Abstract
Objective
Diastolic dysfunction is common in hypertrophic cardiomyopathy (HCM) and hypertensive heart disease (HHD), but its relationships with left ventricular (LV) parameters have not been well studied. Our objective was to assess the relationship of various measures of diastolic function, and maximum left ventricular wall thickness (MLVWT) and left ventricular mass index (LVMI) in HCM, HHD and normal controls using cardiac magnetic resonance imaging (CMR). We also assessed LV parameters and diastolic function in relation to late gadolinium enhancement (LGE) and right ventricular (RV) hypertrophy in HCM.
Methods
41 patients with HCM, 21 patients with HHD and 20 controls were studied. Peak filling rate (PFR), time to peak filling (TPF), MLVWT and LVMI were measured using CMR. LGE and RV morphology were assessed in HCM patients.
Results
MLVWT correlated with TPF in HCM (r = 0.38; p = 0.02), HHD (r = 0.58; p = 0.01) and controls (r = 0.54; p = 0.01); correlation between MLVWT and TPF was weaker in HCM than HHD. LVMI did not correlate with diastolic function. In HCM, LGE extent correlated with MLVWT (τ = 0.41; p = 0.002) and with TPF (τ = 0.29; p = 0.02). The HCM patients with RV hypertrophy had higher MLVWT (p < 0.001) and TPF (p = 0.03) than patients without RV hypertrophy.
Conclusion
MLVWT correlates with diastolic function (TPF) in HCM, HHD and controls. LVMI did not show significant correlation with TPF. The diastolic dysfunction in HCM is not entirely explained by wall thickening. LGE and RV involvement are associated with worse LV diastolic function, suggesting that these may be markers of more severe underlying myocardial disarray and fibrosis that contribute to diastolic dysfunction.
Keywords: Diastolic function, Hypertension, Hypertrophic cardiomyopathy, Left ventricle, Magnetic resonance imaging
1. Introduction
Diastolic dysfunction indicates abnormal mechanical properties of the myocardium and includes slow or delayed myocardial relaxation, abnormal left ventricular distensibility and impaired left ventricular filling.1 Diastolic dysfunction is one of the early manifestations in hypertrophic cardiomyopathy (HCM) and hypertensive heart disease (HHD), which could be subclinical. Diastolic dysfunction with preserved ejection fraction accounts for approximately 50% of patients with heart failure2 with substantial morbidity and mortality. Accurate non- invasive assessment of diastolic function could be helpful in elucidating pathophysiology, predicting adverse outcome, patient monitoring, and assessing treatment response. For these reasons, there exist several measures of left ventricular diastolic function that are used in clinical practice and research. Cardiac magnetic resonance imaging (CMR) provides excellent assessment of maximum left ventricular wall thickness (MLVWT), left ventricular mass (LVM) and focal fibrosis shown as late gadolinium enhancement (LGE). Recent advances in image-processing software have facilitated measurements of left ventricular (LV) filling parameters and diastolic function. However, the relationships between diastolic function and left ventricular structural parameters in HCM and HHD have not been well studied. Therefore, the objective of our study was to assess the relationship between various measures of diastolic function, and MLVWT and left ventricular mass index (LVMI) in HCM, HHD and normal controls. We also assessed diastolic function in relation to LGE and right ventricular (RV) hypertrophy in HCM.
2. Methods
2.1. Study population
This is a retrospective study of 41 patients with HCM, 21 patients with HHD, and 20 control subjects. The study population were from cardiac MRI database of our tertiary care hospital during the time period January 2006 to November 2013. The study protocol was approved by our institutional research ethics board (Medical Research Ethics Committee number #12-389). All the patients in the HCM group had a confirmed diagnosis of HCM based on CMR morphological features, in addition to other clinical information (previous echocardiography, ECG, or family history). CMR diagnosis of HCM was based on the definition of LV wall thickness ≥15 mm at end-diastole or septal to lateral wall thickness ratio higher than 1.3 in a non-dilated LV, in the absence of a loading condition sufficient to cause the observed abnormality or a ratio between apical to basal LV wall thicknesses ≥1.3.3 Genetic mutations were not assessed. All patients in the HHD group had hypertension diagnosed by the treating physicians. Those with coexisting severe chronic kidney disease in the HHD group did not undergo late enhancement imaging. Control subjects consisted of patients with normal CMR (including late enhancement imaging) and no history of hypertension, heart failure, angina, myocardial infarction, coronary revascularization or cardiomyopathy. Subjects in the control were referred for CMR by cardiologists to exclude structural heart disease for symptoms such as palpitations, syncope, or family history of sudden cardiac death. All the patients were in sinus rhythm and had left ventricular ejection fraction ≥50%. Patients with other coexisting diseases (e.g. pericardial disease, significant primary valvular disease) and patients with suspected amyloidosis (based on other clinical findings) and any CMR findings suggestive of cardiac amyloidosis (e.g. abnormal gadolinium kinetics and diffuse subendocardial LGE) which could potentially affect diastolic filling parameters were excluded from our study.
