CRC tissue and adjacent nontumor controls. A, Venn diagram of the differentially expressed metabolites in different batches of CRC tissues compared with the corresponding adjacent controls. B, metabolic correlation between those differentially expressed metabolites in all four groups and the differentially expressed genes between CRC tissues and adjacent controls.
Prognostic analysis in colorectal cancer tissue samples
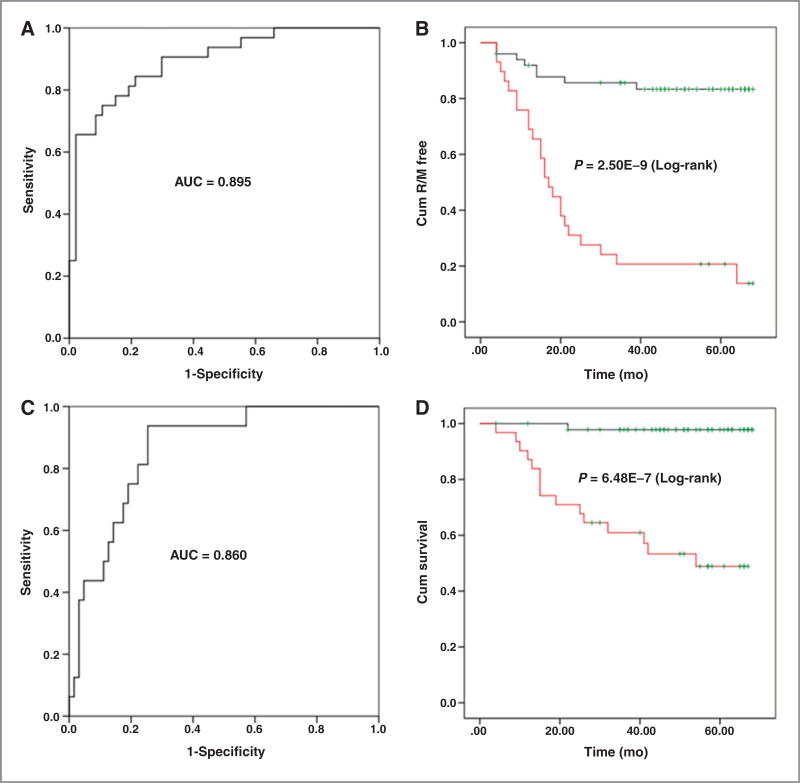
The metabolic signature of the 15 differentially expressed metabolites shown in Fig. 3 was used to statistically predict the 5-year recurrence (including metastasis, R/M) rate of colorectal cancer. For most of the samples in the Shanghai patient cohort, follow-up information for 4 to 5 years after surgery was available. We excluded patients diagnosed with stage IV colorectal cancer (those with metastases; n = 6) from the analysis. In the 79 patients with stage I–III colorectal cancer, 32 (40.51%) had relapsed and 16 (20.25%) had died during the 5-year period after undergoing surgery and receiving standard adjuvant chemotherapy (Supplementary Table S3). A binary logistic regression analysis was then performed R/M results as the dichotomous-dependent variable (0, case free and 1, case) and these 15 differential metabolites plus age and gender as the covariates. The predicted probability values were obtained and saved as a new variable in the SPSS software. Using the predicted probability values as the test variable and R/M as the state variable, an ROC curve was performed and a cutoff predicted probability value (0.499) was selected on the basis of the Youden index [J, J = maxc {sensitivity (c) + specificity (c) − 1}; ref. 15]. On the basis of the cutoff value, these 79 patients with colorectal cancer from the Shanghai cohort were divided into two groups: Patients with predicted probability values above and below the cutoff. Then a Kaplan–Meier analysis was performed for a recurrence rate analysis. The overall time to recurrence for those patients with predicted probability values less than the cutoff value was significantly longer than those with predicted probability values greater than the cutoff value (59.2 vs. 25.9 months; P = 2.50E–9; Fig. 4B). As illustrated in Fig. 4A, the metabolite panel had an area under the curve (AUC) value of 0.895 (95% confidence level, 0.824–0.966) with a sensitivity of 0.750 and a specificity of 0.894, indicating that it provided reasonable accuracy for predicting the recurrence rate of these patients with colorectal cancer more than a 5-year postoperative period.
Figure 4.
