Abstract
Background
Previously, transgenic trichome-bearing (hairy leaf) Brassica napus lines expressing either the Arabidopsis thaliana GL3 gene (line AtGL3+) [1] or the AtGL3 gene in combination with an RNAi construct to down-regulate TTG1 (line K-5-8) [2] were developed. The leaves of these lines exhibited altered insect feeding (flea beetle) and oviposition (diamondback moth) behaviour compared to the non-transgenic semi-glabrous leaves of B. napus cv. Westar. Interestingly, the cotyledons of these lines remained glabrous, but also showed reduced feeding by flea beetles. Here we examine the composition and global transcriptome of the glabrous cotyledons from these transgenic lines to ascertain the mechanism(s) underlying this unexpected phenomenon.
Results
Approximately, 7500 genes were up-regulated in cotyledons of each hairy line, compared with < 30 that were down-regulated. The up-regulated genes included those involved in cell wall synthesis, secondary metabolite production, redox, stress and hormone-related responses that have the potential to impact host plant cues required to elicit defense responses toward insect pests. In particular, the expression of glucosinolate biosynthetic and degradation genes were substantially altered in the glabrous cotyledons of the two hairy leaf lines. The transcriptomic data was supported by glucosinolate and cell wall composition profiles of the cotyledons. Changes in gene expression were much more extreme in the AtGL3+ line compared with the K-5-8 line in terms of diversity and intensity.
Conclusions
The study provides a roadmap for the isolation and identification of insect resistance compounds and proteins in the glabrous cotyledons of these hairy leaf lines. It also confirms the impact of mis-expression of GL3 and TTG1 on types of metabolism other than those associated with trichomes. Finally, the large number of up-regulated genes encoding heat shock proteins, PR proteins, protease inhibitors, glucosinolate synthesis/breakdown factors, abiotic stress factors, redox proteins, transcription factors, and proteins required for auxin metabolism also suggest that these cotyledons are now primed for resistance to other forms of biotic and abiotic stress.
Electronic supplementary material
The online version of this article (10.1186/s12870-018-1277-6) contains supplementary material, which is available to authorized users.
Keywords: Brassica napus, Trichomes, Cotyledons, Glucosinolates, Flea beetle, RNA sequencing
Background
Plants possess a variety of biochemical and morphological defences aimed at deterring insects from herbivory and oviposition. Defences can be either induced upon attack and localized to the site of attack, be systemic, or both (reviewed in [3]). Host plant resistance is categorized into three groups: i) antibiosis, resulting in increased mortality, reduced longevity or reduced reproduction of the insect; ii) antixenosis, affecting the behaviour of the insect and often expressed as non-preference for a resistant plant compared to a susceptible plant; and iii) tolerance, this being the ability of a plant to withstand or recover from insect damage and perform better than a susceptible plant grown under similar conditions [4]. Crops with insect resistance can reduce the accumulation of harmful chemical residues in the environment, as well as provide economic benefits to farmers and seed producers.
Glabrous (smooth) and semi-glabrous (few trichomes) lines of Brassica napus L. and Brassica rapa L. canola (oilseed rape) and Brassica vegetable crops are susceptible to many specialist and generalist insect pests. Flea beetles (FBs) [Phyllotreta cruciferae (Goeze) and Phyllotreta striolata (Fab.)] are specialist Brassica pests in several parts of the world, including Canada [5], India, and Eastern Europe [6, 7]. FBs attack at the crucial seedling stage as well as at more mature stages, such as leaves and developing green pods, where they reduce seed yield and grade. FB have developed resistance to insecticides used for their control (reviewed in [8, 9]). This highlights the importance of developing insect-resistant cultivars to reduce the use of chemical protection methods.
Trichomes (leaf hairs) have evolved as a physical defence against herbivore feeding and oviposition [10], and trichome density and length both negatively impact these processes in many insect species. Trichomes on Arabidopsis thaliana L. develop at the distal end of the developing leaf, thereby protecting the more supple, younger parts of the leaf that are preferred for diamondback moth oviposition and more vulnerable to FB and Psylloides sp. feeding damage [11, 12]. Mature Brassica villosa leaves have an extremely high density of trichomes (~ 4000 cm− 1) and are immune to FB damage in the field as the insects avoid the leaves [13].
Several Arabidopsis thaliana glabrous (lacking trichomes) mutants are also deficient in the production of secondary metabolites, most notably anthocyanins. The link between trichome formation and secondary metabolite production occurs through sharing of components within the Myb/bHLH/TTG1 (MBW) transcriptional regulatory complex. In trichome formation, the basic helix-loop-helix transcription factors GL3, or the similar factor ENHANCER OF GL3, form a regulatory complex with the R2R3-MYB factor GL1 and the WD-repeat protein TTG1 which interacts with GL3 and EGL3. This regulatory complex activates the expression of genes encoding a secondary set of transcription factors comprising GL2, TTG2 and SIM to induce trichome formation. In addition, at least 6 other R3 MYB proteins (CPC, TRY, ETC1, ETC2, ETC3 and TCL1) can replace GL1 to disrupt and/or alter the specificity of the regulatory complex [14–16]. Genes encoding enzymes involved in the later stages of anthocyanin biosynthesis are regulated by a similar complex consisting of TTG1 and GL3, but with different MYB factors [17].
Earlier, we demonstrated that ectopic expression of A. thaliana GL3 dramatically increases trichome formation on B. napus leaves, but compromises plant development [1]. Trichome production is further enhanced, while the developmental abnormalities are alleviated, when the expression of the endogenous TTG1 gene is reduced through the introduction of an RNAi construct [2]. Like hairy B. villosa, FB feeding is reduced on seedlings of the hairy leaf transgenic B. napus lines [1, 2, 18] as the insects do not initiate feeding probes on the trichome-enhanced leaves [2, 19]. The leaves of these lines exhibit large changes to their transcriptomes and growth patterns [2]. Curiously, the glabrous cotyledons from the hairy-leaf transgenic B. napus lines also showed highly reduced FB feeding (30–50%) [18]. It was suggested that the antixenotic effect could be due to altered plant architecture as the cotyledons in both transgenic lines are initially vertically oriented before becoming horizontally oriented as the plant develops. However, they could not rule out the possibility that a change in biochemical composition was also involved. Attraction and stimulation of feeding in FBs is governed principally by glucosinolates and/or their breakdown products from the host plant [20]. This suggested that the composition and gene expression patterns of the AtGL3 transgenic glabrous cotyledons may also have been altered so as to impact FB behaviour. Here, we examine the composition of glabrous cotyledons of the two transgenic hairy B. napus lines: one hairy leaf line expressing the AtGL3 gene (AtGL3+) and one ultra-hairy leaf line (K-5-8) expressing AtGL3 and down-regulated in the expression of the BnTTG1 gene. We demonstrate that the glabrous cotyledons of these lines exhibited altered secondary metabolite (anthocyanins and glucosinolates) and lignin content, as well as altered expression of genes specifying secondary metabolite biosynthesis and degradation, cell wall biosynthesis, hormones, and redox proteins which may have contributed to changes in host plant cues for insect pests.
Methods
Plant material
Untreated seeds of three plant entries were used in this study: the semi-glabrous leaf B. napus cv. Westar (parent line), a homozygous hairy-leaf AtGL3 transgenic B. napus developed from a cv. Westar parental background (line AtGL3+, 1), and a homozygous ultra-hairy transgenic leaf B. napus (line K-5-8) having highly reduced BnTTG1 expression within the AtGL3+ B. napus background [2]. Seeds were sterilized and placed onto solid MS media. Cotyledons for glucosinolates (GS), cell wall component analysis, and RNA sequencing were harvested from 7-day old seedlings grown in three replicates in magenta jars (10 seeds/replicate) under insecticide-free conditions in a controlled plant growth chamber (22/18o C; 16 h photoperiod; 60–80 μmoles.m− 2.s− 1). Cotyledons for qRT-PCR of trichome genes were grown at 22/24o C under a 16 h photoperiod with light at 400 μE. m− 2.s− 1. Cotyledons used to measure phenotype changes, anthocyanin content, and qRT-PCR of anthocyanin genes were grown for 10 days under continuous light (400 μE. m− 2.s− 1).
Cotyledon composition analysis
Seven-day-old cotyledons were extracted and glucosinolates (GS) converted to desulfoglucosinolates (DS-GS) based on the AOCS Official Method Ak 1–92. Specifically, freeze-dried cotyledons (~ 0.1 g) were agitated with steel rods (25 × 8 mm) on an Eberbach reciprocating shaker for 10 min at 280 rpm, and then 3 ml of methanol and 1 ml of 0.2 mM benzyl GS were added and shaking continued for 60 min. After centrifugation at 2300 g for 15 min, 3 ml of supernatant was loaded onto 0.3 ml pre-swollen DEAE-Sephadex resin (~ 30 mg) in Bio-Spin micro-columns (Bio-Rad, Mississauga Canada). The resin was rinsed with 1.5 ml of 2% acetic acid, 1.8 ml of water and 1.2 ml of 20 mM sodium acetate at pH 4.0, and then 100 μl of a purified sulfatase solution was added to the resin and the micro-columns sealed and incubated at 20 °C overnight. DS-GS were eluted with 1.2 ml of water, filtered, and separated using a Waters UPLC-PDA-TQD system and a BEH Shield RP18 column (2.1 × 50 mm; 1.7 μm) (100% water at 0.8 mL/min) for 0.3 min, followed by a linear gradient of 0% to 25% acetonitrile (v/v) over 6.7 min. The DS-GS were quantified at 229 nm and identified by monitoring the characteristic loss of 162.2 mass units using MS/MS constant neutral loss scans.
Cell wall carbohydrates and lignin were measured on purified cell wall residue (CWR) as per methods in UpdeGraff [21], Brinkman et al. [22], and Aung et al. [23]. CWR was extracted in phosphate buffer with Triton X-100 using 10 mg of seven-day-old cotyledons. Lignin content was analysed on CWR based on the thioglycolate-alkaline hydrolysis assay [22] and quantified using UV/VIS spectrophotometry at 280 nm and a calibration curve developed with commercial lignin (Sigma-Aldrich, Oakville, ON, Canada). Total acid-releasable cellulosic glucose was determined from CWR using a commercial cellulose standard (Sigma-Aldrich) and anthrone reagent spectrophotometry at 280 nm after sugar and starch removal based on the method of Theander et al. [24].