2.2. Cardiac magnetic resonance imaging
All the scans were performed with a 1.5 T MR scanner (Intera, Philips Medical systems). The imaging protocol consisted of standard 2-chamber, 3-chamber, 4-chamber and short-axis steady state free precession (SSFP) cine imaging and LGE imaging. Contiguous short axis SSFP cine images were obtained from apex to base of left ventricle with 8 mm slice thickness (no interslice gap), 3.6–3.9/1.9 ms TR/TE, 60° flip angle, 11–16 turbofactor, 28–34 × 28–34 cm field of view, 256 × 256 acquisition matrix and 25 phases. The temporal resolution was approximately 50 ms. LGE imaging was performed 8–15 min after intravenous gadolinium-DTPA (Magnevist, Bayer) or gadobenate dimeglumine (Multihance, Bracco) contrast administration (0.2 mmol/kg). Inversion recovery prepared breath hold images were obtained with 8 mm slice thickness, 3.8/1.3 ms TR/TE, 15° flip angle,180 × 160 acquisition matrix and 28–34 × 28–34 cm field of view.
2.3. Image analysis
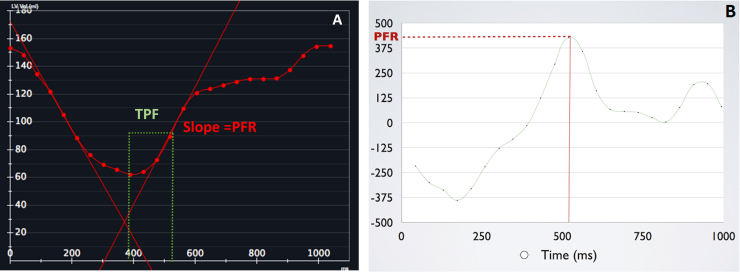
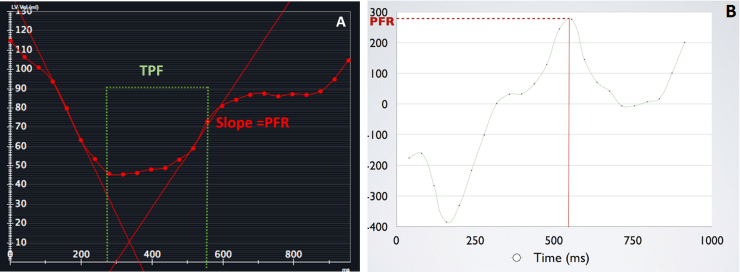
Semi-automated endocardial contouring of the left ventricular slices from apex to base was performed using commercially available software (CVi 42; Circle Cardiovascular Imaging) to generate left ventricular time-volume curve (TVC) and its first derivative curve to obtain early peak filling rate (PFR), PFR/end-diastolic volume (PFR/EDV) and time to early peak filling rate (TPF) for assessment of diastolic function.4, 5 A radiologist with two years of dedicated cardiac radiology experience reviewed all contours and revised them if required. TVC (Fig. 1A) is a graphic representation of the change in the LV volume during cardiac cycle plotted against time. The first derivative of TVC (Fig. 1B) is a graphic representation of the instantaneous filling rates during cardiac cycle plotted against time. The PFR is the maximal change in LV volume per unit time, which is the highest positive slope in the volume curve (Fig. 1A and B). PFR occurs during early ventricular diastole. Time to peak filling rate is the time interval from end systole to peak filling rate (Fig. 1A and B). Diastolic dysfunction is characterized by decreased peak filling rate and prolonged time to peak filling rate (Fig. 2A and B).
Fig. 1.
Time-volume curve (A) and its first derivative curve (B) of a control subject. These demonstrate peak filling rate and time to peak filling rate. PFR is the highest upward slope of TVC. TPF is the time interval between end systole and PFR.
Fig. 2.
TVC (A) and its first derivative curve (B) of a patient with hypertrophic cardiomyopathy and diastolic dysfunction. The PFR is reduced and TPF is prolonged as compared to control (1A and B).