ROC curves and Kaplan–Meier curves for disease recurrence and survival rate in samples from the patient cohorts from Shanghai using 15 differentially expressed metabolites. A, an ROC curve using disease recurrence/metastasis (R/M) as the variable in the patient cohort from Shanghai; B, Kaplan–Meier curve comparing disease recurrence in the patient cohort from Shanghai with lower predicted P values (black line) and higher predicted probability values (red line); C, an ROC curve using overall survival as the variable in the patient cohort from Shanghai; D, Kaplan–Meier curve comparing overall survival for the patient cohort from Shanghai with lower predicted P values (black line) and higher predicted P values (red line).
We also assessed the ability of this metabolic signature to predict the survival of the 79 patients with colorectal cancer from the Shanghai cohort 5 years after surgery. As illustrated in Fig. 4C, the AUC reached 0.860 (95% confidence level, 0.771–0.949) with a sensitivity of 0.938 and a specificity of 0.746. The overall survival time for those patients with predicted probability values (determined using the same method as described above) less than the cutoff value (0.186) was significantly longer than those with predicted probability values greater than the cutoff value (67.0 vs. 44.7 months; P = 6.48E–7; Fig. 4D).
Discussion
Metabolic polymorphisms in human carcinogenesis derived from altered oncogenic expression, variable hypoxia levels, and the utilization of different carbon sources may produce diverse metabolic phenotypes and treatment responses. Metabolomic phenotyping of many types of cancers, such as prostate cancer, breast cancer, and colorectal cancer (16–18), has provided important biomarker findings, which unfortunately are rarely replicated across similar studies primarily due to the different analytical and clinical protocols used in the studies. As a result, very few metabolic hallmarks in a given cancer type have been discovered, confirmed, and validated by use of this approach or other investigational modalities.
In this study, we identified a panel of 15 differential metabolites in colorectal cancer tumors in four cohorts of patients with colorectal cancer. We used this panel of metabolites to analyze the metabolomics data that we generated previously from patients with gastric cardiac cancer (40 pairs of tissue samples) using the sample analytical platform and protocols. It seemed that this panel of markers was not able to separate between the tumor and nontumor tissues for gastric cardiac cancer (Supplementary Text 2 and Supplementary Fig. S3B), suggesting that they constitute a distinct metabolic signature of colorectal cancer. Using this panel of 15 metabolites, we are able to distinguish patients with colorectal cancer with better prognostic outcomes, i.e., longer time-to-recurrence (52.9 vs 25.9 months; P = 2.50E-9) and better 5-year survival rate (67.0 vs 44.7 months; P = 6.48E-7) earlier recurrence and lower survival rates. In addition, our result also revealed that the ratio of hypoxanthine to aspartate (Hyp/Asp) showed great potential for prognostic analysis. As shown in Supplementary Text 3 and Supplementary Fig. S4, patients with lower ratio of Hyp/Asp had better outcomes in the following treatment compared with those patients with higher Hyp/Asp ratios. Compared with the 15 metabolite panel, the ratio of two metabolites may be simpler and more clinically applicable although it showed lower sensitivity and AUC value for recurrence prediction, and lower specificity and AUC value for survival prediction than the panel (Supplementary Text 3 and Supplementary Fig. S4A and S4B).
The metabolomics approach has allowed us to unveil several key metabolic variations coexisting in colorectal cancer cells to support their proliferation, despite their varied genetic mutations and pathologic stages. This may explain the ineffectiveness of chemotherapeutic agents that target only a single metabolic enzyme or a specific regulatory pathway. Recent cancer metabolomics studies have revealed important metabolic variations to sustain the fast growth of various cancer cells (19), some of which have already been used as therapeutic targets (such as L-asparaginase for leukemia; ref. 20).
The Warburg effect is known to be a characteristic feature of cancer metabolism, which describes increased rate of glycolysis followed by lactic acid fermentation during tumor growth (21). Coincide with the result of the Warburg effect, we found significantly higher levels of lactate in all colorectal cancer samples analyzed from the 4 patient cohorts. An elevated level of lactate in tissue samples and serum samples compared with their nontumor counterparts was also observed in recent colorectal cancer metabolomics studies (12, 18, 22). Lactate dehydrogenase A (LDHA), which is a key enzyme that catalyzes pyruvate to lactate, was also found to be upregulated in colorectal cancer tissues from our study, confirming that an increase in lactate levels plays an integral role in colorectal cancer metabolism.
