Abstract
Introduction:
Leber’s Optic Hereditary Neuropathy (LHON) is a common cause of teenaged blindness in both eyes for which there is currently no effective treatment. In 1871, the German ophthal-mologist Theodor Leber was the first to describe the clinical characteristics of his namesake disease, and through unremitting efforts over the past 100 years, researchers have continued to increase their under-standing of LHON. In recent years, using gene therapy, several groups have obtained breakthroughs in the treatment of the disease.
Conclusion:
In this article, we will review the challenging journey that researchers faced towards our current understanding of LHON, and describe the transition of gene therapy research for LHON from the bench to bedside.
Keywords: Leber’s optic hereditary neuropathy, Gene therapy, Translational medicine, Mitochondrial disease, MT-ND4, Allotopic expression technology
1. Leber’s Hereditary Optic Neuropathy
1.1. Overview
Leber′s Hereditary Optic Neuropathy (LHON) is a hereditary disease causing blindness in both the eyes in teenagers [1, 2]. It was first reported in 1858, and in 1871, the German ophthalmologist T. Leber was the first to describe the clinical characteristics of this disease, which is named after him [3]. In 1972, Erickson proposed that LHON was a classical maternally inherited disease [4] because most patients are male [5]. The clinical presentation of LHON is the simultaneous or sequential painless loss of vision with either an acute or subacute onset, which is accompanied with a deficiency in the central visual field and defects in color vision [6-8]. There are no effective treatments for LHON and once the disease manifests, only a minority of affected individuals will self-recover, whereas most patients will eventually be completely blind. Because of the severity of the condition and its early onset in life, LHON has been the focus of researchers, clinicians, and healthcare professionals who have been searching since its discovery for an understanding of its etiology and underlying mechanism as well as efforts towards developing an effective treatment of the disease.
The completion of the Human Genome Project was a major advancement in medicine enabling researchers to identify and characterize the causes of many diseases. In fact, some researchers have used bioengineering techniques to study the etiology and mechanisms of LHON at the molecular level. In 1988, Wallace et al. [9] found a mutation in MT-ND4 (m.11778G>A) in mitochondrial DNA (mtDNA) that was associated with LHON. This discovery was a major breakthrough as it determined the main etiopathology of LHON, as a mitochondrial disorder. The identification of this mutation was followed by the discovery of other mtDNA mutations, such as m.3460G>A [10] and m.14484T>C [11]. Subsequently, researchers have found over 50 mtDNA mutations that are associated with LHON, and our understanding of the pathogenic mechanisms of LHON continues to improve.
The aim of medical research is to benefit patients conferring improved quality of life both for themselves as well as for their family members. Human eyes are confined spheres and this unique structural feature is a major reason that eyes are a favorable site for gene therapy. Because our understanding of the pathogenic causes and mechanisms of LHON has been continuously increasing, we believe that the time has arrived towards using gene therapy for the treatment of LHON. However, despite an improved understanding of the disease, there is still a distance required for a breakthrough in gene therapy taking it from the bench and translating it to the bedside as an effective clinical treatment approach. In 2007, three groups in the United States and the United Kingdom successfully carried out clinical trials on gene therapy for Leber congenital amaurosis (LCA) [12, 13] providing satisfactory results [14, 15]. These studies opened a new chapter and provided references in the use of gene therapy demonstrating its potential as a valid treatment option of eye diseases. Globally, there are many groups including ourselves who are pursuing translational medicinal research in LHON to bridge this distance from the bench to the bedside [16-26] and bring hope to the many patients with LHON and their families in the next 10 years.
1.2. Epidemiology
LHON can occur at any age group, but occurs mostly in teenagers [27], with a modal age of onset between 15-20 years [28]. The disease has two major characteristics consisting of incomplete penetrance and occurring mainly in males. Epidemiological studies in Europe found that the incidence of LHON varies between countries [29, 30]. The highest incidence for LHON was reported in Northeastern England with an incidence of approximately 1 in 25 000 and a gene mutation rate of 1.18/10 000, whereas the Netherlands and Finland reported lower incidences of 1 in 39 000 and 1 in 50 000, respectively [31-34]. To date, there are no data from large-sample epidemiological studies in Asia.
1.3. Mutation Sites
Since Wallace et al. [35] found the first mutation for LHON in MT-ND4 (m.11778G>A) in 1998, 50 mtDNA mutations have been associated with the disease. These mutations can be classified as primary or multi-site mutations.
1.3.1. Primary Mutations
Primary mutations specifically occur in families with LHON and any one of which can cause the disease. Common primary mutations include m.11778G>A, m.3460G>A, and m.14484T>C, and some reports have shown that 90% of patients with LHON were because of mutations in one of these three sites [1, 2, 36]. Therefore, the detection of these three mutations has become the gold standard in LHON diagnosis. The frequency of these mutations in patients with LHON varies by ethnicity. In Asia, the frequency of m.11778G>A, m.3460G>A, and m.14484T>C in LHON are 87%-92.9%, 1.4%-4%, and 5.7%-9%, respectively. In the mainland China, m.11778G>A is the most common mutation found in approximately 90% of patients, of which 80% were male [37, 38]. The frequency of m.11778G>A in the Middle East is similar to that found in Asia [39], whereas in Europe, the frequency of m.11778G>A, m.3460G>A, and m.14484T>C is 50%-70%, 15%-25%, and 15%, respectively. In addition to these three common primary mutations, other lower-frequency primary mutations such as, m.3635G>A have been reported [40].
