Abstract
Background:
Craniocervical Dissections (CCD) are a crucial emergency state causing 20% of strokes in patients under the age of 45. Although DSA (digital substraction angiography) is regarded as the gold standard, noninvasive methods of CT, CTA and MRI, MRA are widely used for diagnosis.
Aim:
Our aim is to illustrate noninvasive imaging findings in CCD.
Conclusion:
Emphasizing on diagnostic pitfalls, limitations and mimicking diseases.
Keywords: Craniocervical dissection, DSA, MRA, MRI, CTA, CT
1. INTRODUCTION
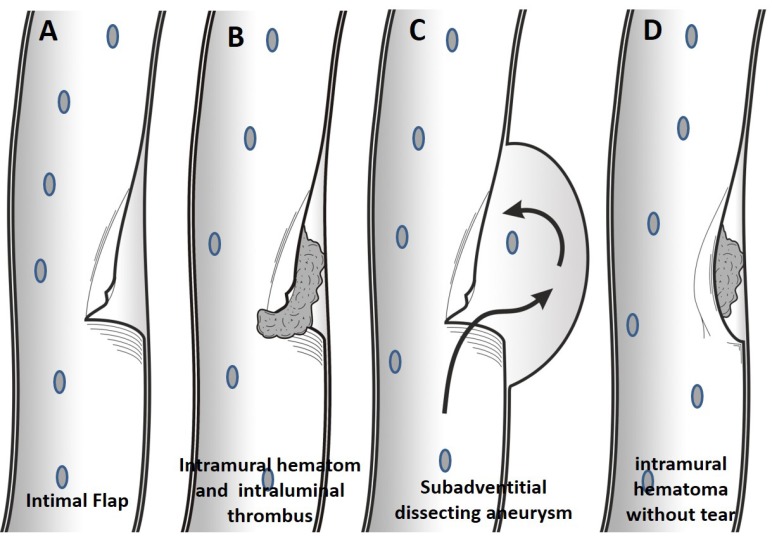
Dissections are characterized by penetration of blood into the arterial wall through a tear, which develops in one or more layers of the wall, producing an intramural hematoma [1]. Arterial wall tears can be subintimal or subadventitial. In subintimal dissection, tear occurs between the intimal and medial layers of the arterial wall, and penetrating blood between these layers leads to stenosis. In subadventitial dissection, tear occurs between the medial and adventitial layers of the arterial wall and may cause aneurysmal dilation in the wall of artery. Intramural hematoma may also occur without a tear in the wall of an artery, especially in arteriopathic vessels with hemorrhage originating from vaso vasorum [2] (Fig. 1). By increasing the width of the vessel wall tear, stenosis and aneurysm formation are more likely to occur following dissection [3-8].
Fig. (1).
In dissection with intimal flap tear without thrombus formation, patient will be presenting only with pain (A). More extensive tear will result in intraluminal and subintimal thrombus formation, therefore, thromboembolic event is more common in this group (B). In dissecting aneurysm, the wall tear will extend into subadventitial region, and the adventitia will be weakened and widened, resulting in the subadventitial aneurysm limited by the adventitia. (C). Without intimal tear, intramural thrombus may occur by leakage from vaso vasorum within media layer in arteriopathic patients (D).
This article reviews the common findings in CCD and potential pitfalls related to image interpretation and other mimicking conditions affecting the craniocervical arteries are also described. Radiologic findings of our cases were presented in the light of the literature review. The literature review was prepared by searching databases such as Web of Science, Embase, and PubMed and citation indexes.
2. Ethiopathologies
Craniocervical Dissections (CCD) account for 20% of strokes in patients under the age of 45 years of age. It is considered to often occur spontaneously, without obvious cause, with an annual incidence of 2.5-3:10,000 [1, 9, 10]. CCDs are the second most common source of large-artery cerebrovascular disease after atherosclerosis. Mean age in vertebral artery dissection is 51 years and in carotid dissections, 43 years. CCD is considered to be either spontaneous or traumatic in origin. Although a large number of spontaneous dissections are idiopathic, underlying pathologies such as hypertension, atherosclerosis, fibromuscular dyplasia, Ehlers-Danlos syndrome type IV, Marfan syndrome, osteogenesis imperfecta type I, alfa-1-antitrypsin deficiency, cystic medial necrosis, autosomal dominant polycystic renal disease, medial mucoid degeneration, polyarteritis nodosa, Behçet’s disease, migraine, transient postinfectious arteriopathy and long styloid process may also be found [3, 7, 11, 12]. Recent infection may be a trigger for dissection, due to recognized seasonal variation of spontaneous CCD, with a peak in autumn and winter [13]. Usually a trivial trauma (e.g. coughing, vomiting, sneezing, fast head turning, neck massage) precedes the spontaneous dissection in a susceptible individual with an underlying arteriopathy. Traumatic dissection in the order of frequency is a complication of car accidents (40-50%), assaults to head and neck region (10-20%), car crashes (12-18%), falls (5-15%) and hanging from the neck to commit suicide (5%). Falling of a child with an object in his mouth is the most common cause of dissection inpediatric population. In traumatic injuries, hyperextension or rotation of the neck, direct blunt vascular trauma, intraoral trauma or vascular lacerations due to surrounding bone fragments are mechanisms for dissections [14-18]. Classification of blunt cerebrovascular injury resulting in dissection and associated stroke rates has been reported by Colorado Universtiy and is widely used [14, 15] (Table 1).
Table 1.
Denver dissection grading system in blunt blunt cerebrovascular injury and associated stroke rates. A grade I injury heals regardless of therapy. 70% of grade II dissections progress whilst on heparin therapy. Only 8% of pseudoaneurysms (grade III) healed with heparin, about 89% resolves after endovascular stenting. Occluded carotid arteries (grade IV) does not recanalize in the early post-injury period. Grade V injuries (transections) are lethal and refractory to intervention. Stroke risk increases with injury grade.
| Grade | Type of Dissection | Stroke in Carotid Artery Dissection (%) | Stroke in Vertebral Artery Dissection (%) |
|---|---|---|---|
| I | Luminal irregularity or dissection with <25% luminal narrowing | 3 | 19 |
| II | intimal flap or intramural hematoma with luminal narrowing ≥ 25% or intraluminal thrombus | 11 | 40 |
| III | Pseudoaneurysm | 33 | 13 |
| IV | Total occlusion | 44 | 33 |
| V | Transection and free bleeding | 100 | N/A |
3. Anatomic Distribution
CCDs are rare, with an estimated annual incidence of 5 cases per 100.0000 in the population [7, 12]. Dissection of carotid arteries (68%) is more frequent than dissections of vertebral arteries (27%). While most of the time the cause of spontaneous dissections is idiopathic, vertebrobasilar dissections are often related with hypertension [3, 19, 20]. In carotid artery dissections, mobile segments are frequently affected and dissection usually involves 2-3 cm distal to the carotid bulb. In 17% of cases, carotid artery dissection extends to intracranial segment. Spontaneous VA dissections predominantly occur in V2 (35%) extending throughout the transverse foramina or in V3 (34%) looping around C1 and C2 vertebrae. 10% of VA dissections extends intracranial. V2 segment is injured because of longitudinal strain; V3 segment is injured secondary to rotation and torsion movements [3, 21-25].
Spontaneous CCDs are more frequently seen in the extracranial segments of carotid and vertebral arteries due to the fact that these parts are more mobile and closer to the bony structures (cervical vertebrae, styloid process). There is no sex related predominance in spontaneous extracranial dissections, whereas intracranial dissections are more commonly seen in males. Spontaneous intracranial dissections constitute only 12% of CCDs. In intracranial dissections, likelihood of aneurysm formation and extravascular bleeding is more common when compared to those of extracranial dissections. Because the medial and adventitial layers of intradural arteries are thin, they have no external elastic lamina; have less vaso vasorum and thicker internal elastic lamina. However, dissecting aneurysm is frequently a finding of extracranial artery dissection. Aneurysm in CCDs is saccular in appearance, therefore, it often is called as pseudoaneurysm or false aneurysm. In fact, aneurysm is limited to adventitia layer; therefore dissecting aneurysm is a more accurate description. In 1/3 of extracranial dissections, dissecting aneurysm is seen [8, 26, 27]. Intracranial dissection is the cause of 3% of intracranial aneurysms and 6% of the non-traumatic subarachnoid hemorrhages [3]. Intradural carotid dissections have mostly ischemic symptoms because of intramural hematoma and stenosis. Intradural vertebrobasilar dissections are more prone to subarachnoid hemorrhage. The cause of spontaneous intracranial dissections is mostly idiopathic but the relationship of intradural vertebrobasiler dissections with hypertension is strong [20]. In pediatric age group, spontaneous craniocervical dissections frequently involve intracranial arteries and particularly, the carotid system [18].
4. CLINICAL PRESENTATION
CCDs causing high-grade stenosis or occlusion lead to hemodynamic infarcts involved in artery territory or in the presence of thrombus extending into artery lumen and dissecting aneurysm multiple thromboembolic infarcts may occur. In dissections, thromboembolic stroke mechanism is more dominant. Ischemic stroke is more frequent in the first two weeks, especially in acute phase (first 3 days). Ischemic cerebral symptoms can accompany 55% of carotid dissections and 72% of vertebral artery dissections. In vertebral artery dissections, ischemic symptoms occur in brain stem, cerebellum and posterior cerebral artery territory, and in internal carotid artery dissections, ischemic symptoms more frequently occur in middle cerebral artery territory [3, 4, 25, 29].
