Abstract
This article reviews important features to improve the diagnosis of congenital heart disease (CHD) by applying ultrasound in prenatal cardiac screening. As low and high-risk pregnancies for CHD are subject to routine obstetric ultrasound, the diagnosis of structural heart defects represents a challenge that involves a team of specialists and subspecialists on fetal ultrasonography. In this review, the images highlight normal anatomy of the heart as well as pathologic cases consistent with cardiac malposition and isomerism, septal defects, pulmonary stenosis/atresia, aortic malformations, hypoplastic left ventricle, conotruncal anomalies, tricuspid dysplasia, and Ebstein’s anomaly, and univentricular heart, among other congenital cardiovascular defects. Anatomical details of most CHD in fetuses were provided by two-dimensional (2D) ultrasound with higher quality imaging, enhancing diagnostic accuracy in a variety of CHD. Moreover, the accuracy of the cardiac defects in obstetrics ultrasound improves the outcome of most CHD, providing planned delivery, aided genetic counseling, and perinatal management.
Keywords: Prenatal diagnosis, Ultrasound imaging, Echocardiography, Congenital heart disease
1. Introduction
Congenital heart disease (CHD) are the most common form of birth defects. The incidence of CHD is about eight to 10 per 1000 (0.8%–1%) live-born, full-term births, and it could be 10 times higher in preterm infants (8.3%).1, 2 Furthermore, in early gestation, this incidence is even higher as certain CHDs are complex and have been show to result in fetal demise. In fact, 50%–60% of the CHD will require surgical correction and of these, 25% are critical with CHD a leading cause of infant mortality.3, 4 In this setting, the survival, extensive medical care, and developmental disabilities depend on the time of the diagnosis, on the delay of the treatment, and on the severity of the CHD. Therefore, early fetal diagnosis of a treatable CHD has been shown to reduce the risk of perinatal morbidity and mortality.5
Cardiovascular development involves a complex process in which genetic and environmental factors are involved. Considering that approximately 49% of pregnancies are unplanned, women may not take precautionary actions against environmental factors.6 The detection of CHD by fetal echocardiography when referred by a suspicion of cardiac abnormality on routine obstetric ultrasound is up to 40% in low-risk populations. However, risk factors are identified in only 10% of CHD. In this scenario, the heart should be examined in detail on a routine sonographic scanning.
In the sonographic prenatal diagnosis of CHD, the fetal heart remains a challenge that involves sonographers, obstetricians, radiologists, and fetal medicine subspecialists. High risk for cardiac defects and the suspicion of a cardiac abnormality on obstetric ultrasound, even in low-risk populations, are indications for referral for performance of a detailed fetal echocardiogram. This manuscript reviews important aspects to improve the prenatal screening for CHD focusing on ultrasound clues to enable the diagnosis of the cardiac defects; drawing the management of cardiac defects in utero and the delivery plan strategies were also approached in this study.
2. Prenatal screening
2.1. How to screen the fetal heart
The fetal cardiac screening by ultrasound can detect a high proportion of cases of CHD. However, when the prenatal screening was based on the visualization of the four-chamber view, it was inadequate to detect many cases of CHD, especially conotruncal and outflow defects (ex: transposition of the great vessels, tetralogy of Fallot, double-outlet right ventricle, truncus arteriosus, and outlet septal defects). When the evaluation of outflow tracts was added to the four-chamber view, the sensitivity of ultrasound screening for CHD increased from approximately 30% to 69%–83%.7 Currently, the three vessels (3 V) and 3 V with trachea (3VT) views were added to the standard four-chamber and outflows views in order to improve the detection of CHD.7, 8 The latter one enabled the detection of lesions such as coarctation of the aorta, right aortic arch, double aortic arch, and vascular rings, achieving a prenatal detection rate of congenital heart disease to up 90%. The average time to obtain the cardiac views was just over 2 min, but in approximately one third of cases, the cardiac examination was postponed by 15–20 min due to unfavorable fetal lie (anterior spine).9 A fetal echocardiogram should be performed if the CHD is suspected on the obstetric cardiac of screening, or if there is a recognized increased risk (maternal, fetal and/or familial factors) for CHD >2% to 3%. Fetal echocardiography may be considered when risk is estimated at 1% to 2%, and when risk approaches that of the general population (≤ 1%), this exam is not indicated.10
2.2. Upper abdomen and four-chamber views
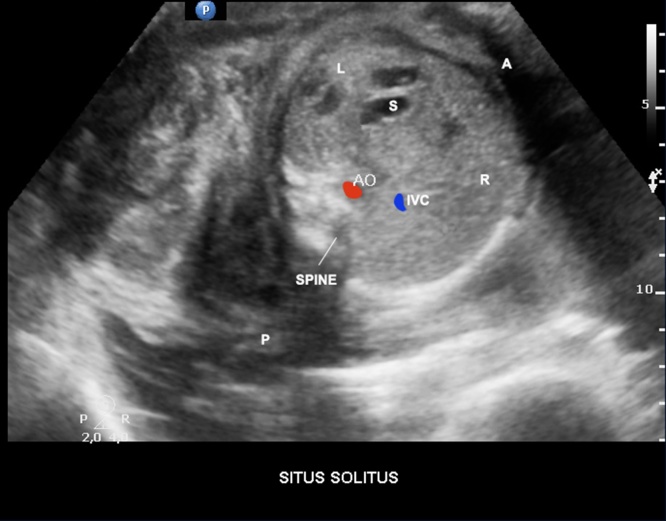
The examination of the upper abdomen (cross-sectional plane) of the fetus by echocardiography provides the distinction between the left and the right sides of the fetus. When the situs is normal (solitus), the aorta and stomach are located on the left side and the inferior vena cava and liver are placed on the right. Therefore, situs solitus is the normal arrangement of thoracic and abdominal organs (Fig. 1). In general, more complex CHD are associated with abnormalities of the situs. Furthermore, the umbilical vein and the hepatic veins can be visualized in upper abdomen view.
Fig. 1.
Upper abdomen view showing situs solitus. AO, aorta; IVC, inferior vena cava; L, fetal left; R, fetal right; P, fetal posterior; A, fetal anterior; S, stomach.
The four-chamber view is the most important plane. This approach enables the evaluation of the main cardiac structures, the position, the size (1/3 of the thorax), the contractility and the rhythm of the heart. In normal levocardia, 2/3 of the heart is left-sided with the axis pointing to the left. Cardiac axis is at 45+/−20° and abnormal axis is associated with chromosomal anomaly, abnormal displacement of the heart (diaphragmatic hernia or space- occupying lesion), and many CHD, especially in conotruncal anomalies and univentricular hearts.11 Cardiomegaly can be evaluated by the global size of the heart and in small fetuses this should be done by cardiothoracic ratio (CTr = cardiac area/thorax area). The size of the left and right chambers is similar. However, in the third trimester, mild over right-left asymmetry can be a normal variant. Besides the size, the morphological and functional characteristics of each chamber (atria, ventricles, and atrioventricular valves) can be analyzed. The left atrium (LA) is normally located most posteriorly (near descending aorta) and it is identified by the finger-like appendage. Furthermore, the LA is characterized by the presence of the foramen ovale flap and its connection with the pulmonary veins. The right atrium (RA) has a pyramidal appendage with a broad base and receives the vena cava.