For calculation of LVMI, epicardial contours were semi-automatically drawn from the apex to basal short axis SSFP images in end diastole. The LVM was calculated by multiplying the volume of myocardium measured by CMR with the specific gravity of the myocardium 1.05 g/ml,4 and LVMI was indexed to body surface area. Papillary muscles were not included in LVM measurement. We reviewed the short axis and long axis end diastole SSFP images and its imaging planes to identify the optimal plane and segment with maximal wall thickness, and manually measured MLVWT.
LGE was categorized according to the severity and extent of involvement on an arbitrary scale of 0 to 3. The absence of LGE was scored as 0. LGE involving an equivalent of one or less than one myocardial segment (based on the LV 17-segment model), 2–3 segments, and more than 3 segments were scored as 1, 2, and 3, respectively.
RV morphology of HCM patients was evaluated by reviewing the end-diastolic short axis and 4-chamber cine SSFP images to determine the presence of RV wall thickening (more than 5 mm) and the regional distribution of RV wall thickening. In addition, left atrial area was measured on 4-chamber cine SSFP images and mid longitudinal left atrial diameter was measured on 2-chamber cine SSFP images during LV end-systole.
2.4. Statistical analysis
Continuous variables are shown as median with interquartile range. Differences between the HCM, HHD and control groups were analyzed using Kruskal-Wallis test. Further, pairwise comparisons of relevant parameters were performed using Mann-Whitney test, with correction for multiple comparisons by the Hochberg method. Relationships between variables of interest were examined by scatterplots. The non-parametric Spearman’s correlation coefficients (r) were calculated to examine the relationships between MLVWT, LVMI, LGE, and parameters of diastolic function, without assuming normality and linearity. In addition, we calculated the non-parametric Kendall tau-b correlation coefficient (τ) since there were ties for LGE (a 4-level ordinal variable). Statistical analysis was performed using SPSS 21.0 (IBM). P value of less than 0.05 was considered significant.
3. Results
3.1. Demographic characteristics
Table 1 summarizes the demographic characteristics of HCM, HHD and control groups. Age, gender and body surface area did not differ between HCM, HHD and control groups.
Table 1.
Demographics, left ventricular and left atrial parameters of the subjects.
| Hypertrophic cardiomyopathy (n = 41) | Hypertensive heart disease (n = 21) | Control (n = 20) | p value | |
|---|---|---|---|---|
| Age(years) | 49(45–57) | 57(47–61) | 52(44–56) | 0.31 |
| Males | 34(82.9%) | 18(85.7%) | 14(70%) | 0.38 |
| Body Surface Area(m2) | 1.96(1.75–2.20) | 1.93(1.79–2.05) | 1.93(1.79–2.06) | 0.71 |
| Heart Rate(bpm) | 65(61–68) | 69(63–77) | 64(58–77) | 0.23 |
| LVEDVI(ml/m2) | 80(74–87) | 90(73–101) | 83(75–91) | 0.21 |
| LVESVI(ml/m2) | 29(25–34) | 34(28–40) | 33(28–37) | 0.23 |
| LVEF(%) | 64(59–67) | 62(60–64) | 62(59–64) | 0.45 |
| LVM(g) | 173(136–204) | 161(148–193) | 110(101–132) | <0.001 |
| LVMI(g/m2) | 88(75–101) | 85(81–93) | 60(52–66) | <0.001 |
| MLVWT(mm) | 17(14–20) | 13(12–15) | 9(7–10) | <0.001 |
| PFR(ml/s) | 414(349–536) | 395(356–528) | 445(372–532) | 0.87 |
| PFR/EDV(/s) | 2.67(2.28–3.26) | 2.68(2.24–3.05) | 2.90(2.60–3.16) | 0.82 |
| TPF(ms) | 173(147–203) | 172(164–219) | 151(136–163) | 0.01 |
| LA area(cm2) | 29(26–33) | 25(20–28) | 25(23–29) | 0.003 |
| Longitudinal LA(mm) | 51 (48–54) | 58(53–61) | 47(43–52) | <0.001 |
Data are shown as median (interquartile range).
bpm, beats per minute; LVEDVI, left ventricular end-diastolic volume index; LVESI, left ventricular end-systolic volume index; LVEF, left ventricular ejection fraction; LVM, left ventricular mass; LVMI, left ventricular mass index; MLVWT, maximum left ventricular wall thickness; PFR, early peak filling rate; PFR/EDV, PFR/end-diastolic volume; TPF, time to PFR; LA, left atrium.