In CCDs without significant luminal stenosis, local signs and symptoms including head, neck, or facial pain; horner syndrome; pulsatile tinnitus; and cranial nerves palsies can be seen. In carotid artery dissections, face, head and neck pain (frontotemporal), and in vertebral artery dissections, head and neck pain (occipital) may be present. Pain resolves within one week in 90% of cases. Rare clinical manifestations include visual loss or retinal hemorrhages in carotid artery dissections; cervical spinal cord ischemia or radiculopathy in vertebral artery dissections. Vertebral artery dissections lead to radiculopathy by disrupting the blood supply to the nerve, or by narrowing the neural foramen due to dissecting aneurysm. Horner syndrome can occur in patients with carotid dissection, because of the compression of sympathetic fibers by the enlarged artery. Vertebral artery dissections are more symptomatic compared to carotid artery dissections. Stroke is common in extracranial in vertebral artery dissections, while subarachnoid bleeding is more prevalent in intracranial in vertebral artery dissections [3, 4, 25, 29].
5. IMAGING TECHNIQUES AND FINDINGS
DSA is still widely regarded as the gold standard method in diagnosis of CCDs; however, other noninvasive, easy to reach methods including CT, CTA, MRI and MRA, in which arterial wall can be well depicted, play an important role in the diagnosis of dissection. DSA is often used for demonstration of intraluminal thrombus, high-grade stenosis, evaluation of collateralization in total occlusion and endovascular treatment. Although ultrasound and doppler ultrasound can be used in the diagnosis of extracranial arterial dissections, diagnosis should be confirmed with MRI or MRA [3, 30-32].
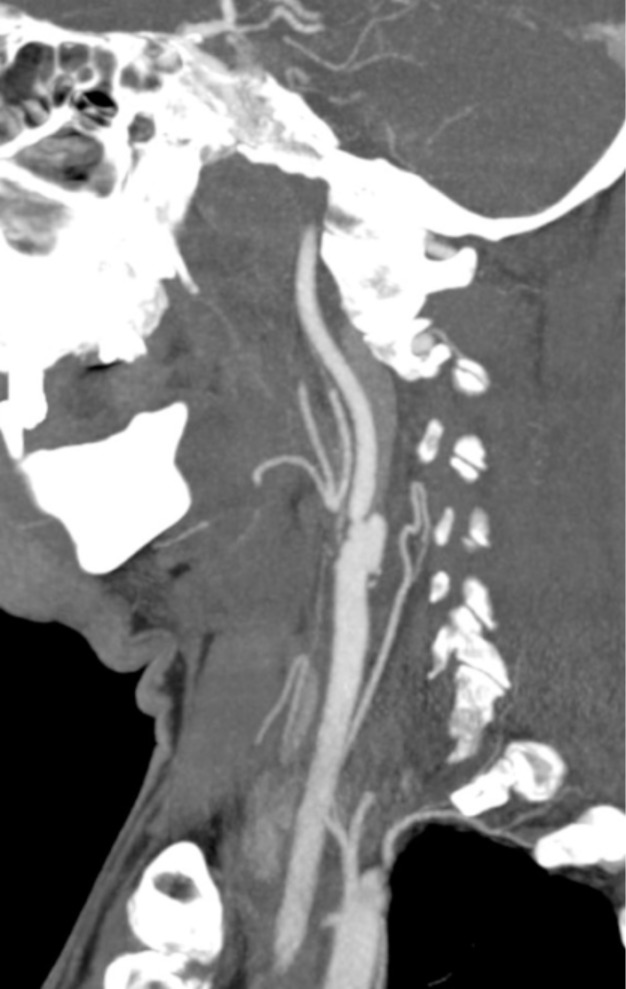
Diagnostic radiological findings in patients with CCDs on MRI, MRA and CT, CTA include total occlusion, total ICA occlusion sparing bulbus (tapering stenosis, flame sign), short segment stenosis (string sign), focal stenosis and distal dilatation (string and pearl sign), filling defect, pseudoaneurysm, double lumen appearance, intimal flap, eccentric thickening of the arterial wall, crescent sign on MRI secondary to intramural thrombus, target sign on CTA, narrowed eccentric lumen with increase in the external diameter of the artery, partial recanalization of the artery, and dissection induced thromboembolic or hemodynamic infarctions [3, 30-32] (Figs. 2-15).
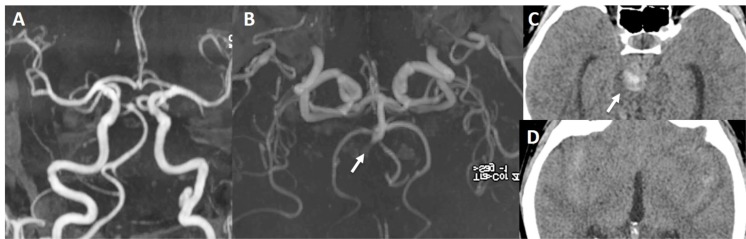
Fig. (2).
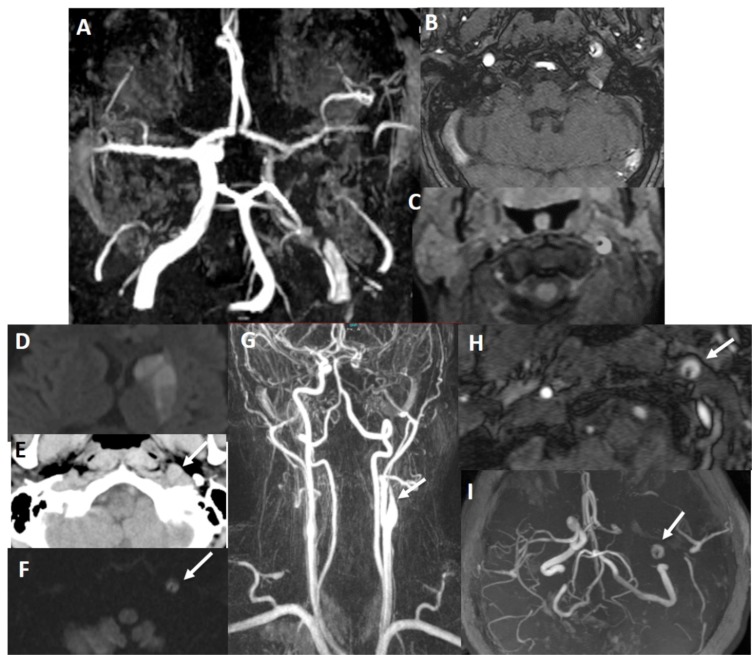
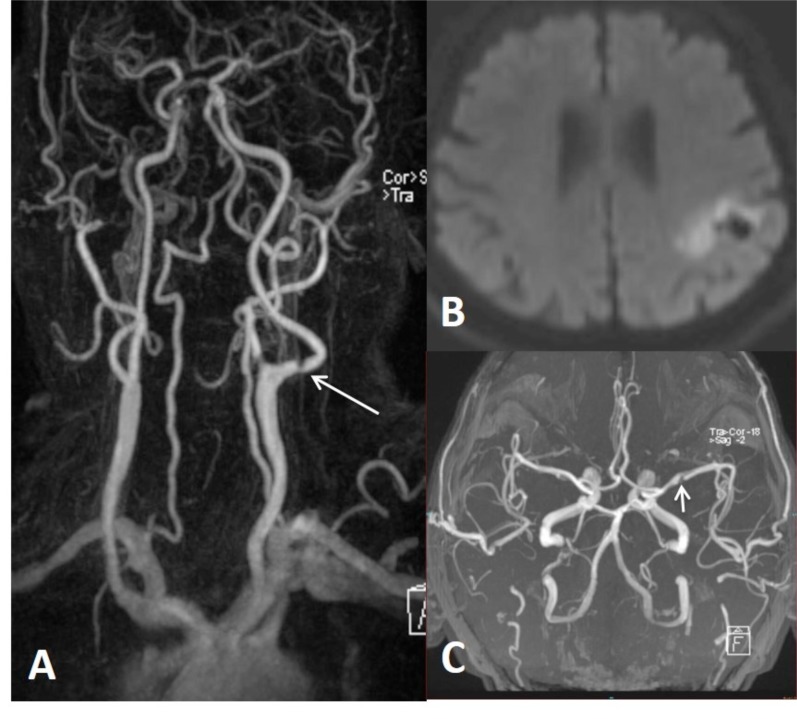
There is false double lumen appearance of left petrous ICA on MIP (A) and source (B) views of 3D TOF MRA. Fat suppressed T1 image show crescent sign of hyperintense intramural thrombus, increased external diameter of left ICA with eccentric luminal narrowing (C). Not: intramural thrombus is seen hyperintense on 3D TOF MRA, giving false appearance of double lumen. In another patient with left sided acute striatal stroke (D), arrows show left ICA as enlarged and hyperdense on CT (E) associated with diffusion signal changes on DWI (F). On contrast enhanced neck MRA, flame sign of left ICA is seen with very limited flow distally (arrow on G) and associated enlarged external diameter of left ICA with crescent sign and eccentric luminal narrowing appearing in both source (arrow on H) and MIP (arrow on I) images of 3D-TOF brain MRA. Findings are compatible with dissection of left ICA at the skull base where the enlargement of the vessel is present.