1.3.2. Multi-site Mutations
Multi-site LHON mutations are found in families and can also occur in the normal population. However, the frequency of LHON in family members with an affected individual is higher than that found in the general population. Multi-site mutations such as m.3394T>C cannot cause LHON alone, but may confer a phenotypic effect through cooperation with a primary causative LHON mutation [41]. The other multi-site mutations may result in phenotypic variability in affected patients. We have been interested in multi-site mutations and have performed related research.
1.4. Clinical Presentation
The clinical presentation of LHON is the simultaneous or sequential onset of acute or subacute painless vision loss in both the eyes. The course of the disease is classified according to disease progression into presymptomatic, acute, and atrophic stages. Patients are normally diagnosed in the presymptomatic stage during family screening without obvious clinical presentations. In the acute stage, the vision of patients rapidly decreases [42] and vision acuity reaches a nadir at 4-6 weeks based on common indices or hand motion. Simultaneous or sequential onset of vision loss in both eyes occurs in 25% and 75% of patients, respectively [43]. During this stage, most patients present with a classical triad consisting of a swelling of the nerve fiber layer around the optic disc, circumpapillary telangiectatic microangiopathy (Fig. 1), and the absence of fluorescent leakage in fluorescein angiography. Visual field and color vision testing can show deficiency in the central visual field (Fig. 2) and color vision defects, respectively. During the atrophic stage, the main pathological processes are optic nerve atrophy and thinning of the retinal nerve fiber layer with no other specific clinical manifestations. It has been reported that some patients with LHON experience vision recovery including recovery of the visual field and color vision [44]. This stage may be considered a recovery stage; however, such patients are in minority. Several studies have found that vision recovery is associated with specific mtDNA mutations. Those patients with LHON caused by m.14484T>C were the most likely to experience vision recovery followed by patients harboring m.3460G>A. In contrast, patients with m.11778G>A had the least vision recovery [2, 11, 28, 45]. This variable recovery response in patients with different mutations may be because of differences in the condition of the eye and of the body itself during disease onset.
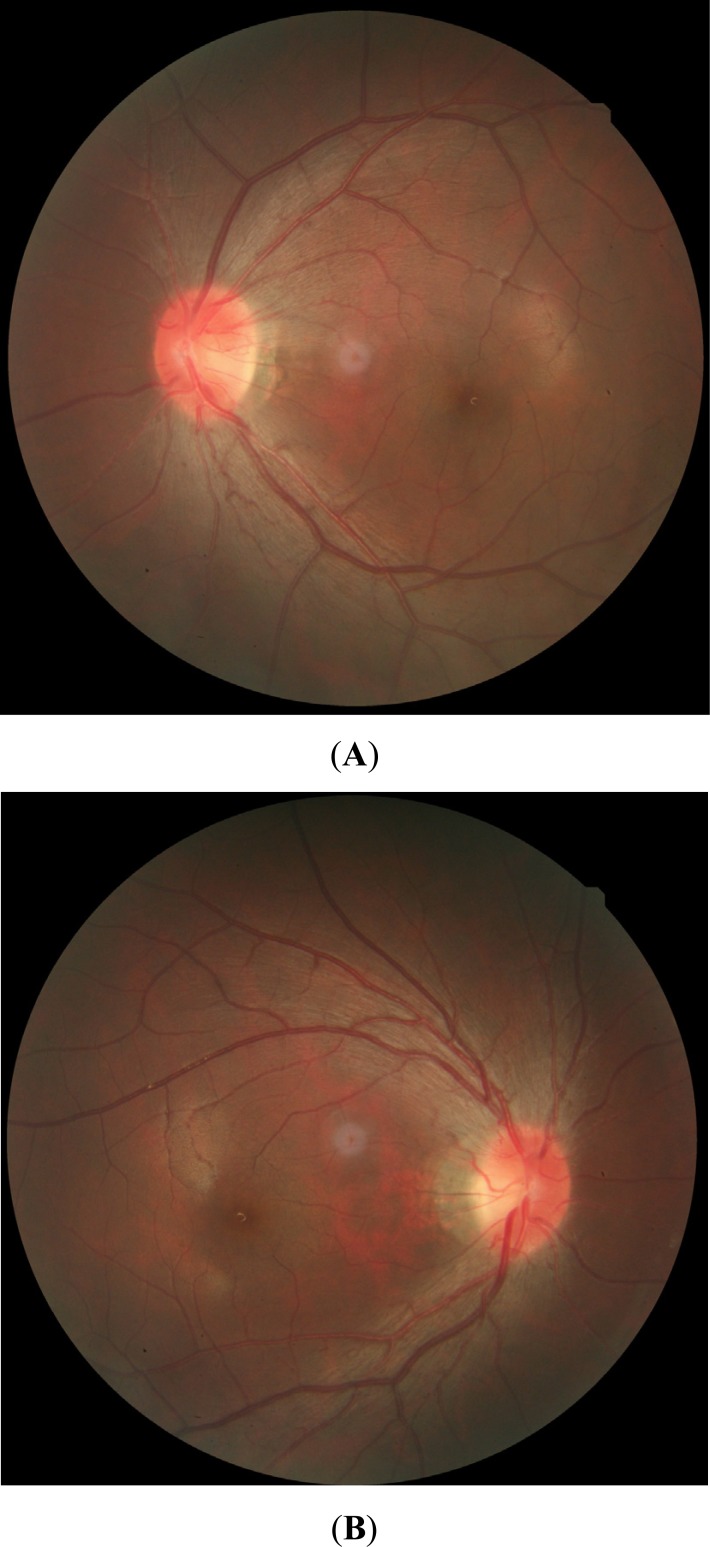
Fig. (1).
