Abstract
The fractalkine receptor chemokine (C-X3-C motif) receptor 1 (CX3CR1) and its highly se-lective ligand CX3CL1 mediate chemotaxis and adhesion of immune cells, which are involved in the pathogenesis and progression of numerous inflammatory disorders and malignancies. The CX3CL1/CX3CR1 axis has recently drawn attention as a potential therapeutic target because it is in-volved in the ontogeny, homeostatic migration, or colonization of renal phagocytes. We performed a Medline/PubMed search to detect recently published studies that explored the relationship between the CX3CL1/CX3CR1 axis and renal diseases and disorders, including diabetic nephropathy, renal allograft rejection, infectious renal diseases, IgA nephropathy, fibrotic kidney disease, lupus nephritis and glo-merulonephritis, acute kidney injury and renal carcinoma. Most studies demonstrated its role in promot-ing renal pathopoiesis; however, several recent studies showed that the CX3CL1/CX3CR1 axis could also reduce renal pathopoiesis. Thus, the CX3CL1/CX3CR1 axis is now considered to be a double-edged sword that could provide novel perspectives into the pathogenesis and treatment of renal diseases and disorders.
Keywords: Fractalkine, CX3CL1, Chemokine receptor, CX3CR1, Renal disease, Kidney transplantation
1. INTRODUCTION
1.1. CX3CL1/CX3CR1 Axis
Chemokine (C-X3-C motif) Receptor 1 (CX3CR1) is ubiquitously expressed in most tissues on mononuclear and circulatory lymphatic leucocytes [1]. Fractalkine, also known as CX3CL1, is the only ligand of CX3CR1, which is a sole chemokine that not only involves a chemoattractant function but also assists CX3CR1+ cells to adhere; thus, the CX3CL1/CX3CR1 axis is a new type of leukocyte-migration controller [2]. CX3CL1 is mainly produced by the endothelium and has two forms (membrane and soluble). Membrane CX3CL1 is an adhesion molecule, but soluble CX3CL1 is a chemoattractant for CX3CR1+ cells [3].
Leukocytes can tether and roll on endothelium in canonical pathway, by which leukocytes are exposed to the surface of the local endothelium, which produces chemokines by binding to glycosaminoglycans. When chemokines contact their homologous receptors, signal transduction activates integrins to form firm adhesions between rolling leukocytes and the endothelial surface. Finally, leukocytes change their shape and start diapedesis through the endothelium, arriving in the tissue [4]. It has been clearly demonstrated that CX3CR1 selectively exists on different lineages of cytotoxic leukocytes with high contents of intracellular perforin, granzyme B and death-signaling Fas ligand, which could help leukocytes undergo transendothial migration to infiltrate into inflamed tissues [5].
In humans, CX3CL1 is mainly expressed on the tubular epithelium, especially during inflammation. Concomitantly, CX3CR1-expressing monocytes and T cells are ubiquitously expressed in inflammatory renal tissues in patients [6, 7]. How does the inflammatory response link to the CX3CL1/CX3CR1 axis? When microbes, injuries and non-self-antigens stimulate the kidney, innate immune cells respond via special receptors, pattern recognition receptors (PRRs) [8]. Their ligands include Pathogen-Associated Molecular Patterns (PAMPs) [9], damage- or Danger-Associated Molecular Patterns (DAMPs) [10] and allogeneic non-self-associated patterns [11]. All of the above effects are able to initiate the inflammatory cascade and produce inflammatory cytokines. The CX3CL1/CX3CR1 axis is activated by inflammatory cytokines, such as interferon-γ (INF-γ), interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α), IL-10, and IL-6 [12, 13]. CX3CL1 is mainly produced by glomerular endothelial cells and the tubular epithelium and can be detected in many other cells, such as podocytes, mesangial cells, renal tumor cells and stromal cells [14-16]. CX3CR1 is a G-protein coupled receptor that is expressed on CD8+ T lymphocytes [17], mast cells [18], Natural Killer (NK) cells [19], Dendritic Cells (DCs) [20], platelets [21], renal cancer cells [22], vascular smooth cells [23], tubular cells [24] mesenchymal cells [25], and monocytes/macrophages [26, 27]. After CX3CL1 conjugates with CX3CR1, the CX3CL1/CX3CR1 axis can initiate a cascade via several signaling pathways in the kidney, including ROS/MAPKS, Raf/MEK1/2-ERK1/2-Akt/PI3K, and nuclear factor of kappa light polypeptide gene enhancer in B cells 1 (NF-kB). CX3CL1/CX3CR1 axis directly up-regulates mesangial cell expansion via Reactive Oxygen Species (ROS) and Mitogen-Activated Protein Kinase (MAPK) diabetic nephropathy [28]. The CX3CL1/ CX3CR1 axis also activates vascular smooth muscle cell proliferation through Phosphatidylinositol-3 Kinase (PI3K), Akt and NF-kB [29]. According to Yao’s observation, CX3CR1 was expressed in human clear cell Renal Cell Carcinoma (RCC) cell lines, and only membrane positive cells were responsible for CX3CL1‐induced cell migration. Extracellular signal-Related Kinases (ERK1/2) and PI3K/Akt were triggered by soluble CX3CL1 dependent on time [22]. These concepts are summarized schematically in Fig. (1).
Fig. (1).
The general process of CX3CL1/CX3CR1 axis cascade. (1) Stimulation process: stranger pattern (microbes), danger pattern (injuries) and allogeneic non-self pattern stimulate the kidney. (2) Inflammatory factors release process: INF-γ,TNF-α, IL-1, MCP-1, IL-10, IL-6 are produced by (1). (3) CX3CL1 expression process: fractalkine is produced and increasingly expresses by endothelium, tubular epithelium, podocytes, mesangial cells, renal tumor cell, and stromal cells. (4) CX3CR1 conjugation process: fractalkine would conjugate with CX3CR1+ cells such as monocytes/macrophages, NK, γδ T cells, CD8+ T cells, DC, NKT, mast cells, cancer cells, vascular smooth cells, tubular cells, mesenchymal cells, and platelets. (5) CX3CL1/CX3CR1 axis effect process: CX3CL1/CX3CR1 axis would produce effect via ROS/MAPKS, Raf/MEK1/2-ERK1/2-Akt/PI3K, NF-kB pathways.
2. CX3CL1/CX3CR1 AXIS IN NEPHROPATHIES
In many renal diseases, CX3CL1 expression and CX3CR1+ cells are evident in patients. A major observation regarding the role of CX3CL1/CX3CR1 axis was reported in renal disease in the context of ischemic Acute Renal Failure (ARF). Oh and colleagues recently reported the up-regulation of CX3CL1 in the renal endothelium and the protective effect of macrophage depletion during ischemic ARF in mice by blocking CX3CR1 on macrophages via specific blocking antibodies, thereby decreasing macrophage infiltration into the ischemic kidney. Similarly, decreased renal fibrosis after Ischemia-Reperfusion Injury (IRI) was achieved through CX3CR1-blocking antibodies [30]. Furthermore, CX3CL1 is known to play a critical role in glomerulonephritis by acting as a chemoattractant and adhesion molecule [31]. Indeed, the majority of leukocytes that infiltrate the kidney during glomerulonephritis or other nephropathies were shown to express CX3CR1. Moreover, Inoue et al. designed a potent CX3CL1 antagonist that delayed onset and progression of lupus nephritis in MRL/lpr mice, once again underlining the major therapeutic potential associated with CX3CR1 antagonism [32]. In a recent study, Lakkis et al. found the mainstream of recipient DCs which could replace donor DCs in heart and kidney grafts are non-conventional CD11b+CD11c+ DCs that produce IL-12 and originate from non-classical monocytes, which highly express CX3CR1 [33]. Nevertheless, Engel et al. revealed that CX3CR1 could reduce kidney fibrosis through preventing retention of profibrotic macrophages locally [27]. Chousterman et al. reported that inflammatory monocytes played a protective role in sepsis based on the CX3CR1 pathway [26]. Thus, the CX3CL1/CX3CR1 axis is considered to be a double-edged sword.