In cancer metabolism, glycolysis is a preferred pathway for generating the metabolic intermediates used during de novo biosynthesis to support cell proliferation (see review in ref. 23). This can result in higher levels of free fatty acids (FFA) and nuclear acids related metabolites such as myristic acid, palmitoleic acid, and hypoxanthine in tumor tissues as was observed in our study. The higher level of ATP citrate lyase (ACLY) in the cytosol is important for generating substrates of fatty acid synthase (FASN), such as acetyl-CoA. FASN and stearoyl-CoA desaturase (SCD) are key enzymes for unsaturated fatty acid synthesis, such as palmitoleic acid (24). The higher expression levels of ACLY, FASN, and SCD in colorectal cancer samples found in our study suggest that de novo fatty acid synthesis is increased in these tumors. In addition to glycolysis, the pentose phosphate pathway (PPP) may also be activated and provide components for nucleic acid and fatty acid synthesis (ribose-5-phosphate and NADPH, respectively). Here, we found that transaldolase 1 (TALDO1), which is a key enzyme involved in PPP, was significantly increased in colorectal cancer tissue.
In addition to the generation of acetyl-CoA through the ACLY-mediated hydrolysis of citrate, oxaloacetate is another product that can be further converted to malate by malate dehydrogenase. Malic enzyme 1 (ME1), which catalyzes malate to pyruvate with the concomitant conversion of NADP+ to NADPH, was observed with significantly higher expression level in colorectal cancer tissues compared with nontumor controls in this study. As NADPH is essential for fatty acid synthesis, the increased transformation from malate to pyruvate may compensate for the consumption of NADPH during the fast growth of tumor cells.
β-Alanine was found to be the most significantly altered metabolite in the tumor tissues, as indicated in the Z-score plots. This metabolite was also previously identified as a metabolite marker in colorectal cancer tissues (5). Glutamate decarboxylase 1 (GAD1), which catalyzes aspartate to β-alanine, was also higher in colorectal cancer tissues compared with adjacent nontumor tissues. Our gene expression analysis revealed that pantothenate kinase 1 (PANK1), which is responsible for catalyzing the first and rate-limiting step of CoA biosynthesis (25), had significantly higher expression in colorectal cancer tissues. The higher level of cysteine (a substrate in CoA synthesis) in colorectal cancer samples observed in this study suggests that accelerated synthesis of CoA play a role in colorectal cancer–related morbidity. The gene encoding 4-aminobutyrate aminotransferase (ABAT), which catalyzes the conversion of β-alanine to malonic semialdhyde, had higher expression in colorectal cancer tissues, suggesting that β-alanine is metabolized to malonic semialdhyde for subsequent fatty acid synthesis through malonyl-CoA. Taken together, the increased need for acetyl-CoA and malonyl-CoA in fatty acid synthesis may contribute to the increased production of β-alanine in colorectal cancer tissues.
Reactive oxygen species (ROS), as by-products of cellular metabolism, are associated with the increased metabolic activities in tumor cells (26). In fact, large amount of ROS was reported to be produced by several types of human tumor cells (27). Tumor cells may undergo metabolic transformation to adapt to accelerated anabolic metabolism as well as elevated ROS levels during tumorigenesis. In addition, there was also a marked increase in glutamate, glycine, and cysteine, which are three precursors of GSH. The γ-glutamyl cycle controls the synthesis and degradation of GSH, and the intermediate, 5-oxoproline, which is an important factor in the pathway, was also significantly higher in colorectal cancer tissues compared with adjacent nontumor tissues.
Ophthalmate was previously reported to be a biomarker for oxidative stress and indicative of GSH consumption through the activation of γ-glutamyl cysteine synthetase (GCS; ref. 28). Ophthalmate can be catalyzed by GCS and GSH synthetase (GSS) from 2-aminobutyric acid, glutamate, and glycine (29). In our study, 2-aminobutyric acid and GSS were significantly elevated in colorectal cancer tissues, suggesting that ophthalmate synthase activity in colorectal cancer tissues is increased. Interestingly, a recent metabolomics study also detected higher levels of 2-aminobutyric acid in primary epithelial ovarian cancer compared with normal ovary tissue (30).