Fundus color images during the acute stage of LHON. (A, B) Swelling of the nerve fiber layer around the optic disc, circumpapillary telangiectatic microangiopathy, and presence of the optic nerve boundary can be observed. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper.)
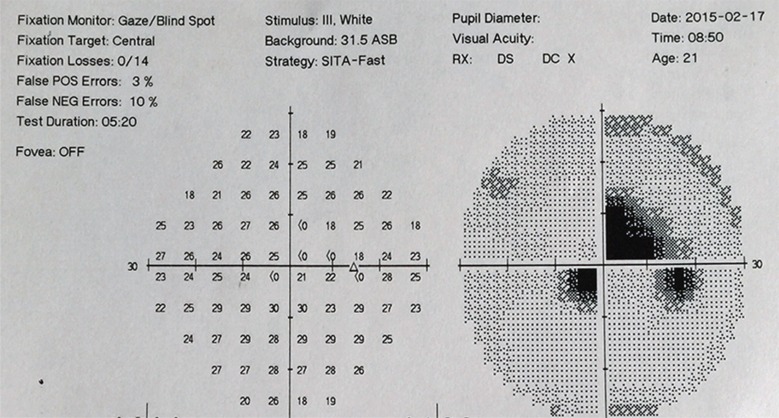
Fig. (2).
Visual field presentation during the acute stage of LHON. Note deficiency of the central visual field.
2. Basic research on LHON
Mitochondria are organelles in eukaryotic cells with independent genetic systems. The complete mtDNA genome consists of 16 569 base pairs in a double-stranded, close-loop structure and 37 genes that encode polypeptides associated with oxidative phosphorylation as well as 2 rRNA, 22 tRNA, and 13 RNA encoding for polypeptides associated with oxidative phosphorylation [46]. Although the mitochondrial genome can operate independently, it is largely regulated by nuclear genes. Therefore, mutations in either mitochondrial and/or nuclear-related genes involved in mitochondrial function can lead to defects and cause mitochondrial-related diseases [47].
Mitochondria are the location where oxidative phosphorylation occurs inside eukaryotic cells, providing 95% of the energy needed for an organism’s metabolism. Mitochondria can also participate in cellular apoptosis. It is a major source of intracellular reactive oxygen species (ROS) and plays an indispensable function in maintaining optic nerve function. Because of its important biological functions, mutations in mtDNA can impair mitochondrial function that leads to an increased energy demand and damage to ROS-sensitive retinal ganglion cells. The result is vision impairment and is likely the underlying mechanism of LHON [28, 48-50]. Researchers have found that 95% of patients with LHON are associated with one of the three primary mtDNA mutations, m.11778G>A, m.3460G>A, or m.14484T>C, and therefore, these mutations are considered necessary for LHON pathogenesis [51-53]. In addition, these primary mutations are found in genes that encode complex I of the respiratory chain. Hence, current research on LHON has focused on identifying mutations in complex I-related mitochondrial genes, specifically ND1 to ND6 (also known as mitochondrially encoded NADH:ubiquinone oxidoreductase core subunits 1 to 6), with a particular focus on MT-ND4, which is the site of the most commonly reported mutation in patients with LHON, m.11778G>A [54]. Studies have found that mutations, at this site, result in decreased activity of respiratory complex I that leads to a slowing of cellular respiration and decrease in production efficiency, which eventually leads to damage of the optic nerve [55-58].
Because the MT-ND4 11778G>A point mutation is the most common causative mutation found in patients with LHON, it has been the focus of previous gene therapy efforts. In 1998, Guy et al. [59] attempted gene therapy using allotopic expression technology [60] to transfer normal (wild type) MT-ND4 into cells, which will then be translated into functional protein. NADH-ubiqinone oxidoreductase chain 4 (ND4) was imported into mitochondria rather than to its typical target, the nucleus, by addition of a mitochondrial targeting sequence (MTS) to the capsid [61] and the imported wild type MT-ND4 resulted in recovery of production efficiency in some damaged mitochondria, which achieved the aim of restoring cellular function and confirmed that gene therapy is theoretically feasible. To overcome the limitations of in vitro cell experiments, some researchers [62] constructed animal models and used allotopic expression technology to transfer both wild type MT-ND4 and mutated MT-ND4 containing the 11778G>A point mutation into the eyes of rabbits. They found that mutated MT-ND4 resulted in damage to retinal ganglion cells, whereas no such effects were observed with wild type MT-ND4. In 2009, Guy et al. [63] used injection into the vitreous cavities of eyes to successfully transfer copies of MT-ND4 into mice using constructed AVV-ND4 vectors. They found that injection of wild type MT-ND4 did not cause damage to mouse optic nerves, a finding that confirmed the intravitreal injection of drugs was a safe approach for gene therapy.