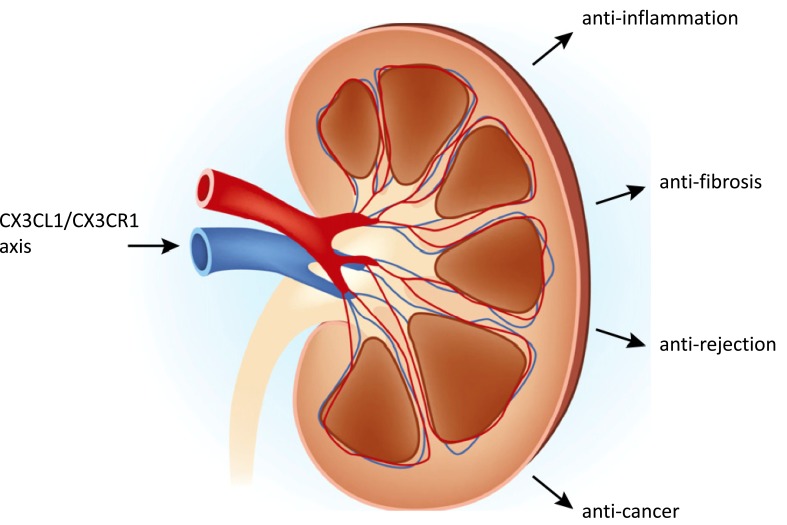
Pondering the relationship between the kidney disease therapy and the CX3CL1/CX3CR1 axis immediately brings to mind anti-inflammation, anti-fibrosis, anti-rejection and anti-cancer components (Fig. 2). We discuss the role of CX3CL1/CX3CR1 axis in kidney diseases and disorders in detail below to provide novel perspectives into the pathogenesis and treatment of renal diseases and disorders.
Fig. (2).
The relationship between the kidney diseases therapy and the CX3CL1/CX3CR1 axis. Anti-inflammation, anti-fibrosis, anti-rejection and anti-cancer are the four main purposes to figure out CX3CL1/CX3CR1 axis. (The original kidney picture was copied and modified from Yatim KM, Lakkis FG. A brief journey through the immune system. Clin J Am Soc Nephrol 2015; 10(7): 1274-1281.)
2.1. Diabetic Nephropathy
Diabetic nephropathy is the leading cause of end-stage renal disease (ESRD) worldwide [34]. CX3CL1/CX3CR1 axis in diabetic renal disease was firstly assessed by CX3CR1 Knockout (KO) mice twelve weeks after inducing diabetes. The function of the CX3CL1/CX3CR1 pathway was first investigated using a diabetic model, Streptozotocin (STZ)-injected CX3CR1 gene knockout mice. Macrophage aggression and renal Extracellular Matrix (ECM) accumulation were aggravated in diabetic mice [35]. CX3CL1 triggers vascular smooth muscle cell proliferation via PI3K/Akt and NF-kB [29] and stimulates the Janus kinase and Stat pathways in severe acute pancreatitis of rat [36]. ROS and MAPK were shown to be the signal molecules that were induced by CX3CL1, and blockade of ROS and MAPK successfully prevented synthesis of the mesangial ECM induced by CX3CL1 [28].
2.2. Allograft Function and Rejection
A small sample-size, half-quantified, retrospective study displayed that the CX3CL1 [15] and CX3CR1 [6] levels were higher in a transplanted kidney with a pathological diagnosis of acute cellular rejection than that with no sign of rejection. Hoffmann’s work studied the expression of CX3CR1 in biopsies of 174 renal allografts. The results revealed that only a minor percentage of CX3CR1+ cells was detectable in normal kidney, while CX3CR1 was generally expressed in grafts that were pathologically diagnosed as acute tubulointerstitial and vascular allograft rejection. Additionally, in acute tubulointerstitial rejection, CX3CR1+ cells infiltrated the interstitial tissues and were mainly located around the peritubular capillaries, and they were also found in the glomeruli in a few patients. In acute vascular rejection, CX3CR1+ cells were ubiquitously detectable in the subendothelial regions of the arteries [37]. In fact, only a minor portion of CX3CR1+ cells could express CD4 and CD8 in allogeneic renal transplantation tissue. However, the majority of CX3CR1+ cells were CD68+ mononuclear cells and CD209+ DCs. Because successive macrophages infiltrated both the glomeruli and interstitium of the grafts, which could cause fibrosis, fibrosis is also included in the pathogenesis of chronic kidney transplant rejection [38].
A recent work by our team showed that host DCs played a pivotal role in kidney allograft rejection, and most of the host DCs originated from non-classical monocytes (CX3CR1high), which revealed that CX3CR1 might play a significant role in the formation of monocyte-derived DCs (mono-DCs) in kidney transplants [33]. In our paper, these CX3CR1high mono-DCs formed stable, cognate interactions with effector T cells in the graft, as visualized by a multiphoton intravital microscope. We are presently investigating whether this cell-cell interaction can be attenuated by CX3CR1 blockade or gene knockout (unpublished data). Zhang et al. found that CX3CR1 and CX3CL1 were good predictors for diagnosing Acute Rejection (AR) in allogeneic renal transplant patients. They tested the CX3CL1 and CX3CR1 serum levels at day -1 and every other day (5 times in total) post-kidney transplantation. In the AR group with a pathological diagnosis, the serum levels of CX3CL1 and CX3CR1 were notably higher than those in the non-AR group. Moreover, in the AR group, high expression of serum CX3CL1 and CX3CR1 was positively associated with the onset time of AR. Moreover, in patients with increased CX3CL1 and CX3CR1 serum levels, 3 patients had pathological diagnoses of early AR but no sign of increased serum creatinine and proteinuria, which indicated that the serum CX3CL1 and CX3CR1 levels were more sensitive than increased serum creatinine at diagnosing early AR [39].
Delayed Graft Function (DGF), defined as a need for dialysis during the first 7 days after transplantation, is a common post-renal transplantation complication [40]. Dabrowska-Zamojcin et al. reported a relationship between the CX3CR1 gene V249I (rs3732379) Single-Nucleotide Polymorphism (SNP) and allograft function in the kidney. In their paper, DGF was diagnosed in 39.16% of individuals with the CC genotype, 22.73% with the CT genotype and 23.53% with the TT genotype. Therefore, the CC genotype was considered as an independent and substantial predictor of DGF [41].
As described in the studies mentioned above, in chronic renal allograft rejection, the levels of CX3CL1 and CX3CR1 were increased. Because microcapillary inflammation is a significant feature of chronic renal allograft rejection, Park et al. tested whether monocytes attached mesangial cells via the CX3CL1/CX3CR1 axis in a Lipopolysaccharide (LPS)-stimulated medium ex vivo. They found that siRNA against CX3CL1 or CX3CR1 effectively inhibited LPS-induced monocyte-mesangial cell attachment. In mesangial cells stimulated by LPS, the mRNA levels of CX3CL1 and CX3CR1 were improved and CX3CL1 protein synthesis was increased. In summary, it can be concluded that monocytes attach mesangial cells via the CX3CL1/CX3CR1 axis; moreover, the CX3CL1/ CX3CR1 axis could provide the evidence to the development of inflammation, causing chronic renal allograft rejection [42].
