SUMMARY
Setting
Haiti has the highest burden of tuberculosis (TB) in the Americas, with an estimated prevalence of 254/100,000 population. GHESKIO conducted active case finding (ACF) for TB at the household level in nine slums in Port-au-Prince.
Objective
We report on the prevalence of undiagnosed TB detected through GHESKIO’s ACF campaign.
Design
From August 1, 2014 to July 31, 2015, we conducted a retrospective cohort analysis using GHESKIO’s ACF campaign data. All individuals who reported chronic cough (cough ≥2 weeks) were tested for TB at GHESKIO, and those who were ≥10 years of age were included in the analyses.
Results
Of 104,097 individuals screened in the community, 5598 (5%) reported chronic cough and satisfied the study inclusion criteria. A total of 1110 (20%) were diagnosed with active TB disease (prevalence of 1066/100,000). Of the 5472 (98%) patients tested for HIV, 528 (10%) were HIV-positive; 143 (3%) patients were diagnosed with both diseases.
Conclusion
Household-level screening for cough, with TB and HIV testing for symptomatic patients, was a high-yield strategy, detecting a prevalence of undiagnosed disease exceeding national estimates by more than four-fold for TB, and by five-fold for HIV.
Keywords: Xpert MTB/RIF, tuberculosis, HIV, active case finding, Haiti
INTRODUCTION
Tuberculosis (TB) is a major cause of morbidity and mortality worldwide. In 2015, an estimated 10.4 million people developed active TB, but only 6.1 million new cases were reported to the World Health Organization (WHO).1 Major reasons for missed cases include insufficient knowledge of TB symptoms, delays in seeking care, limited effectiveness of passive case finding and smear microscopy, and lack of access to TB services.1,2 In TB-endemic countries, active case finding (ACF) has been recommended for those at highest risk of TB to reduce delays in diagnosis and treatment initiation, improve outcomes, and reduce transmission.3,4
The WHO conditionally recommends systematic screening for active TB for geographically-defined subpopulations with high levels of undetected TB (1% prevalence or higher), and for other subpopulations with poor access to health care, such as residents of urban slums.3 However, in many resource-limited settings, national guidelines still recommend health center-based TB case finding, and ACF has not been widely implemented in slums.5–9 Furthermore, though Xpert MTB/RIF testing (Cepheid, Sunnyvale, CA) has been shown to increase case detection rates in other populations, WHO does not provide specific recommendations regarding the use of Xpert MTB/RIF for ACF at the household level.8–12
Haiti is the poorest country in the Americas, and one of the poorest in the world.13 Haiti has the highest TB incidence in the region (194/100,000 population), with 16,431 new cases diagnosed in 2015 and an estimated case detection rate of 79%.1 The estimated HIV prevalence in Haiti is 1.7%; 16% of TB patients are co-infected with HIV.14 During the study period, 30,521 patients were diagnosed with HIV nationally at 195 HIV testing centers.15
In January of 2010, Haiti sustained a devastating earthquake with total damages estimated at 7.8 billion USD.16 About 1.5 million people lost their homes and were moved to internally-displaced persons camps for three or more years before being relocated to the Port-au-Prince slums, where they continue to suffer from crowding, poor nutritional status, and limited access to sanitation and health services.16 Post-earthquake, the number of reported TB cases increased nationally, which is attributed to a higher case detection rate, and/or a rise in TB burden post-earthquake.16
In 2011, the Haitian Group for the Study of Kaposi’s Sarcoma and Opportunistic Infections (GHESKIO) conducted a pilot project of ACF in a slum in Port-au-Prince, and found the prevalence of undiagnosed TB (1165/100,000) was nearly five-fold higher than national estimates.16 In 2013, an ACF project in the same slum found a TB prevalence of 1920/100,000.17 In 2014, GHESKIO received funding under Wave 4 of the TB REACH initiative of the WHO Stop TB Partnership to extend ACF to nine slums in Port-au-Prince.18 In this report, we describe the prevalence of TB disease and HIV co-infection in these communities; study findings will inform national TB policies.