These studies provided the basis in theory for the feasibility, efficacy, and safety of gene therapy for LHON.
3. Clinical treatment for LHON
Since the discovery of LHON, various methods and techniques including traditional Chinese medicine, western medicine, acupuncture, infrared, stem cells, and biological agents, especially idebenone [64, 65] have been attempted for the treatment of LHON. A multicenter clinical study of idebenone treatment for LHON was conducted in Europe in 2007, but upon its conclusion in 2011, their results showed no significant effect [66, 67].
Stem cells therapy was another approach considered to have good potential [68]. However, key issues remain unsolved in stem cell therapy of LHON, and that there is still some distance before clinical applications may be realized.
4. Gene therapy for LHON
In gene therapy for LHON, normal (wild type) unmutated MT-ND4 is transferred into the cells of a patient that contain mutated MT-ND4. This wild type gene will then be encoded into properly functioning ND4 that will be imported into mitochondria to replace the mutated ND4. This replacement rescues the physiological function of the protein in its role in cellular respiration, thereby achieving the aim of treating LHON. ND4, ND1, and ND2 form complex I in mitochondria (Fig. 3) and repairing ND4 enables resumption of normal physiological functions inside mitochondria.
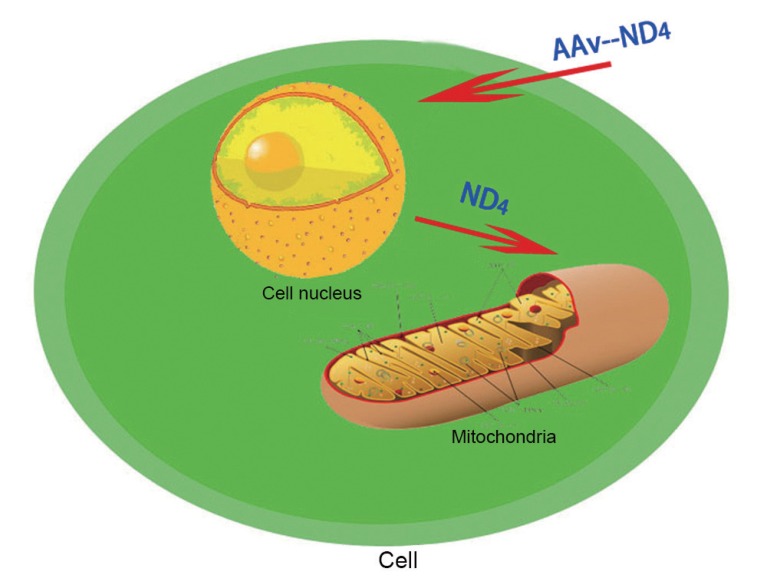
Fig. (3).
Allotopic expression of AAV2-ND4 effector mechanism. AAV2 transports normal (wild type) MT-ND4 into nodular cells, which is translated into ND4 in the cytoplasm. Under the guidance of a MTS, ND4 is imported into mitochondria to carry out its biological functions.
In 2011, we performed the first LHON gene therapy study in the world by injecting 0.05 mL of AAV2-ND4 in the vitreous cavity of a single eye, and completed the treatment of another eight patients in 2 years [22]. In the subsequent 36-month follow-up period, none of the nine patients experienced serious adverse events either in their eye or in their entire body. Using measures of best-corrected visual acuity (BCVA) compared to baseline, six of the nine patients exhibited improvement. Based on visual field tests compared to baseline measurements, we found that seven patients exhibited progress. From results of other observational markers, such as retinal nerve fiber layer thickness and visual evoked potential (VEP), all patients showed improvements compared to baseline measures before therapy.
In 2014, Guy et al. [69] performed a prospective, open-label clinical trial where his team designed, constructed, and injected AAV2-P1ND4-v2 into the vitreous cavity of a single eye. The five patients recruited in their study were divided into groups and given low, medium, or high therapeutic doses. After a 6-month follow-up, no serious adverse events because of treatment were observed in any of the patients. In addition, two patients exhibited significant improvements in visual acuity, whereas no significant changes were found in the other three patients.
In 2015, GenSight Biologics carried out a clinical trial on LHON gene therapy by injecting GS0101 into the vitreous cavity of a single eye in 15 patients. In the 48-week follow-up period, they found improved visual acuity in patients with a disease course less than 2 years, whereas patients with a disease course greater than 2 years did not exhibit any significant change.
Those studies showed that gene therapy for LHON may be achievable and effective based on current scientific progress.
CONCLUSION AND Prospects
After decades of exploration and development, gene therapy is gradually maturing for clinical use. Over the past decade, clinical research of gene therapy for eye disease has flourished and some studies have achieved initial success [70-74]. Ophthalmology has entered an era of gene therapy.