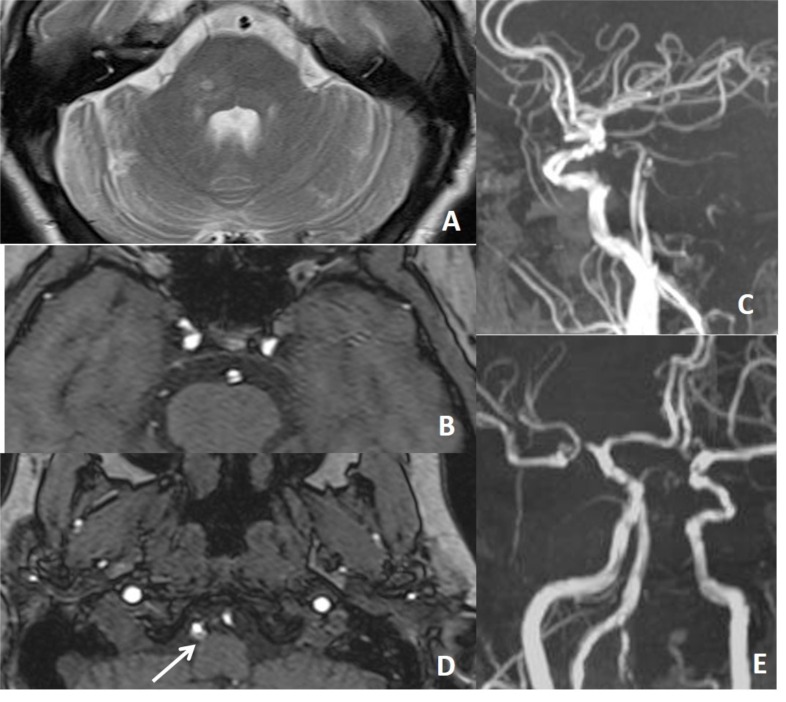
Fig. (15).
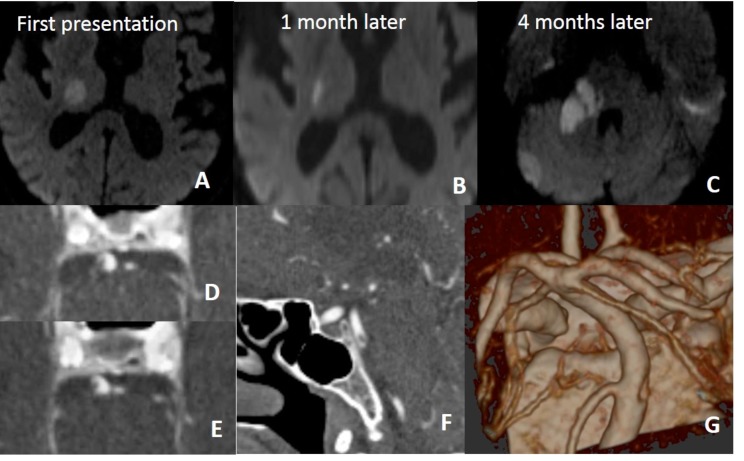
In a patient with recurrent infarction within the posterior circulation, brain and neck MRA (not shown here) repeated a few times were all normal. On 4th months, CTA was performed for vertebrobasiler system evaluation. There is dissection of intimal flap type tearing of basiler artery on CTA (D, E, F, G).
6. MRI and MRA Findings
The most common finding of dissection on MRI is crescent-shaped intramural hematoma (Figs. 2, 3 and 14). Intramural hematoma is easily distinguished from the surrounding tissue on fat-suppressed T1-weighted images. However, intramural hematoma is bright on T1-weighted images in its subacute stage. MRI signal characteristics of intramural hematoma change with time after the intial injury. Intramural hematoma has different T1 and T2 weighted MR signal poperties in hyperacute (< 24 hours), acute (1-3 days), ealy subacute (> 3 days), late subacute (> 7 days) and chronic (> 14 days) stages. Within the first 3 days, hematoma is isointense on T1-weighted images. Appearance of T1 hyperintensity may extend in 7 days. After 3 days and up to 2 months, thrombus becomes hyperintense on T1-weighted images. In contrast, T2 signal changes are hyperintense for the entire first two months. After 6 to 12 months, the signal of intramural hematoma becomes isointense to the surrounding structures. If intramural hematoma is homogenous on MRI, this supports spontaneous dissection in only one time, whereas if intramural hematoma is heterogeneous, this supports recurrent hemorrhages. Luminal stenosis, eccentric lumen (mural thickening with displacement of vessel lumen off midline) and increased external diameter of the artery are other findings (Figs. 2, 3, 4, 6 and 7). Increased external diameter of the artery is observed more commonly on 3D-TOF MRA (95%) than MRI (68%). Short segment stenosis may not be helpful in differential diagnosis because this finding may also be seen in carotid stenosis. Also, subadventitial dissections (Fig. 8), by expanding the vessel wall and compressing the surrounding the tissues, may not lead to vessel stenosis [3, 30-32]. In acute dissections of carotid arteries at the skull base, DWI can be hyperintense due to blood products within intramural thrombus (Figs. 2 and 9).
Fig. (3).
Distal cervical ICA is narrowed (A) with target sign (arrow on B) on CTA, compatible with dissection. Intramural thrombus is hard to see on T1W image (C), but hyperintense on T2W (D) and fat suppressed T1W (E) images (arrows), giving crescent sign on MRI. Thin annular enhancement of vessel wall with eccentric luminal narrowing gives target sign on CTA. Thin annular enhancement of vessel wall is due to the contrast enhancement of the adventitial layer via vaso vasorum.
Fig. (14).
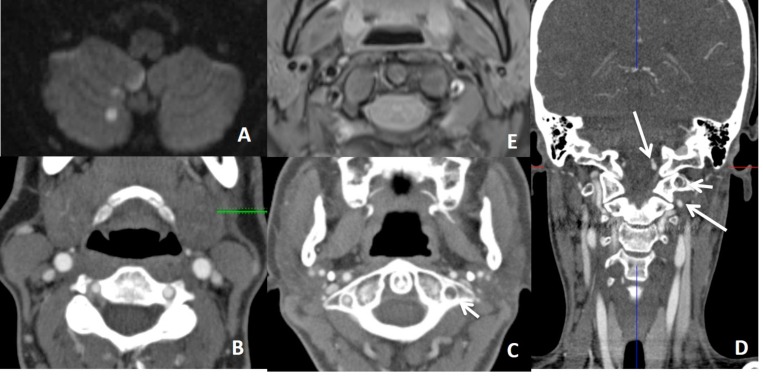
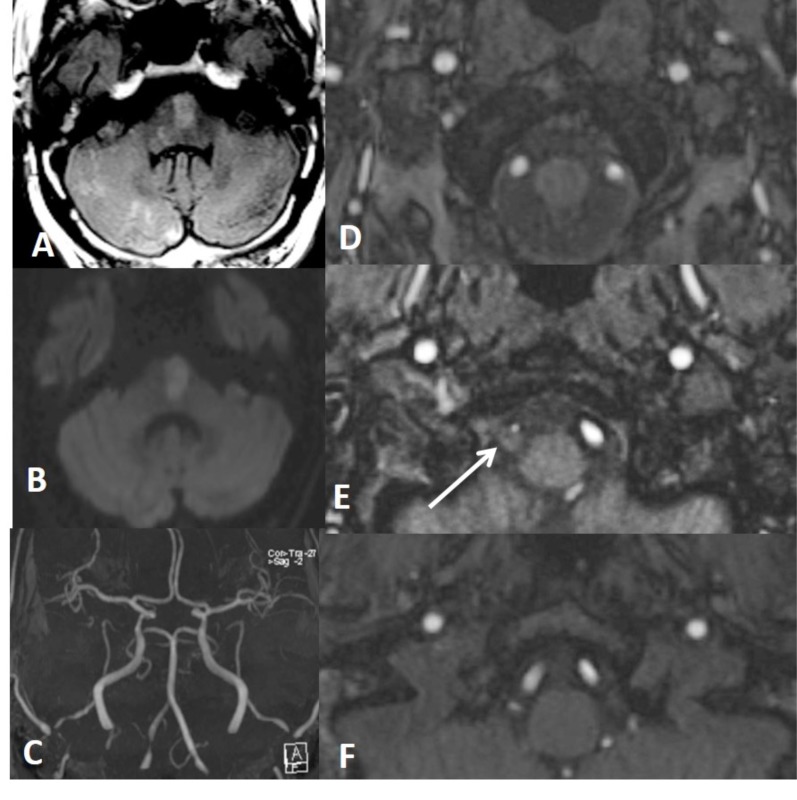
In a patient with thromboembolic infarction within right cerebellum (A), CTA (B, C, D) shows normal caliber of left VA at the proximal part of the V3 and V4 but narrowed at the distal part of the V3 (short arrow showing distal V3 on C) (long arrows showing proximal V3 and V4 on D). Distal V3 narrowing (C) is associated with intramural hematoma (crescent sign) on fat sat T1W images (E). Findings are suggestive of distal V3 dissection.
Fig. (4).
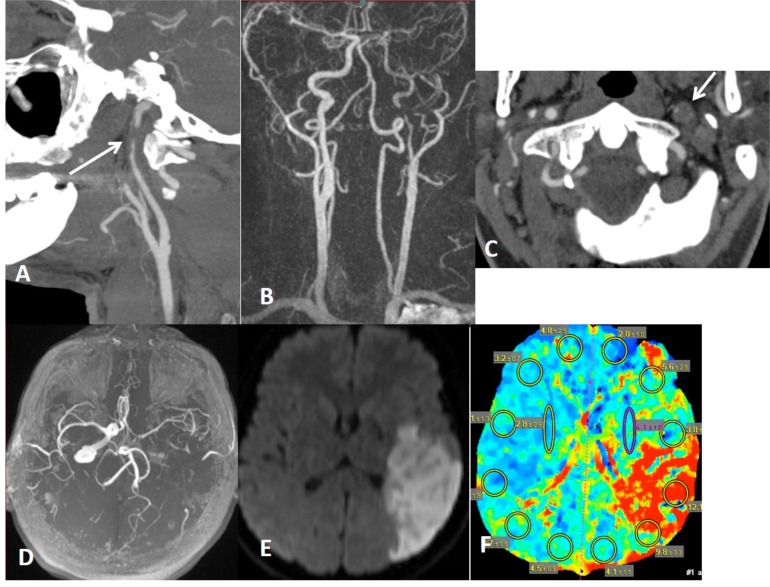
Left distal cervical ICA dissection with focal stenosis (string sign) on CTA (arrow on A) and MRA (B) with target sign on CTA (arrow on C). There is limitation flow in the left MCA territory on MRA (B and D) with hemodynamic infartcion on DWI (E) associated with penumbra on CT perfusion (F).