Cotyledon RNA sequencing
Total RNA was extracted from cotyledons of seven-day-old seedlings using an RNAeasy Mini Kit with contaminating gDNA being removed using RNAse-free DNAse™ (Qiagene Inc., ON Canada). RNA samples were quantified and RNA integrity determined using an RNA6000 nano assay in an Agilent 2100 Bioanalyzer™ (Agilent Technologies, Palo Alto, CA USA). RNA library preparation and sequencing were carried out using the Illumina TrueSeq RNA sample preparation platform v.2 with multiplex labeling following the manufacturer’s protocols. Details on cDNA library development, RNA sequencing, and data analysis were identical to those found in Alahakoon et al. [2]. Cotyledon gene expression changes in the two transgenic hairy leaf lines relative to cv. Westar cotyledon expression were then introduced into MAPMAN for allocation into 36 functional barcode index numbered (BIN) categories [25].
Quantitative-reverse transcription PCR (qRT-PCR) analysis
qRT-PCR was conducted on 10-day-old cotyledons to test AtGL3 expression and the summed transcripts of all homeologues for each of five B. napus regulatory genes (BnGL1, BnGL2, BnGL3, BnTTG1 and BnTRY) encoding proteins known to be involved in the trichome MYB-βHLH − WD40 tri-protein initiation complex as outlined in Alahakoon et al. [2].
Statistical analysis
Cotyledon composition and qRT-PCR data were analyzed with either one-way or two-way ANOVA using a MIXED model in SAS 9.2 [26] or a t-test. Assumptions of ANOVA were tested using a Normality test (Shapiro-Wilk), an Equal variance test (Brown Forsythe) and a Levenes test. Means were compared using a Tukey test or pairwise using a Dunn’s method in SAS 9.2, and treatments were declared significant at P ≤ 0.05 and trends declared at P ≤ 0.1. Read counting and statistical analysis of the RNA-seq data were carried out using Cuffdiff in the Cufflinks software package [27].
Results
Cotyledon composition
As with true leaves, the glabrous AtGL3+ cotyledons were much smaller than Westar and K-5-8 cotyledons (Fig. 1 insert), reflecting the smaller stature and lower vigor of the AtGL3+ line, while the K-5-8 line produced much larger cotyledons and subsequently plants with higher vigour [2, 18]. We compared the biochemical composition of the cotyledons expressing AtGL3 to that of the wild-type line to determine if particular specific secondary metabolites or polymers contributing to cell fortification could be correlated with altered insect behaviour. Anthocyanins are particularly useful in young plants as a means of insect defense as they alter the spectral properties of the plant, potentially making it less visible, and increase their phenolic content [28]. AtGL3+ cotyledons also exhibited more red coloration on the abaxial surface than Westar cotyledons, while the K-5-8 cotyledon abaxial surface was green when grown indoors under continuous light to stress the cotyledons (Fig. 1a insert). The red coloration was due to increased anthocyanin (Fig. 1a) and an increase in transcription of three anthocyanin genes (BnANS, BnDFR and BnGST) in AtGL3+ cotyledons was noted compared to the other two lines (Fig. 1b).
Fig. 1.

Total seedling anthocyanins and qRT-PCR of anthocyanin gene expression in B. napus cv. Westar and transgenic lines (AtGL3+ and K-5-8) grown under 24 h continuous light. Panel A: Anthocyanins. Insert shows colour and morphology on the abaxial surface of the cotyledon. Panel B: qRT-PCR of anthocyanin genes. Expression of individual genes is relative to that of glabrous B. napus cv. Westar (set at 1), which has been normalized to the expression of the B. napus Act2 gene. A Tukey test was used to detect significant differences in total anthocyanins or expressed genes between the plant lines. Means (n = 3) + standard error with different letters differ significantly (p ≤ 0.05). FW = fresh weight
Glucosinolates are sulfur-linked glucosides commonly found in species within the Brassicaceae/Cruciferae [29]. Feeding initiation in crucifer specialists, such as FB, are influenced by glucosinolates and their breakdown products derived from the host plant [20]. The level of GSs in glabrous cotyledons of the two transgenic lines and Westar was 1000-fold lower than GS levels in the seed (data not shown). Total GS levels in K-5-8 cotyledons were not significantly different from those of Westar, while total GS in the AtGL3+ line was lower (Fig. 2a). The GS profile of the lines expressing AtGL3 was also found to be different from that of Westar and different from one another. Progoitrin (2-OH-3-butenyl-GS), 4-hydroxyglucobrassicin (4-hydroxy-3-indolylmethyl-GS) and glucobrassicin (indol-3-ylmethyl-GS) were the most abundant cotyledon GSs. While progoitrin levels in the transgenic lines were similar to Westar, the level was significantly higher in the K-5-8 line when compared to AtGL3+. The level of glucoraphanin (4-methylsulfinylbutyl-GS) was not significantly different among the lines, whereas the level of gluconapin (3-butenyl-GS) was significantly higher in the transgenic lines and further elevated in the AtGL3+ line. 4-hydroxyglucobrassicin levels were reduced with the K-5-8 lines having only 60% of that in Westar. Glucobrassicin levels in the transgenic lines were statistically similar to Westar; however, the K-5-8 cotyledons had significantly less of this GS than those of AtGL3+. Both types of transgenic cotyledons had lower 4-methoxy-glucobrassicin (4-methoxy-indol-3-ylmethyl-GS) levels compared to Westar with the AtGL3+ having even lower levels than the K-5-8 line.
Fig. 2.
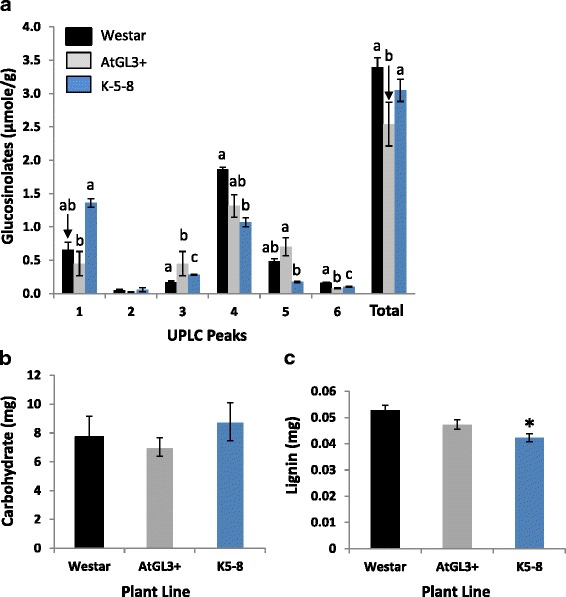
Composition of 7-day-old glabrous cotyledons. a Glucosinolates. UPLC peaks represent: 1, progoitrin (2-OH-3-butenyl-GS); 2, glucoraphanin (4-methylsulfinylbutyl-GS); 3, 3-butenyl-GS; 4, 4-hydroxy-3-indolylmethyl-GS; 5, 4-methoxy-3-indolylmethyl-GS; 6, 4-methoxy-glucobrassicin (4-methoxy-indol-3-ylmethyl-GS). Different letters indicate pairwise significance difference of the means (± SD) for each line within each compound type (p < 0.05). b Cell wall residue carbohydrates. c Cell wall residue lignin. Asterisk (*) indicates significance difference of the means (± SD) relative to cv. Wester (p < 0.05)
Changes in the composition of the plant cell wall (e.g. cellulose, hemicellulose, pectin and lignin) serves to fortify plant tissues and can make them more difficult to consume and more difficult from which to extract nutrition [30]. Cotyledon cell wall carbohydrate was similar between the two transgenic lines and cv. Westar (Fig. 2b). Lignin extracted from the cell wall residue was similar between Westar and the AtGL3+ line, but was significantly reduced in K-5-8 cotyledons (Fig. 2c).
Cotyledon transcriptomes related to insect host cues, metabolism and regulation
The glabrous cotyledons of the K-5-8 and AtGL3+ lines were as resistant to FB feeding as those from pesticide-treated Westar [18]. To further explore these phenomena, we conducted RNA-Seq analysis to identify transcription patterns in cotyledons which might affect insect behaviour. Cotyledons of the AtGL3+ line and K-5-8 showed up-regulation of 7924 and 7286 genes relative to Westar cotyledons, respectively, of which 4477 were common (Fig. 3). Curiously, fewer than 30 genes were down-regulated in either the hairy line or the ultra-hairy line relative to Westar (Fig. 3; Additional file 3: Figure S1 and Additional file 4: Figure S2). These genes could be organized within 36 MAPMAN functional categories (BINS), with the largest number of up-regulated genes falling within 17 functional categories (Fig. 3; Additional file 1: Table S1). Categories with moderate (ca. 20–200) numbers of up-regulated genes relative to Westar cotyledons, included those specifying photosynthesis (BIN #1), carbohydrates (BINs #2, 3), glycolysis (BIN #4), TCA/organic acid transformation (BIN #8), cell wall synthesis (BIN #10), lipid and amino acid metabolism (BIN #11, 13), metal handling (BIN #15), secondary metabolism (BIN #16), hormones (BIN #17), stress (BIN #20), redox (BIN #21), nucleotide-related (BIN #23), DNA-related (BIN #28), signalling (BIN #30), cell organization, etc. (BIN #31), development (BIN #31), and transport (BIN #34) (Fig. 3; Additional file 3: Figure S1 and Additional file 4: Figure S2). Categories with a large number of up-regulated genes (> 500) included those without known functions (BIN #26), RNA-related (BIN #27), protein-related (BIN #29) and miscellaneous functions (BIN #35; e.g. cytochrome P450 enzymes, carbohydrases, lipases) (Fig. 3). Moreover, ~ 25% of all genes that exhibited different expression patterns in the transgenic cotyledons could not be assigned a function (BIN #35).
Fig. 3.