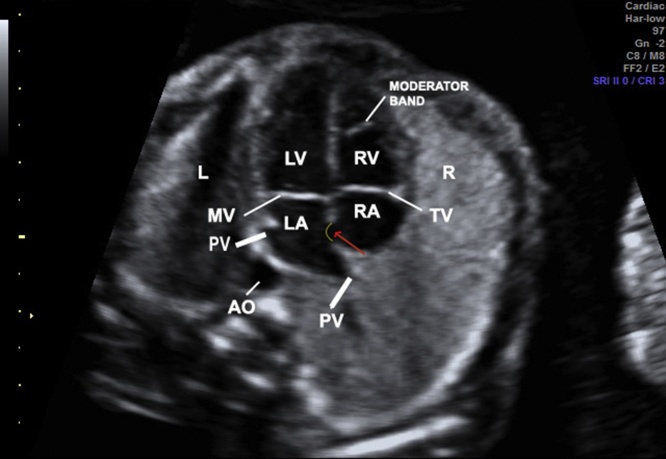
Different to the atria which communicate each other with the foramen ovale, the ventricles are separated by the interventricular septum. The muscular part of the septum is the lower two-thirds and the membranous one is the part of the interventricular septum adjacent to the aortic, mitral and tricuspid (septal cusp) valves. On suspicion of a ventricular septal defect (VSD), the heart should be examined using a lateral view allowing the drop-out effect due to the insonation angle by a four-chamber view. In a retrosternal location, the right ventricle (RV) is trabeculated with the presence of the moderator band and the lumen is shorter than the left ventricle (LV) lumen (Fig. 2). The LV is posterior to the RV, reaches the apex of the heart and its lumen is longer than the RV. Furthermore, the tricuspid valve inserts slightly more apical than the mitral valve.
Fig. 2.
Image showing the four-chamber view. RV with the moderator band, tricuspid valve more apical than mitral valve, LA with the presence of forame ovale flap (red arrow), and pulmonary veins draining into LA. L, fetal left; R, fetal right; LA, left atrium; LV, left ventricle; RV, right ventricle; PV, pulmonary vein; TV, tricuspid valve; MV, mitral valve; AO, aorta. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Qualitative assessment of the chambers and quantitative assessment of the atrioventricular valves should be done by four-chamber view. In addition, the cardiac function and rhythm assessment should be performed.
2.3. Left and right ventricular outflow tracts
The outflow tracts view (five-chamber view) can be obtained from the four-chamber view by sliding the transducer to the fetal head that enables the identification of the origin of the great arteries. In a normal RV outflow view (RVOT), the pulmonary trunk can be visualized arising from RV and crossing the ascending aorta. To optimize the five-chamber view, focusing on the analysis of the continuity of the ventricular septum to the aorta, and even the ascending aorta, the transducer has been rotated to the fetal right shoulder. The evaluation of LV outflow view (LVOT) and RVOT helps to identify outflow septal ventricular defects and conotruncal anomalies.
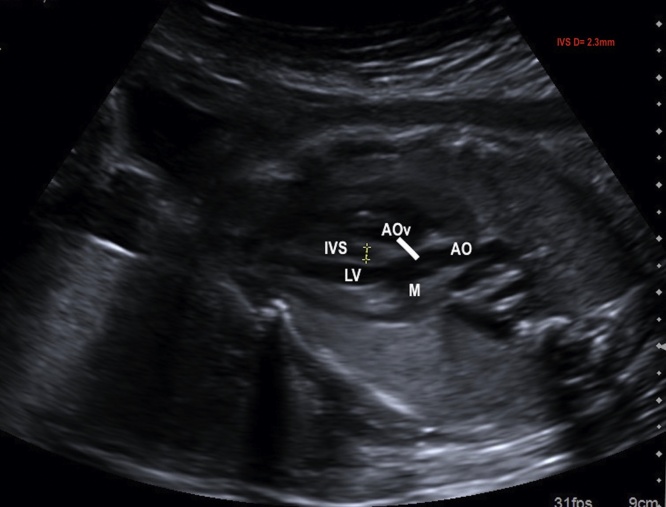
The LVOT confirms the aorta arising from the morphological LV as a vessel continuing the outflow ventricular septum as well the aortic-mitral valve continuity. In this view, the aortic valve can be recognized and a detailed evaluation of its size and mobility can be performed. Even, the thickness of the septum is measured by the five chamber (LVOT) view (IVS raging between 2 and 4 mm during gestation) (Fig. 3).
Fig. 3.
Image showing the five-chamber view. Outflow LV tract (LVOT): aorta arising from LV, aortic valve and the thickness of the interventricular septum (2.3 mm). AO, aorta; LV, left ventricle; AOv, aortic valve; M, mitral valve; IVS, interventricular septum; D, diameter.
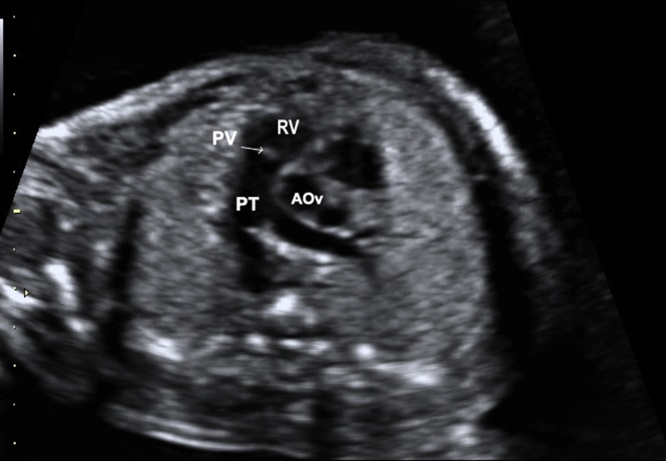
In the RVOT view, the pulmonary artery crosses over the ascending aorta and becomes the vessel on the left. However, when the great vessels are transposed (TGA), the aorta and pulmonary arteries run in parallel and don’t cross over each other. The great arteries short axis view can be obtained by tilting from four- to five-chambers view and confirms the pulmonary trunk (PT) arising from RV (Fig. 4). In this view, the bifurcation of the right and left pulmonary arteries for the PT is best visualized, and also the size and mobility of the pulmonary valve can be assessed.
Fig. 4.
Image showing RV outflow short-axis (RVOT). The pulmonary trunk arises from RV. PV, pulmonary valve; PT, pulmonary trunk; RV, right ventricle; AOv, aortic valve.