Fig. (6).
Contrast enhanced MRA show stenosis of distal cervical ICA (arrow on A). Axial T2W shows hypointense true lumen of right ICA surrounded by hyperintense pseudolumen (arrow on B). Hyperintensity is due to sluggish blood flow or thrombosis within pseudolumen.
Fig. (7).
In a patient with right sided visual loss there is focal narrowing of right ICA distal to bulbus (A) and increased external diameter and concentric mural thickening of right ICA comparing the left one (arrows on B), compatible with right ICA dissection. In this case, true lumen is not eccentric, almost centrally located. There is sluggish blood flow in the distal right ICA (C).
Fig. (8).
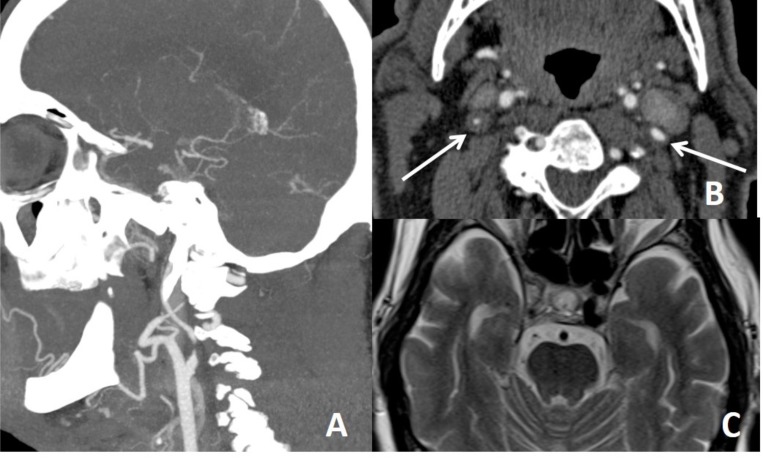
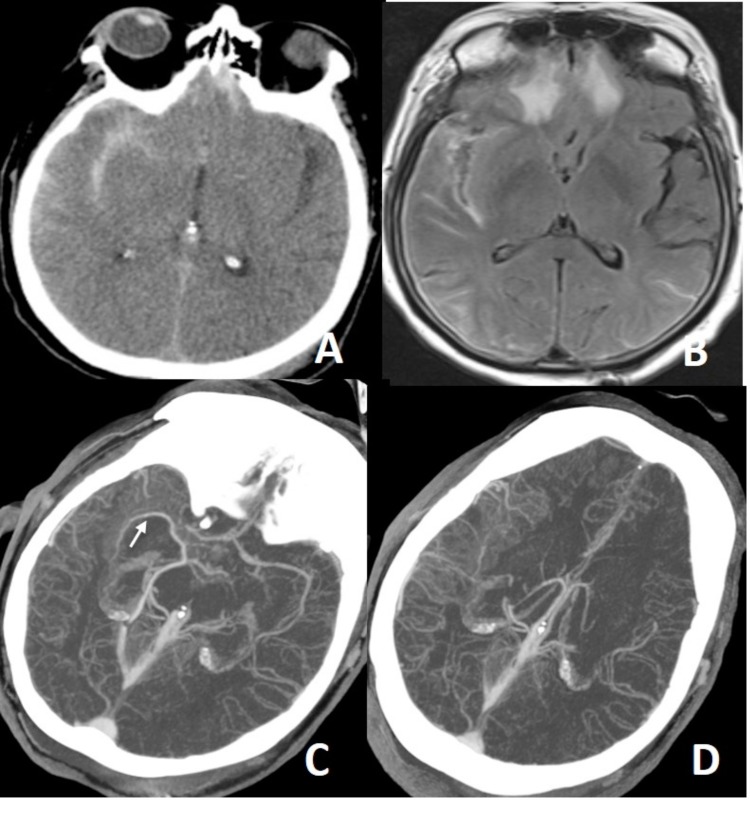
Dissecting aneurysm of left distal cervical ICA on MRA. Dissecting aneurysm is a finding of extracranial dissection, observed in 1/3 of extracranial dissections. Intracranial dissections mostly result in subarachnoid bleeding. Dissecting anurysm of basilar tip (arrow on B) in another patient results in extraaxial hematoma surrounding the dissected vessel (arrow on C) and diffuse subarachnoid bleeding (D).
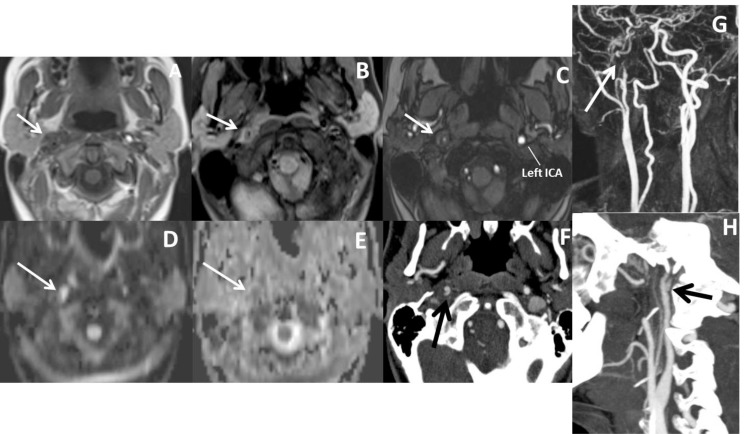
Fig. (9).
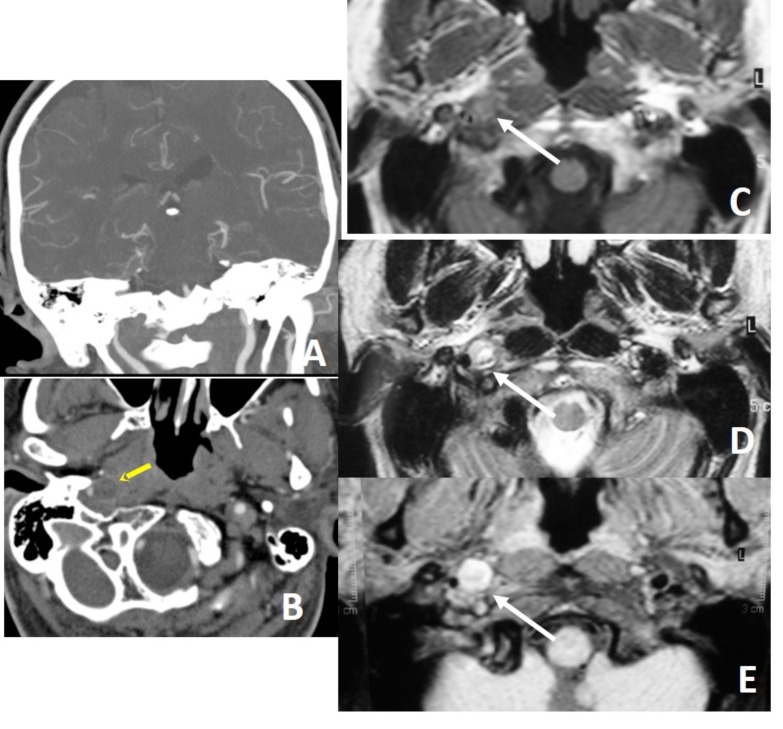
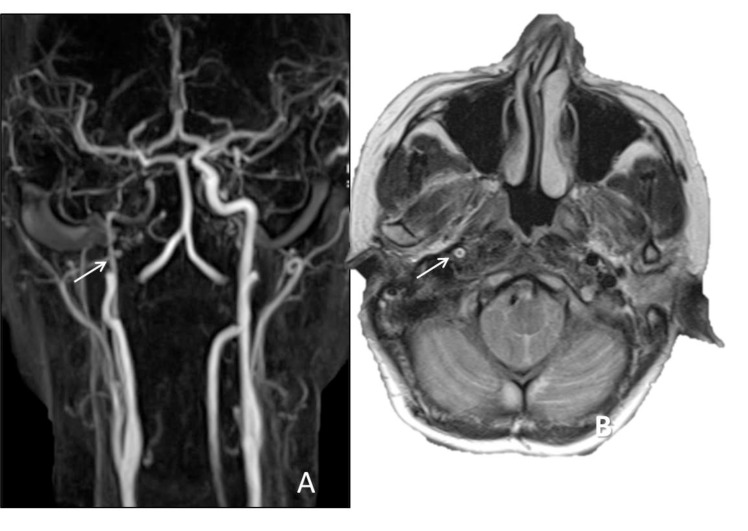
In a patient with right sided Horner syndrome short white arrows show increase in external diameter of distal cervical ICA on axial T1 (A), hyperintensity in the wall of the vessel secondary to intramural thrombus on fat-sat axial T1A (B), eccentric luminal narrowing and mural thickening on source image of 3D- TOF MRA (C), diffusion changes related to blood product within the wall of the vessel on DWI (D) and corresponding ADC map (E), contrast extravasation from the narrowed lumen into thickened vessel wall on axial CTA (F), narrowed right ICA at the skull base on contrast enhanced MRA (G) and dissecting aneurysm on sagittal MIP of CTA (H).