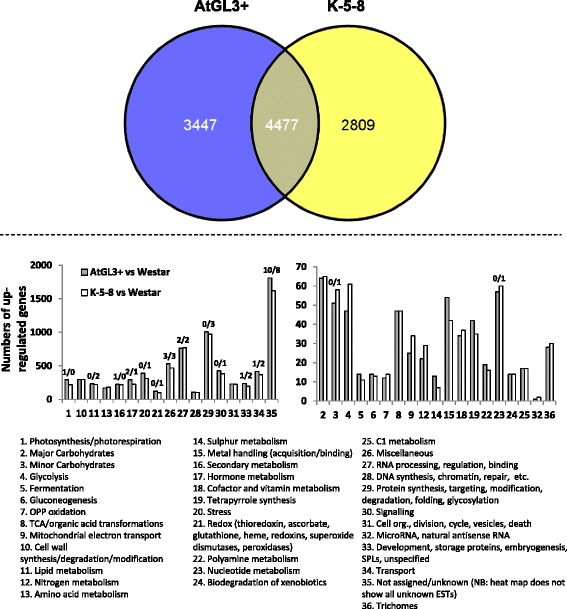
Overview of changes in glabrous cotyledon gene expression in 7-day-old hairy leaf (AtGL3+) and ultra-hairy leaf (K-5-8) B. napus lines relative to cv. Westar. Upper Panel: Venn diagram showing the number of up-regulated genes. Lower Panel: Mapman categories. The graphs show different data graphed on expanded or smaller Y axes. #/# above some pairs of bars indicate the number of down-regulated genes in that bin
Within the functional categories above, a suite of genes potentially involved in insect resistance and specifying tissue toughness (wax, cell wall carbohydrates/proteins and lignin synthesis), metal handling, flavonoid glycosylation, as well as phenylpropanoid, alkaloid, and cyanogenic glycoside synthesis, were strongly up-regulated in glabrous cotyledons of both the AtGL3+ hairy leaf line and the K-5-8 ultra-hairy leaf line relative to Westar cotyledons (Table 1, Additional file 1: Table S1; Additional file 5: Figure S3). Genes involved in GS biosynthesis and degradation were also up-regulated in both types of transgenic cotyledons, but a much larger number of these genes tended to be represented in transcriptomes of AtGL3+ cotyledons than in K-5-8 cotyledons (Fig. 4; Additional file 2: Table S2). In particular, genes encoding proteins involved in core biosynthesis of methionine-based, indole and benzyl GSs, including cytochrome P450s and a sulfotransferase (SOT18), as well as a MYB factor, were > 2-fold up-regulated in the AtGL3+ line, although genes encoding SOT18 and an aliphatic acconitase were highly up-regulated in the K-5-8 line (Fig. 4; Additional file 1: Table S1 and Additional file 2: Table S2). Moreover, a large number of genes involved in GS degradation, including those encoding myrosinase, a number of myrosinase-associated proteins (MAP), a myrosinase binding protein (MBP), an AOP1 oxidoreductase, and nitrile-specifier and epithio-specifier proteins were also up-regulated more strongly in the AtGL3+ line (Fig. 4). Finally, a large number of stress response genes were up-regulated in both types of transgenic cotyledons, including those responsive to biotic, drought and salt stress, those encoding pathogenesis-related (PR) proteins, PR-related protease inhibitors, heat shock proteins, and redox proteins known to assist with protection against reactive oxygen species (ROS), as well as those involved in hormonal control of growth and development (Additional file 2: Table S2; Additional file 6: Figure S4 and Additional file 1: Table S1). Genes encoding a wide variety of metabolism and transcription factors, or enzymes involved in protein modification and degradation, were strongly up-regulated in both AtGL3+ and K-5-8 cotyledons (Additional file 6: Figure S4 and Additional file 7: Figure S5).
Table 1.
Cotyledon genes (common to two hairy lines) with potential to impact host plant responses to flea beetles and diamondback moth
| Fold change relative to Westar | ||||
|---|---|---|---|---|
| Gene ID | Sub-categories | Description | AtGL3 line | K-5-8 linea |
| Metal Handling | ||||
| bo7g039050 | general | SBP3 (selenium-binding protein 3) | 1.07E+301 | 1.07E+301 |
| bo9g123330 | binding, chelation, storage | NAS2 (NICOTIANAMINE SYNTHASE 2) | 3.96E+08 | 3.92E+09 |
| bo4g040020 | " | ATSERAT2;1, SAT5, SAT1 (O-SERINE ACETYLTRANSFERASE 2;1) | 8.99 | 352.70 |
| bra002851 | " | NAS2 | NAS2 (NICOTIANAMINE SYNTHASE 2) | 47.80 | 77.58 |
| bra015594 | " | MT1C; copper ion binding | 4.42 | 10.20 |
| bra009595 | " | MT2A, ATMT-K, ATMT-1 (METALLOTHIONEIN 2A) | 3.23 | 7.38 |
| bo5g008330 | " | MT1C; copper ion binding | 4.41 | 7.17 |
| Wax | ||||
| bo8g102710 | synthesis | KCS4 (3-KETOACYL-COA SYNTHASE 4) | 3.33 | 5.83 |
| bra033983 | " | YBR159, KCR1 | YBR159; ketoreductase/oxidoreductase | 4.59 | 2.81 |
| bra004034 | " | CUT1, POP1, CER6, G2, KCS6 (3-KETOACYL-COA SYNTHASE 6) | 29.07 | 2.58 |
| bo7g019710 | " | KCS9 (3-KETOACYL-COA SYNTHASE 9) | 1.67 | 2.42 |
| bra024749 | " | CUT1, POP1, CER6, G2, KCS6 (3-KETOACYL-COA SYNTHASE 6) | 2.83 | 1.51 |
| Cell Wall | ||||
| bra011899 | modification | ATEXLA2, EXPL2, ATHEXP BETA 2.2 (EXPANSIN-LIKE A2) | 9.17 | 12.64 |
| bra024848 | " | EXGT-A3, XTH27, hydrolase, xyloglucan:xyloglucosyl transferase | 3.29 | 4.03 |
| bo3g018260 | precursor synthesis | UXS3 (UDP-GLUCURONIC ACID DECARBOXYLASE 3) | 4.72 | 18.03 |
| bra006722 | " | " | 3.87 | 9.62 |
| bra021798 | " | ATCSLB03, ATCSLB3 cellulose synthase/ glycosyl transferase | 6.43 | 5.18 |
| bo1g037390 | " | ATCSLG2 cellulose synthase/ glycosyl transferase | 3.73 | 3.17 |
| bra016440 | cell wall proteins | unknown protein | 8.59 | 101.30 |
| bo4g194100 | " | proline-rich extensin-like family protein | 5.50E+04 | 11.24 |
| bra037731 | " | " | 33328.67 | 7.21 |
| bra026268 | " | hydroxyproline-rich glycoprotein family protein | 20.79 | 5.06 |
| bo1g051380 | " | " | 9.65 | 4.97 |
| bra003200 | degradation.mannan-xylose-arabinose-fucose | PMR6 (powdery mildew resistant 6); pectate lyase | 5.41E+12 | 190.90 |
| bra024089 | " | MERI5B, MERI-5, SEN4 (meristem-5); hydrolyzing glycosyl bonds | 3.13 | 66.59 |
| bo4g108130 | " | pectinesterase family protein | 6.87 | 43.88 |
| bra009234 | " | ATPGIP1 (POLYGALACTURONASE INHIBITING PROTEIN 1); binding | 44.74 | 37.33 |
| bo2g127040 | " | pectinacetylesterase, putative | 3.30 | 19.53 |
| bra010038 | " | ATBXL1 (BETA-XYLOSIDASE 1); hydrolyzing O-glycosyl compounds | 1.70 | 9.03 |
| bo2g013480 | degradation.pectate lyases/polygalacturonases | glycoside hydrolase family 28 protein | 5.78 | 7.76 |
| bo6g058470 | " | PMR6 (powdery mildew resistant 6); pectate lyase | 1.96E+05 | 4.92 |
| Lignin | ||||
| bo3g024650 | biosynthesis | ATC4H, C4H, CYP73A5 (CINNAMATE-4-HYDROXYLASE) | 2717.78 | 39.35 |
| bra036480 | " | HCT (SHIKIMATE/QUINATE HYDROXYCINNAMOYL COA-TRANSFERASE) | 2.72 | 2.68 |
| Flavonoids | ||||
| bra018364 | dihydroflavonols | UGT71D1 (UDP-GLUCOSYL TRANSFERASE 71D1) | 7.75 | 12.33 |
| bra037386 | " | " | 7.15 | 9.32 |
| bo9g003740 | " | " | 65.69 | 6.15 |
| Phenylpropanoids | ||||
| bra028893 | phenylpropanoids | transferase family protein | 14.84 | 17.57 |
| bra029364 | " | NIC2 (NICOTINAMIDASE 2); catalytic nicotinamidase | 5.46 | 8.84 |
| bo3g167180 | " | O-methyltransferase family 2 protein | 10.07 | 8.41 |
| bo7g064020 | " | transferase family protein | 12.06 | 4.93 |
| Alkaloids | ||||
| bra003263 | N misc. alkaloid-like | strictosidine synthase family protein | 416.91 | 3.94 |
| bo6g054230 | " | " | 17.29 | 2.92 |
| Cyanogenic glucosides | ||||
| bra014956 | cyanase | CYN (CYANASE); DNA binding / cyanate hydratase/ hydro-lyase | 7.06 | 6.21 |
aCotyledon values are arranged from highest expression to lowest expression within each functional category using the K-5-8 line.
Fig. 4.

Changes in expression of genes related to glucosinolate biosynthesis and degradation in 7-day-old cotyledons grown under a 16/8 h diurnal light cycle. Figure shows GS UPLC peaks and gene expression changes (up-regulation) for the AtGL3+ line (A) and the K-5-8 line (K), each relative to B. napus cv. Westar levels. Br, Brassica rapa A-genome homeologue. Bo, Brassica oleracea C-genome homeologue. Gene IDs and expression levels can be viewed in Table 2
In general, more genes in each category were upregulated in the AtGL3+ line than in the K-5-8 line relative to Westar, for example, in hormones (BIN #17), stress (BIN #20), miscellaneous (BIN #26), protein (BIN #29), signalling (BIN #30), development (BIN #33), and transport (BIN #34) (Fig. 3; Additional file 1: Table S1). Up-regulated genes that were common to both hairy leaf lines often exhibited a greater level of expression in the AtGL3+ line than in the K-5-8 line (Tables 1, 2, and 3). This included genes involved in cell wall synthesis (cell wall proteins, pectate lyases, and lignin synthesis), and stress-response genes encoding chlorophyllase (COR1), PR protease inhibitors, and a SENESCENCE ASSOCIATED GENE 20, while the same genes were only moderately up-regulated within K-5-8 cotyledons. Up-regulated genes that were unique to AtGL3+ and K-5-8 cotyledons were linked to sulphur metabolism, metal-handling, secondary metabolism (anthocyanins, dihydroflavonols, carotenoids, non-mevalonate isoprenoids, alkaloids, phenylpropanoids, lignin, GS synthesis and GS degradation), hormones (auxin, brassinosteroids, cytokinin, ethylene, gibberellic acid, and jasmonate), and stress responses (general, biotic, PR proteins, protease inhibitors, defensins, heat shock, touch or wounding, drought and salt, and non-specified abiotic stress) (Additional file 1: Table S1). In addition, more redox-related genes potentially impacting the levels of reactive oxygen species (ROS) were up-regulated in AtGL3+ cotyledons than in K-5-8 cotyledons relative to Westar, although up-regulated redox-related genes unique to K-5-8 cotyledons had very strong transcriptional responses. In addition, genes involved in aspects of development, lipid metabolism, amino acid metabolism, photosynthesis, heat shock, and cold-response were more frequently and more strongly up-regulated in K-5-8 cotyledons (Additional file 1: Table S1).