STUDY POPULATION AND METHODS
Setting
GHESKIO is a Haitian non-governmental organization, and the largest provider of HIV and TB care in the Caribbean, treating up to 700 patients per day for HIV and/or TB. In 2015, GHESKIO diagnosed 2437 people with TB and 3115 with HIV, accounting for 15% and 10% of the national total, respectively (Table 1).15 All care is provided free of charge.
Table 1.
Largest Diagnostic Sites for Tuberculosis and HIV in Haiti
| Tuberculosis Testing Centers | HIV Testing Centers | ||
|---|---|---|---|
| Site Name | Number of TB Diagnoses* |
Site Name | Number of HIV Diagnoses |
| GHESKIO | 2437 | GHESKIO | 3115 |
| Sanatorium de Port-au-Prince | 643 | Centre Médical Charles Colimon | 1119 |
| CDS de Ka Soleil | 489 | Hôpital Dumarsais Estimé | 671 |
| Mennonite Croix des Bouquets | 426 | Hôpital de l'Université d'Etat d'Haiti | 642 |
| Grace Children’s Hospital | 364 | SSPE de Saint-Marc | 549 |
| Hôpital Immaculée Conception | 315 | Hôpital Universitaire de Mirebalais | 534 |
| Sanatorium de Sigueneau | 290 | ICC Grace Children's Hospital | 513 |
| Hôpital Nos Petits Freres et Soeurs | 273 | Hôpital Universitaire Justinien | 503 |
| Hôpital Sainte Therese | 267 | Hôpital Universitaire la Paix | 466 |
TB cases diagnosed during 2015; HIV cases diagnosed August 1, 2014 to July 31, 2015.
The TB REACH project was conducted in nine slum neighborhoods of Port-au-Prince (Figure 1), where socio-economic conditions are among the worst in the Americas. Most residents live in extreme poverty, enduring crowded conditions with limited access to basic sanitation and medical services.19,20 Approximately 130,000 people live in the slum neighborhoods where screening activities were conducted.21
Figure 1.
Map of the Nine Port-au-Prince, Haiti Slums Screened for Chronic Cough Through TB REACH
Key: 1-Delmas; 2-Cite Soleil; 3-La Saline; 4-Village de Dieu; 5-Cite Plus; 6-Cite Kabrit; 7-Cite l’Eternel; 8-Martissant; Not pictured: Carrefour
Active case finding for tuberculosis at the household level
The ACF campaign was conducted from August 1, 2014 to July 31, 2015. Eighty community health workers (CHWs), who were recruited from the catchment area, worked in teams of 8–10 persons per slum. CHWs went door-to-door in the neighborhoods to verbally administer a short questionnaire designed to collect socio-demographic information, TB history of individuals and households, and a household roster for each home screened, and to query individuals for chronic cough, defined as cough ≥2 weeks in duration. CHWs received monthly stipends, as well as phone cards and transportation fees to accompany and communicate with patients and GHESKIO staff. Prior to commencement of screening activities, CHWs participated in a two-week training, which included instruction on TB screening. Meetings were held with community, school, and religious leaders to answer questions regarding the ACF campaign and encourage residents to participate.
TB screening and diagnostic algorithm
Patients who reported chronic cough were referred to GHESKIO’s TB REACH clinic, which consisted of one nurse, one CHW, and one physician. A nurse recorded vital signs and a physician conducted a physical examination. Patients with physician-confirmed cough were referred for on-site same-day chest radiograph (CXR) and for sputum testing, regardless of CXR results. In accordance with National TB Program guidelines, smear microscopy was performed on a spot specimen, and Xpert MTB/RIF testing was performed on an early-morning specimen.22 Patients were taught how to expectorate sputum, and were instructed to return to GHESKIO the next day with their early morning specimen. Sputum testing was conducted off-site; specimens were refrigerated upon collection, packed into a TB specimen cooler, and transported to the centralized GHESKIO biosafety-level 3 laboratory across Port-au-Prince. Spot sputum specimens were stained by the Ziehl-Neelsen method and examined by microscopy for the presence of acid-fast bacilli. The Xpert MTB/RIF test was performed directly on clinical samples without prior extraction according to the manufacturer’s instructions. Approximately 1% of Xpert results were indeterminate; these specimens were re-tested. Patients were referred for blood draw the same day for HIV rapid antibody testing (Determine, Alere, Waltham, MA).