3.2. LV structure and function
In HCM patients, the morphologic phenotype was asymmetric septal hypertrophy in 21 patients (51%), apical hypertrophy in 17 patients (41%) and mid ventricular hypertrophy in 3 patients (7%). Four patients in the HCM group had systolic anterior motion of anterior mitral valve leaflet, secondary mitral regurgitation and significant left ventricular outflow tract obstruction. 26.8% of HCM group were on beta blockers and 7.3% were on calcium channel blockers. The majority of the patients (62% and 76%, respectively) in the HHD group had LVMI above the normal range (>81 g/m2 for males and >79 g/m2 for females) and maximum left ventricular wall thickness of ≥12 mm, with a concentric hypertrophy pattern. In the HHD group, 38.1% were on diuretic(s), 38.1% were on beta blocker, 28.6% were on calcium channel blocker, 28.6% were on angiotensin converting enzyme inhibitor, and 19.0% were on angiotensin receptor blocker; 57% were on 2 or more medications.
Table 1 summarizes the LV structural and functional parameters of the subjects in HCM, HHD and control groups. There were significant differences in MLVWT, LVM and LVMI between the three groups (p < 0.001). On pairwise comparisons, MLVWT was significantly higher in HCM as compared to the other two groups (p < 0.001). MLVWT in the HHD group was significantly higher than in the control group (p < 0.001). LVM and LVMI were significantly higher in HCM (p < 0.001) and HHD (p < 0.001) as compared to the control group, but not significantly different between HCM and HHD (p = 0.41 and p = 0.94).
There were significant differences in TPF (p = 0.01) between the three groups, with significantly more prolonged TPF in HCM (p = 0.01) and HHD (p = 0.004) as compared to the control group. However, there was no significant difference in TPF between the HCM and HHD groups (p = 0.38). PFR/EDV also did not differ between the groups (p = 0.82).
3.3. Relationship between MLVWT, LVM, LGE, and diastolic function
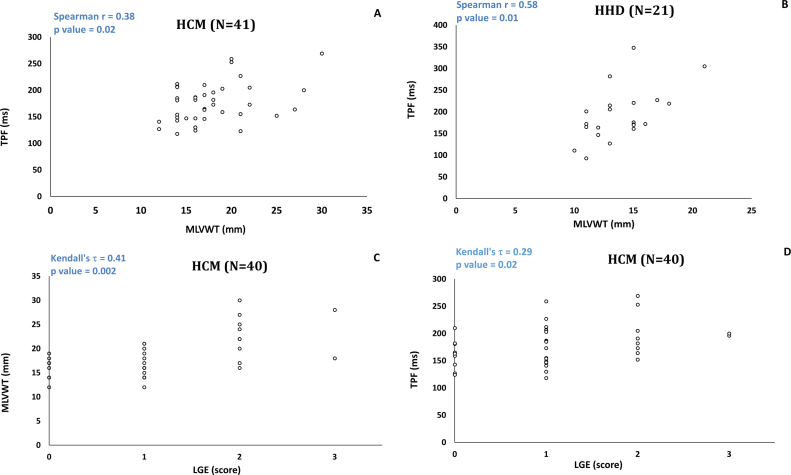
Fig. 3A demonstrates the relationship of MLVWT and diastolic function in HCM in a scatterplot. The MLVWT showed weak to moderate positive correlation with TPF (r = 0.38; p = 0.02) and weak to moderate negative correlation with PFR/EDV (r = −0.37; p = 0.02). Fig. 3B demonstrates the relationship between MLVWT and diastolic function in HHD. There was moderate positive correlation between MLVWT and TPF (r = 0.58; p = 0.01). There was no significant correlation between MLVWT and PFR/EDV. There was no significant correlation between LVMI and diastolic function in the HCM or HHD group.
Fig. 3.
Scatterplot demonstrating a weak to moderate correlation between MLVWT and TPF in HCM group (A). Scatterplot demonstrating a moderate to strong correlation between MLVWT and TPF in HHD group (B). Scatterplot demonstrating a moderate correlation between LGE and MLVWT in HCM group (C). Scatterplot demonstrating a weak to moderate correlation between LGE and TPF in HCM group (D).
In the control group, the MLVWT showed moderate positive correlation with TPF (r = 0.54; p = 0.01) and moderate negative correlation with PFR/EDV (r = −0.49; p = 0.03) There was no significant correlation between LVMI and diastolic function in the control group.