2.3. Infection- and Sepsis-induced Renal Diseases
As chemokines play key roles in regulating leukocyte migration, they are widely considered as possible therapeutic targets in sepsis. Raspé and his colleagues found increased levels of CX3CL1/CX3CR1 mRNA in sepsis models induced by Cecum Ligation and Puncture (CLP), and increased levels were also observed in plasma 24 and 48 hours after CLP. Furthermore, CLP-induced down-regulation of CX3CR1 in the kidney was reversed by pre-treatment with the selective NF-kB inhibitor Pyrrolidine Dithiocarbamate (PDTC). These results suggested that expression of the related ligands and receptors induced by CLP led to reversed regulation (up-regulation of CX3CL1 and down-regulation of CX3CR1), which might be regulated by the transcription factor NF-kB, probably through abridged release of inflammatory cytokines [43].
Chousterman et al. showed that inflammatory monocytes had protective effects on renal sepsis through a CX3CR1-dependent adhesion mechanism. During sepsis with multiple pathogenic bacteria, inflammatory monocytes migrated from bone marrow, approached the renal cortex endothelial cells and stimulated monocytosis in several hours by increasing CX3CR1-related adhesion. Deficiency of CX3CR1 increased kidney damage and reduced mice mortality, which was related to the migration of monocytes. They also confirmed that the protective function of CX3CR1 was related to the reduction of inflammatory monocyte adhesion and IL-1ra secretion by Ly6Chigh monocytes. In human diseases, CX3CR1 also took part in the pathogenesis of sepsis, and a study of CX3CR1 gene polymorphism showed the I249 CX3CR1 allele was correlated with more monocytes adhesion and less renal injury [26].
In acute systemic infection, Cytomegalovirus (CMV) spreads throughout the organism. Vascular endothelial cells are the main candidate host cells that promote virus transmission of the vascular pathway. CMV-infected endothelial cells are also believed to play a critical role in the inflammatory response in acute infection [44]. This is why CX3CL1+ endothelial cells are the critical site of CMV latency and reoccurrence. At the same time, latent CMV infected patients with ESRD is related to the expansion of circulation, late differentiation, and cytotoxicity of CD4+ T cells.
These effects are characterized by the lack of the costimulator marker CD28 from the cell surface (CD4+CD28null) [45]. In Shabir’s study, CD4+CD28null T cells were mainly located in the patients with CMV infection and were amplified after transplantation. Therefore, improving CX3CR1+ CD4+CD27-CD28null cells was of biological significance to CMV cellular immunity [46].
2.4. IgA Nephropathy
IgA nephropathy (IgAN) is the most common form of primary glomerulonephritis throughout the world and has a large impact on 20%-50% of patients with glomerulonephritis. Between 30%-40% of patients with IgAN develop ESRD in 2 decades [47]. Consequently, IgAN is considered as a foremost cause of ESRD in many countries. Kim and his colleagues recently found that mice treated with anti-APRIL (a proliferation-inducing ligand) antibodies decreased evolution of IgAN, serum IgA levels, and glomerular IgA accumulation, and also decreased inflammation mediated by CX3CL1/CX3CR1-related activation of monocytes [48].
Tonsillectomy has been reported to be one of the applicable therapies for IgAN patients [49]. In IgAN patients, an over-immune response of Tonsillar Mononuclear Cells (TMCs) to microbial DNA causes IFN-γ-mediated up-regulation and enhanced production of B-cell-Activation Factor (BAFF), resulting in boosted production of IgA [50]. Otaka et al. recently presented an interesting study on the correlation between IgAN and tonsils. They analyzed the expression of CX3CR1 in tonsillar mononuclear cells of IgAN patients. Immunohistochemical analysis concluded that the distribution of CX3CR1+ cells in the inter-follicular region of the tonsillar area in IgAN patients was superior to that of non-IgAN patients.
Flow cytometry revealed that expression of CX3CR1+ CD8+ T cells in the tonsil was considerably increased in IgAN patients. Chemotaxis of CX3CL1 in the tonsillar mononuclear cells of IgAN patients was meaningfully increased. CX3CR1 had a highly significant effect on the expression of CD8+ T cells in IgAN patients. The toxicity of T cells after tonsillectomy was obviously decreased, and the hematuria was also relieved [17].
Cox et al. unveiled that high level of CX3CR1 on circulatory T cell subsets, for example, γδ T cells, which adjusted IgA production in mucosa. CX3CR1 was also found to be up-regulated on NK cells, NKT cells, CD8+ T cells. Ex vivo, in Peripheral Blood Mononuclear Cells (PBMCs) of IgAN patients, but not from those with membranous or membranoproliferative glomerulonephritis, LPS-induced CX3CR1 expression was increased, implying an endogenous tendency of IgAN PBMCs expressing CX3CR1 [51, 52].
2.5. Fibrotic Kidney Disease
Some studies have shown that the CX3CL1/CX3CR1 axis might play a role in tubulointerstitial fibrosis, without considering the primary diseases. Koziolek et al. found that expression of CX3CR1 was higher in the kidneys of patients with renal fibrosis compared with those of patients with non-fibrotic, non-inflammatory nephropathies [53]. Peng and his colleagues showed that the CX3CL1–CX3CR1 pair acted as a destructive pathway in obstructed renal fibrosis, which appeared to operate by promoting Ly6C-CX3CR1high macrophage accumulation in the kidney [54]. CX3CL1-CX3CR1 pair promoted Ly6C-CX3CR1high macrophage survival and maintenance in the obstructed kidney. Nevertheless, these results also showed that the CX3CL1–CX3CR1 pair was able to replace monocyte trafficking or differentiation in the obstructed kidneys. Remarkably, a study by Engel et al. based on their previous study verified that CX3CR1 deficiency could attenuate renal fibrosis. However, through the DC-independent mice fibrotic model of Unilateral Ureteral Obstruction (UUO), renal fibrosis was unpredictably severer.
Macrophages were more plentiful in the nonappearance of CX3CR1 and formed more profibrotic mediators in the kidney via flow cytometry, such as Transforming Growth Factor-β (TGF-β) [27].
Chronic Kidney Disease (CKD) could accelerate the progress of organ impairment [55]. At stage 5 of CKD, basal activation of leukocytes has been associated with micro-inflammation and atherosclerosis. Uremic retention solutes and kidney-replacing treatment result in many indispensable alterations of leukocyte function, such as in ROS, apoptosis, chemotaxis and cytokine secretion, validating that leukocytes play a crucial role in vasculopathy of CKD [56]. CX3CR1+ CD16+ cells therefore favorably enter the endothelium and regions of inflammation by locally expressed CX3CL1, which has been shown to be up-regulated in chronic inflammatory situations [57]. Zaza et al. showed that the transcriptome profile of circulatory mononuclear cells (involving CX3CR1+ cells) was differentiated in patients of renal replacement therapy [58].
In the inflammatory response of the kidneys, CX3CL1 secreted from proximal tubular cells played a pivotal role in leukocyte enrollment and retaining to the interstitial tissue [59]. Additionally, these polymorphisms are also investigated in glucometabolic-, cardiovascular- and obesity-associated diseases [60, 61]. Shah et al. confirmed that CX3CR1 deficiency had a modest preventive impact on insulin resistance [62]. The II genotype and I allele frequencies of the CX3CR1 V249I polymorphism were discovered notably more frequently in Chronic Renal Failure (CRF), CRF with diabetes mellitus and atherosclerosis than control groups [63].