MRI and MRA are recommended imaging methods employed for the diagnosis of spontaneous dissections. MRI and MRA demonstrated excellent sensitivity of 87-99%, compared with DSA in the diagnosis of carotid dissection. However, in VA dissections, this sensitivity reduced to 60%. The sensitivity of MRI and MRA in the diagnosis of VA dissections is low as the calibers of vertebral arteries are small and flow-related enhancement of paravertebral veins mimics crescent sign. In suspicion of VA dissection, surface coils can be used. To prevent signals from venous structures during the MRI, cranial presaturation bands may be placed. Contrast-enhanced MRA can provide better results than 3D-TOF MRA. If the suspicion of VA dissection continues, patient should be directed to CTA (Figs. 14 and 15). The biggest disadvantage of MRI and MRA is the difficulty in differentiating intramural thrombus from intraluminal thrombus. MRA displays arterial stenosis higher than it is in both length and diameter. CTA is more successful than MRA in the diagnosis of VA dissections. Compared to DSA, the sensitivity and specificity of CTA in cervical VA dissections have been reported as 74-98% and 84-100%. In comparison between MR, MRA and CT, CTA, both modalities have a similar sensitivity in the diagnosis of carotid dissections; whereas in VA dissections, CT, CTA is more superior to MR, MRA [3, 30-34].
7. CT and CTA Findings
On pre-contrast CT, enlarged external diameter of the artery and hyperdense intramural thrombus can be seen (Fig 2). Narrowed eccentric lumen, mural thickening and thin annular enhancement of vessel wall are defined as “target lesion” on CTA (Figs. 3 and 4). Thin annular enhancement of vessel wall is due to the contrastive enhancement of the adventitial layer via vaso vasorums. However, target sign is seen very rarely. In fact, narrowed eccentric arterial lumen and mural thickening are the most reliable CTA findings. Other signs of dissections are short segment stenosis, total occlusion, dissecting aneurysm, filling defect, intimal flap, focal stenosis and dilatation (string and pearl sign) and tapering stenosis (flame sign) (Figs. 4, 7, 9, 14 and 15). Carotid occlusions show tapering stenosis whereas vertebral occlusions may show blunt ending that gives false appearance of thrombotic occlusion. Moreover, intimal flap is a rare finding and is mostly seen in carotid dissection. While the biggest advantages of CT and CTA are short examination time and higher resolution than MR and MRA, while disadvantages of CT and CTA are ionizing radiation, administration of iodinated contrast material and inability to detect ischemia as MRI does [3, 30-32].
8. USG and Doppler USG
Ultrasonography can be used to screen only the midcervical segments of carotid or vertebral arteries; the proximal, distal cervical, and intracranial portions of the arteries cannot be adequately imaged. However, this modality is less reliable than DSA, CTA or MRA. The USG findings of arterial dissection are a double lumen that is separated by an echogenic intimal flap and an intramural hematoma as an eccentric echogenicity that surrounds a relatively narrowed arterial lumen. Dissections of distal cervical ICA should be suspected on Doppler USG by decreased velocities in the carotid bulb with either high resistance due to arterial stenosis or a biphasic pattern that suggests an occlusion. On dissections of VA, diagnosis is nonspecific and can be suggested only in the appropriate clinical settings. Since it is hard to visualize intraforaminal segments of the midcervical VA, diagnosis is made by indirect hemodynamic findings demonstrating the absence of arterial flow or low blood velocities in the dissected artery, and a compensatory increased blood flow in the contralateral vertebral artery [28, 30].
Transcranial Doppler is reported helpful in monitoring cerebral microemboli that were present in 59% of a series of ICA dissections. The presence of microemboli in ICA dissections is a risk factor for cerebral ischemia and authors suggested that the patients identified through Transcranial Doppler should be provided appropriate treatment [35, 36].
9. New Image Techniques to Evaluate the Arterial Wall
The source images of 3D TOF MRA and fat saturated 2D T1WI SE sequences can provide direct visualization of subacute intramural hematoma as a high-signal structure due to their short T1 features. Recently, 3D black-blood fat-saturated T1 FSE (SPACE/CUBE/VISTA) sequence is favored over 2D FS T1 SE sequence due to its shorter acquisition time, larger coverage area, very good fat saturation and excellent delineation of intramural hematoma in the acute and subacute phases of CCDs [37, 38].
More recently, 3D SNAP MR sequences has been proposed, instead of multisequence imaging protocol with 2 separate sequences showing MRA and MR vessel wall. A simultaneous non-contrast angiography and intraplaque hemorrhage (SNAP) MR imaging technique acquires noncontrast MRA and vessel wall images simultaneously in a single scan. It has been proposed for the evaluation of vessel wall for vulnerable carotid atherosclerotic plaques, intraplaque hemorrhage and intramural hematoma. The vessel wall images derived from the 3D SNAP sequence carry heavy T1-weighting, fast scanning, large longitudinal coverage and isotropic high resolution. SNAP is recommended as an ideal candidate for quick and reliable identification of dissection, particularly for tortuous craniocervical arteries with longer lesions [37, 38].
10. Differential Diagnosis and Pitfalls
Vasculitis, ulcerated atheroslerotic plaques, preocclusive appearence proximal to thrombotic occlusions, and hypoplasia of ICAs may give false appearence similar to dissection (Figs. 16-21). The appearance of the lesion site, the site itself, as well as the presence of calcifications, can help in differentiating dissections from atherosclerotic vascular disease (Figs. 19 and 21). Carotid artery dissection typically involves the postbulbar segment while atherosclerosis is likely to be in the carotid bifurcation. Vertebral artery dissection tends to occur at the points of entry into and exit from the foramina transversaria—most commonly, at the C1-2 and C6-7 levels or at the intradural segment, rather than at the origin of the vertebral artery, where atherosclerosis is most common. Moreover, associated clinical symptoms (e.g., oculosympathetic paresis or cranial nerve paralysis) and other angiographic findings (e.g., pseudoaneurysm) further help in differentiation. Blunted end of occlusion is more suggestive of intraluminal thrombus rather than intramural thrombus of dissection (Fig. 21).
Fig. (16).
Vasculitis is big mimicker of dissection. On the other hand, vasculitis the one of causes of dissection. In a patient with Behcet’s disease, there is brain stem infarction (A) and intimal flap type dissection of arcus aorta (B) and bilateral CCAs (C, D) on CTA. All lumens in dissected arcus aorta is filling (B). Both two lumens in the right CCA is filling while filling lumen (true lumen) is very thin and other (false lumen) is is thrombosed in the left CCA (C, D). In another patient with osteogenesis imperfecta type 1, there is infarction within the left ACA territory (E) with focal narrowing and dialation (string and pearl sign) of left A2 on the volume rendering CTA (F), consisting with dissection of proximal part of left A2. In another patient with Behcet’s disease, vessel wall irregularities with saccular dilatations (pseudoaneurysms) are seen along the both ICAs and right VA (H). Pseudoaneurysm of the celiac trunk is seen in the same patient (I).
Fig. (21).
Thrombotic occlusion of left ICA on CTA (A, B) and DSA (C) are seen. Blunted ends of occlusion are against the dissection.
Fig. (19).
Ulcerated atherosclerotic plaque may give false appearance of pseudoaneurysm.
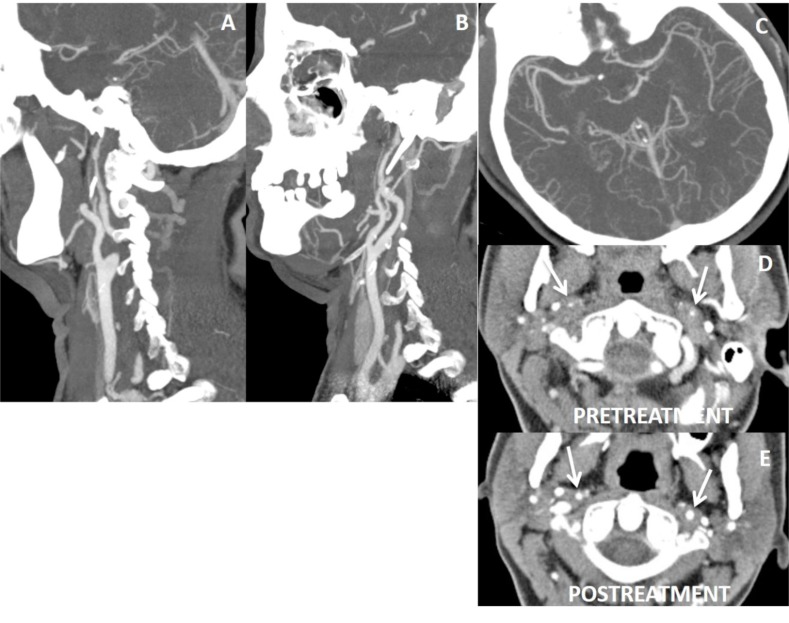
Vaculitis may cause luminal narrowing and increase in outer diameter of the vessel. Thickness of vessel wall is irregular (beads on a string) and in the form of long segment involvement; and also similar findings in other vascular structures rather than carotis-vertebral artery system can be present. In addition, arterial dissection is a common finding in vasculitic diseases (Figs. 16 and 17). In ICA dysgenesis, looking for any asymmetry of bony carotid canal at the skull base will help to distinguish ICA hypoplasia from dissection (Fig. 18) [30-32]. Vasospasm can also mimic arterial dissection in trauma cases, and vasospasm is self limiting, reversible event that favorably responds to vasodilators in a short time although dissected segment requires weeks to months to be resolved [39]. In thrombotic distal ICA occlusion, preocclusive appearance of ICA proximal to thrombotic occlusions may mimic dissection due to its reduced lumen. Absence of associated intramural thrombus or increase in external diameter of proximal ICA will distinguish tapering appearance of proximal ICA from that of the dissection (Fig. 20).