Reviewing the research progress of gene therapy for LHON, the progression from concept to clinical testing has been gradual from the discovery of LHON gene mutation sites to the feasibility of gene therapy, from building the AAV2-ND4 construct to determine the effectiveness of cell and animal experiments, and finally, from clinical treatment studies to determine clinical observation indicators to gene therapy implementation. In the future, large-sample, multicenter, randomized controlled trials are needed to validate the safety and efficacy of gene therapy for LHON in every stage.
During our investigation, the findings from three independent research teams showed improvement in visual acuity in both eyes after injecting AAV2-ND4 in the vitreous cavity of a single eye in patients, a finding that astonished everyone. To answer this question, researchers returned to the laboratory. The results of animal experiments in one study [19] showed that injection of AAV2-ND4 in a single eye with resulting improvements in visual acuity in both eyes may be because there is a degree of axonal connectivity between the two optic nerves, which results in both eyes receiving the injected construct.
This process illustrates the need in the present day where specialization is increasing, and it is only through close cooperation and collaboration between molecular biologists, clinicians, and healthcare professionals that we can make the transition from bench to bedside for gene therapy for LHON. Laboratory and clinical research should be integrated using clinical problems as a guide for laboratory research, and furthermore, using clinical research and testing to validate research findings from the laboratory. Only through this concerted effort, we can make the successful transition from bench to bedside for the eventual benefit for all patients.
Consent for Publication
Not applicable.
ACKNOWLEDGEMENTS
We would like to express our appreciation to everyone who was involved in the drafting and preparation of the manuscript.
CONFLICT OF INTEREST
This research was supported by the National Nature Science Foundation of China (Grant #81271015, #81770969) and the 2016 Huazhong University of Science and Technology Independent Innovation Fund Major Project (2016 YXZD022). The authors report no conflict of interest.
REFERENCES
- 1.Man P.Y.W., Turnbull D.M., Chinnery P.F. Leber hereditary optic neuropathy. J. Med. Genet. 2002;39(3):162–169. doi: 10.1136/jmg.39.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Man P.Y.W., Griffiths P.G., Brown D.T., et al. The epidemiology of Leber hereditary optic neuropathy in the North East of England. Am. J. Hum. Genet. 2003;72(2):333–339. doi: 10.1086/346066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leber T.H. Über hereditäre und congenital-angelegte Sehnervenleiden. Graefes Arch. Clin. Exp. Ophthalmol. 1871;17(2):249–291. [Google Scholar]
- 4.Erickson R.P. Leber’s optic atrophy, a possible example of maternal inheritance. Am. J. Hum. Genet. 1972;24:348–349. [PMC free article] [PubMed] [Google Scholar]
- 5.Puomila A., Hamalainen P., Kivioja S., et al. Epidemiology and penetrance of Leber hereditary optic neuropathy in Finland. Eur. J. Hum. Genet. 2007;15:1079–1089. doi: 10.1038/sj.ejhg.5201828. [DOI] [PubMed] [Google Scholar]
- 6.Newman N.J., Wallace D.C. Mitochondria and Leber’s hereditary optic neuropathy. Am. J. Ophthalmol. 1990;109(6):726–730. doi: 10.1016/s0002-9394(14)72445-6. [DOI] [PubMed] [Google Scholar]
- 7.Carelli V., La Morgia C., Valentino M.L., et al. Retinal ganglion cell neurodegeneration in mitochondrial inherited disorders. Biochim. Biophys. Acta. 2009;1787(5):518–528. doi: 10.1016/j.bbabio.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 8.Brown M.D., Wallace D.C. Spectrum of mitochondrial-DNA mutations in Lebers hereditary optic neuropathy. Clin. Neurosci. 1994;2(2):138–145. [Google Scholar]
- 9.Wallace D.C., Singh G., Lott M.T., et al. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science. 1988;242(4884):1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- 10.Huoponen K., Vilkki J., Aula P., et al. A new mtDNA mutation associated with Leber hereditary optic neuroretinopathy. Am. J. Hum. Genet. 1991;48:1147–1153. [PMC free article] [PubMed] [Google Scholar]
- 11.Mackey D. A variant of Leber hereditary optic neuropathy characterized by recovery of vision and by an unusual mitochondrial genetic etiology. Am. J. Hum. Genet. 1992;51:1218–1228. [PMC free article] [PubMed] [Google Scholar]