2.4. Three vessels and three vessels and trachea views
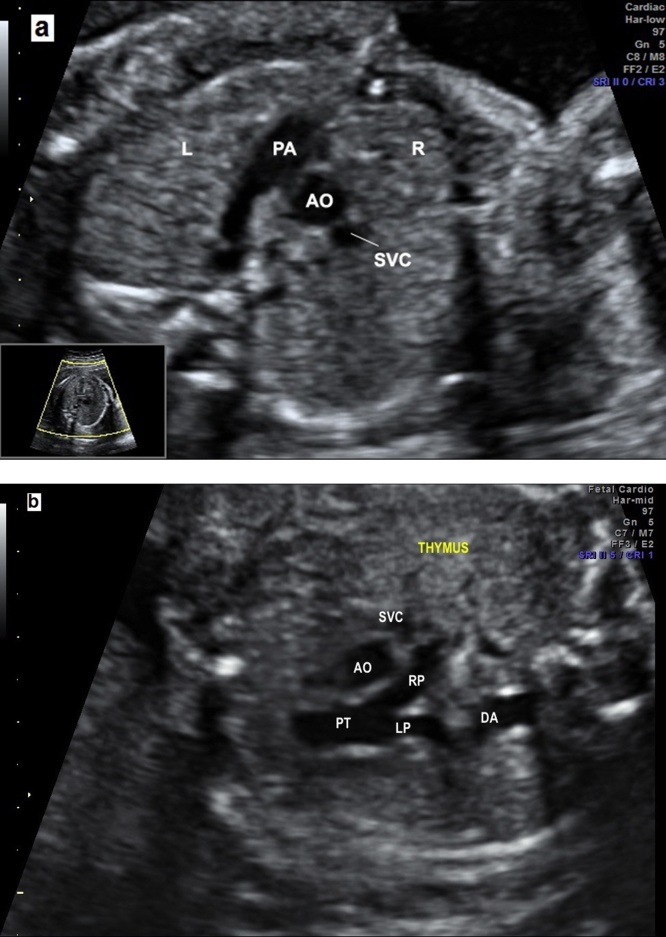
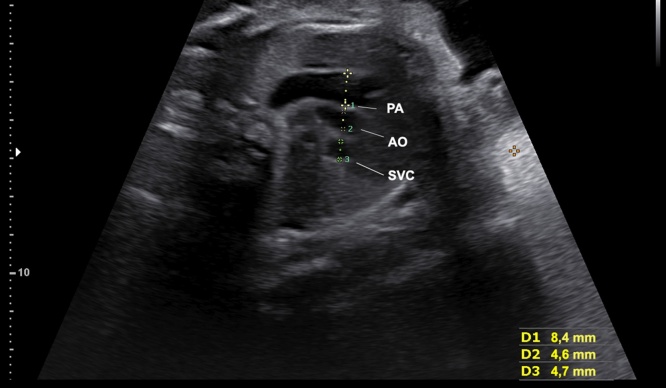
The three vessels (3 V) view is obtained from the four-chamber view by moving the transducer in the direction of the upper fetal and the pulmonary trunk; the arch with aortic isthmus and superior vena cava (SVC) can be visualized.7 The caliber of the pulmonary trunk is slightly larger than aorta, whereas the SVC is the smaller and posterior of the arteries. The PT, the right pulmonary artery, and the ductal arch can be seen. The aortic and ductal arches are located on the left of the trachea on a V-shaped configuration.8 The trachea is recognized as an echogenic structure on the right side of the arteries and anterior to the spine. The three vessels and trachea (3VT) view enables the diagnosis of coarctation of the aorta, right aortic arch, double aortic arch and vascular rings. The thymus can be visualized in the front of the three vessels as a less echogenic structure which is important to detect defects associated with the 22q11.2 deletion syndrome, facial abnormalities, absent or hypoplastic thymus, and hypocalcaemia.12 The measurement of the thymic-thoracic ratio (TT-ratio) is a feasible and useful tool in fetuses with cardiac defects (Fig. 5).13
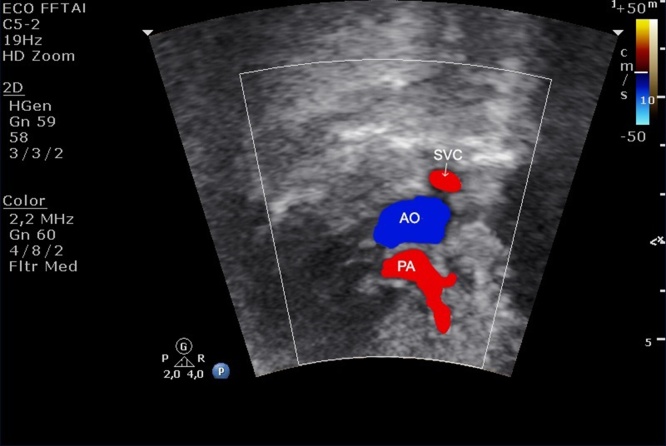
Fig. 5.
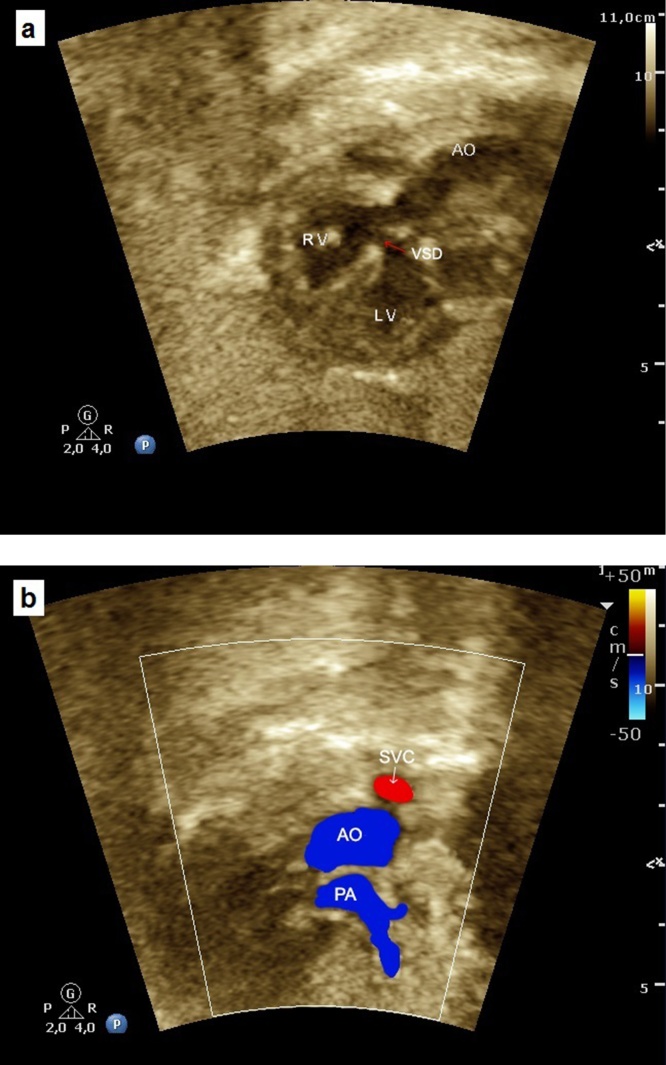
(a) The three vessels view showing the relationship and size of the pulmonary artery, aorta and superior vena cava (three vessels). The pulmonary artery, to the left, is slightly larger than aorta and the superior vena cava is the smallest and posterior vessel. PA, pulmonary artery; AO, aorta; SVC, superior vena cava; R, right; L, left. (b) The three vessels view showing the aorta and ductus arteriosus located to the left to trachea. The thymus can be visualized in the front of three vessels. PT, pulmonary trunk; LP, left pulmonary artery; RP, right pulmonar artery; AO, aorta; SVC, superior vena cava; T, trachea.
3. Fetal congenital heart diseases
3.1. Cardiac malpositions and isomerism
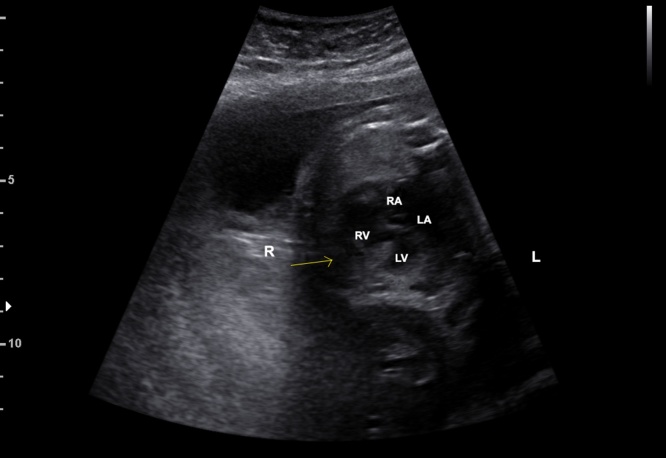
In normal conditions, the heart is on the left side of the thorax (levocardia), with situs solitus for the visceral and atrial arrangement. The first step in the assessment of the cardiac position and situs is to identify the fetal position and the transducer orientation.14 In the normal upper abdomen view, the heart points to the left anterior thoracic cavity with a normal base-axis of 45°, left-sided stomach and descending aorta and right-sided liver and inferior vena cava. Cardiac malpositions include mesocardia, dextrocardia, dextroposition, and even an ectopia cordis in which the heart is displaced outside the thorax.14 Dextrocardia is present when the heart points to the right side of the thorax and dextroposition occurs when the heart is only displaced to the right chest preserving the normal cardiac left axis (Fig. 6). Dextrocardia with situs inversus is known as mirror-image dextrocardia, in which the inferior vena cava (IVC) and liver are on the left and the aorta and stomach are on the right. Dextrocardia with situs inversus totalis has less incidence of cardiac malformations than there dextrocardia with situs solitus. Dextroposition is caused by a displacement of the heart due to a congenital diaphragmatic hernia, left-sided fluid, or masses.14
Fig. 6.