LGE was present in 31 of 40 (76%) patients with HCM. One of the HCM patients did not have LGE imaging as the scan was prematurely terminated due to patient discomfort. LGE was patchy midwall and at the RV insertion points in the septum. 3 patients had isolated enhancement of the RV insertion points in the septum. There was moderate correlation between LGE and MLVWT (τ = 0.41, p = 0.002) (Fig. 3C) and weak to moderate correlation between LGE and LVMI (τ = 0.28; p = 0.03). There was weak to moderate positive correlation between LGE and TPF (τ = 0.29, p = 0.02) in HCM (Fig. 3D). There was no significant correlation between LGE and PFR/EDV (τ = −0.07; p = 0.60).
3.4. Right ventricular hypertrophy and left atrium in hypertrophic cardiomyopathy
36.6% of the patients with HCM had hypertrophy involving the right ventricle. The most common finding was hypertrophy of the RV apex. Table 2 summarizes MLVWT, LVMI, and diastolic function in HCM patients with and without RV hypertrophy. The HCM patients with RV hypertrophy had significantly higher MLVWT and TPF than patients without RV hypertrophy.
Table 2.
Left ventricular parameters and morphological right ventricular involvement in hypertrophic cardiomyopathy.
| Parameters | Hypertrophic cardiomyopathy with morphological right ventricular involvement (n = 15) | Hypertrophic cardiomyopathy without morphological right ventricular involvement (n = 26) | p value |
|---|---|---|---|
| MLVWT(mm) | 21(17–25) | 16(14–17) | <0.001 |
| LVMI(g/m2) | 89(80–116) | 84(73.8–94.5) | 0.07 |
| PFR(ml/s) | 414(350–551) | 408(344–536) | 0.90 |
| PFR/EDV(/s) | 2.57(2.10−3.35) | 2.78(2.29−3.20) | 0.74 |
| TPF(ms) | 196(164–207) | 161(143–186) | 0.03 |
Data are shown as median (interquartile range).
MLVWT, maximum left ventricular wall thickness; LVMI, left ventricular mass index; PFR, early peak filling rate; PFR/EDV, PFR/end-diastolic volume; TPF, time to PFR.
Table 1 shows the median (interquartile range) left atrial area and longitudinal left atrial diameter in the HCM, HHD and control groups. We did not find any significant correlation between left atrial parameters and MLVWT, LVMI, PFR/EDV or TPF in the HCM or HHD groups (all p values >0.10).
4. Discussion
The present study shows that the TPF was significantly prolonged in HCM and HHD as compared to controls. MLVWT and TPF correlated positively in HCM, HHD and controls. In HCM patients, the scar burden and MLVWT correlated positively with TPF. HCM patients with RV hypertrophy had significantly higher MLVWT and TPF than patients without RV hypertrophy. To our knowledge, this is the first study evaluating the relationship between LV parameters and LV filling parameters using CMR in HCM, HHD and controls, and the relationship of RV hypertrophy and diastolic dysfunction in HCM.
Diastolic function can be evaluated by various techniques using imaging modalities such as radionuclide ventriculography, cardiac catheterization, echocardiography and magnetic resonance imaging. Mitral inflow pattern by echocardiography is the most commonly used diastolic parameter probably because of ease of assessment, wide availability and superior temporal resolution.6, 7 However, echocardiography has limitations due to limited acoustic window, limited field of view, poor endocardial definition, errors in angle and limited reproducibility.6, 8 The pressure-volume loop measurements with conductance catheter during transient vena caval occlusion have technical and theoretical limitations, and are not used in routine clinical practice.9 LV filling profile was primarily used in nuclear imaging for assessment of diastolic function. However, the measurement of left ventricular volume using nuclear cardiology is not as accurate as CMR. CMR gives the best available assessment of LV volume, as well as LV morphology10, 11 which is particularly relevant for HCM. The temporal resolution of CMR is also better than nuclear study. Nevetheless, CMR derived diastolic parameters using LV filling profile has not been widely used in clinical practice, because of longer post-processing time and limited availability of automated LV analysis software.7, 11 Thus, all measures of diastolic function have inherent limitations.
The relationship of CMR derived PFR and TPF to traditional echocardiography parameters have been previously assessed.12, 13, 14 Kawaji et al. assessed CMR derived normalized PFR, TPF and diastolic volume recovery in 101 patients with and without diastolic dysfunction based on echocardiography. They found that decreased PFR, longer TPF and greater diastolic volume recovery were associated with echocardiographic evidence of diastolic dysfunction.13 Kudelka et al. studied nine patients with thick LV wall and reported that CMR can detect diastolic abnormalities in patients without echocardiographic evidence of diastolic dysfunction.12 Mendoza et al. explored the relationship between echocardiographic severity of diastolic dysfunction and multiple CMR derived diastolic indices including filling parameters in post myocardial infarction patients, and concluded that reduced PFR and prolonged TPF reflect one aspect of diastolic dysfunction. PFR and TPF do provide specific information about certain aspects of the complex diastolic phenomenon.14 The best available normal ranges of CMR parameters of diastolic function in literature is published by Maceira et al. in 200615, 16, although these were arbitrarily defined based on mean ±2 standard deviation, in a relatively small number of subjects within each age group.