Fig. (17).
40 year’s old female with fibromuscular dysplasia, irregularities with luminal narrowing of both ICAs (A, B) and left M1 (C) are present on CTA. Axial CTA shows diffuse concentric thickening of wall of ICAs with luminal narrowing (arrows on D). Fat saturated T1W images did not reveal any hyperintensity suggesting intramural hematoma (not shown here). Following steroid treatment, wall thickening of ICAs lessens and their luminal diameter gets increased comparing to the pretreatment appearances (arrows on E). Note is that multiple vessels and long segment involvement are suggestive of vasculitis.
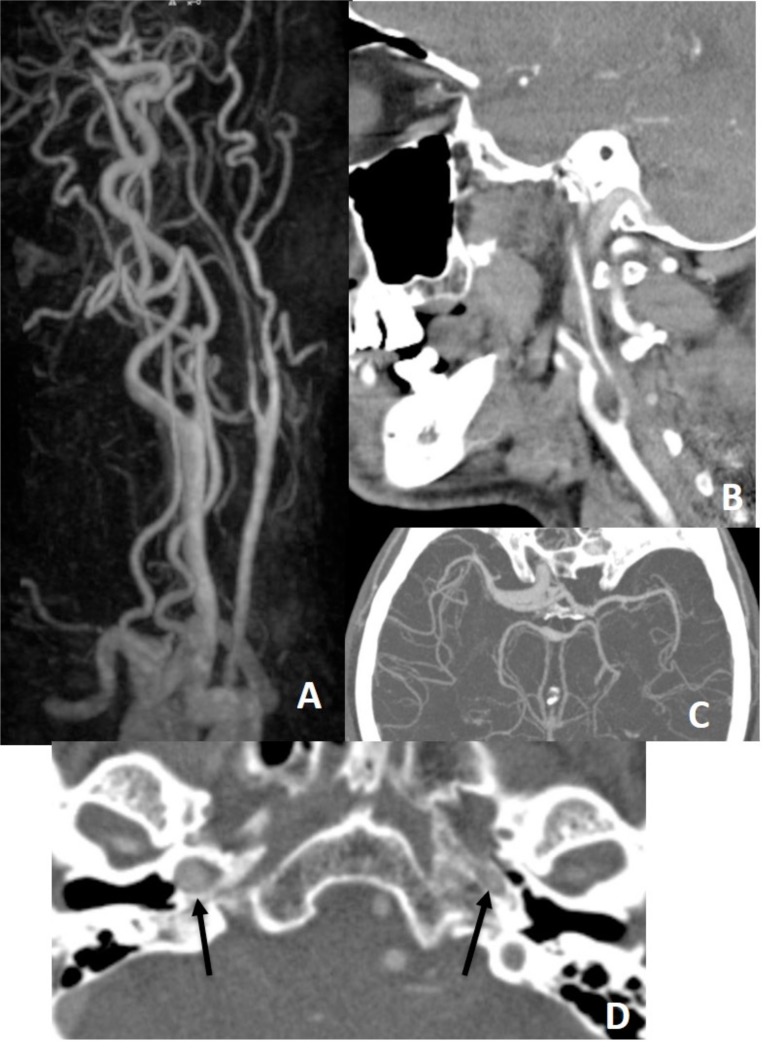
Fig. (18).
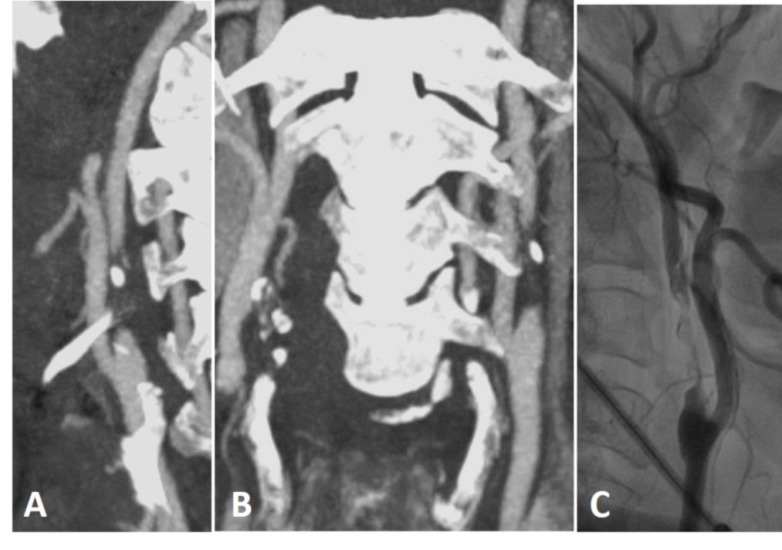
There is narrowing of left ICA on MRA (A) and CTA (B) with limitation of left MCA flow on CTA (C), but on CTA, bony contours (arrows on D) of ICAs at the skull base demonstrate left ICA being hypoplastic congenitally.
Fig. (20).
False tapering sign appearance of left ICA (A). There is thrombotic occlusion of left carotid terminus extending into M1 segment (B, C). After endovascular thrombolysis (D, E), left ICA return to normal. In fat suppressed T1 obtained before thrombolysis did not yield neither mural T1 hyperintensity nor increase in external diameter of left ICA comparing the right ICA (arrows on F) that would suggest dissection, but intramural hematoma will not be hyperintense in acute stage, external diameters of ICAs are more helpfull in this stage.
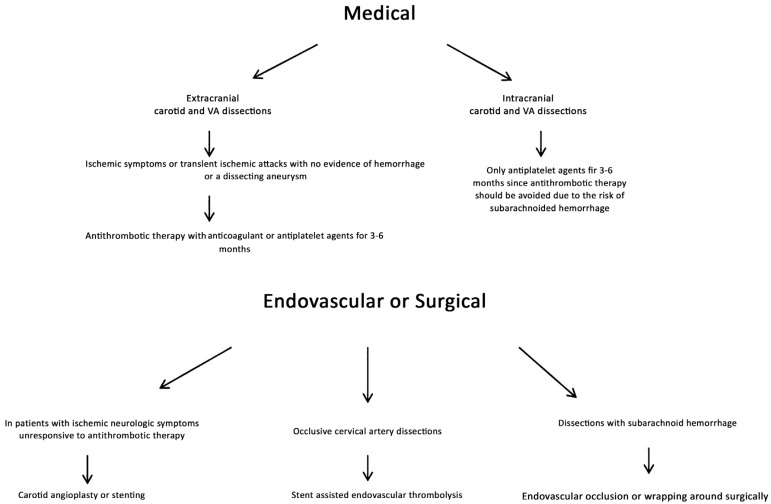
11. Treatment Approaches
There is no general consensus regarding optimal management of CCDs, but the choice among medical, endovascular, and surgical options may depend on the type of injury, anatomic location, the mechanism of injury, coexisting injuries, and comorbid conditions. Cervical spine immobilization, which is usually appropriate, should be performed in the setting of any significant traumatic injury that could involve the neck. Dissections may heal without causing any symptoms. In extracranial carotid and VA dissections, patients with ischemic symptoms or transient ischemic attacks and without any evidence of hemorrhage or a dissecting aneurysm, antithrombotic therapy with anticoagulant or antiplatelet agents for 3-6 months is the mostly widely used treatment [18, 40-42]. With MRI and MRA, by assessing the recanalization of the artery, treatment period can be changed. Recanalization of the lumen and reduction of the stenosis are more common in vertebral artery dissections than carotid artery dissections and seem to occur within 6 months after injury [4, 5, 7]. In intracranial dissections (especially in vertebral artery dissections), due to the risk of subarachnoid hemorrhage, antithrombotic therapy should be avoided. In patients in whom ischemic neurologic symptoms do not respond to antithrombotic therapy, carotid angioplasty or stenting may be considered [40]. In occlusive cervical artery dissections, stent assisted endovascular thrombolysis is being extensively performed in patients [43, 44]. Dissections presenting with subarachnoid hemorrhage are usually treated with surgery. The artery may be occluded by endovascularly or by wrapping it around surgically (Fig. 22). Rebleeding risk of previously ruptured dissecting aneuryms is more common than saccular aneurysms [3, 45].
Fig. (22).
The flow chart shows treatment approaches in CCDs.
Conclusion
DSA has been accepted as golden standard in the diagnosis of CCDs; however, nowadays, MRI, MRA and CT, CTA are more widely used imaging modalities. These techniques have the advantages of demonstrating luminal stenosis and intramural hematoma; therefore, they have up to 99% accuracy and sensitivity. Although endovascular methods have been developed in the treatment of CCDs, the use of antithrombotic therapy and follow-up of patients with MRI and MRA are more appropriate. If MRI, MRA are normal, the suspicion of dissection is still present especially in posterior circulation, CTA should be employed. If there is difficulty in differentiating between thrombotic occlusions and dissection, CTA and DSA should be performed in order.
Consent for Publication
Not applicable.
Fig. (5).
Intimal flap type dissection of left ICA on contrast enhanced MRA (arrow on A) distal to bulbus with thromboembolic infarction in left MCA territory on DWI (B). Intimal flap type dissection of left M1 on brain MRA (C) in another patient with a history of right sided paresis, difficulty in speaking, visual problem and tinnitus 11 days ago.