In addition to GSS, the expression level of several genes associated with GSH metabolism, including GSH peroxidase 1 (GPX1), GSH reductase (GSR), gamma-glutamylcyclotransferase (GGCT), and glutathione S-transferase pi 1 (GSTP1), was also significantly higher in colorectal cancer samples compared with nontumor controls, whereas aminopeptidase N (ANPEP) was significantly lower, respectively. GPX and GSR catalyze the transformation between reduced and oxidized GSH (known as the GSH redox cycle), which directly reflects cellular GSH homeostasis. The GSH redox cycle is also coupled with the NADP+/NADPH transformation. As discussed above, to meet the requirement of increased de novo fatty acid synthesis, NADPH production may be elevated in tumor cells through the activation of PPP, as evidenced by the elevated expression of TALDO1, and the metabolism of malate to pyruvate, as evidenced by elevated expression of ME1. GGCT catalyzes the degradation of gamma-glutamyl dipeptides to 5-oxoproline and L-amino acids (31). Therefore, the elevated expression level of GGCT may result in a higher level of 5-oxoproline and 2-aminobutyric acid in colorectal cancer tissues, which was observed in this study. A higher expression level of GGCT was also recently suggested to be a potential biomarker for several cancers, including colorectal cancer (32). ANPEP catalyzes the degradation of cysteinylglycine to cysteine and glycine, and was significantly lower in colorectal cancer tissues compared with control tissues, which is consistent with previous reports (33). GSTP1 was also found to be highly expressed in colorectal cancer tissues, which was previously reported to occur during human colon carcinogenesis in correlation with K-ras mutation (34). Taken together, these data show that several metabolites involved in cellular antioxidation activity are overexpressed in colorectal cancer tumors, indicating a robust metabolic adaptation to increased oxidative stress in these cells.
Increased oxidative stress is usually associated with increased oxidation of fatty acids, which may result in an accumulation of 3-hydroxybutyrate. We did not detect an increase in 3-hydroxybutyrate in the colorectal cancer samples from our study; however, increased 3-hydroxybutyrate was detected in the serum samples of patients with colorectal cancer from one of our previous studies (12). These results suggest that higher fatty acid degradation may occur in circulating biofluids, but not in tumor cells, which further supports the hypothesis of enhanced metabolic adaptation of tumor cells to oxidative stress. Increased oxidative stress was reported to induce dysregulation of the osmotic control in astrocytes, which resulted in a loss of myo-inositol (35). Therefore, a lower concentration of myo-inositol in the colorectal cancer tissues may result from the higher oxidative stress.
The kynurenine pathway is one of the main metabolic pathways of tryptophan metabolism, which is first catalyzed by indoleamine 2,3-dioxygenase (IDO; ref. 36). Elevated expression of IDO was suspected as a mediator of tumor immune tolerance, which may help tumor cells avoid immune attack (37). Significant increase of IDO gene expression was also observed in the colorectal cancer tissues compared with adjacent controls in our study. The increased expression of IDO may result in a higher level of kynurenine as was observed in our study. The kynurenine pathway would finally generate nicotinamide adenosine dinucleotide (NAD) from tryptophan. The activated kynurenine pathway may generate more NAD for the electron transport chain to meet the fast growth of tumor cells.
In summary, we identified a distinct metabolic signature with 15 metabolite markers from colorectal cancer tissue samples, which can be used to predict outcomes with surgical and chemotherapy treatment in patients with colorectal cancer. The metabolic aberrations identified at gene expression level indicate a robust metabolic adaptation to sustain increased proliferation in colorectal cancer cells. Such a metabolic adaptation in colorectal cancer extends beyond the Warburg effect that only addresses the increased energy requirements through a preferred glycolysis process. We found that these metabolic changes in colorectal cancer provide support to the increased needs of energy, macromolecular precursors, as well as the maintenance of redox balance under strong oxidative stress.
Supplementary Material
Translational Relevance.
Cancer cells undergo metabolic transformation to sustain fast cell growth and proliferation. This transformation would result in different metabolic phenotypes in cancer cells compared with their control counterparts. Identifying these differential metabolites would be helpful in understanding cancer biology as well as in developing diagnostic and prognostic markers. We performed a comprehensive study that analyzed colorectal cancer samples collected from four independent cohorts in China and the United States. A panel of 15 differential metabolites was identified from these four cohorts, and demonstrated the ability to predict 5-year survival rate of patients with colorectal cancer after standard surgical and medical treatment. These metabolite markers hold great potential for further development of colorectal cancer prognostic markers and/or therapeutic targets.
Acknowledgments
Grant Support
This work was funded by the following grants: the National Basic Research Program of China (no. 2007CB914700); the National Natural Science Foundation of China (no. 81001055); the Shanghai Rising Star Program of the Science and Technology Commission of Shanghai Municipality (no. 10QA1401400); and the National High Technology Research and Development Program of China (no. 2012AA02A506).