2.6. Lupus Nephritis and Glomerulonephritis
Systemic Lupus Erythematosus Erythema (SLE) is an autoimmune disease that mostly affects women at young ages and can affect a variety of organs and systems. If the kidney is involved, it is called Lupus Nephritis (LN), which is a serious complication of SLE, and is one of the highest causes of morbidity and mortality in SLE patients [64]. In LN, CX3CL1 is mostly expressed on the glomerular endothelium, but mesangial cells and the interstitial microvasculature can also secrete it. CX3CR1 is expressed on macrophages and is essential for their migration [65].
In an inducible lupus model in which SCID mice are injected with hybridomas derived from MRL/lpr mice [66], diseased kidneys display up-regulated CX3CL1, which subsequently attracts macrophages. Cros et al. reported the occurrence of CD16+ monocytes within the glomerular blood vessels of LN patients, which correspond to the high CX3CR1+ expressing monocytes [67]. Taken together, this evidence indicates that targeting CX3CL1 signaling would be worthwhile for decreasing monocyte recruitment to the kidney, thus interfering with this pathway in LN pathogenesis.
DCs exist in almost all organs, playing a leading role in maintaining organ homeostasis and inducing the immune response against the invasion of pathogens [68, 69]. Hochheiser et al. determined that CX3CR1 could be defined as the specific “Homing Receptor” of the kidney DCs. DCs were notably abridged in CX3CR1 KO mice kidneys, which was not detected in other organs, excluding the small intestine. The symptoms of Glomerulonephritis (GN) were improved in the CX3CR1 KO mouse model due to the CX3CR1-dependent reduction of DCs, especially in the cortex of kidney. Conversely, immune defenses had no significant effect on the most frequent kidney infection, bacterial pyelonephritis, in CX3CR1 KO mice [70]. In summary, CX3CR1 is believe to be a therapeutic target for GN because CX3CR1 selectively affected the DCs in the kidneys and did not make mice more susceptible to bacterial renal infections.
2.7. Kidney Injury
Monocytes exist in two main forms, termed as, classical and non-classical. Evidence has shown that non-classical monocytes (CX3CR1high CCR2− Ly6C−) perform a specialized form of immune homeostasis, as stimulators of acutely inflamed responses and making contribution to tissue remodeling [71, 72]. Finsterbusch et al. observed cell-cell contacts of CX3CR1high monocytes and neutrophils in both non-inflammatory and inflammatory glomeruli by multiphoton and confocal intravital microscopy. In the model of induced glomerular inflammation, the network of neutrophils and CX3CR1high monocytes showed that the retention time of these cells in was prolonged in glomerular capillaries, and there was a tendency to produce ROS, which eventually resulted in kidney injury [73, 74]. In this study, inflammatory monocytes during the initial adhesion to the glomerular capillary required LFA-1 and CX3CR1. However, the neutrophils in the glomerular capillary were immediately arrested, which did not required the prior rolling process, but did require the function of P-selectin, a classical adhesion molecule, for leukocyte rolling [75]. Inflammation triggers TNF production of monocytes in the kidney, which can lead to kidney damage by prolonging the dwell time of neutrophils and activating the release of ROS from neutrophils. TNF can also rapidly produce ROS by stimulating the activity of NAPDH oxidase in neutrophils [76]. Thus, in the acute inflammatory glomeruli, the deficit of CX3CR1 and consequent decrease in monocyte retention were correlated with decreased neutrophil activation.
IRI is the main reason of acute and chronic renal dysfunction or failure. In renal IRI, invasive leukocytes and renal parenchyma cells, just like tubular epithelium, can produce chemokines [77]. In Stroo’s IRI model, the expression level of CX3CR1 was appreciably improved a week after operation and the invasion of macrophages also reached its highest peak, which indicated that CX3CR1 mediates macrophage trafficking to the kidney [78]. Furuichi et al. [79] revealed elevated expression of CX3CR1 occurred at a later stage of renal IRI and that the absence of CX3CR1 could lead to a decrease in invasion of macrophage peak a week after operation. Nevertheless, Oh et al. [30] determined that early ischemic Acute renal Tubular Necrosis (ATN) was slightly correlated with a CX3CR1-dependent process, similar to serum creatinine. In the early stage of ischemia reperfusion, inhibition of CX3CR1 decreased the ATN score and macrophage infiltration.
2.8. Renal Tumor and Metastasis
In addition to their function in the immune system, chemokines and their receptors also play crucial roles in tumor initiation, progression, and metastasis [80]. Previous studies have found that tumor cells originated from diverse cancer express distinct chemokine receptors, and thus may cause diverse metastatic abilities [81]. Clear Cell RCC (CCRCC), as the main classification of RCC, is an aggressive and refractory cancer [82]. Consequently, it is imperative to identify the key proteins that regulate CCRCC metastasis. Yao et al. demonstrated that CX3CR1 could be expressed on a RCC cell line, and activation of CX3CL1 could contribute to cancer cell migration and activation. ERK1/2 and Akt were phosphorylated after CX3CL1 secretion and participated in CX3CL1-induced cell movement. In addition, high expression of CX3CR1 was associated with poor prognosis of CCRCC [22]. This research provides insights into that CX3CR1 promotes chemotaxis of tumor cells, and CX3CL1/CX3CR1 axis might play a certain role in tumor metastasis in the kidney.
Chemokine receptors contribute to cancer cell metastasis and must meet the following criteria. First, matched chemokines must be expressed on the host site depending on the metastatic profile of the targeting cancer [83]. Therefore, CX3CL1-expressing tissue can be the priority target of CX3CR1 expression in circulating renal cancer cells. Second, chemokines must be able to promote adhesion ability of tumor cells to endothelium and migration to target sites [84]. Membrane-CX3CL1 combined with CX3CR1 is rapid and resolute, which leads to captive and arrested leukocytes even in normal blood flow. Finally, the chemotaxis process requires expression of the tumor cell chemokine receptor [20].
Liu and his colleagues reported higher CX3CL1 expression in serum samples of patients with spinal metastases originated from kidney cancer. For one, it is reasonable to assume that endothelium distributed in bone marrow sinusoids can express membrane-associated CX3CL1, which could supply attachment sites for cancer cells expressing CX3CR1 in circulation. For another, membrane-associated CX3CL1 could be detached into the bone marrow and circulation. Thus, a concentration gradient of chemokines has been established, which could attract CX3CR1+ tumor cells from the peripheral circulation into the spine. However, immunohistochemical staining for expression of CX3CR1 in spinal metastases samples from the kidney showed negative staining [85]. The reason why CX3CR1 is not detectable might be attributed to Epithelial-Mesenchymal Transition (EMT) [86]. In the course of EMT, CX3CR1 is only expressed in mesenchymal cells because of changes in cell properties, and it tends to infiltrate and metastasize. Another conceivable explanation would be that tumor cells without expression or with little expression of CX3CR1 are more probable to spread to sites of metastasis rather than in situ. If neoplastic cells within the primary mass lose any part of the chemokine axis (ligand or receptor), they are less engaged and free to spread and metastasize [64]. Thus, higher CX3CL1 concentrations in bone marrow and plasma are needed to enroll lower CX3CR1-expressing tumor cells. In addition, the antitumor effects of the CX3CL1/CX3CR1 axis are mostly related to the attraction of cytotoxic CD8+ T lymphocytes and NK cells. CX3CR1 is usually expressed in cytotoxic lymphocytes of peripheral blood. These cells release intracellular perforin and granzyme B [5]. Hong et al. found that CX3CL1 secreted into the tumor environment directly recruits CX3CR1-expressing NK cells to tumor tissues, and then, these NK cells could lyse cancer cells. Removal of these NK cells result in the disappearance of the anti-tumor immune response, confirming the function of CX3CL1/ CX3CR1 axis in the NK cell-mediated anti-tumor process. They also found that CD8+ T cells play an essential role in the CX3CL1-induced anti-tumor effect as cance specific Cytotoxic T Lymphocytes (CTL). In addition, in tumor tissues, they observed that CD4+ T cells can give rise into T helper 1 cells and enhance the ability of CTL to kill tumor cells through secretion of interferon gamma [87].