Table 2.
Strongly up-regulated stress-responsive genes changes common to transgenic hairy B. napus cotyledons
| Expression relative to Westar | |||
|---|---|---|---|
| ID | Description | AtGL3 line | K-5-8 line |
| Biotic Stress | |||
| bo5g027670 | CORI1, ATHCOR1, ATCLH1 (CORONATINE-INDUCED 1); chlorophyllase | 5.30E+07 | 21.57 |
| bra008224 | pathogenesis-related thaumatin family protein | 2.83 | 14.46 |
| bo2g069600 | MLP28 (MLP-LIKE PROTEIN 28) | 174.64 | 13.00 |
| bra007947 | " | 119.90 | 11.52 |
| bra016785 | RPS5 (RESISTANT TO P. SYRINGAE 5); nucleotide binding | 7.66 | 8.11 |
| bra008667 | glycosyl hydrolase family 81 protein | 6.06 | 7.46 |
| bo5g149860 | CHAT (acetyl CoA:(Z)-3-hexen-1-ol acetyltransferase) | 46.71 | 5.28 |
| bra011734 | ATRCCR | ACD2 (ACCELERATED CELL DEATH 2); red chlorophyll catabolite reductase | 4.47 | 4.47 |
| bo9g163710 | glycosyl hydrolase family 81 protein | 7.85 | 3.96 |
| bo07027s010 | HRT, RCY1, RPP8 (RECOGNITION OF PERONOSPORA PARASITICA 8); binds nucleotides | 3.46 | 3.84 |
| bra009184 | NHL3 | 3.83 | 3.57 |
| bo7g087120 | avirulence induced gene (AIG) protein, putative | 3.49 | 3.26 |
| Biotic Stress Signalling | |||
| bra031065 | TIFY10A | JAZ1 (JASMONATE-ZIM-DOMAIN PROTEIN 1); protein binding | 5.35E+14 | 2.49E+08 |
| bo8g102890 | " | 369.12 | 66.76 |
| bo5g027170 | " | 4.17 | 12.21 |
| Biotic Stress Regulation of Transcription | |||
| bra027377 | RSH2 (RELA-SPOT HOMOLOG 2); GTP diphosphokinase | 4.97 | 5.09 |
| bo5g131760 | " | 3.24 | 3.25 |
| PR proteins General | |||
| bo8g091760 | disease resistance protein (TIR-NBS-LRR class), putative | 5.81 | 6.67 |
| bra005378 | disease resistance family protein | 3.09 | 6.40 |
| bo6g007620 | molecular_function unknown; LOCATED IN: endomembrane system | 3.94 | 5.00 |
| bo1g048080 | disease resistance protein (NBS-LRR class), putative | 1.23 | 3.75 |
| PR proteins Protease Inhibitors | |||
| bo6g010170 | trypsin and protease inhibitor family protein / Kunitz family protein | 165.56 | 3.67 |
| bo6g010250 | " | 11.99 | 3.54 |
| bo6g010100 | " | 113.13 | 3.33 |
| bra015999 | " | 78.81 | 3.25 |
| bra037702 | trypsin inhibitor, putative | 18.46 | 1.40 |
| bra016073 | trypsin and protease inhibitor family protein / Kunitz family protein | 49.47 | 1.07 |
| Wounding | |||
| bra034157 | WI12, SAG20 (SENESCENCE ASSOCIATED GENE 20) | 1.07E+301 | 1.07E+301 |
| bra029887 | " | 2.93E+05 | 4.53E+04 |
| bra010381 | wound-responsive protein-related | 6697.92 | 76.02 |
| bo7g111980 | " | 5.51 | 10.78 |
| bo8g054160 | " | 6.39 | 9.56 |
| Abiotic Stress General | |||
| bo01463s030 | benzodiazepine receptor-related | 1.90E+04 | 4.85E+05 |
| bra021442 | " | 798.78 | 32627.32 |
| bra007841 | unknown protein | 388.29 | 266.92 |
| bo8g099690 | " | 96.36 | 115.05 |
| bo4g154720 | " | 2.96 | 6.57 |
| Heat responsive | |||
| bo1g138440 | DNAJ heat shock N-terminal domain-containing protein | 1.25E+15 | 7.51E+23 |
| bo9g026330 | DNAJ heat shock protein, putative | 6642.31 | 1.68E+11 |
| bra037247 | " | 7768.93 | 1.62E+10 |
| bo7g117750 | DNAJ heat shock N-terminal domain-containing protein (J11) | 1.46E+08 | 7.57E+09 |
| bra039384 | DNAJ heat shock N-terminal domain-containing protein | 1238.27 | 1.74E+06 |
| bra011656 | DNAJ heat shock N-terminal domain-containing protein (J11) | 216.64 | 2.14E+04 |
| bo1g005990 | " | 415.52 | 1.04E+04 |
| bra034691 | DNAJ heat shock N-terminal domain-containing protein | 12.28 | 139.25 |
| bra017744 | DNAJ heat shock N-terminal domain-containing protein (J11) | 18.63 | 77.88 |
| bra020505 | DNAJ heat shock protein, putative | 8.76E+04 | 24.75 |
| bo5g132640 | DNAJ heat shock N-terminal domain-containing protein | 5.58 | 23.31 |
| bra011735 | ATHSF4 | HSF4 (HEAT SHOCK FACTOR 4); DNA binding /transcription repressor | 7.14E+04 | 18.15 |
| bra018216 | 17.6 kDa class I small heat shock protein (HSP17.6C-CI) (AA 1-156) | 47.14 | 12.92 |
| bo2g158600 | DNAJ heat shock protein, putative | 788.57 | 7.39 |
| bo8g066630 | Hsp70b (heat shock protein 70B); ATP binding | 6.69 | 7.32 |
| bo4g169420 | 17.6 kDa class I small heat shock protein (HSP17.6B-CI) | 13.24 | 7.18 |
| bo8g097710 | DNAJ heat shock N-terminal domain-containing protein | 3.07 | 5.16 |
| bra016644 | Hsp70b (heat shock protein 70B); ATP binding | 4.04 | 4.31 |
| bo1g134560 | DNAJ heat shock N-terminal domain-containing protein | 8.46 | 3.95 |
| bo2g029130 | heat shock protein-related | 4.61 | 3.81 |
| bra004457 | DNAJ heat shock N-terminal domain-containing protein | 2.72 | 3.75 |
| bra003592 | J8; heat shock protein binding / unfolded protein binding | 4.72 | 3.70 |
| bra020419 | heat shock protein-related | 4.29 | 3.60 |
| bo9g176930 | DNAJ heat shock N-terminal domain-containing protein | 4.42 | 3.46 |
| bo3g091720 | HSC70-1 (HEAT SHOCK COGNATE PROTEIN 70-1); ATP binding | 4.25 | 3.29 |
| bo8g102330 | DNAJ heat shock family protein | 4.19 | 3.10 |
| Cold responsive | |||
| bra017742 | CSDP1 (cold shock domain protein 1); RNA/single/double-stranded DNA binding | 1160.12 | 131.40 |
| bo7g117730 | " | 1.31E+14 | 8.31 |
| bra013087 | WCOR413-LIKE, FL3-5A3 | COR413-PM1 | 14.61 | 2.84 |
| Drought and salt responsive | |||
| bo3g052160 | early-responsive to dehydration protein-related / ERD protein-related | 177.05 | 1.43E+11 |
| bra039623 | ATCOAD (4-phosphopantetheine adenylyltransferase) | 4.62 | 5.42 |
| bo6g004950 | QUA2 | TSD2 (TUMOROUS SHOOT DEVELOPMENT 2); methyltransferase | 3.50 | 3.64 |
| bo4g154160 | hydrophobic protein, putative / low temperature-salt responsive | 3.15 | 3.24 |
| Abiotic Stress unspecified | |||
| bo8g052730 | PHOS34 | universal stress protein (USP) family protein | 9.63 | 35.62 |
| bo2g069600 | MLP28 (MLP-LIKE PROTEIN 28) | 174.64 | 13.00 |
| bo7g111350 | PHOS34 | universal stress protein (USP) family protein | 6.84 | 12.10 |
| bra007947 | MLP28 (MLP-LIKE PROTEIN 28) | 119.90 | 11.52 |
| bra008745 | universal stress protein (USP) family protein | 4.45 | 4.83 |
| bo5g002660 | ozone-responsive stress-related protein, putative | 4.21 | 4.15 |
| bra022721 | PHOS34 | universal stress protein (USP) family protein | 4.15 | 3.76 |
| bo9g165720 | " | 3.08 | 3.60 |
| bo3g039740 | " | 3.31 | 3.05 |
| bo6g020120 | " | 3.27 | 2.94 |
aCotyledon values are arranged from highest expression to lowest expression within each functional category using the K-5-8 line.
Table 3.