Footnotes
Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors' Contributions
Conception and design: G. Cai, B. Zhou, S. Cai, L.X. Xu, W. Jia, S. Zheng, Y. Yen, W. Jia
Development of methodology: G. Cai, G. Xie, Y. Yen
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): Y. Qiu, G. Cai, B. Zhou, D. Li, A. Zhao, H. Li, D. Xie, W. Ge, Y. Yen, W. Jia
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): Y. Qiu, G. Cai, B. Zhou, G. Xie, Y. Yen, W. Jia
Writing, review, and/or revision of the manuscript: Y. Qiu, G. Xie, H. Li, Y. Yen, W. Jia
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): G. Cai, B. Zhou, A. Zhao, S. Cai, C. Huang, Z. Zhou, W. Jia, S. Zheng, Y. Yen, W. Jia
Study supervision: Y. Yen, W. Jia
References
- 1.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 3.Wellen KE, Thompson CB. Cellular metabolic stress: considering how cells respond to nutrient excess. Mol Cell. 2010;40:323–32. doi: 10.1016/j.molcel.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tennant DA, Duran RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer. 2010;10:267–77. doi: 10.1038/nrc2817. [DOI] [PubMed] [Google Scholar]
- 5.Denkert C, Budczies J, Weichert W, Wohlgemuth G, Scholz M, Kind T, et al. Metabolite profiling of human colon carcinoma–deregulation of tca cycle and amino acid turnover. Mol Cancer. 2008;7:72. doi: 10.1186/1476-4598-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirayama A, Kami K, Sugimoto M, Sugawara M, Toki N, Onozuka H, et al. Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res. 2009;69:4918–25. doi: 10.1158/0008-5472.CAN-08-4806. [DOI] [PubMed] [Google Scholar]
- 7.Beyoglu D, Imbeaud S, Maurhofer O, Bioulac-Sage P, Zucman-Rossi J, Dufour JF, et al. Tissue metabolomics of hepatocellular carcinoma: tumor energy metabolism and the role of transcriptomic classification. Hepatology. 2013;58:299–38. doi: 10.1002/hep.26350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocha CM, Barros AS, Gil AM, Goodfellow BJ, Humpfer E, Spraul M, et al. Metabolic profiling of human lung cancer tissue by 1h high resolution magic angle spinning (hrmas) nmr spectroscopy. J Proteome Res. 2010;9:319–32. doi: 10.1021/pr9006574. [DOI] [PubMed] [Google Scholar]
- 9.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 10.Calvert PM, Frucht H. The genetics of colorectal cancer. Ann Intern Med. 2002;137:603–12. doi: 10.7326/0003-4819-137-7-200210010-00012. [DOI] [PubMed] [Google Scholar]
- 11.Pan L, Qiu Y, Chen T, Lin J, Chi Y, Su M, et al. An optimized procedure for metabonomic analysis of rat liver tissue using gas chromatography/time-of-flight mass spectrometry. J Pharm Biomed Anal. 2010;52:589–96. doi: 10.1016/j.jpba.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 12.Qiu Y, Cai G, Su M, Chen T, Zheng X, Xu Y, et al. Serum metabolite profiling of human colorectal cancer using gc-tofms and uplc-qtofms. J Proteome Res. 2009;8:4844–50. doi: 10.1021/pr9004162. [DOI] [PubMed] [Google Scholar]
- 13.Cheng Y, Xie G, Chen T, Qiu Y, Zou X, Zheng M, et al. Distinct urinary metabolic profile of human colorectal cancer. J Proteome Res. 2012;11:1354–63. doi: 10.1021/pr201001a. [DOI] [PubMed] [Google Scholar]
- 14.Jansson J, Willing B, Lucio M, Fekete A, Dicksved J, Halfvarson J, et al. Metabolomics reveals metabolic biomarkers of crohn's disease. PLoS ONE. 2009;4:e6386. doi: 10.1371/journal.pone.0006386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–5. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–4. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 17.Giskeodegard GF, Grinde MT, Sitter B, Axelson DE, Lundgren S, Fjosne HE, et al. Multivariate modeling and prediction of breast cancer prognostic factors using mr metabolomics. J Proteome Res. 2010;9:972–9. doi: 10.1021/pr9008783. [DOI] [PubMed] [Google Scholar]
- 18.Chan EC, Koh PK, Mal M, Cheah PY, Eu KW, Backshall A, et al. Metabolic profiling of human colorectal cancer using high-resolution magic angle spinning nuclear magnetic resonance (hr-mas nmr) spectroscopy and gas chromatography mass spectrometry (gc/ms) J Proteome Res. 2009;8:352–61. doi: 10.1021/pr8006232. [DOI] [PubMed] [Google Scholar]