- 12.Maguire A.M., Simonelli F., Pierce E.A., et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bainbridge J.W., Smith A.J., Barker S.S., et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 14.Hauswirth W.W., Aleman T.S., Kaushal S., et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: Short-term results of a phase I trial. Hum. Gene Ther. 2008;19(10):979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hufnagel R.B., Ahmed Z.M., Correa Z.M., et al. Gene therapy for Leber congenital amaurosis: Advances and future directions. Graefes Arch. Clin. Exp. Ophthalmol. 2012;250:1117–1128. doi: 10.1007/s00417-012-2028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi H., Gao J., Pei H., et al. Adeno-associated virus- mediated gene delivery of the human ND4 complex I subunit in rabbit eyes. Clin. Experiment. Ophthalmol. 2012;40(9):888–894. doi: 10.1111/j.1442-9071.2012.02815.x. [DOI] [PubMed] [Google Scholar]
- 17.Cui G., Ding H., Xu Y., et al. Applications of the method of high resolution melting analysis for diagnosis of Leber’s disease and the three primary mutation spectrum of LHON in the Han Chinese population. Gene. 2013;512(1):108–112. doi: 10.1016/j.gene.2012.09.110. [DOI] [PubMed] [Google Scholar]
- 18.Pei H., Wan X., Hu W., et al. Construction and detection of a novel type of recombinant human rAAV2/2-ND4. Eye Sci. 2013;28(2):55–59. [PubMed] [Google Scholar]
- 19.Shuo Y, Heng H, Zhu Y, et al. Chemical and material communication between the optic nerves in rats[J]. Clin Exp ophthalmol . 2015;43(8):742–8. doi: 10.1111/ceo.12547. [DOI] [PubMed] [Google Scholar]
- 20.Wan X., Pei H., Zhao M., et al. Efficacy and safety of rAAV2-ND4 treatment for Leber’s hereditary optic neuropathy. Sci. Rep. 2016;6:21587. doi: 10.1038/srep21587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ran R., Yang S., He H., et al. A retrospective analysis of characteristics of visual field damage in patients with Leber’s hereditary optic neuropathy. Springerplus. 2016;5(1):1–6. doi: 10.1186/s40064-016-2540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shuo Y., Ma S., Wan X., et al. Long-term outcomes of gene therapy for the treatment of Leber’s hereditary optic neuropathy. EBioMedicine. 2016;10:258–268. doi: 10.1016/j.ebiom.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shuo Y., Hong Y., Ma S., et al. Evaluation of Leber’s hereditary optic neuropathy patients prior to a gene therapy clinical trial. Medicine (Baltimore) 2016;95(40):e5110. doi: 10.1097/MD.0000000000005110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feuer W.J., Schiffman J.C., Davis J.L., et al. Gene therapy for leber hereditary optic neuropathy: Initial results. Ophthalmology. 2016;123(3):558–570. doi: 10.1016/j.ophtha.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jing G., Hui S., Han P. Comparison of immunosuppressive effects and ND4 expression among different immunosuppressive strategies following AAV2-ND4 gene treatment for Leber hereditary optic neuropathy. Acta Medicinae Universitatis Scientiae et Technologiae Huazhong. 2013;2:14. [Google Scholar]
- 26.Guy J, Feuer WJ, Davis JL, et al. Gene therapy for leber hereditary optic neuropathy: Low- and medium-dose visual results. . Ophthalmology. 2017 doi: 10.1016/j.ophtha.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Testa F., Maguire A.M., Rossi S., et al. Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital Amaurosis type 2. Ophthalmology. 2013;120(6):1283–1291. doi: 10.1016/j.ophtha.2012.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fulda S., Kufer M.U., Meyer E., et al. Sensitization for death receptor- or drug-induced apoptosis by re-expression of caspase-8 through demethylation or gene transfer. Oncogene. 2001;20(41):5865. doi: 10.1038/sj.onc.1204750. [DOI] [PubMed] [Google Scholar]
- 29.Yu-Wai-Man P., Griffiths P.G. Mitochondrial optic neuropathies - disease mechanisms and therapeutic strategies. Prog. Retin. Eye Res. 2011;30:81–114. doi: 10.1016/j.preteyeres.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu-Wai-Man P., Griffiths P.G., Hudson G., et al. Inherited mitochondrial optic neuropathies. J. Med. Genet. 2009;46(3):145–158. doi: 10.1136/jmg.2007.054270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chinnery P.F., Johnson M.A., Wardell T.M., et al. The epidemiology of pathogenic mitochondrial DNA mutations. Ann. Neurol. 2000;48(2):188–193. [PubMed] [Google Scholar]
- 32.Man P.Y.W., Griffiths P.G., Brown D.T., et al. The epidemiology of Leber hereditary optic neuropathy in the North East of England. Am. J. Hum. Genet. 2003;72(2):333–339. doi: 10.1086/346066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spruijt L., Kolbach D.N., de Coo R.F., et al. Influence of mutation type on clinical expression of Leber hereditary optic neuropathy. Am. J. Ophthalmol. 2006;141(4):676–682. doi: 10.1016/j.ajo.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Puomila A., Hamalainen P., Kivioja S., et al. Epidemiology and penetrance of Leber hereditary optic neuropathy in Finland. Eur. J. Hum. Genet. 2007;15(10):1079–1089. doi: 10.1038/sj.ejhg.5201828. [DOI] [PubMed] [Google Scholar]