We defined a pulmonary TB case according to the WHO definition, which included bacteriologically-confirmed and clinically-diagnosed TB cases.23 TB treatment was initiated according to national guidelines.22 If the physician determined that the symptoms and CXR were highly suspicious for TB, same-day treatment was initiated without waiting for sputum test results. The remaining patients were given an appointment within 5 days; those with positive sputum smear or Xpert MTB/RIF tests initiated treatment on the day their results were provided, and others determined not to have TB were further evaluated and treated appropriately. Patients who did not present for TB testing, or who did not return with their early morning specimen, were phoned by a CHW; home visits were conducted for those not reachable by phone.
Data collection and analysis
Data from the household questionnaires were entered into a central electronic database. Individuals meeting these three criteria were included in the analyses: (1) reported chronic cough, (2) were tested at GHESKIO for TB and (3) were ≥10 years of age. De-identified data were exported to an Excel database (Microsoft, Redmond, WA). Data were then imported into Stata version 14 (StataCorp LP, College Station, Texas) for analysis. Children <10 years of age were excluded due to differences in presentation and diagnostic methods, compared with adults.
We calculated the prevalence of undiagnosed TB and HIV among coughing patients who received TB testing. We determined that TB and HIV were not diagnosed previously by self-report and by a review of GHESKIO’s EMR. We conducted multivariable logistic regression to determine predictors of TB and HIV, controlling for socio-demographic variables (Table 2). The number needed to screen (NNS) was calculated as the number of persons screened divided by the number of persons diagnosed with TB. The number needed to test (NNT) was calculated as the number of persons tested divided by the number of persons diagnosed with TB. The study was approved by Institutional Review Boards at all participating institutions.
Table 2.
Baseline Characteristics of Patients Tested for Tuberculosis, by HIV Status (N=5598)
| Variable | HIV-Positive (n=528) |
HIV-Negative (n=4899) |
HIV Missing or Indeterminate (n=171) |
Total (n=5598) |
|---|---|---|---|---|
| Female sex – no. (%) | 303 (57) | 2945 (60) | 118 (69) | 3366 (60) |
| Age (years) – no. (%) | ||||
| 10 to 17 years | 13 (2) | 548 (11) | 7 (4) | 568 (10) |
| 18 to 39 years | 331 (63) | 2669 (54) | 112 (65) | 3112 (56) |
| ≥40 years | 184 (35) | 1682 (34) | 52 (30) | 1918 (34) |
| Annual income <$US 150 – no. (%) | 350 (66) | 3489 (71) | 114 (67) | 3953 (71) |
| Education – no. (%) | ||||
| No school | 138 (26) | 914 (19) | 27 (16) | 1079 (19) |
| Primary school | 226 (43) | 1718 (35) | 56 (33) | 2000 (36) |
| Secondary school and higher | 161 (30) | 2254 (46) | 88 (51) | 2503 (45) |
| Baseline CD4 count – no. (%) | ||||
| <200 cells/mm3 | 124 (23) | |||
| 200 to 350 cells/mm3 | 73 (14) | |||
| 351 to 500 cells/mm3 | 67 (13) | |||
| >500 cells/mm3 | 89 (17) | |||
| Marital Status – no. (%) | ||||
| Single | 278 (53) | 2725 (56) | 84 (49) | 3087 (55) |