Our study shows that compared with LVMI, MLWVT correlates better with diastolic function in HCM, HHD and controls. The correlation in the controls might reflect subclinical diastolic dysfunction in cases with relatively thicker wall. The correlation between MLVWT and diastolic function in HHD was stronger than in HCM (0.58 vs 0.38), although MLVWT was significantly higher in HCM as compared to HHD. This implies that myocardial properties at the microscopic level such as myocyte disarray, interstitial fibrosis and replacement fibrosis are likely contributing to diastolic dysfunction beyond MLVWT in HCM.
Several previous studies have assessed the relationship of diastolic function, and MLVWT and LVM in HCM.17, 18, 19, 20 While some of these studies showed that diastolic function decreases with increasing left ventricular hypertrophy, others showed no definite correlation between diastolic function and left ventricular hypertrophy.17, 18, 19 Ciampi et al. studied the relationship of LVH diastolic function in 21 cases of HCM using invasive cardiac catheterization. They showed that Wigles score is the only index that correlated with diastolic function. No significant correlation was found between MLVWT, sum of maximum wall thickness in 4 ventricular segments, number of hypertrophied segments, and diastolic function.19 Spirito et al. in a study of 52 cases with HCM and 22 controls using 2 dimensional and M mode echocardiography demonstrated worse diastolic dysfunction with extensive LVH than mild LVH.17 Our study demonstrates positive correlation between MLVWT and TPF but no correlation between LVMI and TPF. Of our 41 patients with HCM, 13 (32%) had normal LV mass index. The MLVWT in these patients were abnormal and ranged from 14 to 21 mm. These data are consistent with the notion that although the myopathic process is not restricted to the abnormally thickened segments, MLVWT may reflect its severity at the microscopic level.
LGE in CMR is a reflection of histopathological abnormality which includes myocyte disarray and fibrosis.5, 21 Similar to the previously published data,22, 23 LGE was seen in 75% of our HCM cases. Our study showed moderate positive correlation between LGE, and MLVWT and LVM index, and weak to moderate positive correlation between LGE and TPF in HCM. The positive correlation between LGE and TPF found in HCM patients further emphasizes the contribution of histopathological changes to diastolic dysfunction. Motoyasu et al. reported strong correlation between the extent of LGE and diastolic dysfunction as assessed by PFR/EDV using CMR in HCM patients. They did not assess TPF, and there was no significant correlation between LGE and LVM.4
LGE imaging assesses focal fibrosis well as chelated gadolinium, which is an extracellular contrast agent, shows high volume distribution in the regions of focal fibrosis with resultant reduction in T1 relaxation and high signal. However, diffuse myocardial fibrosis might be uniformly nulled. Hence, this may underestimate abnormality within the cardiomyocytes which could contribute to diastolic dysfunction.24 Direct measure of T1 (T1 mapping) can assess diffuse myocardial fibrosis, which alters extra cellular volume (ECV) and has shown to be significantly different in normal versus various disease conditions including HCM.8, 25 ECV quantification using CMR has already been shown to predict prognosis in some disease conditions such as diabetes.26
It is important to explore the relationship of RV hypertrophy to LV parameters and diastolic function in HCM patients. 37% of our HCM cases had RV hypertrophy, which is similar to RV hypertrophy in one third of patients reported by Maron et al.27 Our HCM patients with RV hypertrophy had significantly higher MLVWT than patients without RV hypertrophy. McKenna et al. also found moderate correlation between right and left ventricular thickness in a study of 73 patients with HCM.28 To our knowledge, previous studies have not assessed LV diastolic function in relation to RV involvement. We found that HCM patients with RV hypertrophy have worse LV diastolic function than those without RV hypertrophy.
Our study provides pathophysiological insights in to LV diastolic dysfunction and parameters affecting LV diastolic function. Better understanding of disease process is helpful in closely monitoring disease progression, predicting adverse outcome and exploring new options of therapy, especially considering the absence of lifesaving therapy in diastolic heart failure, unlike systolic heart failure. This may also guide future research in to diastolic dysfunction in HCM.