- 35.Bu-Amero K.K., Bosley T.M., Bohlega S., et al. Complex I respiratory defect in LHON plus dystonia with no mitochondrial DNA mutation. Br. J. Ophthalmol. 2005;89(10):1380–1381. doi: 10.1136/bjo.2005.072819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan S.E., Ryan F., Barton D., et al. Development and validation of a novel PCR-RFLP based method for the detection of 3 primary mitochondrial mutations in Leber’s hereditary optic neuropathy patients. Eye Vis. (Lond.) 2015;2(1):18. doi: 10.1186/s40662-015-0028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huoponen K., Vilkki J., Aula P., et al. A new mtDNA mutation associated with Leber hereditary optic neuroretinopathy. Am. J. Hum. Genet. 1991;48(6):1147–1153. [PMC free article] [PubMed] [Google Scholar]
- 38.Howell N., Bindoff L.A., McCullough D.A., et al. Leber hereditary optic neuropathy: Identification of the same mitochondrial ND1 mutation in six pedigrees. Am. J. Hum. Genet. 1991;49(5):939–950. [PMC free article] [PubMed] [Google Scholar]
- 39.Mashima Y., Yamada K., Wakakura M., et al. Spectrum of pathogenic mitochondrial DNA mutations and clinical features in Japanese families with Leber’s hereditary optic neuropathy. Curr. Eye Res. 1998;17(4):403–408. doi: 10.1080/02713689808951221. [DOI] [PubMed] [Google Scholar]
- 40.Zhang A.M., Jia X., Guo X., et al. Mitochondrial DNA mutation m.3635G>A may be associated with Leber hereditary optic neuropathy in Chinese. Biochem. Biophys. Res. Commun. 2009;386(2):392–395. doi: 10.1016/j.bbrc.2009.06.051. [DOI] [PubMed] [Google Scholar]
- 41.Zhang M. Mitochondrial haplogroup M9a specific variant NDI T3394C may have a modifying role in the phenotypic expression of the LHON-associated ND4 G11778A mutalion. Mol. Genet. Metab. 2010;10l(2-3):192–199. doi: 10.1016/j.ymgme.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 42.Man P.Y.W., Turnbull D.M., Chinnery P.F. Leber hereditary optic neuropathy. J. Med. Genet. 2002;39(3):162–169. doi: 10.1136/jmg.39.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meunier I., Lenaers G., Hamel C., et al. Hereditary optic neuropathies:from clinical signs to diagnosis. J. Fr. Ophtalmol. 2013;36(10):886–900. doi: 10.1016/j.jfo.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Nikoskelainen E.K., Huoponen K., Juvonen V., et al. Ophthalmologic findings in Leber hereditary optic neuropathy, with special reference to mtDNA mutations. Ophthalmol. 1996;103(3):504–514. doi: 10.1016/s0161-6420(96)30665-9. [DOI] [PubMed] [Google Scholar]
- 45.Klopstock T., Yu-Wai-Man P., Dimitriadis K., et al. A randomized placebo-controlled trial of idebenone in Leber’s hereditary optic neuropathy. Brain. 2011;134(9):2677–2686. doi: 10.1093/brain/awr170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DiMauro S., Schon E.A. Mitochondrial respiratory-chain diseases. N. Engl. J. Med. 2003;348(26):2656–2668. doi: 10.1056/NEJMra022567. [DOI] [PubMed] [Google Scholar]
- 47.DiMauro S., Davidzon G. Mitochondrial DNA and disease. Ann. Med. 2005;37(3):222–232. doi: 10.1080/07853890510007368. [DOI] [PubMed] [Google Scholar]
- 48.Koilkonda RD, Guy J. Koilkonda RD, Guy J. Leber's hereditary optic neuropathy-gene therapy: From benchtop to bedside. J Ophthalmol. 2011;2011(2011):16. doi: 10.1155/2011/179412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brady S.T., Lasek R.J., Allen R.D. Video microscopy of fast axonal transport in extruded axoplasm:a new model for study of molecular mechanisms. Cytoskeleton (Hoboken) 1985;5(2):81–101. doi: 10.1002/cm.970050203. [DOI] [PubMed] [Google Scholar]
- 50.Chinnery P.F., Andrews R.M., Turnbull D.M., et al. Leber hereditary optic neuropathy: Does heteroplasmy influence the inheritance and expression of the G11778A mitochondrial DNA mutation? Am. J. Med. Genet. A. 2001;98(3):235–243. doi: 10.1002/1096-8628(20010122)98:3<235::aid-ajmg1086>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 51.Qu J., Li R., Zhou X., et al. The novel A4435G mutation in the mitochondrial tRNAMet may modulate the phenotypic expression of the LHON-associated ND4 G11778A mutation. Invest. Ophthalmol. Vis. Sci. 2006;47(2):475–483. doi: 10.1167/iovs.05-0665. [DOI] [PubMed] [Google Scholar]
- 52.Qu J., Li R., Zhou X., et al. Cosegregation of the ND4 G11696A mutation with the LHON-associated ND4 G11778A mutation in a four generation Chinese family. Mitochondrion. 2007;7(1):140–146. doi: 10.1016/j.mito.2006.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liang M., Guan M., Zhao F., et al. Leber’s hereditary optic neuropathy is associated with mitochondrial ND1 T3394C mutation. Biochem. Biophys. Res. Commun. 2009;383(3):286–292. doi: 10.1016/j.bbrc.2009.03.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou X., Zhang H., Zhao F., et al. Very high penetrance and occurrence of Leber’s hereditary optic neuropathy in a large Han Chinese pedigree carrying the ND4 G11778A mutation. Mol. Genet. Metab. 2010;100(4):379–384. doi: 10.1016/j.ymgme.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johns D.R., Neufeld M.J., Park R.D., et al. An ND-6 mitochondrial DNA mutation associated with leber hereditary optic neuropathy. Biochem. Biophys. Res. Commun. 1992;187(3):1551–1557. doi: 10.1016/0006-291x(92)90479-5. [DOI] [PubMed] [Google Scholar]