| Married/co-habiting | 200 (38) | 1911 (39) | 80 (47) | 2191 (39) |
| Previously married | 47 (9) | 250 (5) | 7 (4) | 304 (5) |
RESULTS
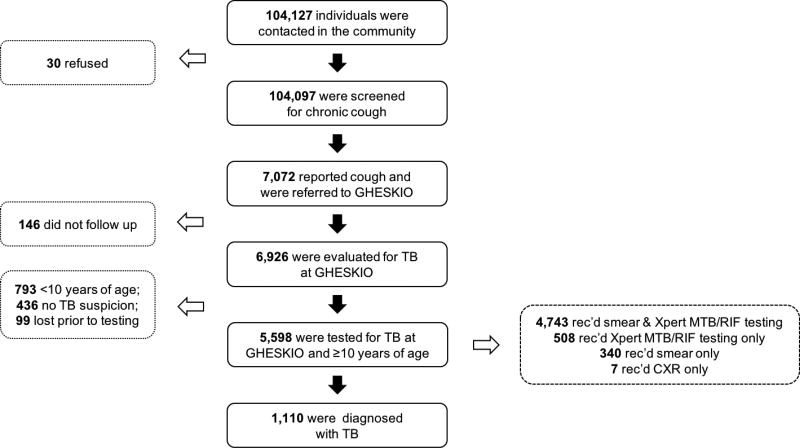
From August 1, 2014 to July 31, 2015, 104,127 residents were queried about participation in the ACF campaign, and 104,097 agreed to provide information, and were screened for chronic cough. Of these, 7072 (7%) reported chronic cough and were referred to GHESKIO, and 5598 (5%) satisfied the study inclusion criteria and were included in the analyses (Figure 2). Of the 1474 patients who were not included in the analyses, 146 never presented to GHESKIO for evaluation, 793 were <10 years of age, 436 did not have cough at the time of physician evaluation, and 99 were lost to care prior to receiving TB testing. Among the 5598 patients included in the analyses, the median age was 31 (interquartile range [IQR]: 22 to 46), 3366 (60%) were female, and 3079 (55%) had no school or primary school only (Table 2). HIV testing was completed for 5472 (98%) patients, and 528 (10%) were HIV-positive. Median CD4 count was 311 cells/mm3 (IQR: 128 to 503).
Figure 2.
Flow Chart Describing the Number of Patients Tested for Tuberculosis
Diagnostic yield of active case finding for tuberculosis
Among the 528 HIV-positive patients tested for TB, 143 (27%) were diagnosed with active disease. Of these, 114 (80%) were bacteriologically-confirmed, meaning they were smear and/or Xpert MTB/RIF positive (SS+/Bac+). Forty-four patients (39% of SS+/Bac+ cases) were smear-negative and Xpert MTB/RIF positive (Table 3).
Table 3.
Method of TB Diagnosis, by HIV Status (n=1110)
| Method of TB Diagnosis | HIV-Positive | HIV- Negative |
HIV Missing or Indeterminate |
Total |
|---|---|---|---|---|
| Smear and Xpert MTB/RIF positive – no. (%) | 53 (37) | 571 (61) | 12 (38) | 636 (57) |
| Smear negative and Xpert MTB/RIF positive – no. (%) | 44 (31) | 203 (22) | 7 (22) | 254 (23) |
| Smear positive and Xpert MTB/RIF negative – no. (%) | 0 (0) | 29 (3) | 1 (3) | 30 (3) |
| Smear positive; Xpert MTB/RIF not done – no. (%) | 5 (3) | 23 (2) | 0 (0) | 28 (3) |
| Xpert MTB/RIF positive; smear not done – no. (%) | 12 (8) | 35 (4) | 5 (16) | 52 (5) |
| Clinically diagnosed – no. (%) | 29 (20) | 74 (8) | 7 (22) | 110 (10) |
| Total TB Cases Diagnosed | 143 | 935 | 32 | 1110 |
Of the 4899 HIV-negative patients tested for TB, 935 (19%) were diagnosed with active disease. Of these, 861 (92%) were bacteriologically-confirmed. Two hundred three patients (24% of SS+/Bac+ cases) were smear-negative and Xpert MTB/RIF positive. An additional 32 of 171 (19%) patients with indeterminate or unknown HIV status were diagnosed with TB; 25 (78%) had bacteriologically-confirmed disease (Table 3).
Out of the 5598 patients tested for TB, 1110 (20%) were diagnosed with active disease. Of these, 1000 (90%) were bacteriologically-confirmed. Two hundred fifty-four patients (25% of SS+/Bac+ cases) were smear-negative and Xpert MTB/RIF positive. Rifampin resistance was detected in 16 (1.4%) patients who were diagnosed with TB. Out of the population queried for cough, the overall prevalence of undiagnosed TB was 1.1%; the NNS was 94 and the NNT was five for every patient diagnosed with TB.