It is important to view our study within the context of its limitations. We assessed diastolic function by measuring PFR/EDV and TPF obtained using TVC and its first derivative curve. We did not correlate CMR findings with echocardiography which is the commonly used and standardized tool for diastolic function. Other CMR measures of LV diastolic function such as mitral valve flow mapping or pulmonary venous flow mapping by phase contrast imaging, left atrial volume assessment with left atrial cine SSFP stack, myocardial motion velocity by phase contrast imaging and LV strain rate and torsion recovery rate with myocardial tagging were not assessed in our study.10 It would have been ideal to have normal volunteers as control group rather than subjects with normal CMR, even though they did not have any significant known underlying disease conditions such as hypertension, heart failure, angina, myocardial infarction or cardiomyopathy. Another drawback of our study is that patient’s symptoms, brain natriuretic peptides, grades of severity and duration of disease conditions were not assessed. Cardiac medications could have affected diastolic function. Among our patients with hypertension, there might be other co-existing conditions which could contribute to LV hypertrophy. These were beyond the scope of this study. HCM and hypertension may co-exist and its individual contribution to diastolic dysfunction cannot be precisely quantified. Blood pressure measurements were not verified independently. The clinical importance of the significant but small between-group differences in TPF remains to be determined. Finally, the relatively small number of patients in our study might have limited the power to detect weak correlations. The numbers for each subtype of HCM were too small to allow meaningful comparison.
5. Conclusion
MLVWT correlated with diastolic function (TPF) in HCM, HHD and controls. LVMI did not show significant correlation with TPF. Diastolic dysfunction in HCM is not entirely explained by wall thickness. LGE and RV involvement are associated with worse diastolic function, suggesting that these may be surrogate markers of more severe underlying myocardial disarray and fibrosis that contribute to diastolic dysfunction.
Conflicts of interest and sources of funding
This research received no grant from any funding agency in the public, commercial or not-for profit sectors. The authors declare that there is no conflict of interest.
References
- 1.Gaasch W.H., Zile M.R. Left ventricular diastolic dysfunction and diastolic heart failure. Annu Rev Med. 2004;55:373–394. doi: 10.1146/annurev.med.55.091902.104417. [DOI] [PubMed] [Google Scholar]
- 2.Yancy C.W., Jessup M., Bozkurt B. ACCF/AHA guideline for the management of heart failure. J Am Coll Cardiol. 2013;62(16):147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Noureldin R.A., Liu S., Nacif M.S. The diagnosis of hypertrophic cardiomyopathy by cardiovascular magnetic resonance. J Cardiovas Magn Reson. 2012;14:17. doi: 10.1186/1532-429X-14-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motoyasu M., Kurita T., Onishi K. Correlation between late gadolinium enhancement and diastolic function in hypertrophic cardiomyopathy assessed by magnetic resonance imaging. Circ J. 2008;72:378–383. doi: 10.1253/circj.72.378. [DOI] [PubMed] [Google Scholar]
- 5.Małek Ł.A., Chojnowska L., Kłopotowski M. Left ventricular diastolic function assessed with cardiovascular magnetic resonance imaging and exercise capacity in patients with non-obstructive hypertrophic cardiomyopathy. Kardiol Pol. 2009;67:1–6. [PubMed] [Google Scholar]
- 6.Duarte R., Fernandez G. Assessment of left ventricular diastolic function by MR: why, how and when. Insights Imaging. 2010;1:183–192. doi: 10.1007/s13244-010-0026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caudron J., Fares J., Bauer F. Evaluation of left ventricular diastolic function with MR imaging. Radiographics. 2011;31:239–261. doi: 10.1148/rg.311105049. [DOI] [PubMed] [Google Scholar]
- 8.Maceira A.M., Mohiaddin R.H. Cardiovascular magnetic resonance in systemic hypertension. J Cardiovasc Magn Reson. 2012;14:28. doi: 10.1186/1532-429X-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaasch W.H., Little W.C. Assessment of left ventricular diastolic function and recognition of diastolic failure. Circulation. 2007;116:591–593. doi: 10.1161/CIRCULATIONAHA.107.716647. [DOI] [PubMed] [Google Scholar]
- 10.Paelinck B.P., Lamb H.L., Bax J.J. Assessment of diastolic function by cardiovascular magnetic resonance. Am Heart J. 2002;144:198–205. doi: 10.1067/mhj.2002.123316. [DOI] [PubMed] [Google Scholar]