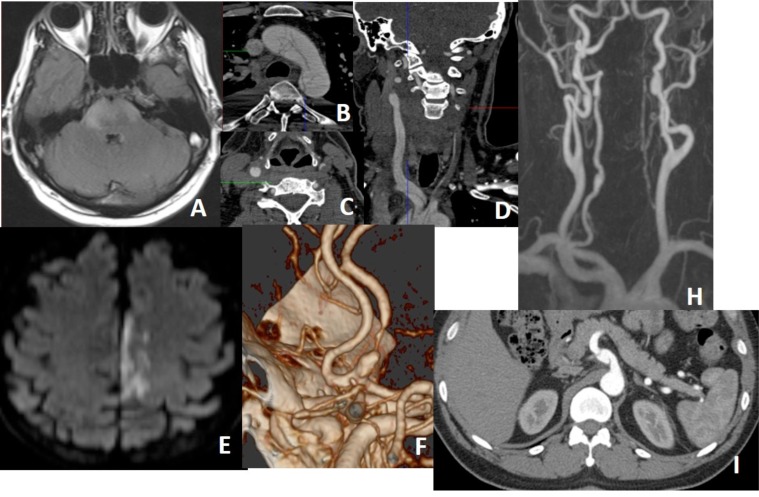
Fig. (10).
In a patient with a history of trauma, there is massive subarachnoid bleeding in right sylvian fissure (A) and parenchymal contusions in right temporal and frontal lobes (B). Axial MIP CTA images (C, D) show occlusion of right M1 segment and increased leptomeningeal-pial collateralization in the right MCA territory. Findings are compatible with postraumatic grade 5 dissection. Not: the arrow on C show middle cerebral vein spilling into the basal vein of Rosenthal.
Fig. (11).
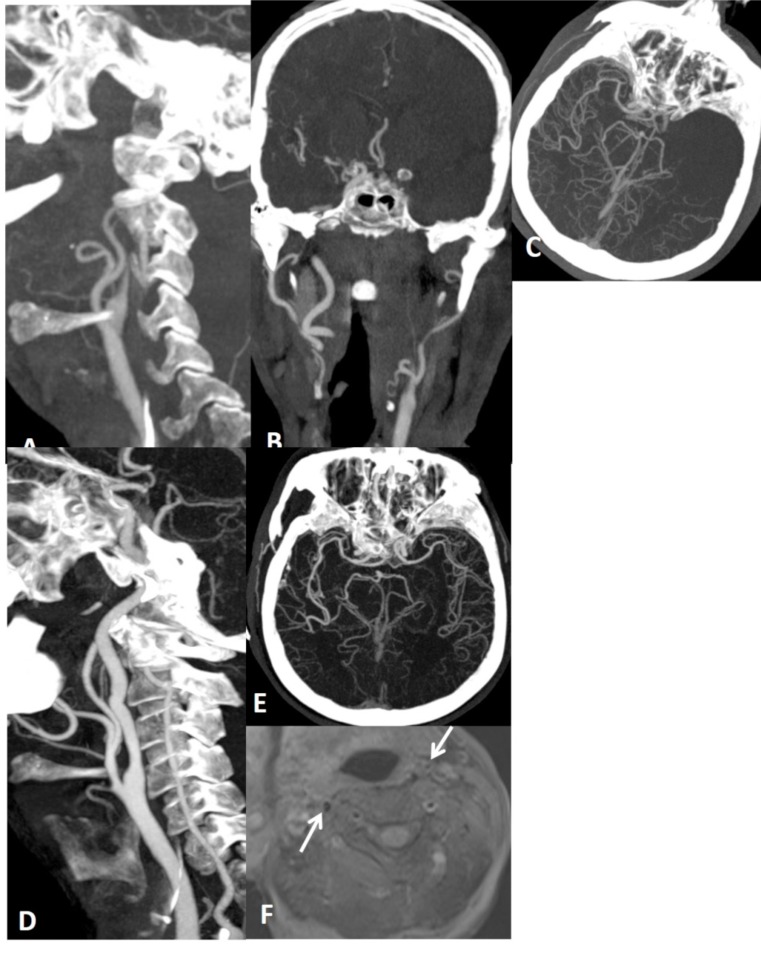
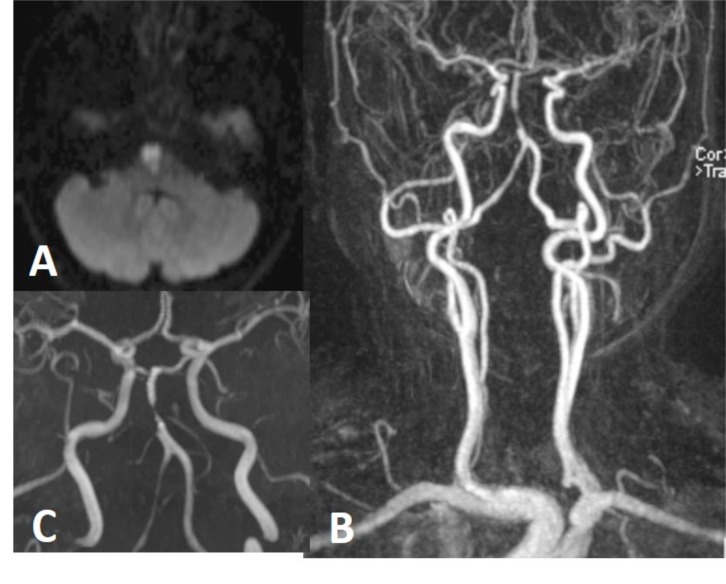
6 months earlier neck and brain MRA performed for tinnitus was normal (not shown here). Upon the presentation of brain stem acute infarction (A), neck (B) and brain (C) MRA were repeated showing focal narrowing (string sign), compatible with basilar artery dissection.
Fig. (12).
Axial Flair (A) and DWI (B) show different ages of thromboembolic infarctions in the brain stem and right cerebellum. Focal narrowing of right VA is seen on a MIP image of brain MRA (C). Source images of 3D TOF MRA from caudal to cranial (D-F) show eccentric luminal narrowing associated with increased external diameter of right VA due to intramural hematoma which is seen minimally hyperintense on E (arrow).
Fig. (13).
In a patient with recurrent thromboembolic infarction within the posterior circulation (A), brain MRA shows double lumen appearance of basiler artery. Flow is present in both lumen (B, C). Dissection is extending into right VA with hyperintense mural thickening (arrow on D), better seen in source image (D) than MIP image (E) of 3D TOF MRA.
Acknowledgements
Declared none.
LIST OF ABBREVIATIONS
- CCD
Craniocervical Dissections
- CTA
Computerized Tomography Angiography
- DSA
Digital Subtraction Angiography
- ICA
Internal Carotid Artery
- MRA
Magnetic Resonance Angiography
- USG
Ultrasonography
- VA
Vertebral Artery
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Nagumo K. N akamori A, Kojima S. Rinsho Shinkeigaku. 2003;43:313–321. [Spontaneous intracranial internal carotid artery dissection: 6 case reports and a review of 39 cases in the literature]. [PubMed] [Google Scholar]
- 2.Volker W., Besselmann M., Dittrich R., et al. Generalized arteriopathy in patients with cervical artery dissection. Neurology. 2005;64:1508–1513. doi: 10.1212/01.WNL.0000159739.24607.98. [DOI] [PubMed] [Google Scholar]
- 3.Fusca M.R., Harrigan M.R. Cerebrovascular dissections--a review part I: Spontaneous dissections. Neurosurgery. 2011;68:242–257. doi: 10.1227/NEU.0b013e3182012323. [DOI] [PubMed] [Google Scholar]
- 4.Benninger D.H., Gandjour J., Georgiadis D., et al. Benign long-term outcome of conservatively treated cervical aneurysms due to carotid dissection. Neurology. 2007;69:486–487. doi: 10.1212/01.wnl.0000266633.67387.2e. [DOI] [PubMed] [Google Scholar]
- 5.Caso V., Paciaroni M., Corea F., et al. Recanalization of cervical artery dissection: influencing factors and role in neurological outcome. Cerebrovasc. Dis. 2004;17:93–97. doi: 10.1159/000075775. [DOI] [PubMed] [Google Scholar]
- 6.Menon R.K., Norris J.W. Cervical arterial dissection: Current concepts. Ann. N. Y. Acad. Sci. 2008;1142:200–217. doi: 10.1196/annals.1444.015. [DOI] [PubMed] [Google Scholar]
- 7.Lee V.H., Brown R.D., Jr, Mandrekar J.N., et al. Incidence and outcome of cervical artery dissection: A population-based study. Neurology. 2006;67:1809–1812. doi: 10.1212/01.wnl.0000244486.30455.71. [DOI] [PubMed] [Google Scholar]
- 8.Guillon B., Brunereau L., Biousse V., et al. Long-term follow-up of aneurysms developed during extracranial internal carotid artery dissection. Neurology. 1999;53:117–122. doi: 10.1212/wnl.53.1.117. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez-Sabin J. Stroke in teenagers. Rev. Neurol. 1997;25:919–923. [PubMed] [Google Scholar]
- 10.Fullerton H.J., Johnston S.C., Smith W.S. Arterial dissection and stroke in children. Neurology. 2001;57:1155–1160. doi: 10.1212/wnl.57.7.1155. [DOI] [PubMed] [Google Scholar]
- 11.Raser J.M., Mullen M.T., Kasner S.E., et al. Cervical carotid artery dissection is associated with styloid process length. Neurology. 2011;77:2061–2066. doi: 10.1212/WNL.0b013e31823b4729. [DOI] [PubMed] [Google Scholar]
- 12.Schievink W.I., Roiter V. Epidemiology of cervical artery dissection. Front Neurol. Neurosci. 2005;20:12–15. doi: 10.1159/000088125. [DOI] [PubMed] [Google Scholar]
- 13.Piacaroni M., Georgiardis D., Arnold M., et al. Seasonal variability in spontaneous cervical artery dissection. J. Neurol. Neurosurg. Psychiatry. 2006;77:677–679. doi: 10.1136/jnnp.2005.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biffl W.L., Moore E.E., Offner P.J., et al. Blunt carotid arterial injuries: Implications of a new grading scale. J. Trauma. 1999;47:845–853. doi: 10.1097/00005373-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Biffl W.L., Moore E.E., Offner P.J., et al. Blunt carotid and vertebral arterial injuries. World J. Surg. 2001;25:1036–1043. doi: 10.1007/s00268-001-0056-x. [DOI] [PubMed] [Google Scholar]
- 16.Fusco M.R., Harrigan M.R. Cerebrovascular dissections: A review. Part II: Blunt cerebrovascular injury. Neurosurgery. 2011;68:517–530. doi: 10.1227/NEU.0b013e3181fe2fda. [DOI] [PubMed] [Google Scholar]
- 17.Stein D.M., Boswell S., Sliker C.W., et al. Blunt cerebrovascular injuries: does treatment always matter? J. Trauma. 2009;66:132–143. doi: 10.1097/TA.0b013e318142d146. [DOI] [PubMed] [Google Scholar]
- 18.Stence N.V., Fenton L.Z., Goldenberg N.A., et al. Craniocervical arterial dissectionin children: Diagnosis and treatment. Curr. Treat. Options Neurol. 2011;13:636–648. doi: 10.1007/s11940-011-0149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohkuma H., Suzuki S., Ogane K. Dissecting aneurysms of intracranial carotid circulation. Stroke. 2002;33:941–947. doi: 10.1161/01.str.0000013564.73522.05. [DOI] [PubMed] [Google Scholar]
- 20.Ro A., Kageyama N., Abe N., et al. Intracranial vertebral artery dissection resulting in fatal subarachnoid hemorrhage: Clinical and histopatho-logical investigations from a medicolegal perspective. J. Neurosurg. 2009;110:948–954. doi: 10.3171/2008.11.JNS08951. [DOI] [PubMed] [Google Scholar]