Predictors of Tuberculosis and HIV
In multivariable analysis, predictors of TB included HIV infection (OR: 1.49; 95% CI: 1.20–1.85), male sex (OR: 2.34; 95% CI: 2.04–2.69), and single marital status (OR: 1.21; 95% CI: 1.04–1.42). TB was less likely among individuals from 10 to 17 (OR: 0.40; 95% CI: 0.30–0.53) and over 39 years of age (OR: 0.46; 95% CI: 0.39–0.56) (Table 4). Predictors of HIV infection included male sex (OR: 1.14; 95% CI: 1.04–1.26), and being single (OR 1.12; 95% CI: 1.01–1.25) or previously married (OR 1.45; 95% CI: 1.20–1.76). HIV infection was less likely among individuals from 10 to 17 (OR: 0.43; 95% CI: 0.34–0.55) and over 39 years of age (OR: 0.76; 95% CI: 0.68–0.86), and among those with at least some secondary education (OR: 0.61; 95% CI: 0.53–0.70) (Table 5).
Table 4.
Predictors of Tuberculosis by Multivariable Logistic Regression Analysis
| Variable | Reference Category | aOR with 95% CI* | p-value |
|---|---|---|---|
| Positive HIV status | Negative HIV status | 1.49 (1.20–1.85) | <0.001 |
| Male sex | Female sex | 2.34 (2.04–2.69) | <0.001 |
| Age | |||
| Age 10–17 years | Age 18–39 years | 0.40 (0.30–0.53) | <0.001 |
| Age 40 years and over | Age 18–39 years | 0.46 (0.39–0.56) | <0.001 |
| Education | |||
| Primary school | No school | 0.99 (0.80–1.23) | 0.938 |
| At least some secondary school | No school | 0.87 (0.70–1.08) | 0.206 |
| Income >$US 150 per year | Income ≤$US 150/Year | 1.06 (0.90–1.24) | 0.495 |
| Marital status | |||
| Previously married | Currently married | 0.66 (0.43–1.00) | 0.051 |
| Single | Currently married | 1.21 (1.04–1.42) | 0.016 |
Adjusted odds ratio with 95% confidence intervals
Table 5.
Predictors of HIV by Multivariable Logistic Regression Analysis
| Variable | Reference Category | aOR with 95% CI | p-value |
|---|---|---|---|
| Male sex | Female sex | 1.14 (1.04–1.26) | 0.008 |
| Age | |||
| Age 10–17 years | Age 18–39 years | 0.43 (0.34–0.55) | <0.001 |
| 40 years and over | Age 18–39 years | 0.76 (0.70–0.86) | <0.001 |
| Education | |||
| Primary school | No school | 0.93 (0.81–1.05) | 0.235 |
| At least some secondary school | No school | 0.61 (0.53–0.70) | <0.001 |
| Income >$US 150 per year | Income ≤$US 150/Year | 1.06 (0.96–1.18) | 0.264 |
| Marital status | |||
| Previously married | Currently married | 1.45 (1.20–1.76) | <0.001 |
| Single | Currently married | 1.12 (1.01–1.25) | 0.031 |
Adjusted odds ratio with 95% confidence intervals
DISCUSSION
We detected a high prevalence of TB (1066/100,000) among individuals screened for chronic cough in the slums of Port-au-Prince, indicating that these slums are hot spots for TB transmission. This TB prevalence is over four-fold higher than national estimates (244/100,000) and 30-fold higher than the Americas region (36/100,000).1 More cases of TB were diagnosed through this ACF project than in all but one of Haiti’s 261 TB clinics. The actual burden of TB in these neighborhoods is likely even higher than we detected, because cough was used as an indicator for testing rather than other TB symptoms or radiographic abnormalities.3,24,25 It is possible that the burden of TB has increased after the devastating earthquake of 2010, but there are no pre-earthquake data for comparison. This project was only feasible due to TB REACH funding – additional funding is necessary to expand ACF activities to other slums in Port-au-Prince.