A malignant tumor after organ transplantation, such as post-transplantation lymphoproliferative disease (PTLD), is a foremost reason of recipient mortality without allograft dysfunction [88]. There are two ordinary SNPs situated in the coding sequence of the CX3CR1 gene, V249I and T280M, which were correlated with reduced numbers of CX3CL1 attached sites, attenuated cell adhesion, and reduced signaling and chemoattractant ability [89, 90]. In Courivaud’s cohort study, they showed that 622 renal transplant recipients and demonstrated that I249M280 homozygotes had an independent increased risk of cancer. Thus, CX3CR1 gene polymorphisms were correlated with a higher rate of tumor occurrence in renal transplant recipients [14].
CONCLUDING REMARKS AND THERAPEUTIC PROSPECTS
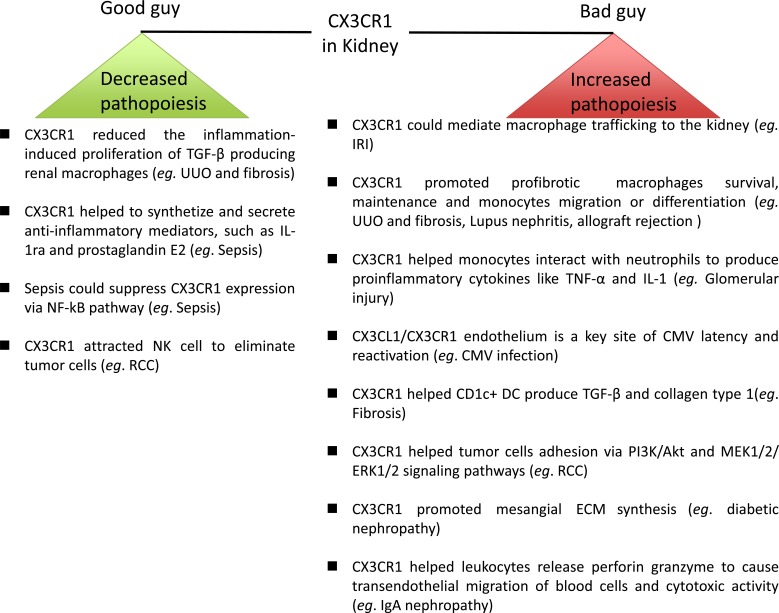
CX3CR1 is usually considered to be a negative factor, increasing injury in kidney diseases. However, this view is more controversial now because CX3CR1 can be a positive factor under some circumstances. Regarding the mechanisms of increasing or decreasing renal pathopoiesis, several explanations are summarized as follows (Fig. 3). Increasing pathopoiesis mechanisms: 1) CX3CR1 could mediate macrophage trafficking; 2) CX3CR1 promoted profibrotic macrophage survival and maintenance, as well as monocyte migration or differentiation; 3) CX3CR1 helped monocytes interact with neutrophils to produce proinflammatory cytokines, such as TNF-α and IL-1; 4) the CX3CL1/CX3CR1 endothelium is a key site of CMV latency and reactivation; 5) CX3CR1 helped CD1c+ DC produce TGF‐β and collagen type 1; 6) CX3CR1 helped tumor cell adhesion via the PI3K/Akt and MEK1/2/ERK1/2 signaling pathways; 7) CX3CR1 promoted mesangial ECM synthesis; and 8) CX3CR1 helped leukocytes release perforin and granzyme to cause transendothelial migration of blood cells and cytotoxic activity. Decreasing pathopoiesis mechanisms: 1) CX3CR1 reduced inflammation-induced proliferation of TGF-β producing renal macrophages; 2) CX3CR1 helped to synthesize and secrete anti-inflammatory mediators, such as IL-1ra and prostaglandin E2; 3) sepsis suppressed CX3CR1 expression via the NF-kB pathway; and 4) CX3CR1 attracted NK cells to eliminate tumor cells.
Fig. (3).
CX3CR1 as enhancers and regulators of pathopoiesis in kidney disease.
Although we discussed the dual function of the CX3CL1/CX3CR1 axis in the kidney diseases and disorders described above, many pathologies cannot be involved in one single disease. For instance, IRI and fibrosis could occur in the different stages of the kidney transplantation process. IRI mainly occurs in the stage of innate immunity of transplantation [91]. CX3CR1+ mononuclear cells, including DC, macrophages and CD4+ T cells, migrate into the kidney following IRI [92]. Human renal allograft chronic rejection is associated with endothelial cell damage, chronic vascular changes, and interstitial inflammation and fibrosis. The CX3CL1/CX3CR1 axis could contribute to the whole pathogenesis of renal allograft chronic rejection [93].
Therefore, we believe that both features should provide valuable opportunities in the future to inhibit renal injury in an accurate and safe manner. Despite potential future obstacles and disappointments, there is an urgent need to devise methods to manipulate the CX3CL1/ CX3CR1 axis due to its major and unique potential as a therapeutic target in inflammation, fibrosis, neoplasms, and transplantation rejection.
Consent for Publication
Not applicable.