Image showing the fetal heart pointing (yellow arrow) to the right side of fetal thorax (dextrocardia). R, fetal right; L, fetal left; RA, right atrium; RV, right ventricle; LA, left atrium; LV, left ventricle. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Conditions with incomplete lateralization of the organs are known as heterotaxy syndromes or isomerisms or situs ambigus. Left atrial isomerism is a condition with double left-sided structures (double left-sided shaped atria and lungs), and an absence of the RA and IVC. The IVC is interrupted with an azygous continuation in almost all cases of left isomerism (Fig. 7). As the right sinus node is absent, the risk of bradycardia due to heart block is increased in left isomerism. Right atrial isomerism is the reverse of left atrial isomerism (double right-sided shaped atria and lungs). Therefore, LA is absent and the pulmonary veins are abnormal. Total anomalous venous pulmonary drainage is found in about 80% of right isomerism and the IVC is usually present. Additionally, asplenia is often associated, resulting in a long-term risk for infections.15 The upper abdomen view provides the means of diagnosing the right or left isomerism in most patients. In left isomerism, the azygos vein can be seen as a venous structure posterior to the aorta in upper abdomen view and in right isomerism, the IVC and aorta are located on the same side of the spine (right sided).
Fig. 7.
Upper abdomen view showing left isomerism with interrupted inferior vena cava with azygos continuation (dilated azygos and aorta side-by-side).
3.2. Anomalous pulmonary venous return
During the embryonic development, the common pulmonary vein empties into the LA and then four independent pulmonary veins are incorporated into the atrium. The anomalous return may be partial (3 or <3 pulmonary veins) or total (all four veins). Anomalous pulmonary venous return (APVR) is frequently associated with heterotaxy syndrome. In total APVR, there is no connection between the pulmonary veins and LA. At first step, the diagnosis of ventricular asymmetry with RV dominance and a small LA size with the presence of an additional vessel between the aorta and LA in four-chamber view should increases the suspicion of total APVR.16 Dilated coronary sinus or dilation of the IVC can be present, depending on the point of the anomalous pulmonary vein return. Indeed, the absence of pulmonary venous flow into the LA by color Doppler confirms the diagnosis. Total APVR is classified into four groups, according to the level of the anomalous connection: 1- supracardiac; 2- cardiac; 3- infracardiac; and 4- mix levels of connection. Total APVR requires surgical correction after birth and, in most cases, in the first weeks of life. The delivery should be planned in a hospital with pediatric cardiology and cardiac surgery backup.16
Partial APVR may be overlooked in utero, especially when it is an isolated defect and less than three veins are involved. Scimitar syndrome represents an unusual form of partial APVR in which the right-sided pulmonary veins return to the IVC, just above or below the diaphragm. Scimitar is frequently associated with dextrocardia and right pulmonary hypoplasia caused by a pulmonary sequestration. Prenatal diagnosis is possible, mainly using 3D power Doppler imaging, and enables identification of a collateral vessel arising from the descending aorta and supplying a portion of the right lung. Postnatally, neonatal surgical intervention is rarely required in partial APVR.
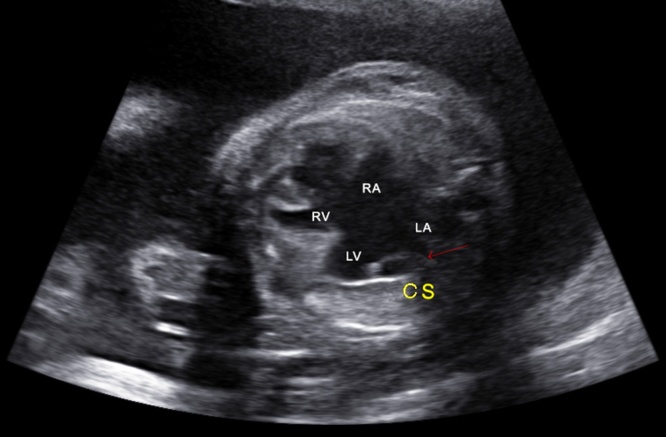
Persistent left superior vena cava (SVC) is the most common variation of the systemic venous. The 3VV enables the diagnosis of left SVC by showing a supernumerary vessel to the left of the pulmonary trunk and arterial duct. An enlarged sinus coronary can be present, as in general the left SVC drains into it (Fig. 8). Isolated, persistent left superior vena cava has no clinical significance after birth, however, it can be associated with left-heart obstructive diseases, conotruncal anomalies and AVSD.17 Therefore, the prenatal diagnosis of left SVC requires a detailed evaluation of the heart.
Fig. 8.
Image showing an atrioventricular septal defect with an enlarged sinus coronary (red arrow) due to the left SVC draining into it. CS, coronary sinus; RA, right atrium; RV, right ventricle; LA, left atrium; LV, left ventricle. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Septal defects
Intracardiac shunt malformations are the most common congenital cardiac defects leading to left-right shunt after birth. Atrial, ventricular, and atrioventricular septal defects are included in this group. These defects can be associated with other cardiac malformations and, depending on their magnitude, are responsible for heart failure postnatally, however with no hemodynamic significance during fetal life.
3.4. Atrial septal defect
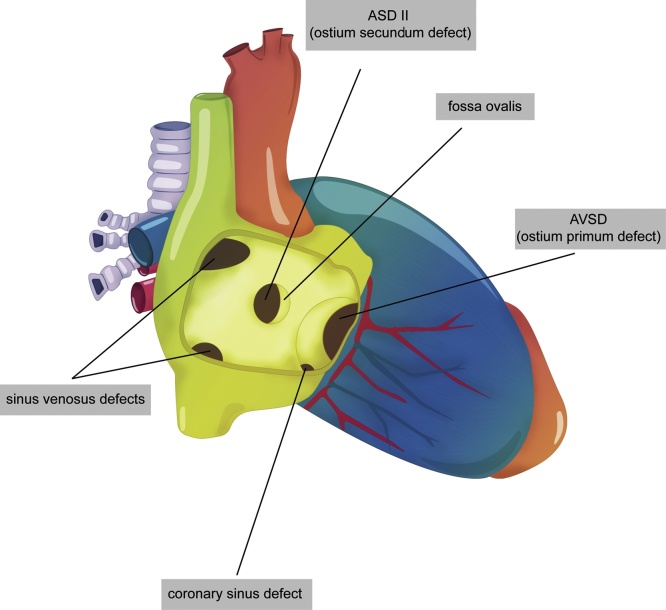
Atrial septal defect (ASD) is a common heart malformation occurring in about 10% to 15% of CHD after birth, and results from an abnormal embryologic development of the atrial septum.18 In general, ASD occurs sporadically with a recurrence risk of 7% to 10%. Less frequently, ASD is associated with bone malformations and syndromes such as Holt-Oram, Noonan and Treacher Collins. Furthermore, there is a familial form of secundum ASD that has been shown to be caused by gene mutations (GATA4 and NKX2-5 genes).19 During fetal life the ASD is well tolerated and leads to left-right shunt postnatally, with symptoms depending on the size and the type of the atrial defect.20 Indeed, it may be isolated or associated with other cardiac defects as a part of complex CHD. The types of ASD are: 1-secundum ASD; 2- primum ASD; 3- sinus venosus ASD; 4- coronary sinus ASD (Fig. 9).