ACF studies conducted in slums in Brazil, Uganda, and Bangladesh have also found a prevalence of undetected TB that far exceeds national estimates, providing further evidence of the importance of ACF in slums.5–7 Future ACF interventions may be most effective if Xpert MTB-RIF is used as the first-line TB test, due to higher rates of bacteriologic confirmation of TB with this strategy. We found that among SS+/Bac+ cases, 39% of HIV-positive and 25% of HIV-negative patients were smear-negative but Xpert MTB-RIF positive. This has been demonstrated in other studies as well.8–12,26
We found an HIV prevalence of 10% among patients who reported cough, which is over five-fold higher than national prevalence estimate.14 Using limited additional resources, more patients were diagnosed with HIV through this ACF project than in 189 of Haiti’s 195 HIV testing centers.15 Other studies have also found high rates of HIV among patients with TB symptoms, underscoring the importance of combined HIV and TB testing in countries with high burdens of both diseases, as recommended by the WHO.3,27
The likelihood of TB infection was higher among individuals infected with HIV, and of male gender, single status, and ages 18 to 39 years. Similarly, the likelihood of HIV infection was higher among males and individuals of single status or history of being previously married. These results are consistent with those of other studies which show that males have higher rates of TB.1,28–30 Multiple studies have also demonstrated that males are more likely to present with advanced AIDS, highlighting the importance of household level ACF strategies for earlier HIV diagnosis.31–33
We attribute the high participation rate in this study to the employment of CHWs from the affected communities, which helped to establish early rapport with residents and community leaders.34 Educational activities also increased awareness of signs of active disease. Nearly all patients who reported cough to the CHWs were confirmed to have cough by a physician, and the few who presented without cough were offered HIV testing and/or referred for other free services within GHESKIO.
This study was conducted in slum neighborhoods, which may limit the generalizability of the findings to other settings. This study is also limited by our inability to measure the averted delay in diagnosis and impact of ACF on TB transmission, and to determine whether those diagnosed with TB would have presented for testing without ACF.35 In addition, we screened only for chronic cough. This likely resulted in the detection of the most highly infectious patients; however, due to the low sensitivity of cough and potential bias of self-report we may under-estimate the true prevalence of undiagnosed disease.24,25 Furthermore, we did not screen for extra-pulmonary TB; such patients may have a higher rate of mortality. Future directions for research include a comparison of outcomes between patients diagnosed through active and passive case finding, and a cost-effectiveness analysis of ACF for TB at the household level in urban slums.
CONCLUSIONS
The prevalence of TB and HIV detected through ACF in slums of Port-au-Prince, Haiti, were four-fold and five-fold higher than national estimates, respectively. By conducting ACF for both diseases at the household level, we diagnosed more TB than 99% of TB clinics and more HIV than 97% of HIV testing centers in Haiti. The use of Xpert MTB/RIF testing increased the proportion of patients with bacteriologically-confirmed TB. If active case finding for TB and HIV were expanded to other slum populations in Haiti as part of routine programmatic activities, long-term benefits could potentially include an increase in TB case detection rate, and a decrease in TB transmission.