Acknowledgements
This study was supported by the New Xiangya Talent Project of the Third Xiangya Hospital of Central South University (JY201629).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Julia V., Staumont-Salle D., Dombrowicz D. Role of fractalkine/CX3CL1 and its receptor CX3CR1 in allergic diseases. Med. Sci. (Paris) 2016;32:260–266. doi: 10.1051/medsci/20163203010. [DOI] [PubMed] [Google Scholar]
- 2.Stromberg A., Olsson K., Dijksterhuis J.P., Rullman E., Schulte G., Gustafsson T. CX3CL1--a macrophage chemoattractant induced by a single bout of exercise in human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016;310:R297–R304. doi: 10.1152/ajpregu.00236.2015. [DOI] [PubMed] [Google Scholar]
- 3.Liu W., Jiang L., Bian C., et al. Role of CX3CL1 in diseases. Arch. Immunol. Ther. Exp. (Warsz.) 2016;64:371–383. doi: 10.1007/s00005-016-0395-9. [DOI] [PubMed] [Google Scholar]
- 4.Springer T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura M., Umehara H., Nakayama T., et al. Dual functions of fractalkine/CX3C ligand 1 in trafficking of perforin+/granzyme B+ cytotoxic effector lymphocytes that are defined by CX3CR1 expression. J. Immunol. 2002;168:6173–6180. doi: 10.4049/jimmunol.168.12.6173. [DOI] [PubMed] [Google Scholar]
- 6.Segerer S., Hughes E., Hudkins K.L., Mack M., Goodpaster T., Alpers C.E. Expression of the fractalkine receptor (CX3CR1) in human kidney diseases. Kidney Int. 2002;62:488–495. doi: 10.1046/j.1523-1755.2002.00480.x. [DOI] [PubMed] [Google Scholar]
- 7.Yadav A.K., Lal A., Jha V. Association of circulating fractalkine (CX3CL1) and CX3CR1(+)CD4(+) T cells with common carotid artery intima-media thickness in patients with chronic kidney disease. J. Atheroscler. Thromb. 2011;18:958–965. doi: 10.5551/jat.8722. [DOI] [PubMed] [Google Scholar]
- 8.Oberbarnscheidt M.H., Lakkis F.G. Innate allorecognition. Immunol. Rev. 2014;258:145–149. doi: 10.1111/imr.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beutler B.A. TLRs and innate immunity. Blood. 2009;113:1399–1407. doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kono H., Rock K.L. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhuang Q., Lakkis F.G. Dendritic cells and innate immunity in kidney transplantation. Kidney Int. 2015;87:712–718. doi: 10.1038/ki.2014.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraticelli P., Sironi M., Bianchi G., et al. Fractalkine (CX3CL1) as an amplification circuit of polarized Th1 responses. J. Clin. Invest. 2001;107:1173–1181. doi: 10.1172/JCI11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallquist C., Paulson J.M., Hylander B., Lundahl J., Jacobson S.H. Increased accumulation of CD16+ monocytes at local sites of inflammation in patients with chronic kidney disease. Scand. J. Immunol. 2013;78:538–544. doi: 10.1111/sji.12115. [DOI] [PubMed] [Google Scholar]
- 14.Courivaud C., Bamoulid J., Loupy A., et al. Influence of fractalkine receptor gene polymorphisms V249I-T280M on cancer occurrence after renal transplantation. Transplantation. 2013;95:728–732. doi: 10.1097/TP.0b013e31827d61cb. [DOI] [PubMed] [Google Scholar]
- 15.Cockwell P., Chakravorty S.J., Girdlestone J., Savage C.O. Fractalkine expression in human renal inflammation. J. Pathol. 2002;196:85–90. doi: 10.1002/path.1010. [DOI] [PubMed] [Google Scholar]
- 16.Kim K.W., Vallon-Eberhard A., Zigmond E., et al. In vivo structure/function and expression analysis of the CX3C chemokine fractalkine. Blood. 2011;118:e156–e167. doi: 10.1182/blood-2011-04-348946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otaka R., Takahara M., Ueda S., Nagato T., et al. Up-regulation of CX3CR1 on tonsillar CD8-positive cells in patients with IgA nephropathy. Hum. Immunol. 2017;78:375–383. doi: 10.1016/j.humimm.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Papadopoulos E.J., Fitzhugh D.J., Tkaczyk C., et al. Mast cells migrate, but do not degranulate, in response to fractalkine, a membrane-bound chemokine expressed constitutively in diverse cells of the skin. Eur. J. Immunol. 2000;30:2355–2361. doi: 10.1002/1521-4141(2000)30:8<2355::AID-IMMU2355>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Hamann I., Unterwalder N., Cardona A.E., et al. Analyses of phenotypic and functional characteristics of CX3CR1-expressing natural killer cells. Immunology. 2011;133:62–73. doi: 10.1111/j.1365-2567.2011.03409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitching A.R. Dendritic cells in progressive renal disease: some answers, many questions. Nephrol. Dial. Transplant. 2014;29:2185–2193. doi: 10.1093/ndt/gfu076. [DOI] [PubMed] [Google Scholar]
- 21.Schafer A., Schulz C., Eigenthaler M., et al. Novel role of the membrane-bound chemokine fractalkine in platelet activation and adhesion. Blood. 2004;103:407–412. doi: 10.1182/blood-2002-10-3260. [DOI] [PubMed] [Google Scholar]
- 22.Yao X., Qi L., Chen X., Du J., Zhang Z., Liu S. Expression of CX3CR1 associates with cellular migration, metastasis, and prognosis in human clear cell renal cell carcinoma. Urol. Oncol. 2014;32:162–170. doi: 10.1016/j.urolonc.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Martynowicz H., Janus A., Nowacki D., Mazur G. The role of chemokines in hypertension. Adv. Clin. Exp. Med. 2014;23:319–325. doi: 10.17219/acem/37123. [DOI] [PubMed] [Google Scholar]
- 24.Furuichi K., Kaneko S., Wada T. Chemokine/chemokine receptor-mediated inflammation regulates pathologic changes from acute kidney injury to chronic kidney disease. Clin. Exp. Nephrol. 2009;13:9–14. doi: 10.1007/s10157-008-0119-5. [DOI] [PubMed] [Google Scholar]
- 25.Ge J., Guo L., Wang S., et al. The size of mesenchymal stem cells is a significant cause of vascular obstructions and stroke. Stem Cell Rev. 2014;10:295–303. doi: 10.1007/s12015-013-9492-x. [DOI] [PubMed] [Google Scholar]
- 26.Chousterman B.G., Boissonnas A., Poupel L., et al. Ly6Chigh Monocytes Protect against Kidney Damage during Sepsis via a CX3CR1-Dependent Adhesion Mechanism. J. Am. Soc. Nephrol. 2016;27:792–803. doi: 10.1681/ASN.2015010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engel D.R., Krause T.A., Snelgrove S.L., et al. CX3CR1 reduces kidney fibrosis by inhibiting local proliferation of profibrotic macrophages. J. Immunol. 2015;194:1628–1638. doi: 10.4049/jimmunol.1402149. [DOI] [PubMed] [Google Scholar]
- 28.Park J., Song K.H., Ha H. Fractalkine increases mesangial cell proliferation through reactive oxygen species and mitogen-activated protein kinases. Transplant. Proc. 2012;44:1026–1028. doi: 10.1016/j.transproceed.2012.03.045. [DOI] [PubMed] [Google Scholar]
- 29.Chandrasekar B., Mummidi S., Perla R.P., et al. Fractalkine (CX3CL1) stimulated by nuclear factor kappaB (NF-kappaB)-dependent inflammatory signals induces aortic smooth muscle cell proliferation through an autocrine pathway. Biochem. J. 2003;373:547–558. doi: 10.1042/BJ20030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh D.J., Dursun B., He Z., et al. Fractalkine receptor (CX3CR1) inhibition is protective against ischemic acute renal failure in mice. Am. J. Physiol. Renal Physiol. 2008;294:F264–F271. doi: 10.1152/ajprenal.00204.2007. [DOI] [PubMed] [Google Scholar]
- 31.Ito Y., Kawachi H., Morioka Y., et al. Fractalkine expression and the recruitment of CX3CR1+ cells in the prolonged mesangial proliferative glomerulonephritis. Kidney Int. 2002;61:2044–2057. doi: 10.1046/j.1523-1755.2002.00369.x. [DOI] [PubMed] [Google Scholar]