Trichome-related glabrous cotyledon genes in the hairy leaf AtGL3+ and ultra-hairy leaf K-5-8 lines relative to glabrous leaf B. napus cv. Westar
| ID | Bin Name | Description | Expression relative to Westar | |
|---|---|---|---|---|
| AtGL3 line | K-5-8 line | |||
| ESTs common to both transgenic lines | ||||
| bo5g002440 | Positive initiation | AN (ANGUSTIFOLIA); protein binding | 5.39 | 1.63 |
| bra024875 | " | RGA1 (REPRESSOR OF GA1-3 1); protein binding / transcription factor | 3.29 | 1.59 |
| bo9g070200 | " | RGA1 (REPRESSOR OF GA1-3 1); protein binding / transcription factor | 1.49 | 0.73 |
| bra007766 | " | FDH, KCS10 (3-KETOACYL-COA SYNTHASE 10); acyltransferase | 1.51 | 0.42 |
| bo1g116200 | Positive branching | DER1, LSR2, ENL2 | ACT2 (ACTIN 2); structural constituent of cytoskeleton | 1.53 | 1.46 |
| bra020572 | " | TUA6; structural constituent of cytoskeleton | 1.54 | 0.73 |
| bra039648 | " | TUA6; structural constituent of cytoskeleton | 1.48 | 0.55 |
| bo1g054880 | " | SPK1 (SPIKE1); GTP binding / GTPase binding / guanyl-nucleotide exchange factor | 1.51 | 0.47 |
| bra009451 | Multicellular trichomes | SIM (SIAMESE); cyclin-dependent protein kinase inhibitor | 1978.24 | 11.69 |
| bo9g178800 | " | SIM (SIAMESE); cyclin-dependent protein kinase inhibitor | 935.76 | 7.64 |
| bra027928 | Less developed | unknown protein | 1.45 | 0.42 |
| bo1g046580 | " | MRH5, GPDL2 | SHV3 (SHAVEN 3); glycerophosphodiester phosphodiesterase/ kinase | 1.14 | 0.37 |
| bra026409 | " | MRH5, GPDL2 | SHV3 (SHAVEN 3); glycerophosphodiester phosphodiesterase/ kinase | 1.13 | 0.35 |
| bo9g054590 | " | unknown protein | 1.44 | 0.24 |
| ESTs unique to the AtGL3+ line | ||||
| bra033258 | Positive initiation | AN (ANGUSTIFOLIA); protein binding | 14.72 | NA |
| bra017443 | " | RGA1 (REPRESSOR OF GA1-3 1); protein binding / transcription factor | 1.47 | NA |
| bra029388 | Distorted | EMB3009 (embryo defective 3009); transferase/ transferase, transferring acyl groups | 1.07E+301 | NA |
| bo7g097360 | " | EMB3009 (embryo defective 3009); transferase/ transferase, transferring acyl groups | 1.05E+59 | NA |
| bra004054 | Positive branching | TPS6 | ATTPS6; alpha,alpha-trehalose-phosphate synthase (UDP-forming)/ transferase | 3.29 | NA |
| bo3g071760 | " | DER1, LSR2, ENL2 | ACT2 (ACTIN 2); structural constituent of cytoskeleton | 1.68 | NA |
| bra022356 | " | DER1, LSR2, ENL2 | ACT2 (ACTIN 2); structural constituent of cytoskeleton | 1.64 | NA |
| bo5g117040 | " | DER1, LSR2, ENL2 | ACT2 (ACTIN 2); structural constituent of cytoskeleton | 1.55 | NA |
| bra037560 | " | DER1, LSR2, ENL2 | ACT2 (ACTIN 2); structural constituent of cytoskeleton | 1.48 | NA |
| bra008705 | Negative branching | ATMIXTA | ATMYB16 (MYB DOMAIN PROTEIN 16); DNA binding / transcription factor | 5.94 | NA |
| bo5g021100 | Endoreduplication | CYCA2;3 (CYCLIN A2;3); cyclin-dependent protein kinase regulator | 1.39 | NA |
| bo5g038710 | Trichome size | ATSAC1 (suppressor of actin 1); phosphatidylinositol-4,5-bisphosphate 5-phosphatase | 7.73 | NA |
| bo6g027740 | Less developed | MRH5, GPDL2 | SHV3 (SHAVEN 3); glycerophosphodiester phosphodiesterase/ kinase | 38.59 | NA |
| ESTs unique to the K-5-8 line | ||||
| bo8g115250 | Positive initiation | HDG2 (HOMEODOMAIN GLABROUS 2); DNA binding / transcription factor | NA | 0.43 |
| bo8g067530 | " | HDG12 (HOMEODOMAIN GLABROUS 12); transcription factor | NA | 0.27 |
| bra015401 | " | HDG2 (HOMEODOMAIN GLABROUS 2); DNA binding / transcription factor | NA | 0.13 |
| bo6g029470 | Positive branching | TPS6 | ATTPS6; alpha,alpha-trehalose-phosphate synthase (UDP-forming)/ transferase | NA | 1.65 |
| bo6g107020 | " | TUA6; structural constituent of cytoskeleton | NA | 0.63 |
| bo9g022270 | " | PKCBP, KCBP | ZWI (ZWICHEL); calmodulin binding / microtubule motor | NA | 0.57 |
| bra018825 | " | TUA6; structural constituent of cytoskeleton | NA | 0.53 |
| bra016164 | Negative initiation | MYBL2 (ARABIDOPSIS MYB-LIKE 2); DNA binding / transcription factor | NA | 1.56 |
| bo3g149420 | " | UPL3 | KAK (KAKTUS); ubiquitin-protein ligase | chr4:18041031-18049292 REVERSE' | NA | 0.68 |
| bo2g012520 | Negative branching | ATMIXTA | ATMYB16 (MYB DOMAIN PROTEIN 16); DNA binding / transcription factor | NA | 0.36 |
| bo3g010230 | " | ATMIXTA | ATMYB16 (MYB DOMAIN PROTEIN 16); DNA binding / transcription factor | NA | 0.29 |
| bo9g164230 | " | ATMIXTA | ATMYB16 (MYB DOMAIN PROTEIN 16); DNA binding / transcription factor | NA | 0.18 |
| bra024337 | Trichome size | HYS1 | CPR5 (CONSTITUTIVE EXPRESSION OF PR GENES 5) | NA | 1.94 |
| bo2g029050 | " | FLP1, YRE, CER3, WAX2 | CER3 (ECERIFERUM 3); binding / catalytic/ iron ion binding /oxidoreductase | NA | 0.56 |
| bra020412 | " | FLP1, YRE, CER3, WAX2 | CER3 (ECERIFERUM 3); binding / catalytic/ iron ion binding /oxidoreductase | NA | 0.41 |
| bo9g133540 | " | FLP1, YRE, CER3, WAX2 | CER3 (ECERIFERUM 3); binding / catalytic/ iron ion binding /oxidoreductase | NA | 0.20 |
aCotyledon values are arranged from highest expression to lowest expression within each functional category using the K-5-8 line
Expression of trichome genes in glabrous cotyledons of hairy-leaf and ultrahairy-leaf lines
The transgenic cotyledons remained glabrous despite the expression of AtGL3 or in conjunction with the manipulation of BnTTG1 expression (2). Hence, transcript levels for all known trichome genes were compared in both types of transgenic cotyledons to those of cv. Westar. According to RNA sequencing, no trichome genes were down-regulated in the glabrous transgenic cotyledons relative to cv. Westar cotyledons, and up-regulation of most transcripts involved trichome genes that should impact trichome structure (Table 3). Overall, the greatest change in transcript level relative to cv. Westar cotyledons included two SIAMESE (SIM) genes (bra009451 and bo9g178890) specifying multi-cellular trichomes that were also more highly expressed in the AtGL3+ line than in the K-5-8 line (Table 3). As well, two embryo defective 3009 genes (EMB3009) encoding acyl transferases involved in trichome shape were highly up-regulated in the AtGL3+ line, while SAC1 (affecting trichome size), SHAVEN3, and the MYB16 MIXTA genes were also up-regulated in this line (Table 3). A paralogue of the BrRGA1 gene was uniquely up-regulated in the AtGL3+ cotyledons and two RGA1 genes were commonly up-regulated in both transgenic lines.
Well-known trichome genes coding for the MBW tri-protein initiation complex were not differentially expressed in 7-day-old cotyledons. Only one well-known trichome regulatory gene, ANGUSTIFOLIA (AN, affecting trichome initiation), had a significantly different transcript profile in AtGL3+ or K-5-8 cotyledons compared with cv. Westar (Table 3). qRT-PCR with 10-day-old cotyledons showed that five well-known MBW trichome genes were weakly expressed. Although the BnGL2 trichome-initiation gene was expressed at a higher level in AtGL3+ cotyledons compared with K-5-8 and Westar cotyledons, BnGL3 and BnTRY were expressed at lower levels, while changes for BnTTG1 (which was manipulated) occurred only in the K-5-8 line (Fig. 5) as expected.
Fig. 5.

qRT-PCR analysis of expression levels of five trichome regulatory genes encoding elements of the MBW tri-protein complex in glabrous cotyledons of 10-day-old hairy leaf (AtGL3+) and ultra-hairy leaf (K-5-8) B. napus lines relative to cv. Westar (W). Level of BnACT2 and AtGL3 are provided as controls. Plants were grown under a 16/8 h diurnal light cycle
Discussion
This study examined the composition of glabrous cotyledons from a hairy leaf line derived from the introduction of the AtGL3 gene (line AtGL3+) into B. napus [1] and an ultra-hairy leaf line derived by repression of BnTTG1 within the AtGL3+ B. napus background (line K-5-8) [2]. Expression of AtGL3 induces the formation of trichomes on young leaves of these lines [1, 2] which reduces the insect’s ability to physically interact with the host plant and reduces FB feeding damage; however, the glabrous cotyledons of these lines also deter FB feeding [21]. A comparison of the physiological properties and composition of the cotyledons (Table 4) provides a few clues as to whether a common mechanism(s) might be responsible for the antixenosis. In summary, the cotyledons of the AtGL3+ have an abnormal appearance, which is in keeping with vegetative tissue in this line, but develop normally in the K-5-8 line due to repression of BnTTG1 expression (2). The cotyledons in the wild-type line are horizontally-oriented; however, the cotyledons in both transgenic lines are vertically-oriented and it was suggested that this orientation may be less suitable for FBs to initiate feeding behaviour [19]. Anthocyanin content in the AtGL3+ line was significantly increased, but reduced in the K-5-8 line compared to wild-type. Both lines had similar cell wall carbohydrate contents; however, cell wall lignin was reduced in the K-5-8 line. While total GS content was reduced in the AtGL3+ line, the level of gluconapin was increased and 4-methoxy-glucobrassicin was decreased in both transgenic lines.
Table 4.