Fig. 9.
Types of atrial septal defect (ASD).
A secundum ASD is the most common ASD (70%), located in the middle of the atrial septum. During fetal life, this communication is normal, namely foramen ovale, and has a normal size similar to aortic diameter.21 Consequently, the prenatal diagnosis of secundum ASD is rarely possible, and it can be suspected when a large foramen ovale is identified, especially with an absent or deficient flap. After birth, the flap of the forame ovale produces a functional closure of this communication. The normal fetal forame ovale presents right-to-left shunt (R-L) with velocities ranging from 20 to 40 cm/s and, when restrictive, the velocities flow across it is increased (>100 cm/s) with L-R shunt. Restrictive forame oval is associated with forms of hypoplastic left heart due to the increased LA pressure. In general, small secundum ASD (<6 mm) close spontaneously during the first two years after birth. Larger defects will need closure by catheter intervention or, more rarely, by surgery.
A primum ASD involves the lower part of atrial septum and is caused by an absent fusion of the lower atrial septum to the underlying atrioventricular valve. Therefore, primum ASD involves an abnormality of AV(s) valve(s) and should be categorized as a form of atrioventricular septal defect (AVSD) which will be discussed later on the topic of AVSD.
A sinus venosus ASD is a less common septal defect, located in the posterosuperior (10% of ASD) or posteroinferior portion of the atrial septum, lying at the junction of the SVC or inferior vena cava, respectively. Both are frequently associated with anomalous pulmonary venous return. Coronary sinus ASD is a rare septal defect in which a communication occurs between an unroofed coronary sinus and LA. Persistent left SVC draining into the coronary sinus is almost always present. Both sinus venosus and coronary sinus ASD have not yet been reported in fetal life. Sinus venosus and coronary sinus ASD do not close spontaneously and need to be repaired by surgery postnatally.22
In conclusion, the diagnosis of secundum DSA can be suspected when the foramen ovale is larger than the aortic diameter on four-chamber view, and an echocardiogram should be done after birth to enable this diagnosis. Inversely, when restrictive, the forame ovale is narrowed with increased flow of velocities (>100c/s) or even, it can be an absent.23 In such cases, changes in pulmonary venous flow pattern, such as a reversed wave and the lacking of D wave, can be useful in detecting restrictive foramen ovale.24 The atrial septum restriction flow is associated with poor outcome in cases of left heart hypoplastic syndrome, or aortic or mitral atresia.
3.5. Ventricular septal defect
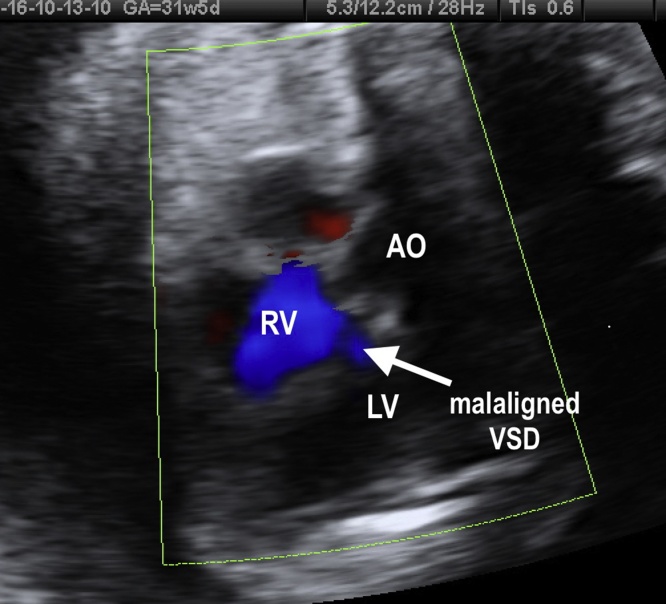
Ventricular septal defect (VSD) is the most common CHD occurring in about 30% of neonates with CHD and 7% to 10% in utero.25 This defect can occur sporadically or in association with TBX5 and GATA4 genes. VSD may be isolated or multiple and is commonly associated with other cardiac defects. In fetal life, the association of VSD with chromosomal anomalies is about 10% to 30%, depending on the type and size of the defect. This rate is significantly higher than after birth, mainly if the defect is large and extends to the inlet septum or, even, when associated with extracardiac defects.26 Ventricular septal defects are classified according to their location on the ventricular septum as: 1- membranous (small segment close to the septal cusp of tricuspid valve and adjacent left heart valves); 2- muscular (lower 2/3 of the septum); and 3- subarterial doubly committed (supracristal). The third one is the least common VSD. It is adjacent to the aortic and pulmonary valves and is caused by the absence of the outlet septum. Membranous and muscular types can also be subclassified according to their area of extension: inlet (close to AV valves implantation); trabecular; and outlet (conus septum) parts of the septum.23 Indeed, the VSD can be termed malaligned when different parts of the septum adjacent to the defect are malaligned to each other (Fig. 10).
Fig. 10.
Fetal echocardiogram showing a malaligned VSD in the four-chamber view. LV, left ventricle; RV, right ventricle; VSD, ventricular septal defect; AO, aorta.
In general, VSD causes no hemodynamic disturbances in utero. Most of all, VSDs evolve to spontaneous closure, even in utero or during the first year of life. The size and location of the defect influence the chance of spontaneous closure. Postnatally, small defects and muscular defects present a greater chance to close spontaneously.27 Surgical intervention may be necessary during the first years of life, when there is congestive heart failure or depending on the size and location of the defect.
In conclusion, the diagnosis of a fetal VSD can be done by a four-chamber view using a lateral view to detect more accurately the bidirectional shunt across the defect. The evaluation of LVOT (five-chamber view) helps to identify outlet defects, mainly membranous outlet VSD. The great arteries short axis view is useful in detecting subarterial doubly-committed VSD and some types of membranous defects. Large defects are greater than the aortic diameter. Three- and four-dimensional ultrasound with inversion flow can be used to detect small defects. The goals of the fetal ultrasound diagnosis of VSD are to define whether the segment of the ventricular septal is involved and to exclude other cardiac anomalies. Malaligned VSD and left-right shunt across the defect should increase the suspicion of left-heart outflow obstructions, such as coarctation of aorta or interrupted aortic arch.
3.6. Atrioventricular septal defects
The atrioventricular septum defect (AVSD) refers to a group of cardiac malformations resulting from a defect of atrioventricular septum that may lead to defects of the interatrial septum (ostium primum ASD) of the interventricular septum (inlet VSD), and the division of the atrioventricular valves. AVSD is also known as atrioventricular canal defect or endocardial cushion defect. The risk of recurrence of AVSD in children of mothers or fathers with this defect is 14% and 2%, respectively.