- 21.Caplan L.R. Dissections of brain-supplying arteries. Nat. Clin. Pract. Neurol. 2008;4:34–42. doi: 10.1038/ncpneuro0683. [DOI] [PubMed] [Google Scholar]
- 22.Menon R.K., Norris J.W. Cervical arterial dissection: Current concepts. Ann. N. Y. Acad. Sci. 2008;1142:200–217. doi: 10.1196/annals.1444.015. [DOI] [PubMed] [Google Scholar]
- 23.Fassett D.R., Dailey A.T., Vaccaro A.R. Vertebral artery injuries associated with cervical spine injuries: A review of the literature. J. Spinal Disord. Tech. 2008;21:252–258. doi: 10.1097/BSD.0b013e3180cab162. [DOI] [PubMed] [Google Scholar]
- 24.Arnold M., Bousser M.G., Fahrni G., et al. Vertebral artery dissection: presenting findings and predictors of outcome. Stroke. 2006;37:2499–2503. doi: 10.1161/01.STR.0000240493.88473.39. [DOI] [PubMed] [Google Scholar]
- 25.Gottesman R.F., Sharma P., Robinson K.A., et al. Clinical characteristics of symptomatic vertebral artery dissection: A systematic review. Neurologist. 2012;18:245–254. doi: 10.1097/NRL.0b013e31826754e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mas J.L., Bousser M.G., Hasboun D., et al. Extracranial vertebral artery dissections: A review of 13 cases. Stroke. 1987;18:1037–1047. doi: 10.1161/01.str.18.6.1037. [DOI] [PubMed] [Google Scholar]
- 27.Leclerc X., Godefroy O., Salhi A., Lucas C., Leys D., Pruvo J.P. Helical CT for the diagnosis of extracranial internal carotid artery dissection. Stroke. 1996;27:461–466. doi: 10.1161/01.str.27.3.461. [DOI] [PubMed] [Google Scholar]
- 28.Mitsumura H., Miyagawa S., Komatsu T., Hirai T., Kono Y., Iguchi Y. Clinical Characteristics of Intracranial Reversed Vertebral Artery Flow Evaluated by Transcranial Color Flow Imaging. J. Stroke Cerebrovasc. Dis. 2015;24(8):1775–1780. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.044. [DOI] [PubMed] [Google Scholar]
- 29.Debette S. Pathophysiology and risk factors of cervical artery dissection: What have we learnt from large hospital-based cohorts? Curr. Opin. Neurol. 2014;27:20–28. doi: 10.1097/WCO.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 30.Rodallec M.H., Marteau V., Gerber S., et al. Craniocervical arterial dissection: Spectrum of imaging findings and differential diagnosis. Radiographics. 2008;28:1711–1728. doi: 10.1148/rg.286085512. [DOI] [PubMed] [Google Scholar]
- 31.Provenzale J.M., Sarikaya B., Hacein-Bey L., et al. Causes of misinterpretation of cross-sectional imaging studies for dissection of the craniocervical arteries. AJR Am. J. Roentgenol. 2011;196:45–52. doi: 10.2214/AJR.10.5384. [DOI] [PubMed] [Google Scholar]
- 32.Provenzale J.M., Sarikaya B. Comparison of test performance characteristics of MRI, MR angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: A review of the medical literature. AJR Am. J. Roentgenol. 2009;193:1167–1174. doi: 10.2214/AJR.08.1688. [DOI] [PubMed] [Google Scholar]
- 33.Bachmann R., Nassenstein I., Kooijman H., et al. Spontaneous acute dissection of the internal carotid artery: High-resolution magnetic resonance imaging at 3.0 tesla with a dedicated surface coil. Invest. Radiol. 2006;41:105–111. doi: 10.1097/01.rli.0000195836.57778.1f. [DOI] [PubMed] [Google Scholar]
- 34.Naggara O., Oppenheim C., Toussaint J.F., et al. Asymptomatic spontaneous acute vertebral artery dissection: Diagnosis by high-resolution magnetic resonance images with a dedicated surface coil. Eur. Radiol. 2007;17:2434–2435. doi: 10.1007/s00330-006-0524-7. [DOI] [PubMed] [Google Scholar]
- 35.Srinivasan J., Newell D.W., Sturzenegger M., Mayberg M.R., Winn H.R. Transcranial Doppler in the evaluation of internal carotid artery dissection. Stroke. 1996;27:1226–1230. doi: 10.1161/01.str.27.7.1226. [DOI] [PubMed] [Google Scholar]
- 36.Babikian V.L., Forteza A.M., Gavrilescu T., Samaraweera R. Cerebral microembolism and extracranial internal carotid artery dissection. J. Ultrasound Med. 1996;15:863–866. doi: 10.7863/jum.1996.15.12.863. [DOI] [PubMed] [Google Scholar]
- 37.Li Q., Wang J., Chen H., et al. Characterization of craniocervical artery dissection by simultaneous MR noncontrast angiography and intraplaque hemorrhage imaging at 3T. AJNR Am. J. Neuroradiol. 2015;36:1769–1775. doi: 10.3174/ajnr.A4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J., Guan M., Yamada K., et al. In vivo validation of simultaneous non-contrast angiography and intraplaque hemorrhage (SNAP) magnetic resonance angiography: An intracranial artery study. PLoS One. 2016;11:e0149130. doi: 10.1371/journal.pone.0149130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janzarik W.G., Ringleb P.A., Reinhard M., Rauer S. Recurrent extracranial carotid artery vasospasms: Report of 2 cases. Stroke. 2006;37:2170–2173. doi: 10.1161/01.STR.0000231646.30541.d8. [DOI] [PubMed] [Google Scholar]
- 40.Brott T.G., Halperin J.L., Abbara S., et al. 2011 ASA/ACCF/AHA/ AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Stroke. 2011;42:e420–e63. doi: 10.1161/STR.0b013e3182112d08. [DOI] [PubMed] [Google Scholar]
- 41.Lyrer P., Engelter S. Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst. Rev. 2010;10:CD000255. doi: 10.1002/14651858.CD000255. [DOI] [PubMed] [Google Scholar]
- 42.Kim Y.K., Schulman S. Cervical artery dissection: Pathology, epidemiology and management. Thromb. Res. 2009;123:810–821. doi: 10.1016/j.thromres.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 43.Mourand I., Brunel H., Vendrell J.F., et al. Endovascular stent-assisted thrombolysis in acute occlusive carotid artery dissection. Neuroradiology. 2010;52:135–140. doi: 10.1007/s00234-009-0597-5. [DOI] [PubMed] [Google Scholar]
- 44.Lavallee P.C., Mazighi M., Saint-Maurice J.P., et al. Stent-assisted endovascular thrombolysis versus intravenous thrombolysis in internal carotid artery dissection with tandem internal carotid and middle cerebral artery occlusion. Stroke. 2007;38:2270–2274. doi: 10.1161/STROKEAHA.106.481093. [DOI] [PubMed] [Google Scholar]
- 45.Yamada M., Kitahara T., Kurata A., et al. Intracranial vertebral artery dissection with subarachnoid hemorrhage: Clinical characteristics and outcomes in conservatively treated patients. J. Neurosurg. 2004;101:25–30. doi: 10.3171/jns.2004.101.1.0025. [DOI] [PubMed] [Google Scholar]