Summary of physiological properties and composition of B. napus cotyledons expressing AtGL3
| A. Insect behaviour and cotyledon biochemistry relative to Westar | |||||||
| Genotype | FB Feeding | Cotyledon Morphology | Cotyledon Orientation | Anthocyanin Accumulation | Cell Wall Carbohydrates | Cell Wall Lignin | |
| AtGL3+a | Decreased | Abnormal | Vertical | Increased | No Difference | No Difference | |
| K-5-8b | Decreased | Normal | Vertical | Decreased | No Difference | Decreased | |
| B. Glucosinolate composition of transgenic lines relative to Westar | |||||||
| Genotype | Total GS | Progoitrinc | Glucoraphaninc | Gluconapinc | 4-hydroxy-glucobrassicinc | Glucobrassicinc | 4-methoxy-glucobrassicinc |
| AtGL3+ | Decreased | No Diference | No Difference | Increased | No Difference | No Difference | Decreased |
| K-5-8 | No Difference | No Difference | No Difference | Increased | Decreased | No Difference | Decreased |
| C. Glucosinolate composition of the transgenic lines relative to one another. | |||||||
| Genotype | Total GS | Progoitrinc | Glucoraphaninc | Gluconapinc | 4-hydroxy-glucobrassicinc | Glucobrassicinc | 4-methoxy-glucobrassicinc |
| AtGL3+ | Decreased | Decreased | No Difference | Increased | No Difference | Increased | Decreased |
| K-5-8 | - | - | - | - | - | - | - |
aAtGL3+, B. napus cv. Westar expressing AtGL3 under direction of CaMV 35S promoter
bK-5-8, B. napus cv. Westar expressing AtGL3 under direction of CaMV 35S promoter as well as a TTG1 RNAi construct
cProgoitrin, 2-hydroxy-3-butenyl-GS; Glucoraphanin, 4-methylsulfinylbutyl-GS; Gluconapin, 3-butenyl-GS; 4-hydroxyglucobrassicin, 4-hydroxy-3-indolylmethyl-GS; Glucobrassicin, 3-indolylmethyl-GS; 4-methoxyglucobrassicin, 4-methoxy-3-indolylmethyl-GS
Role of secondary metabolites in cotyledon resistance
Feeding is highly influenced by GS content for many crucifer specialists; however, there is little consensus as to which GSs stimulate and which deter feeding. Related to the GSs found in cotyledons in the current study, decreased quantities of glucoraphanin and increased levels of progoitrin correlated with increased FB feeding in Sinapis alba [31]. In a broad study of various Brassica species, progoitrin levels were also correlated with stimulation of FB feeding [32]; however, this does not explain the reduced FB feeding on the transgenic cotyledons in this study as progoitrin and glucoraphanin levels were similar to the wild-type line. The same study and studies with the stem FB (Psylliodes chrysocephala) [33, 34] reported that gluconapin is also a feeding stimulant; however, this GS was elevated in cotyledons of both transgenic lines in the current study. Interestingly, glucobrassicin was the most stimulatory of the GS tested in the study with P. chrysocephala [33], but levels of this GS were the same in the transgenic and wild-type lines. To our knowledge, no studies have implicated 4-methoxy-glucobrassicin in affecting FB feeding activity. Future experiments appear to warrant examining individual GS to determine, for example, if applying gluconapin further increases resistance or if adding 4-methoxy-glucobrassicin restores wild-type predation by FB in the transgenic cotyledons.
It has been difficult to extrapolate the response to individual GSs in laboratory studies to damage occurring in the field [35, 36] as this is influenced by the presence of other GSs and other plant secondary metabolites, as well as the myriad GS-derived volatiles that result from the release of myrosinase and associated myrosinase-specifier proteins upon tissue disruption [37]. Communication and recruitment of con-specific insects must also be considered when assessing host susceptibility. In the field, allyl isothiocyanate derived from the GS sinagrin is a strong FB attractant [38]. This volatile also enhances the response of FBs to aggregation pheromone [39], which functions only in the presence of specific host plant volatiles [40]. A role for flavonoids in host recognition and acceptance by many adult insects has also been reported [41, 42]. Moreover, anthocyanins are known to impact insect feeding [28]. Since anthocyanin production was altered in the AtGL3+ cotyledons, these should also be tested for their impact on FB feeding.
Impact of AtGL3 expression on the cotyledon transcriptome
AtGL3+ cotyledon size and colour (small with a dark red abaxial side) differ from that of K-5-8 or Westar, and AtGL3+ plants are smaller and grow less vigorously [2, 18]. Not surprisingly, the alteration of the cotyledon transcriptome in the AtGL3+ plants was more extreme (both in transcript diversity and expression intensity) compared with K-5-8 cotyledons (both lines relative to the Westar cotyledon transcriptome). In contrast, changes to the K-5-8 cotyledon transcriptome were less extreme (relative to AtGL3+ cotyledons), which may reflect the healthier cotyledons that were similar in size to Westar cotyledons and exhibited more vigorous growth [2, 18]. The cell wall of K-5-8 cotyledons was also less lignified suggesting that K-5-8 cotyledons could be in a more vigorous growth phase than the AtGL3+ cotyledons. The red anthocyanin present in AtGL3+ cotyledons growing under continuous light appeared to be part of a stronger and more varied stress response as indicated by the transcriptome data. In addition to the up-regulation of genes directly involved in anthocyanin production, genes involved in hormone synthesis or signalling (jasmonate, auxins, gibberellins, brassinosteroids, and ethylene) were also more strongly up-regulated in the AtGL3+ cotyledons than in those from K-5-8, while genes involved in development, lipid synthesis, amino acid metabolism, and photosynthesis were more strongly up-regulated in K-5-8. These data suggest that AtGL3+ cotyledons are compromised and less able to fuel growth, while K-5-8 cotyledons are primed for better growth characteristics and possibly more able to tolerate FB damage.
Expression of genes involved in trichome formation
Cotyledons of lines expressing AtGL3 remained glabrous; however, RNA sequencing showed that both types of transgenic cotyledons had elevated levels of transcripts for many genes involved in trichome synthesis. SIM, which encodes a cyclin-dependent protein kinase inhibitor specifying multi-cellular trichomes, was up-regulated in these cotyledons. As well, the AtGL3+ line had an elevated level of transcripts for EMB3009 which specifies trichome shape, AUGUSTIFOLIA which is involved in trichome initiation, MIXTA which specifies negative trichome branching, SAC1 which specifies trichome size, and SHAVEN3 which specifies trichome number. These differences may underlie the smaller trichomes present on the AtGL3+ line [2]. Moreover, a paralogue of the BrRGA1 gene was uniquely up-regulated in the AtGL3+ cotyledons, and other RGA1 genes were up-regulated in cotyledons of both transgenic lines. RGA1 (repressor of gibberellic acid 1) is a GA-insensitive DELLA repressor protein that negatively impacts the activity of trichome transcriptional activators [43]. Hence, induction of this repressor could be one of the reasons why the transgenic cotyledons remained glabrous even in the presence of a high AtGL3 transcript levels. Induction of RGA1 also suggests that it could be a cotyledon-specific gene, since these repressors do not appear in the leaf transcriptome of either transgenic line, or in leaves of cv. Westar [2]. The data implies that the expression of the AtGL3 gene in the two transgenic lines may have a measure of positive control by stimulating over-expression of this trichome repressor, ensuring that cotyledons will be maintained as sources of nutrients for young seedlings rather than becoming trichome-bearing leaf-like organs. This is supported by the fact that five trichome regulatory genes related to the MBW initiation complex were only weakly expressed in cotyledons.
Shared components within the MBW regulatory complex link trichome formation and anthocyanin biosynthesis. In trichome formation, GL3 or ENHANCER OF GL3 forms a complex with TTG1 and the MYB protein GL1. This complex activates the expression of genes encoding transcription factors that in turn induce trichome formation. Anthocyanin biosynthesis is regulated by a complex consisting of TTG1 and GL3, but with different MYB factors [17]. In the current study, the chemical profile and changes in the expression of genes in cotyledons expressing AtGL3 alone (AtGL3+) or accompanied by reduced BnTTG1 expression (K-5-8) suggest that the production of other secondary metabolites, such as GSs, may be subject to a similar type of regulation. At least 6 other MYB proteins (CPC, TRY, ETC1, ETC2, ETC3 and TCL1) can replace GL1 and alter the specificity of the regulatory complex [15]. In the future, it would interesting to establish if some type of MBW complex also regulates GS production and what MYB protein(s) might be involved.
Conclusion
An unexpected outcome of manipulating the expression of AtGL3 and BnTTG1 genes was cotyledon resistance to FB, in spite of the fact that this tissue remained glabrous [18, 19]. As such, these glabrous cotyledon transcriptomes represent roadmaps that can lead to the identification of insect-resistance compounds and properties. The altered host interactions could reflect the large up-regulation of genes affecting the synthesis of GSs, other secondary metabolites and tissue structural components, including cell wall carbohydrates, lignin, wax, metal handling systems, flavonoids, phenolics, and indole alkaloids. Quercetin (flavonoid) and chlorogenic acid (phenolic) derivatives accumulate strongly in cotyledons of the crucifer Camelina sativa, protecting them from FB feeding [41], while B. napus cotyledons normally accumulate only traces of these compounds (Gruber unpublished). Seeds of the crucifer Lunnaria annua L. (L. bioennis) accumulate toxic lunarine/lunaridin alkaloids [44], and alkaloids have been shown to deter insects. Plants that accumulate certain metals in the Brassicaceae can also be more resistant to specific insects [45–51]. Finally, the induction of large numbers stress-responsive genes (specifying wounding, abiotic, and biotic stress responses), such as those encoding heat shock proteins, PR proteins, protease inhibitors, glucosinolate synthesis/breakdown factors, abiotic stress factors, redox proteins, transcription factors, and proteins required for auxin metabolism suggest that these cotyledons may now be primed for resistance to other forms of biotic and abiotic stress.
Additional files
Table S1. MAPMAN functional categories of up-regulated genes. Organization of up-regulated genes from AtGL3+ and K-5-8 cotyledons compared to B. napus cv. Westar into 36 functional categories (BINS) according to MAPMAN [24]. (XLS 3406 kb)
Table S2. Genes specifying glucosinolates and their degradation products. Up-regulated genes from AtGL3+ and K-5-8 cotyledons compared to B. napus cv. Westar involved in aspects of glucosinolate biosynthesis or degradation. (DOCX 27 kb)
Figure S1. MAPMAN (heat map) functional overview of changes in gene expression in K-5-8 glabrous cotyledons. MAPMAN (heat map) functional overview of changes in gene expression in glabrous cotyledons in the 10-day-old hairy leaf (K-5-8) B. napus line relative to cv. Westar. The 36 BINs represent MAPMAN sub-cellular function categories. [7497 out of 8037 differentially expressed genes were mapped using this method, with a few genes mapped into more than one category.] The majority of changes involved up-regulated genes. Blue blocks represent individual up-regulated genes. Red blocks represent 29 individual down-regulated genes. The full spectrum of Category 35 genes (unknowns) was too large to fit on the figure. Relative expression intensity scale is in log2, where darkest colour intensity represents log25 and higher/(+ 5) or lower (− 5) relative to Westar. (PPT 484 kb)
Figure S2. MAPMAN (heat map) functional overview of changes in gene expression in AtGL3+ glabrous cotyledons. MAPMAN (heat map) functional overview of changes in gene expression in glabrous cotyledons in the 10-day-old hairy leaf (AtGL3+) B. napus line relative to cv. Westar. The 36 BINs represent MAPMAN sub-cellular function categories. [8186 out of 8841 differentially expressed genes were mapped using this method, with a few genes mapped into more than one category.] The majority of changes involved up-regulated genes. Blue blocks represent individual up-regulated genes. Red blocks represent 29 individual down-regulated genes. The full spectrum of Category 35 genes (unknowns) was too large to fit on the figure. Relative expression intensity scale is in log2, where darkest colour intensity represents log25 and higher/(+ 5) or lower (− 5) relative to Westar. (PPT 293 kb)
Figure S3. MAPMAN heat maps of stress responsive genes in glabrous cotyledons. MAPMAN heat maps of stress responsive genes in glabrous cotyledons of (A) 10-day-old hairy leaf (AtGL3+) and (B) ultra hairy leaf (K-5-8) B. napus lines relative to cv. Westar. Blue and red blocks represent individual up- and down-regulated genes. Relative expression intensity scale is in log2 where ±4 represents ± log24 or greater. (PPT 401 kb)
Figure S4. MAPMAN heat maps of metabolism genes in glabrous cotyledons. MAPMAN heat maps of metabolism genes in glabrous cotyledons of (A) hairy leaf AtGL3+ B. napus and (B) ultra-hairy leaf K-5-8 B. napus, relative to Westar. Maps show numbers of ESTs and expression intensity. Blue blocks represent up-regulated genes. Red blocks represent individual down-regulated genes. Relative expression intensity scale is in log2, where ±5 represents ±log25 or greater. (PPT 287 kb)
Figure S5. MAPMAN heat maps of gene regulation and protein-related genes in glabrous cotyledons. MAPMAN heat maps of gene regulation and protein-related genes in glabrous cotyledons of (A) AtGL3+ B. napus and (B) K-5-8 relative to Westar. Maps show numbers of changed ESTs and expression intensity. Blue blocks represent individual up-regulated genes. Red blocks represent individual down-regulated genes. Relative expression intensity scale is in log2 where ±5 represents ±log24 or greater. (PPT 279 kb)
Acknowledgements
The authors acknowledge the assistance of Wesley Soroka in seed cleaning and L. Grenkow with statistical training. I. Parkin is gratefully acknowledged for sharing data from the B. oleracea genome prior to its publication.