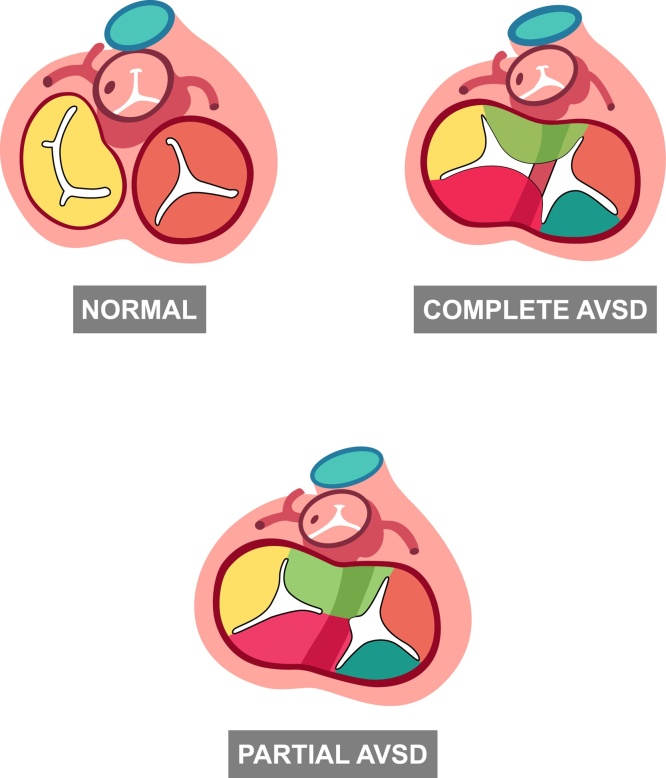
The complete form of AVSD is the most common CHD detectable in utero.28 The complete form of AVSD includes the presence of an ostium primum ASD, an inlet VSD and a common (single) atrioventricular valve. The AVSD is termed partial or incomplete, when a tongue of tissue joins the superior and inferior cusps dividing the common valve into two valves (Fig. 11). The classical partial form of AVSD, also known ostium primum ASD, combines an atrial defect and a cleft of the left valve orifice. However, subtypes of AVSD may include both atrial and ventricular communications.
Fig. 11.
Types of AVSD and normal atrioventricular valves (normal heart). The image shows the partial AVSD with two AV valves and the complete form of AVSD with a single AV valve. AVSD, atrioventricular septal defect; AV atrioventricular valve.
Furthermore, the assessment of the insertion of the common AV valve enables the Rastelli’s AVSD classification (A, B and C types), which is useful to the postnatal surgical approach (Fig. 12).29,30 The analysis of the atrioventricular connection and the size of the ventricles are important tools for the identification of balanced and unbalanced forms of AVSD. Unbalanced AVSD results in ventricular disproportion (hypoplasia of one ventricle) and is typically found in association with heterotaxy syndrome (atrial isomerism).31
Fig. 12.
Fetal echocardiographic image of four-chamber view demonstrating atrioventricular septal defect (AVSD) Rastelli’s type B- see the superior bridging leaflet of atriventricular valve attached over the ventricular septum by an anomalous papillary muscle of the right ventricle (red arrow). LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Complete AVSD is associated with extra-cardiac malformations and syndromes such as trisomies 21 (75% of cases), 18 and 13. Therefore, fetal karyotype should be discussed with parents whenever this diagnosis is made. Moreover, a sequential detailed cardiac examination is indicated to identify additional cardiac anomalies, such as tetralogy of Fallot and double-outlet RV, mainly in fetuses with trisomy 21.31
During the fetal life, the AVSD is well tolerated and the delivery should follow the obstetric routine. Few fetuses will develop congestive heart failure and non-immune hydrops due to severe AV regurgitation and/or complete heart block (atrial isomerism). After birth, complete VSD should be surgically repaired between three and six months of age by the early risk of irreversible arterial pulmonary hypertension.30 In cases of unbalanced DSAVT with hypoplasia of one of the ventricles, the postnatal surgical management will be the same as used for the univentricular heart.
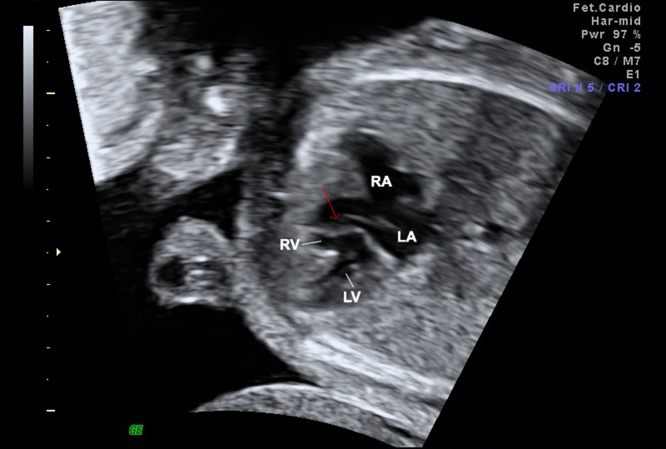
In conclusion, the fetal diagnosis of AVSD can easily be done by four-chamber view. The best diagnosis clues are: the absence of crux of the heart, the presence of the primum ASD and the absence of the usual offset of the AV valves (Fig. 13). In most cases, an inlet VSD is also present. The four-chamber view can be significantly improved by measuring the ratio of atrial to ventricular length (AVL) with a cutoff value of over 0.6.31 Furthermore, this plane is ideal to assess the relationship of AV junction, the size of the ventricles, and AV valve insufficiency. The five-chamber view can identify the characteristics of the LV-outflow view in AVSD: a narrowed and elongated outflow (also known as gooseneck).
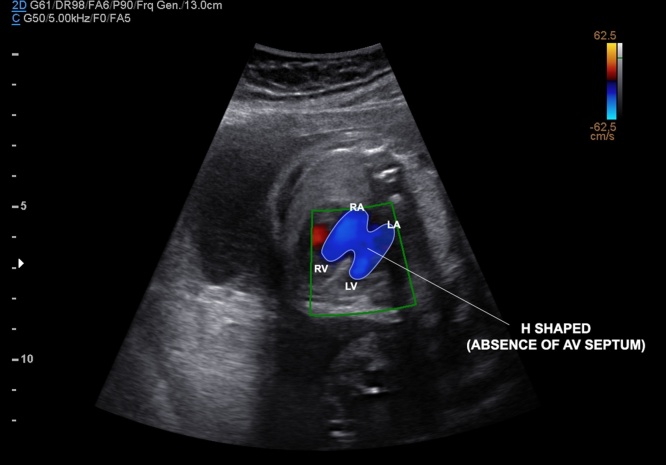
Fig. 13.
Fetal echocardiographic image of four-chamber view showing a balanced complete atrioventricular septal defect (AVSD). During diastole, the common atrioventricular valve is open with the absence of AV septum (H-shaped by color Doppler mapping due to the absence of the AV septum), and large atrial and ventricular defects. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; ASD, primum atrial septum defect; VSD, inlet ventricular septal defect; AV atrioventricular valve.
4. Right heart malformations
4.1. Pulmonary atresia with intact ventricular septum/tricuspid atresia
Pulmonary atresia (PA) with intact ventricular septum account for about 1% to 3% of CHD diagnosed during fetal life and for 2.5% to 4% after birth. The classification includes two types: Type I (75%) − stenotic and competent tricuspid valve (TV) and RV with hypoplasia and Type II − dysplastic and insufficient TV with a normal size RV and an enlarged RA.32
The prenatal diagnosis of PA can be done by the five-chamber view that enables identification of an immobile and thickened pulmonary valve and the presence of a reversed flow in the pulmonary artery from the ductus arteriosus to the pulmonary valve by color Doppler (Fig. 14). The classification of PA varies according to the features of the TV. In cases of PA and TV stenosis, the RV is hypoplastic (Type I), whereas when PA is associated with a dysplastic and incompetent TV (Type 2), the RV is well-developed and the RA enlarged. Ventriculocoronary connections (fistulas) can be observed, more commonly in Type I. In all forms, the PA is smaller than aorta on three-vessel view.32
Fig. 14.
Image showing by color Doppler the presence of reversed flow (arrow) in PA (pulmonary atresia) and a PA with small size in 3 V view. PA, pulmonary artery; AO, aorta.