- 19.Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature. 2012;491:364–73. doi: 10.1038/nature11706. [DOI] [PubMed] [Google Scholar]
- 20.Pieters R, Hunger SP, Boos J, Rizzari C, Silverman L, Baruchel A, et al. L-asparaginase treatment in acute lymphoblastic leukemia: a focus on erwinia asparaginase. Cancer. 2011;117:238–49. doi: 10.1002/cncr.25489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–9. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 22.Piotto MM, Moussallieh FM, Dillmann B, Imperiale A, Neuville A, Brigand C, et al. Metabolic characterization of primary human colorectal cancers using high resolution magic angle spinning 1h magnetic resonance spectroscopy. Metabolomics. 2009;5:292–301. [Google Scholar]
- 23.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paton CM, Ntambi JM. Biochemical and physiological function of stearoyl-coa desaturase. Am J Physiol Endocrinol Metab. 2009;297:E28–37. doi: 10.1152/ajpendo.90897.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong BS, Senisterra G, Rabeh WM, Vedadi M, Leonardi R, Zhang YM, et al. Crystal structures of human pantothenate kinases. Insights into allosteric regulation and mutations linked to a neurodegeneration disorder. J Biol Chem. 2007;282:27984–93. doi: 10.1074/jbc.M701915200. [DOI] [PubMed] [Google Scholar]
- 26.Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881–96. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- 27.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–8. [PubMed] [Google Scholar]
- 28.Soga T, Baran R, Suematsu M, Ueno Y, Ikeda S, Sakurakawa T, et al. Differential metabolomics reveals ophthalmic acid as an oxidative stress biomarker indicating hepatic glutathione consumption. J Biol Chem. 2006;281:16768–76. doi: 10.1074/jbc.M601876200. [DOI] [PubMed] [Google Scholar]
- 29.Orlowski M, Wilk S. Synthesis of ophthalmic acid in liver and kidney in vivo. Biochem J. 1978;170:415–9. doi: 10.1042/bj1700415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fong MY, McDunn J, Kakar SS. Identification of metabolites in the normal ovary and their transformation in primary and metastatic ovarian cancer. PLoS ONE. 2011;6:e19963. doi: 10.1371/journal.pone.0019963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oakley AJ, Yamada T, Liu D, Coggan M, Clark AG, Board PG. The identification and structural characterization of c7orf24 as gamma-glutamyl cyclotransferase. An essential enzyme in the gamma-glutamyl cycle. J Biol Chem. 2008;283:22031–42. doi: 10.1074/jbc.M803623200. [DOI] [PubMed] [Google Scholar]
- 32.Gromov P, Gromova I, Friis E, Timmermans-Wielenga V, Rank F, Simon R, et al. Proteomic profiling of mammary carcinomas identifies c7orf24, a gamma-glutamyl cyclotransferase, as a potential cancer biomarker. J Proteome Res. 2010;9:3941–53. doi: 10.1021/pr100160u. [DOI] [PubMed] [Google Scholar]
- 33.Wiese AH, Auer J, Lassmann S, Nahrig J, Rosenberg R, Hofler H, et al. Identification of gene signatures for invasive colorectal tumor cells. Cancer Detect Prev. 2007;31:282–95. doi: 10.1016/j.cdp.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Miyanishi K, Takayama T, Ohi M, Hayashi T, Nobuoka A, Nakajima T, et al. Glutathione s-transferase-pi overexpression is closely associated with k-ras mutation during human colon carcinogenesis. Gastroenterology. 2001;121:865–74. doi: 10.1053/gast.2001.27982. [DOI] [PubMed] [Google Scholar]
- 35.Brand A, Leibfritz D, Richter-Landsberg C. Oxidative stress-induced metabolic alterations in rat brain astrocytes studied by multinuclear nmr spectroscopy. J Neurosci Res. 1999;58:576–85. [PubMed] [Google Scholar]
- 36.Terentis AC, Thomas SR, Takikawa O, Littlejohn TK, Truscott RJ, Armstrong RS, et al. The heme environment of recombinant human indoleamine 2,3-dioxygenase. Structural properties and substrate-ligand interactions. J Biol Chem. 2002;277:15788–94. doi: 10.1074/jbc.M200457200. [DOI] [PubMed] [Google Scholar]
- 37.Zamanakou M, Germenis AE, Karanikas V. Tumor immune escape mediated by indoleamine 2,3-dioxygenase. Immunol Lett. 2007;111:69–75. doi: 10.1016/j.imlet.2007.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.