- 56.Man P.Y., Turnbull D.M., Chinnery P.F., et al. Leber hereditary optic neuropathy. J. Med. Genet. 2002;39(3):162–169. doi: 10.1136/jmg.39.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown M.D., Trounce I.A., Jun A.S., et al. Functional analysis of lymphoblast and cybrid mitochondria containing the 3460, 11778, or 14484 leber’s hereditary optic neuropathy mitochondrial DNA mutation. J. Biol. Chem. 2000;275(51):39831–39836. doi: 10.1074/jbc.M006476200. [DOI] [PubMed] [Google Scholar]
- 58.Brown M.D. The enigmatic relationship between mitochondrial dysfunction and Leber’s hereditary optic neuropathy. J. Neurol. Sci. 1999;165:1–5. doi: 10.1016/s0022-510x(99)00087-8. [DOI] [PubMed] [Google Scholar]
- 59.Guy J., Qi X., Pallotti F., et al. Rescue of a mitochondrial deficiency causing Leber Hereditary Optic Neuropathy. Ann. Neurol. 2002;52(5):534–542. doi: 10.1002/ana.10354. [DOI] [PubMed] [Google Scholar]
- 60.De Grey A.D.N.J. Mitochondrial gene therapy: An arena for the biomedical use of inteins. Trends Biotechnol. 2000;18(9):394–399. doi: 10.1016/s0167-7799(00)01476-1. [DOI] [PubMed] [Google Scholar]
- 61.Yu H., Mehta A., Wang G., et al. Next-generation sequencing of mitochondrial targeted AAV transfer of human ND4 in mice. Mol. Vis. 2013;19:1482–1491. [PMC free article] [PubMed] [Google Scholar]
- 62.Bonnet C., Kaltimbacher V., Ellouze S., et al. Allotopic mRNA localization to the mitochondrial surface rescues respiratory chain defects in fibroblasts harboring mitochondrial DNA mutations affecting complex I or v subunits. Rejuvenation Res. 2007;10(2):127–144. doi: 10.1089/rej.2006.0526. [DOI] [PubMed] [Google Scholar]
- 63.Koilkonda R.D., Chou T.H., Porciatti V., et al. Induction of rapid and highly efficient expression of the human ND4 complex I subunit in the mouse visual system by self-complementary adeno-associated virus. Arch. Ophthalmol. 2010;128:876–883. doi: 10.1001/archophthalmol.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mashima Y., Hiida Y. Remission of Leber’s hereditary optic neuropathy with idebenone. Lancet. 1992;340:368–369. doi: 10.1016/0140-6736(92)91442-b. [DOI] [PubMed] [Google Scholar]
- 65.Meyerson C., Van Stavern G., McClelland C. Leber hereditary optic neuropathy: Current perspectives. Clin. Ophthalmol. 2015;9:1165. doi: 10.2147/OPTH.S62021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klopstock T., Yu-Wai-Man P., Dimitriadis K., et al. A randomized placebo-controlled trial of idebenone in Leber’s hereditary optic neuropathy. Brain. 2011;134(9):2677–2686. doi: 10.1093/brain/awr170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Newman N.J. Treatment of Leber hereditary optic neuropathy. Brain. 2011;134(9):2447–2450. doi: 10.1093/brain/awr192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vugler A., Carr A.J., Lawrence J., et al. Elucidating the phenomenon of HESC-derived RPE: Anatomy of cell genesis, expansion and retinal transplantation. Exp. Neurol. 2008;214(2):347–361. doi: 10.1016/j.expneurol.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 69.Feuer W.J., Schiffman J.C., Davis J.L., et al. Gene therapy for Leber hereditary optic neuropathy: Initial results. Ophthalmology. 2016;123(3):558–570. doi: 10.1016/j.ophtha.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.MacLaren R.E., Groppe M., Barnard A.R., et al. Retinal gene therapy in patients with choroideremia: Initial findings from a phase 1/2 clinical trial. Lancet. 2014;383(9923):1129–1137. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boye S.E., Boye S.L., Lewin A.S. A comprehensive review of retinal gene therapy. Mol. Ther. 2013;21:509–519. doi: 10.1038/mt.2012.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rakoczy E.P., Lai C.M., Magno A.L., et al. Gene therapy with recombinant adeno-associated vectors for neovascular age-related macular degeneration: 1 year follow-up of a phase 1 randomised clinical trial. Lancet. 2015;386(10011):2395–2403. doi: 10.1016/S0140-6736(15)00345-1. [DOI] [PubMed] [Google Scholar]
- 73.Nguyen Q.D., Schachar R.A., Nduaka C.I., et al. Phase 1 dose-escalation study of a siRNA targeting the RTP801 gene in age-related macular degeneration patients. Eye (Lond.) 2012;26:1099–1105. doi: 10.1038/eye.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moreno-Montañés J., Sádaba B., Ruz V., et al. Phase I clinical trial of SYL040012, a small interfering RNA targeting β-adrenergic receptor 2, for lowering intraocular pressure. Mol. Ther. 2014;22:226–232. doi: 10.1038/mt.2013.217. [DOI] [PMC free article] [PubMed] [Google Scholar]