Funding
Funding was available from the Agriculture Development Fund of the Government of Saskatchewan and SaskCanola. N. Nagubushana and A. Taheri received Visiting Fellowships from the Government of Canadian.
Availability of data and materials
Raw sequencing data files were deposited within NCBI as accession #SRP065063. Seeds of the three lines used in this study are available from D. Hegedus.
Authors’ contributions
MG and PB-S supervised the experiments. AU, AT, NN, MYG, and RZ conducted experiments and analysed data. PB-S, MG, and DD wrote and edited the manuscript. AS provided resources and instrumentation for RNA sequencing. AH provided resources for analysis of cotyledon structural composition. RZ provided resources and conducted glucosinolate analysis. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12870-018-1277-6) contains supplementary material, which is available to authorized users.
Contributor Information
Margaret Gruber, Email: margie.gruber22@gmail.com.
Ushan Alahakoon, Email: UIAlahakoon@dow.com.
Ali Taheri, Email: atahery@gmail.com.
Nayidu Nagubushana, Email: nagabushana@gmail.com.
Rong Zhou, Email: rong.zhou@agr.gc.ca.
Banyar Aung, Email: banyae.ong@gmail.com.
Andrew Sharpe, Email: andrew.sharpe@gifs.ca.
Abdelali Hannoufa, Email: abdelali.hannoufa@agr.gc.ca.
Peta Bonham-Smith, Email: peta.bonhams@usask.ca.
Dwayne D. Hegedus D, Phone: 306-385-9427, Email: dwayne.hegedus@agr.gc.ca
References
- 1.Gruber MY, Wang S, Ethier S, Holowachuk J, Bonham-Smith PC, Soroka J, Lloyd A. “Hairy canola”- Arabidopsis GL3 induces a dense covering of trichomes on Brassica napus seedlings. Plant Mol Biol. 2006;60:679–698. doi: 10.1007/s11103-005-5472-0. [DOI] [PubMed] [Google Scholar]
- 2.Alahakoon U, Taheri A, Naghabushana N, Bonham-Smith PC, Gruber MY. Hairy canola re-visited: down-regulating TTG1 in an AtGL3-enhanced hairy leaf background improves growth, leaf trichome coverage, and metabolite gene expression diversity. BMC Plant Biol. 2016;16:e12. doi: 10.1186/s12870-015-0680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessler A, Baldwin IT. Plant responses to insect herbivory: the emerging molecular analysis. Ann Rev Plant Biol. 2002;53:299–328. doi: 10.1146/annurev.arplant.53.100301.135207. [DOI] [PubMed] [Google Scholar]
- 4.Painter RH. Insect Resistance in Crop Plants 1951. New York: MacMillan.
- 5.Canola Council of Canada. Flea beetles. Canola Encyclopedia. Canola Council of Canada. http://www.canolacouncil.org/canola-encyclopedia/insects [Feb. 10. 2015].
- 6.Crop Protection Compendium. Phyllotreta cruciferae. CABI. http://www.cabi.org/cpc/datasheet/40780 [Feb. 10, 2015].
- 7.Crop Protection Compendium. Phyllotreta striolata. CABI. http://www.cabi.org/cpc/datasheet/40784 [Feb. 2, 2015].
- 8.Tansey JA, Dosdall LM, Keddie BA, Sarfraz RM. Differences in Phyllotreta cruciferae and Phyllotreta striolata (Coleoptera: Chrysomelidae) responses to neonicotinoid seed treatments. J Econ Entomol. 2008;101:159–167. doi: 10.1093/jee/101.1.159. [DOI] [PubMed] [Google Scholar]
- 9.Heimbach U, Müller A. Incidence of pyrethroid-resistant oilseed rape pests in Germany. Pest Management Sci. 2012;69:209–216. doi: 10.1002/ps.3351. [DOI] [PubMed] [Google Scholar]
- 10.Woodman RL, Fernandes GW. Differential mechanical defense: herbivory, evapotranspiration, and leaf-hairs. Oikos. 1991;60:11–19. doi: 10.2307/3544986. [DOI] [Google Scholar]
- 11.Larkin JC, Young N, Prigge M, Marks D. The control of trichome spacing and number in Arabidopsis. Development. 1996;122:997–1005. doi: 10.1242/dev.122.3.997. [DOI] [PubMed] [Google Scholar]
- 12.Handley R, Ekbom B, Agren J. Variation in trichome density and resistance against a specialist insect herbivore in natural populations of Arabidopsis thaliana. Ecol Entomol. 2005;305:284–292. doi: 10.1111/j.0307-6946.2005.00699.x. [DOI] [Google Scholar]
- 13.Palaniswamy P, Bodnaryk RP. A wild Brassica from Sicily provides trichome-based resistance against flea beetles, Phyllotreta cruciferae (Goeze) (Coleoptera: Chrysomelidae) Can Entomol. 1994;126:1119–1130. doi: 10.4039/Ent1261119-5. [DOI] [Google Scholar]
- 14.Payne CT, Zhang F, Lloyd AM. GL3 encodes a bHLH protein that regulates trichome development in Arabidopsis through interaction with GL1 and TTG1. Genetics. 2000;156:1349–1362. doi: 10.1093/genetics/156.3.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang F, Gonzalez A, Zhao M, Payne CT, Lloyd AMA. Network of redundant bHLH proteins in all TTG1-dependent pathways of Arabidopsis. Development. 2013;130:4859–4869. doi: 10.1242/dev.00681. [DOI] [PubMed] [Google Scholar]
- 16.Pesch M, Schultheib I, Klopffleisch K, Uhrig JF, Koegl M, Clemen CS, Simon R, Weidtkamp-Peters S, Hulskamp M. TRANSPARENT TESTA GLABRA1 and GLABRA1 compete for binding to GLABRA3 in Arabidopsis. Plant Physiol. 2015;168:584–597. doi: 10.1104/pp.15.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez A, Zhao M, Leavitt JM, Lloyd AM. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 2008;53:814–827. doi: 10.1111/j.1365-313X.2007.03373.x. [DOI] [PubMed] [Google Scholar]
- 18.Alahakoon U, Adamson J, Grenkow L, Soroka J, Bonham-Smith P, Gruber M. Field growth traits and insect-host plant interactions of two transgenic canola (Brassicaceae) lines with elevated trichome numbers. Can Entomol. 2016;1:1–13. [Google Scholar]
- 19.Soroka JJ, Holowachuk JM, Gruber MY, Grenkow LF. Feeding by flea beetles (Coleoptera: Chrysomelidae; Phyllotreta spp.) is decreased on canola (Brassica napus) seedlings with increased trichome density. J Econ Entomol. 2011;104:125–136. doi: 10.1603/EC10151. [DOI] [PubMed] [Google Scholar]
- 20.Soroka J, Grenkow L. Susceptibility of Brassicaceous plants to feeding by flea beetles, Phyllotreta spp. (Coleoptera:Chrysomelidae) J Econ Entomol. 2013;106:2557–2567. doi: 10.1603/EC13102. [DOI] [PubMed] [Google Scholar]
- 21.Updegraff DM. Semimicro determination of cellulose in biological materials. Anal Biochem. 1969;32:20–24. doi: 10.1016/S0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]
- 22.Brinkmann K, Blaschke L, Polle AJ. Comparison of different methods for lignin determination as a basis for calibration of near-infrared reflectance spectroscopy and implications of lignoproteins. J Chem Ecol. 2002;28:2483–2501. doi: 10.1023/A:1021484002582. [DOI] [PubMed] [Google Scholar]
- 23.Aung B, Gruber MY, Amyot L, Omari K, Bertrand A, Hannoufa A. MicroRNA156 as a promising tool for alfalfa improvement. Plant Biotechnol J. 2015;13:779–790. doi: 10.1111/pbi.12308. [DOI] [PubMed] [Google Scholar]
- 24.Theander O, Aman P, Westerlund E, Andersson R, Pettersson D. Total dietary fiber determined as neutral sugar residues, uronic acid residues, and Klason lignin (the Uppsala method): collaborative study. AOAC Int. 1995;78:1030–1044. [PubMed] [Google Scholar]
- 25.Thimm O, Blasing O, Gibon Y, Nagel A, Meyer S, Kruger P, Selbig J, Muller LA, Rhee SY, Stitt M. MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J. 2004;37:914–939. doi: 10.1111/j.1365-313X.2004.02016.x. [DOI] [PubMed] [Google Scholar]
- 26.SAS Institute, SAS user's guide, version 9.2, SAS Institute. 2008; Cary. https://support.sas.com/documentation/cdl/en/statugintroduction/61750/PDF/default/statugintroduction.pdf
- 27.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and cufflinks. Nat Prot. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karageorgou P, Manetas Y. The importance of being red when young: anthocyanins and the protection of young leaves of Quercus coccifer from insect herbivory and excess light. Tree Physiol. 2006;26:613–621. doi: 10.1093/treephys/26.5.613. [DOI] [PubMed] [Google Scholar]
- 29.Ishida M, Hara M, Fukino N, Kakizaki T, Morimitsu Y. Glucosinolate metabolism, functionality and breeding for the improvement of Brasicaceae vegetables. Breeding Sci. 2014;64:48–59. doi: 10.1270/jsbbs.64.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santiogo R, Barros-Rios J, Malvar RA. Impact of cell wall composition on maize resistance to pests and diseases. Int J Mol Sci. 2013;14:6960–6980. doi: 10.3390/ijms14046960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soroka J, Grenkow L. Susceptibility of brassicaceous plants to feeding by flea beetles, Phyllotreta spp. (Coleoptera: Chrysomelidae) J Econ Entomol. 2013;106:2557–2567. doi: 10.1603/EC13102. [DOI] [PubMed] [Google Scholar]
- 32.Bohinc T, Kosir IJ, Trdan S. Glucosinolates as arsenal for defending Brassicas against cabbage flea beetle (Phyllotreta spp.) attack. Zemdirbyste-Agriculture. 2013;100:199–204. doi: 10.13080/z-a.2013.100.026. [DOI] [Google Scholar]