References
- 1.Global Tuberculosis Report, 2016. World Health Organization; Geneva: 2016. [Accessed May 1, 2017]. at: http://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 2.Lonnroth K, Corbett E, Golub J, et al. Systematic screening for active tuberculosis: rationale, definitions and key considerations. Int J Tuberc Lung Dis. 2013;17(3):289–298. doi: 10.5588/ijtld.12.0797. [DOI] [PubMed] [Google Scholar]
- 3.Systematic Screening for Active Tuberculosis - Principles and Recommendations. World Health Organization; 2013. [Accessed on May 1, 2017]. at: http://apps.who.int/iris/bitstream/10665/84971/1/9789241548601_eng.pdf?ua=1. [PubMed] [Google Scholar]
- 4.Uplekar M, Creswell J, Ottmani SE, Weil D, Sahu S, Lonnroth K. Programmatic approaches to screening for active tuberculosis. Int J Tuberc Lung Dis. 2013;17(10):1248–1256. doi: 10.5588/ijtld.13.0199. [DOI] [PubMed] [Google Scholar]
- 5.Miller AC, Golub JE, Cavalcante SC, et al. Controlled trial of active tuberculosis case finding in a Brazilian favela. Int J Tuberc Lung Dis. 2010;14(6):720–726. [PMC free article] [PubMed] [Google Scholar]
- 6.Sekandi JN, List J, Luzze H, et al. Yield of undetected tuberculosis and human immunodeficiency virus coinfection from active case finding in urban Uganda. Int J Tuberc Lung Dis. 2014;18(1):13–19. doi: 10.5588/ijtld.13.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banu S, Rahman MT, Uddin MK, et al. Epidemiology of tuberculosis in an urban slum of Dhaka City, Bangladesh. PLOS ONE. 2013;8(10):e77721. doi: 10.1371/journal.pone.0077721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khanal S, Baral S, Shrestha P, et al. Yield of intensified tuberculosis case-finding activities using Xpert((R)) MTB/RIF among risk groups in Nepal. Public Health Action. 2016;6(2):136–141. doi: 10.5588/pha.16.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorent N, Choun K, Thai S, et al. Community-based active tuberculosis case finding in poor urban settlements of Phnom Penh, Cambodia: a feasible and effective strategy. PLOS ONE. 2014;9(3):e92754. doi: 10.1371/journal.pone.0092754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Churchyard GJ, Stevens WS, Mametja LD, et al. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet Glob Health. 2015;3(8):e450–457. doi: 10.1016/S2214-109X(15)00100-X. [DOI] [PubMed] [Google Scholar]
- 11.Theron G, Zijenah L, Chanda D, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet. 2014;383(9915):424–435. doi: 10.1016/S0140-6736(13)62073-5. [DOI] [PubMed] [Google Scholar]
- 12.Dierberg KL, Dorjee K, Salvo F, et al. Improved Detection of Tuberculosis and Multidrug-Resistant Tuberculosis among Tibetan Refugees, India. Emerg Infect Dis. 2016;22(3):463–468. doi: 10.3201/eid2203.140732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.International Human Development Indicators, Haiti Country Profile. United Nations Development Program; [Accessed May 1, 2017]. at: http://hdr.undp.org/en/countries/profiles/HTI. [Google Scholar]
- 14. [Accessed May 1, 2017];UNAIDS - Haiti profile. at: http://www.unaids.org/en/regionscountries/countries/haiti.
- 15.Monitoring, Evaluation, and Surveillance Interface. [Accessed May 1, 2017];Haiti. at: http://www.mesi.ht.
- 16.Koenig SP, Rouzier V, Vilbrun SC, et al. Tuberculosis in the aftermath of the 2010 earthquake in Haiti. Bull World Health Org. 2015;93(7):498–502. doi: 10.2471/BLT.14.145649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masur J, Koenig SP, Julma P, Ocheretina O, Duran-Mendicuti M, Fitzgerald DW, Pape JW. Active tuberculosis case finding in Haiti. Am J Trop Med Hyg. 2017 doi: 10.4269/ajtmh.16-0674. published online ahead of in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. [Accessed May 1, 2017];Improving Tuberculosis Case Detection: A compendium of TB REACH case studies, lessons learned and a monitoring and evaluation framework. at: http://www.stoptb.org/assets/documents/reouserces/publications/technival/TB_Case_Studies.pdf.