- 32.Inoue A., Hasegawa H., Kohno M., et al. Antagonist of fractalkine (CX3CL1) delays the initiation and ameliorates the progression of lupus nephritis in MRL/lpr mice. Arthritis Rheum. 2005;52:1522–1533. doi: 10.1002/art.21007. [DOI] [PubMed] [Google Scholar]
- 33.Zhuang Q., Liu Q., Divito S.J., et al. Graft-infiltrating host dendritic cells play a key role in organ transplant rejection. Nat. Commun. 2016;7:12623. doi: 10.1038/ncomms12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whiting D.R., Guariguata L., Weil C., Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 35.Song K.H., Park J., Park J.H., Natarajan R., Ha H. Fractalkine and its receptor mediate extracellular matrix accumulation in diabetic nephropathy in mice. Diabetologia. 2013;56:1661–1669. doi: 10.1007/s00125-013-2907-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang L.Y., Chen P., Xu L.X., Zhou Y.F., Zhang Y.P., Yuan Y.Z. Fractalkine upregulates inflammation through CX3CR1 and the Jak-Stat pathway in severe acute pancreatitis rat model. Inflammation. 2012;35:1023–1030. doi: 10.1007/s10753-011-9406-5. [DOI] [PubMed] [Google Scholar]
- 37.Hoffmann U., Bergler T., Segerer S., et al. Impact of chemokine receptor CX3CR1 in human renal allograft rejection. Transpl. Immunol. 2010;23:204–208. doi: 10.1016/j.trim.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Nankivell B.J., Borrows R.J., Fung C.L., O’Connell P.J., Allen R.D., Chapman J.R. The natural history of chronic allograft nephropathy. N. Engl. J. Med. 2003;349:2326–2333. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Q., Liu Y.F., Su Z.X., Shi L.P., Chen Y.H. Serum fractalkine and interferon-gamma inducible protein-10 concentrations are early detection markers for acute renal allograft rejection. Transplant. Proc. 2014;46:1420–1425. doi: 10.1016/j.transproceed.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 40.Domanski L., Kloda K., Kwiatkowska E., et al. Effect of delayed graft function, acute rejection and chronic allograft dysfunction on kidney allograft telomere length in patients after transplantation: a prospective cohort study. BMC Nephrol. 2015;16:23. doi: 10.1186/s12882-015-0014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dabrowska-Zamojcin E., Dziedziejko V., Safranow K., Kurzawski M., Domanski L., Pawlik A. Association between the CX3CR1 gene V249I polymorphism and delayed kidney allograft function. Transpl. Immunol. 2015;32:172–174. doi: 10.1016/j.trim.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Park J., Song K.H., Ha H. Lipopolysaccharide increases monocyte binding to mesangial cells through fractalkine and its receptor. Transplant. Proc. 2012;44:1029–1031. doi: 10.1016/j.transproceed.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 43.Raspe C., Hocherl K., Rath S., Sauvant C., Bucher M. NF-kappaB-mediated inverse regulation of fractalkine and CX3CR1 during CLP-induced sepsis. Cytokine. 2013;61:97–103. doi: 10.1016/j.cyto.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 44.Adler B., Sinzger C. Endothelial cells in human cytomegalovirus infection: one host cell out of many or a crucial target for virus spread? Thromb. Haemost. 2009;102:1057–1063. doi: 10.1160/TH09-04-0213. [DOI] [PubMed] [Google Scholar]
- 45.Betjes M.G., Huisman M., Weimar W., Litjens N.H. Expansion of cytolytic CD4+CD28- T cells in end-stage renal disease. Kidney Int. 2008;74:760–767. doi: 10.1038/ki.2008.301. [DOI] [PubMed] [Google Scholar]
- 46.Shabir S., Smith H., Kaul B., et al. Cytomegalovirus-associated CD4(+) CD28(null) Cells in NKG2D-dependent glomerular endothelial injury and kidney allograft dysfunction. Am. J. Transplant. 2016;16:1113–1128. doi: 10.1111/ajt.13614. [DOI] [PubMed] [Google Scholar]
- 47.Manno C., Strippoli G.F.M., D’Altri C., Torres D., Rossini M., Schena F.P. A novel simpler histological classification for renal survival in IgA nephropathy: A retrospective study. Am. J. Kidney Dis. 2007;49:763–775. doi: 10.1053/j.ajkd.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 48.Kim Y.G., Alvarez M., Suzuki H., et al. Pathogenic role of a proliferation-inducing ligand (APRIL) in murine IgA nephropathy. PLoS One. 2015:10. doi: 10.1371/journal.pone.0137044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu L.L., Wang L.N., Jiang Y., et al. Tonsillectomy for IgA Nephropathy: A Meta-analysis. Am. J. Kidney Dis. 2015;65:80–87. doi: 10.1053/j.ajkd.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 50.Ramos M.V., Fernandez G.C., Brando R.J.F., et al. Interleukin-10 and interferon-gamma modulate surface expression of fractalkine-receptor (CX(3)CR1) via PI3K in monocytes. Immunology. 2010;129:600–609. doi: 10.1111/j.1365-2567.2009.03181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cox S.N., Sallustio F., Serino G., et al. Activated innate immunity and the involvement of CX3CR1-fractalkine in promoting hematuria in patients with IgA nephropathy. Kidney Int. 2012;82:548–560. doi: 10.1038/ki.2012.147. [DOI] [PubMed] [Google Scholar]
- 52.Eitner F., Floege J. In search of a better understanding of IgA nephropathy-associated hematuria. Kidney Int. 2012;82:513–515. doi: 10.1038/ki.2012.160. [DOI] [PubMed] [Google Scholar]
- 53.Koziolek M.J., Vasko R., Bramlage C., Muller G.A., Strutz F. The CX3C-chemokine fractalkine in kidney diseases. Mini Rev. Med. Chem. 2009;9:1215–1228. doi: 10.2174/138955709789055252. [DOI] [PubMed] [Google Scholar]
- 54.Peng X.G., Zhang J., Xiao Z.C., Dong Y.J., Du J. CX3CL1-CX3CR1 interaction increases the population of Ly6C(-) CX3CR1(hi) macrophages contributing to unilateral ureteral obstruction-induced fibrosis. J. Immunol. 2015;195:2797–2805. doi: 10.4049/jimmunol.1403209. [DOI] [PubMed] [Google Scholar]
- 55.Johansen K.L., Lee C. Body composition in chronic kidney disease. Curr Opin Nephrol Hy. 2015;24:268–275. doi: 10.1097/MNH.0000000000000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brunet P., Gondouin B., Duval-Sabatier A., et al. Does uremia cause vascular dysfunction? Kidney Blood Press. Res. 2011;34:284–290. doi: 10.1159/000327131. [DOI] [PubMed] [Google Scholar]
- 57.Ruth J.H., Volin M.V., Haines G.K., et al. Fractalkine, a novel chemokine in rheumatoid arthritis and in rat adjuvant-induced arthritis. Arthritis Rheum. 2001;44:1568–1581. doi: 10.1002/1529-0131(200107)44:7<1568::AID-ART280>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 58.Zaza G., Granata S., Rascio F., et al. A specific immune transcriptomic profile discriminates chronic kidney disease patients in predialysis from hemodialyzed patients. Bmc Med Genom. 2013;6:17. doi: 10.1186/1755-8794-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chakravorty S.J., Cockwell P., Girdlestone J., Brooks C.J., Savage C.O.S. Fractalkine expression on human renal tubular epithelial cells: potential role in mononuclear cell adhesion. Clin. Exp. Immunol. 2002;129:150–159. doi: 10.1046/j.1365-2249.2002.01906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sirois-Gagnon D., Chamberland A., Perron S., Brisson D., Gaudet D., Laprise C. Association of common polymorphisms in the fractalkine receptor (cx3cr1) with obesity. Obesity (Silver Spring) 2011;19:222–227. doi: 10.1038/oby.2010.125. [DOI] [PubMed] [Google Scholar]