- 33.Barlet E, Parsons D, Williams IH, Clark SJ. The influence of glucosinolates and sugars on feeding by the cabbage stem flea beetle, Psylloides chrysocephala. Entomol Exp Appl. 1994;73:7–83. [Google Scholar]
- 34.Giamoustaris A, Mithen R. The effect of modifying the glucosinolate content of leaves of oilseed rape (Brassica napus spp. oleifera) on its interaction with specialist and generalist pests. Ann Appl Biol. 1995;126:347–363. doi: 10.1111/j.1744-7348.1995.tb05371.x. [DOI] [Google Scholar]
- 35.Bartlet E, Williams IH. Factors restricting the feeding of the cabbage stem flea beetle (Psylliodes chrysocephala) Entomol Exp Appl. 1991;60:233–238. doi: 10.1111/j.1570-7458.1991.tb01543.x. [DOI] [Google Scholar]
- 36.Barlet E, Mithen R, Clark SJ. Feeding of the cabbage stem flea beetle Psylloides chrysocephala on high and low glucosinolate cultivars of oilseed rape. Entomol Exp Appl. 1996;80:87–89. doi: 10.1111/j.1570-7458.1996.tb00892.x. [DOI] [Google Scholar]
- 37.Hopkins RJ, van Dam NM, van Loon JJA. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu Rev Entomol. 2009;54:57–83. doi: 10.1146/annurev.ento.54.110807.090623. [DOI] [PubMed] [Google Scholar]
- 38.Feeny P, Paauwe KL, Demong NJ. Flea beetles and mustard oils: host plant specificity of Phyllotreta cruciferae and P. striolata adults (Coleoptera: Chrysomelidae) Annal Entomol Soc Am. 1970;63:832–841. doi: 10.1093/aesa/63.3.832. [DOI] [Google Scholar]
- 39.Soroka JJ, Bartelt R, Zilkowski BW, Cosse AA. Responses of flea beetle Phyllotreta cruciferae to synthetic aggregation pheromone components and host plant volatiles in field trials. J Chem Ecol. 2005;31:1829–1843. doi: 10.1007/s10886-005-5929-2. [DOI] [PubMed] [Google Scholar]
- 40.Beran F, Mewis I, Srinivasan R, Svoboda J, Vial C, Mosimann H, Boland W, Buttner C, Ulrichs C, Hansson BS, Reinecke A. Male Phyllotreta striolata (F.) produce an aggregation pheromone: identification of male-specific compounds and interaction with host plant volatiles. J Chem Ecol. 2011;37:85–97. doi: 10.1007/s10886-010-9899-7. [DOI] [PubMed] [Google Scholar]
- 41.Onyilagha JC, Gruber MY, Hallett RH, Holowachuk J, Soroka JJ. Constitutive flavonoids deter flea beetle insect feeding in Camelina sativa L. Biochem Syst Ecol. 2012;42:128–133. doi: 10.1016/j.bse.2011.12.021. [DOI] [Google Scholar]
- 42.Simmonds MSJ. Importance of flavonoids in insect-plant interactions: feeding and oviposition. Phytochemistry. 2001;56:245–252. doi: 10.1016/S0031-9422(00)00453-2. [DOI] [PubMed] [Google Scholar]
- 43.Gan Y. Yu H, Peng J Broun P..Genetic and molecular regulation by DELLA proteins of trichome development in Arabidopsis. Plant Physiol. 2007;145:1031–1042. doi: 10.1104/pp.107.104794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henderson FG, Chen KK. The pharmacology of lunarine, the alkaloid of Lunaria biennis. J Amer Pharm Assoc. 1950;39:516–519. doi: 10.1002/jps.3030390914. [DOI] [PubMed] [Google Scholar]
- 45.Broadhurst CL, Chaney RL, Angle JS, Erbe EF, Maugel TK. Nickel localization and response to increasing Ni soil levels in leaves of the Ni hyperaccumulator Alyssum murale. Plant Soil. 2004;265:225–242. doi: 10.1007/s11104-005-0974-8. [DOI] [Google Scholar]
- 46.Isaure MP, Fayard B, Sarret G, Pairis S, Bourguignon J. Localization and chemical forms of cadmium in plant samples by combining analytical electron microscopy and X-ray spectromicroscopy. Spectrochim Acta B At Spectrosc. 2006;61:1242–1252. doi: 10.1016/j.sab.2006.10.009. [DOI] [Google Scholar]
- 47.Küpper H, Lombi E, Zhao FJ, Wieshammer G, McGrath SP. Cellular compartmentation of nickel in the hyperaccumulators Alyssum lesbiacum, Alyssum bertolonii and Thlaspi goesingense. J Exp Bot. 2001;52:2291–2300. doi: 10.1093/jexbot/52.365.2291. [DOI] [PubMed] [Google Scholar]
- 48.Küpper H, Lombi E, Zhao FJ, McGrath SP. Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri. Planta. 2000;212:75–84. doi: 10.1007/s004250000366. [DOI] [PubMed] [Google Scholar]
- 49.McNear DH, Jr, Peltier E, Everhart J, Chaney RL, Sutton S, Newville M, Rivers M, Sparks DL. Application of quantitative fluorescence and absorption-edge computed microtomography to image metal compartmentalization in Alyssum murale. Environ Sci Technol. 2005;39:2210–2218. doi: 10.1021/es0492034. [DOI] [PubMed] [Google Scholar]
- 50.Nagabushana N, Tan Y, Taheri A, Li X, Bjorndahl TC, Nowak J, Wishart DS, Hegedus D, Gruber MY. Brassica villosa, a system for studying non-glandular trichomes and genes in the brassicas. Plant Mol Biol. 2014;85:519–539. doi: 10.1007/s11103-014-0201-1. [DOI] [PubMed] [Google Scholar]
- 51.Salt DE, Prince RC, Pickering IJ, Raskin I. Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol. 1995;109:1427–1433. doi: 10.1104/pp.109.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. MAPMAN functional categories of up-regulated genes. Organization of up-regulated genes from AtGL3+ and K-5-8 cotyledons compared to B. napus cv. Westar into 36 functional categories (BINS) according to MAPMAN [24]. (XLS 3406 kb)
Table S2. Genes specifying glucosinolates and their degradation products. Up-regulated genes from AtGL3+ and K-5-8 cotyledons compared to B. napus cv. Westar involved in aspects of glucosinolate biosynthesis or degradation. (DOCX 27 kb)
Figure S1. MAPMAN (heat map) functional overview of changes in gene expression in K-5-8 glabrous cotyledons. MAPMAN (heat map) functional overview of changes in gene expression in glabrous cotyledons in the 10-day-old hairy leaf (K-5-8) B. napus line relative to cv. Westar. The 36 BINs represent MAPMAN sub-cellular function categories. [7497 out of 8037 differentially expressed genes were mapped using this method, with a few genes mapped into more than one category.] The majority of changes involved up-regulated genes. Blue blocks represent individual up-regulated genes. Red blocks represent 29 individual down-regulated genes. The full spectrum of Category 35 genes (unknowns) was too large to fit on the figure. Relative expression intensity scale is in log2, where darkest colour intensity represents log25 and higher/(+ 5) or lower (− 5) relative to Westar. (PPT 484 kb)
Figure S2. MAPMAN (heat map) functional overview of changes in gene expression in AtGL3+ glabrous cotyledons. MAPMAN (heat map) functional overview of changes in gene expression in glabrous cotyledons in the 10-day-old hairy leaf (AtGL3+) B. napus line relative to cv. Westar. The 36 BINs represent MAPMAN sub-cellular function categories. [8186 out of 8841 differentially expressed genes were mapped using this method, with a few genes mapped into more than one category.] The majority of changes involved up-regulated genes. Blue blocks represent individual up-regulated genes. Red blocks represent 29 individual down-regulated genes. The full spectrum of Category 35 genes (unknowns) was too large to fit on the figure. Relative expression intensity scale is in log2, where darkest colour intensity represents log25 and higher/(+ 5) or lower (− 5) relative to Westar. (PPT 293 kb)
Figure S3. MAPMAN heat maps of stress responsive genes in glabrous cotyledons. MAPMAN heat maps of stress responsive genes in glabrous cotyledons of (A) 10-day-old hairy leaf (AtGL3+) and (B) ultra hairy leaf (K-5-8) B. napus lines relative to cv. Westar. Blue and red blocks represent individual up- and down-regulated genes. Relative expression intensity scale is in log2 where ±4 represents ± log24 or greater. (PPT 401 kb)
Figure S4. MAPMAN heat maps of metabolism genes in glabrous cotyledons. MAPMAN heat maps of metabolism genes in glabrous cotyledons of (A) hairy leaf AtGL3+ B. napus and (B) ultra-hairy leaf K-5-8 B. napus, relative to Westar. Maps show numbers of ESTs and expression intensity. Blue blocks represent up-regulated genes. Red blocks represent individual down-regulated genes. Relative expression intensity scale is in log2, where ±5 represents ±log25 or greater. (PPT 287 kb)
Figure S5. MAPMAN heat maps of gene regulation and protein-related genes in glabrous cotyledons. MAPMAN heat maps of gene regulation and protein-related genes in glabrous cotyledons of (A) AtGL3+ B. napus and (B) K-5-8 relative to Westar. Maps show numbers of changed ESTs and expression intensity. Blue blocks represent individual up-regulated genes. Red blocks represent individual down-regulated genes. Relative expression intensity scale is in log2 where ±5 represents ±log24 or greater. (PPT 279 kb)
Data Availability Statement
Raw sequencing data files were deposited within NCBI as accession #SRP065063. Seeds of the three lines used in this study are available from D. Hegedus.