- 19.Port-au-Prince urban baseline - an assessment of food and livelihood security in Port-au-Prince. USAID; 2009. [Accessed May 1, 2017]. at: http://www.fews.net/sites/default/files/documents/reports/ht_baseline_urban_Port au Prince_en.pdf. [Google Scholar]
- 20.Rouzier V, Severe K, Juste MA, et al. Cholera vaccination in urban Haiti. Am J Trop Med Hyg. 2013;89(4):671–681. doi: 10.4269/ajtmh.13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pape JW, Severe PD, Fitzgerald DW, et al. The Haiti research-based model of international public health collaboration: the GHESKIO Centers. J Acquir Immune Defic Syndr. 2014;65(Suppl 1):S5–9. doi: 10.1097/QAI.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ministère de la Santè Publique et de la population (MSPP) Programme National de Luette contre la Tuberculose (PNLT): Manuel de Normes de la Tuberculose en Haiti. Port-au-Prince, Haiti: MSPP; 2013. [Google Scholar]
- 23.Definitions and Reporting Framework for Tuberculosis - Updated December 2014. World Health Organization; [Accessed May 1, 2017]. at: http://apps.who.int/iris/bitstream/10665/79199/1/9789241505345_eng.pdf?ua) [Google Scholar]
- 24.Van't Hoog AH, Onozaki I, Lonnroth K. Choosing algorithms for TB screening: a modelling study to compare yield, predictive value and diagnostic burden. BMC Infect Dis. 2014;14:532. doi: 10.1186/1471-2334-14-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Systematic Screening for Active Tuberculosis: Principles and Recommendations. [Accessed May 1, 2017];Review 2: A Systematic Review of the Sensitivity and Specificity of Symptom- and Chest-Radiography Screening for Active Pulmonary Tuberculosis in HIV-Negative Persons and Persons with Unknown HIV Status. at: http://www.who.int/tb/Review2Accuracyofscreeningtests.pdf?ua=1.
- 26.Calligaro GL, Zijenah LS, Peter JG, et al. Effect of new tuberculosis diagnostic technologies on community-based intensified case finding: a multicentre randomised controlled trial. Lancet Infect Dis. 2017;17(4):441–450. doi: 10.1016/S1473-3099(16)30384-X. [DOI] [PubMed] [Google Scholar]
- 27.Ayles H, Schaap A, Nota A, et al. Prevalence of tuberculosis, HIV and respiratory symptoms in two Zambian communities: implications for tuberculosis control in the era of HIV. PLOS ONE. 2009;4(5):e5602. doi: 10.1371/journal.pone.0005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss MG, Sommerfeld J, Uplekar MW. Social and cultural dimensions of gender and tuberculosis. Int J Tuberc Lung Dis. 2008;12(7):829–830. [PubMed] [Google Scholar]
- 29.Neyrolles O, Quintana-Murci L. Sexual inequality in tuberculosis. PLOS Med. 2009;6(12):e1000199. doi: 10.1371/journal.pmed.1000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLaren ZM, Brouwer E, Ederer D, Fischer K, Branson N. Gender patterns of tuberculosis testing and disease in South Africa. Int J Tuberc Lung Dis. 2015;19(1):104–110. doi: 10.5588/ijtld.14.0212. [DOI] [PubMed] [Google Scholar]
- 31.Cornell M, Schomaker M, Garone DB, et al. Gender differences in survival among adult patients starting antiretroviral therapy in South Africa: a multicentre cohort study. PLOS Med. 2012;9(9):e1001304. doi: 10.1371/journal.pmed.1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawkins C, Chalamilla G, Okuma J, et al. Sex differences in antiretroviral treatment outcomes among HIV-infected adults in an urban Tanzanian setting. AIDS. 2011;25(9):1189–1197. doi: 10.1097/QAD.0b013e3283471deb. [DOI] [PubMed] [Google Scholar]
- 33.Mills EJ, Bakanda C, Birungi J, et al. Male gender predicts mortality in a large cohort of patients receiving antiretroviral therapy in Uganda. J Int AIDS Soc. 2011;14:52. doi: 10.1186/1758-2652-14-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Systematic Screening for Active Tuberculosis: Principles and Recommendations. [Accessed May 1, 2017];Review 4b: Acceptability of Household and Community-Based TB Screening in High Burden Communities. at: http://www.who.int/tb/Review4bAacceptabilityHousehold_CommunityScreening.pdf?ua=1.
- 35.Kranzer K, Afnan-Holmes H, Tomlin K, et al. The benefits to communities and individuals of screening for active tuberculosis disease: a systematic review. Int J Tuberc Lung Dis. 2013;17(4):432–446. doi: 10.5588/ijtld.12.0743. [DOI] [PubMed] [Google Scholar]