- 61.Ting K.H., Ueng K.C., Chiang W.L., Chou Y.E., Yang S.F., Wang P.H. Relationship of genetic polymorphisms of the chemokine, ccl5, and its receptor, ccr5, with coronary artery disease in taiwan. Evid-Based Compl Alt; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shah R., O’Neill S.M., Hinkle C., et al. Metabolic effects of cx3cr1 deficiency in diet-induced obese mice. PLoS One. 2015;•••:10. doi: 10.1371/journal.pone.0138317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bagci B., Bagci G., Huzmeli C., Sezgin I., Ozdemir O. Associations of fractalkine receptor (CX3CR1) and CCR5 gene variants with hypertension, diabetes and atherosclerosis in chronic renal failure patients undergoing hemodialysis. Int. Urol. Nephrol. 2016;48:1163–1170. doi: 10.1007/s11255-016-1293-0. [DOI] [PubMed] [Google Scholar]
- 64.Mohan C., Putterman C. Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis. Nat. Rev. Nephrol. 2015;11:329–341. doi: 10.1038/nrneph.2015.33. [DOI] [PubMed] [Google Scholar]
- 65.Yona S., Kim K.W., Wolf Y., et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasi. Immunity. 2013;38:1073–1079. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakatani K., Yoshimoto S., Iwano M., et al. Fractalkine expression and CD16(+) monocyte accumulation in glomerular lesions: association with their severity and diversity in lupus models. Am J Physiol-Renal. 2010;299:F207–F16. doi: 10.1152/ajprenal.00482.2009. [DOI] [PubMed] [Google Scholar]
- 67.Cros J., Cagnard N., Woollard K., et al. Human CD14(dim) monocytes patrol and sense nucleic acids and Viruses via TLR7 and TLR8 Receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riedel J.H., Paust H.J., Turner J.E., et al. Immature renal dendritic cells recruit regulatory CXCR6(+) invariant natural killer t cells to attenuate crescentic GN. J. Am. Soc. Nephrol. 2012;23:1987–2000. doi: 10.1681/ASN.2012040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heymann F., Meyer-Schwesinger C., Hamilton-Williams E.E., et al. Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury (vol 119, pg 1286, 2009). J. Clin. Invest. 2009;119:2114. doi: 10.1172/JCI38399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hochheiser K., Heuser C., Krause T.A., et al. Exclusive CX(3)CR1 dependence of kidney DCs impacts glomerulonephritis progression. J. Clin. Invest. 2013;123:4242–4254. doi: 10.1172/JCI70143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nahrendorf M., Swirski F.K., Aikawa E., et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Geissmann F., Jung S., Littman D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 73.Devi S., Li A.Q., Westhorpe C.L.V., et al. Multiphoton imaging reveals a new leukocyte recruitment paradigm in the glomerulus. Nat. Med. 2013;19:107–112. doi: 10.1038/nm.3024. [DOI] [PubMed] [Google Scholar]
- 74.Finsterbusch M., Hall P., Li A.Q., et al. Patrolling monocytes promote intravascular neutrophil activation and glomerular injury in the acutely inflamed glomerulus. Proc. Natl. Acad. Sci. USA. 2016;113:E5172–E81. doi: 10.1073/pnas.1606253113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuligowski M.P., Kitching A.R., Hickey M.J. Leukocyte recruitment to the inflamed glomerulus: a critical role for platelet-derived P-selectin in the absence of rolling. J. Immunol. 2006;176:6991–6999. doi: 10.4049/jimmunol.176.11.6991. [DOI] [PubMed] [Google Scholar]
- 76.Morgan M.J., Kim Y.S., Liu Z.G. TNFalpha and reactive oxygen species in necrotic cell death. Cell Res. 2008;18:343–349. doi: 10.1038/cr.2008.31. [DOI] [PubMed] [Google Scholar]
- 77.Segerer S., Nelson P.J., Schlondorff D. Chemokines, chemokine receptors, and renal disease: from basic science to pathophysiologic and therapeutic studies. J. Am. Soc. Nephrol. 2000;11:152–176. doi: 10.1681/ASN.V111152. [DOI] [PubMed] [Google Scholar]
- 78.Stroo I., Stokman G., Teske G.J.D., et al. Chemokine expression in renal ischemia/reperfusion injury is most profound during the reparative phase. Int. Immunol. 2010;22:433–442. doi: 10.1093/intimm/dxq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Furuichi K., Gao J.L., Murphy P.M. Chemokine receptor CX3CR1 regulates renal interstitial fibrosis after ischemia-reperfusion injury. Am. J. Pathol. 2006;169:372–387. doi: 10.2353/ajpath.2006.060043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Strieter R.M., Belperio J.A., Phillips R.J., Keane M.P. CXC chemokines in angiogenesis of cancer. Semin. Cancer Biol. 2004;14:195–200. doi: 10.1016/j.semcancer.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 81.Raman D., Baugher P.J., Thu Y.M., Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256:137–165. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jemal A., Siegel R., Xu J.Q., Ward E. Cancer Statistics, 2010. CA Cancer J. Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 83.Ahn S.Y., Cho C.H., Park K.G., et al. Tumor necrosis factor-alpha induces fractalkine expression preferentially in arterial endothelial cells and mithramycin A suppresses TNF-alpha-induced fractalkine expression. Am. J. Pathol. 2004;164:1663–1672. doi: 10.1016/s0002-9440(10)63725-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fong A.M., Robinson L.A., Steeber D.A., et al. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J. Exp. Med. 1998;188:1413–1419. doi: 10.1084/jem.188.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu W.M., Bian C., Liang Y., Jiang L.B., Qian C., Dong J. CX3CL1: a potential chemokine widely involved in the process spinal metastases. Oncotarget. 2017;8:15213–15219. doi: 10.18632/oncotarget.14773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aparicio L.A., Blanco M., Castosa R., et al. Clinical implications of epithelial cell plasticity in cancer progression. Cancer Lett. 2015;366:1–10. doi: 10.1016/j.canlet.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 87.Xin H., Kikuchi T., Andarini S., et al. Antitumor immune response by CX3CL1 fractalkine gene transfer depends on both NK and T cells. Eur. J. Immunol. 2005;35:1371–1380. doi: 10.1002/eji.200526042. [DOI] [PubMed] [Google Scholar]
- 88.Webster A.C., Craig J.C., Simpson J.M., Jones M.P., Chapman J.R. Identifying high risk groups and quantifying absolute risk of cancer after kidney transplantation: A cohort study of 15183 recipients. Am. J. Transplant. 2007;7:2140–2151. doi: 10.1111/j.1600-6143.2007.01908.x. [DOI] [PubMed] [Google Scholar]
- 89.Faure S., Meyer L., Costagliola D., et al. Rapid progression to AIDS in HIV+ individuals with a structural variant of the chemokine receptor CX(3)CR1. Science. 2000;287:2274–2277. doi: 10.1126/science.287.5461.2274. [DOI] [PubMed] [Google Scholar]
- 90.Yu Y.R.A., Fong A.M., Combadiere C., Gao J.L., Murphy P.M., Patel D.D. Defective antitumor responses in CX3CR1-deficient mice. Int. J. Cancer. 2007;121:316–322. doi: 10.1002/ijc.22660. [DOI] [PubMed] [Google Scholar]
- 91.Kezic A., Stajic N., Thaiss F. Innate immune response in kidney ischemia/reperfusion injury: potential target for therapy. J. Immunol. Res. 2017;2017:6305439. doi: 10.1155/2017/6305439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rogers N.M., Matthews T.J., Kausman J.Y., Kitching A.R., Coates P.T. Review article: Kidney dendritic cells: their role in homeostasis, inflammation and transplantation. Nephrology (Carlton) 2009;14:625–635. doi: 10.1111/j.1440-1797.2009.01200.x. [DOI] [PubMed] [Google Scholar]
- 93.Cao G., Lu Y., Gao R., et al. Expression of fractalkine, CX3CR1, and vascular endothelial growth factor in human chronic renal allograft rejection. Transplant. Proc. 2006;38:1998–2000. doi: 10.1016/j.transproceed.2006.06.081. [DOI] [PubMed] [Google Scholar]