- 11.Okayama S., Nakano T., Uemura S. Evaluation of left ventricular diastolic function by fractional area change using cine cardiovascular magnetic resonance: a feasibility study. J Cardiovasc Mag Reson. 2013;15:87. doi: 10.1186/1532-429X-15-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kudelka A.M., Turner D.A., Liebson P.R. Comparison of cine magnetic resonance imaging and doppler echocardiography for evaluation of left ventricular diastolic function. Am J Cardiol. 1997;80:384–386. doi: 10.1016/s0002-9149(97)00375-5. [DOI] [PubMed] [Google Scholar]
- 13.Kawaji K., Codella N.C.F., Prince M.R. Automatic segmentation of routine clinical cardiac magnetic resonance imaging for assessment of left ventricular diastolic dysfunction. Circ Cardiovasc Imaging. 2009;2:476–484. doi: 10.1161/CIRCIMAGING.109.879304. [DOI] [PubMed] [Google Scholar]
- 14.Mendoza D.D., Codella N.C., Wang Y. Impact of diastolic dysfunction severity on global left ventricular volumetric filling assessment by automated segmentation of routine cardiovascular magnetic resonance. J Cardiovasc Mag Reson. 2010;12:46. doi: 10.1186/1532-429X-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maceira A.M., Prasad S.K., Khan M. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2006;8:417–426. doi: 10.1080/10976640600572889. [DOI] [PubMed] [Google Scholar]
- 16.Kawel-Boehm N., Maceira A., Valsangiacomo-Buechel E.R. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson. 2015;17:29. doi: 10.1186/s12968-015-0111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spirito P., Maron B.J., Chiarella F. Diastolic abnormalities in patients with hypertrophic cardiomyopathy: relation to magnitude of left ventricular hypertrophy. Circulation. 1985;72:310–316. doi: 10.1161/01.cir.72.2.310. [DOI] [PubMed] [Google Scholar]
- 18.Spirito P., Maron B.J. Relation between extent of left ventricular hypertrophy and diastolic filling abnormalities in hypertrophic cardiomyopathy. J Am Coll Cardiol. 1999;15:808–813. doi: 10.1016/0735-1097(90)90278-w. [DOI] [PubMed] [Google Scholar]
- 19.Ciampi Q., Betocchi S., Losi M.A. Effect of hypertrophy on left ventricular diastolic function in patients with hypertrophic cardiomyopathy. Heart Int. 2006;2:106–114. doi: 10.4081/hi.2006.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olivotto I., Maron M.S., Autore C. Assessment and significance of left ventricular mass by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2008;52:559–566. doi: 10.1016/j.jacc.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 21.Maron M.S. Contrast-enhanced CMR in HCM. What lies behind the bright light of LGE and why it now matters. JACC Cardiovasc Imaging. 2013;6:5. doi: 10.1016/j.jcmg.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 22.Kim Y.J., Choi B.W., Hur J. Delayed enhancement in hypertrophic cardiomyopathy: comparison with myocardial tagging MRI. J Magn Reson Imaging. 2008;27:1054–1060. doi: 10.1002/jmri.21366. [DOI] [PubMed] [Google Scholar]
- 23.Abdel-Aty H., Cocker M., Strohm O. Abnormalities in T2-weighted cardiovascular magnetic resonance images of hypertrophic cardiomyopathy: regional distribution and relation to late gadolinium enhancement and severity of hypertrophy. J Magn Reson Imaging. 2009;28:242–245. doi: 10.1002/jmri.21381. [DOI] [PubMed] [Google Scholar]
- 24.Moreo A., Ambrosio G., De Chiara B. Influence of myocardial fibrosis on left ventricular diastolic function: non-invasive assessment by CMR and ECHO. Circ Cardiovasc Imaging. 2009;2(6):437–443. doi: 10.1161/CIRCIMAGING.108.838367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sado D.M., Flett A.S., Banypersad S.M. Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease. Heart. 2012;98:1436–1441. doi: 10.1136/heartjnl-2012-302346. [DOI] [PubMed] [Google Scholar]
- 26.Wong T.C., Piehler K.M., Kang I.A. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J. 2014;35(10):657–664. doi: 10.1093/eurheartj/eht193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maron M.S., Hauser T.H., Dubrow E. Right ventricular involvement in hypertrophic cardiomyopathy. Am J Cardiol. 2007;100:1293–1298. doi: 10.1016/j.amjcard.2007.05.061. [DOI] [PubMed] [Google Scholar]
- 28.McKenna W.J., Kleinebenne A., Nihoyannopoulos P. Echocardiographic measurement of right ventricular wall thickness in hypertrophic cardiomyopathy: relation to clinical and prognostic features. J Am Coll Cardiol. 1988;11:351–358. doi: 10.1016/0735-1097(88)90101-5. [DOI] [PubMed] [Google Scholar]