Tricuspid atresia is defined as an agenesis of the tricuspid valve with no direct communication between the RA and the RV. It is a rare form of CHD (3%–4% in fetal life), with a recurrence risk of 1%.33 This defect is associated with normally related (type I) or transposed arteries (types II and III: D and L transposed arteries) with, or less commonly, without VSD.32 The pulmonary flow could be normal, decreased (pulmonary stenosis), or absent (pulmonary atresia). Additional cardiac anomalies, such as coarctation of the aorta, are present in less than 20% of cases. If the VSD is absent or small, the restricted flow to RV can lead to severe hypoplasia of the RV.
The prenatal diagnosis of tricuspid atresia is easily done by ultrasound. The features of tricuspid atresia in the four-chamber view are: an echogenic and immobile tricuspid valve; absence of flow across the TV on color Doppler during diastole; and a hypoplastic RV with or without VSD. Transposed arteries are present in about 20% of cases. Sequential follow-up cardiac ultrasound is indicated to assess the LV function, the size of the foramen oval and the presence of RV outflow obstruction. Fetal distress and hydrops are related with very small foramen oval.
Neonates with PA with intact ventricular septum require administration of prostaglandin E1 at birth to maintain patent ductus arteriosus until surgery is performed. In cases of tricuspid atresia with a restrictive foramen oval or secundum ASD, this communication should be enlarged by ballon or surgical atrioseptostomy during the first days of life. In both cases, the prenatal diagnosis will improve the perinatal outcome. Posteriorly, the type of surgical management will depend on the type of the anatomy.
4.2. Pulmonary stenosis/tricuspid valve stenosis
In pulmonary valve stenosis the thickness and the reduced motility of the pulmonary valve are variable. Generally, the severity of the pulmonary stenosis is based on the size of the pulmonary valve and the direction flow in the pulmonary artery. Direct evidence of ductal dependence and tricuspid regurgitation are indicative of severe stenosis (critical pulmonary stenosis). In cases of critical stenosis, the anterograde pulmonary flow can be detectable instead of the fully retrograde flow which characterizes PA. In mild-to-moderate forms, the four-chamber view can be normal, even an increased pulmonary artery peak velocity is present. Therefore, an abnormal pulmonary valve echogenicity on the five-chamber view and patients at a risk (rubella syndrome) should be followed by a Doppler flow investigation. In cases of supravalvar pulmonary stenosis, the RV outflow tract obstruction is located in the pulmonary artery instead of being in the pulmonary valve, and is commonly associated with syndromes such as Noonan, Williams, and Alagille.
In the tricuspid stenosis, the tricuspid valve diameter is reduced with thick cusps and restricted diastolic opening. During the gestation, the lack of flow across the valve can decrease the RV development leading to RA dilatation. The four-chamber view with color Doppler enables the diagnosis of the tricuspid stenosis.
4.3. Ebstein's anomaly and tricuspid dysplasia
Ebstein’s anomaly is characterized by the lack of mobility and downward displacement of septal and posterior cusps of TV (gap between the TV and the mitral valve >8 mm). In cases of ventricular inversion, an Ebstein’s anomaly could be observed on the left side. The diagnosis can be suspected on a cardiac screening (four-view chamber) when the RA is enlarged and the thickened cusps of the TV are displaced down and tethered on the septal surface. Ebstein’s anomaly is a rare congenital condition (3% to 7% of fetal CHD), and has been associated with maternal ingestion of benzodiazepines.34
When Ebstein’s anomaly is identified prenatally, the fetus should be closely monitored due to an increased risk of tricuspid insufficiency progression, cardiac dysfunction, and fetal demise. Serial fetal echocardiography is recommended, especially during the third trimester. Depending on the degree of the TV displacement it can result in less or more severe reduction of the functional RV.35 After birth, the RV functional area is helpful to predict prognosis. However, predicting outcomes of fetuses with Ebstein’s anomaly remains a challenge. Fetuses with pulmonary regurgitation, indicating circular shunt physiology, as well early gestational age at the diagnosis, have been associated with poor outcome.
Fetal transplacental digoxin theraphy may improve the cardiac function in cases of heart failure.36 In fetuses with Ebstein’s anomaly and hydrops, the delivery should be performed in a hospital with an intensive and specialized cardiac care team. The relative risks of premature delivery must be carefully discussed as recent studies suggest it is associated with worsening neonatal outcome.36 However, early delivery may be considered in cases of hydrops and uncontrolled arrhythmia.37
Neonatal repair will be required in cases of heart failure or profound cyanosis. In children and adults, the presence of symptoms (cyanosis and paradoxal embolization) is an indication for surgical approach.38, 39
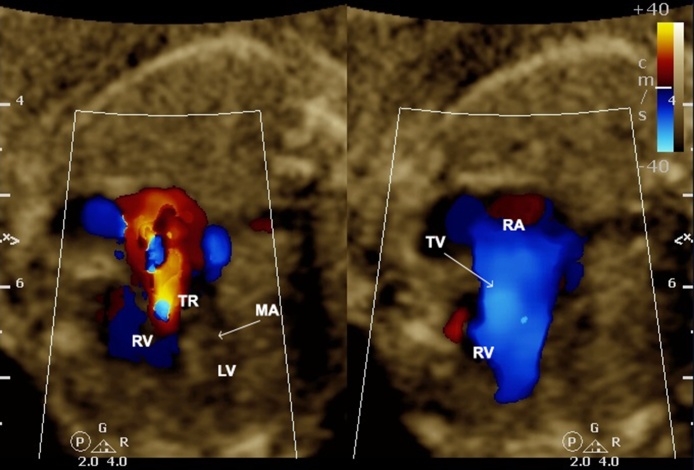
Tricuspid dysplasia can be distinguished from Ebstein’s anomaly on the basis of the normal attachment of the TV. The dysplasia can be isolated or associated with syndromes such as trisomy 21 and CHARGE (coloboma, heart defect, atresia choanae, retarded growth and development, genital hypoplasia, and ear anomalies/deafness) syndrome. The tricuspid dysplasia can be easily diagnosed by the greater echogenicity of the valve (valve deformation) on the four-chamber view of the heart. Furthermore, the color Doppler enables one to identify and quantify the tricuspid regurgitation. Both tricuspid dysplasia and Ebstein’s anomaly are rare congenital TV malformations that lead to the same physiology in utero by the TV regurgitation (Fig. 15). Even in tricuspid dysplasia and Ebstein’s anomaly, fetuses with pulmonary regurgitation (PR) are at high-risk. PR serves as the insult that completes the circular shunt with a systemic flow steal leading to low organ perfusion and ultimately fetal distress.
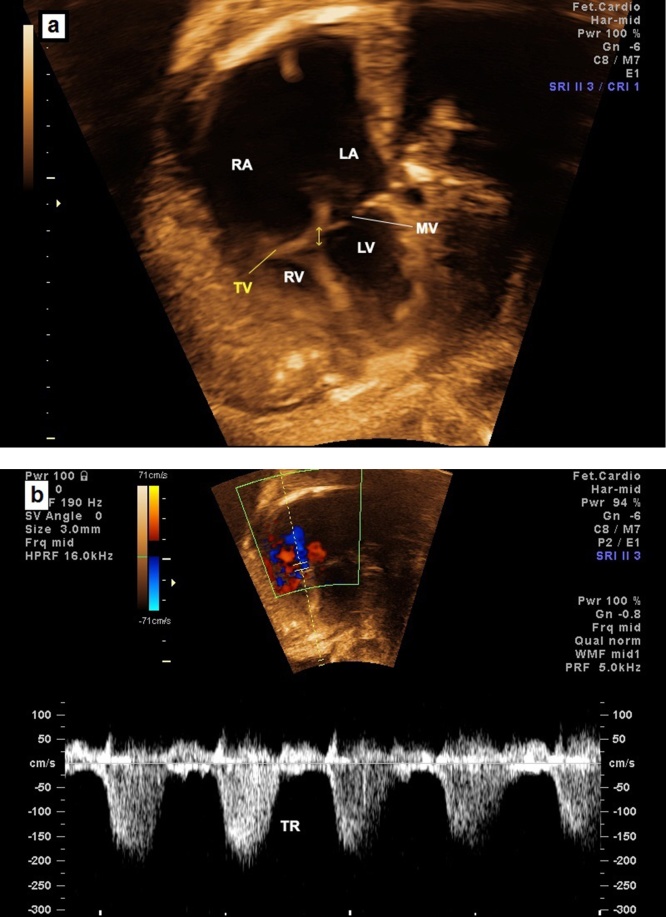
Fig. 15.
Image showing TV dysplasia with normal attachment of the TV (a) and severe tricuspid regurgitation (TR) (b). TV, tricuspid valve; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; TR, tricuspid regurgitation; MV, mitral valve.
5. Left heart anomalies
5.1. Aortic stenosis/mitral stenosis
Valve aortic stenosis is the most common type of aortic stenosis occurring in 60%–70% of patients with aortic stenosis. Supravalvar and subaortic stenosis are rare in fetuses and at least one can be associated with mitral valve disease and coarctation of aorta, also known as Shone syndrome (left-sided heart obstructive lesions). Aortic stenosis occurs in about 3% to 6% of newborns with CHD and is an associated cardiac malformation in about 30% of cases.40
The prognosis and management depend on the degree of the obstruction, the gestational age at the diagnosis, and the presence of associated anomalies. Many cases of severe aortic stenosis evolved at the mid-trimester have shown reduced growth of the left heart structures. Therefore, sequential studies should be performed due to the risk of progressing to a hypoplastic left heart syndrome (HLHS).41
The aortic valve may appear abnormal (thickened with reduced mobility) on the five-chamber view with turbulent color Doppler flow at the valve. If aortic stenosis is moderate to severe, the LV may be normal or mildly hypertrophied with an increased aortic peak Doppler velocity ( > 2 m/s). If the aortic stenosis is critical, the LV is dilated with poor contractility and increased echogenicity (suggesting fibroeslastosis), and the aortic valve is small with Doppler velocity slightly increased ( > 1 to 2 m/s)40 (Fig. 16). Mitral stenosis and mitral regurgitation may be associated. If aortic stenosis is critical stenosis, there will not be full anterograde flow across the aortic valve. In the 3 V view, the reversed flow and the small size of the transverse aorta are clues to the diagnosis. In cases of aortic atresia, no flow across the valve is detectable by color Doppler. The interatrial shunt across the forame oval is left to right, also reversed.
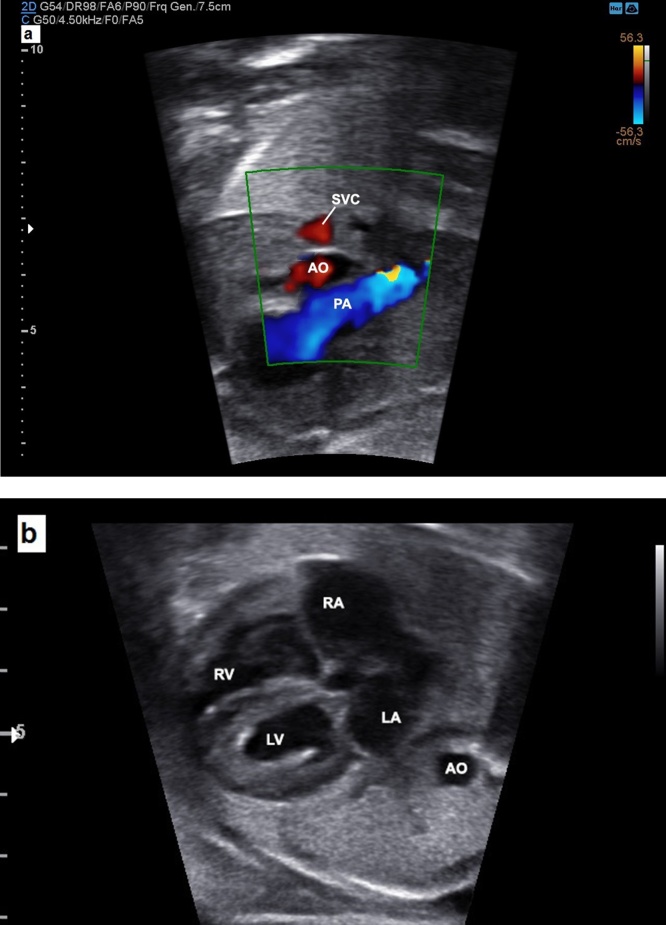
Fig. 16.
Fetal echocardiogram showing the small aorta with reversed flow by three vessels and trachea view in a case of critical aortic stenosis (a) and a dilated LV with endocardial fibro elastosis in a four-chamber view (b). PA, pulmonary artery; AO, descending aorta; SVC, superior vena cava; L, left; R, right; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
In cases of critical aortic stenosis prostaglandin, E1 should be given at birth as the systemic blood perfusion is ductal dependent. Subsequent balloon dilation of the valve or surgery need to be performed. If the LV is hypoplastic, a biventricular repair may not be feasible.
During fetal life, mitral stenosis is associated with a reduced flow to the LV. The LV and aorta are smaller than the RV, and pulmonary artery and the interatrial shunt are reversed (L-R). In the four-chamber view, there is a discrepancy between the ventricles and AV valves. The mitral valve is thickened with small size and reduced mobility. Associated cardiac anomalies, especially other left-sided cardiac lesions, must be excluded.
5.2. Aortic atresia and hypoplastic left ventricle syndrome
The most common sign of aortic atresia is the hypoplastic LV, detectable as an echogenic and dysfunctional chamber in the four-chamber view. In fetuses with classic forms of HLVS (aortic and mitral atresia), there is a markedly abnormal four-chamber view at mid-gestation, with no inflow into the left ventricle (mitral atresia) and a severely hypoplastic LV (Fig. 17). In cases of aortic atresia and mitral stenosis, the LV is more recognized. The degree of hypoplasia of the ascending aorta and reduced size of the LA can be variable according to the form of aortic atresia and HLVS. No anterograde flow across the aortic valve and a reversed flow in the aortic arch are detectable in the 3 V view and the long-axis of aortic arch.
Fig. 17.
Image showing a hypoplastic LV due to mitral atresia and right chambers enlargement with TR. TR, tricuspid regurgitation; LV, left ventricle; RA, right atrium; RV, right ventricle; MA, mitral atresia valve; TV, tricuspid valve.
A restrictive interatrial shunt is associated with poor outcome and the analysis of the pulmonary vein Doppler pattern (reversed a wave and low or absent D wave) is useful. Fetuses with restrictive interatrial shunt may be candidates for percutaneous enlargement of the foramen ovale during the second trimester.42 The right heart should be examined in detail to evaluate if Norwood palliative surgery will be feasible. The HLHS is associated with trisomies (18 and 13) and abnormalities of the central nervous system (CNS).
In fetuses with HLHS the delivery should be planned at a tertiary care center experienced with complex CHD. All newborns with HLHS are ductal dependent for systemic blood flow, so prostaglandin infusion should be initiated. The neonates with HLHS and a restrictive foramen ovale will require atrial septostomy/septectomy after delivery. In general, the surgical management of HLHS requires three palliative surgical procedures: Norwood (or Norwood-Sano) operation or a hybrid approach within the first week after delivery, followed by a Glenn procedure at approximately six months and a Fontan procedure at two to three years. Some heart transplantation may be considered an alternative in cases of newborns with right-sided anomalies or long-term survival with progressive heart failure.43
6. Conotruncal anomalies
6.1. Tetralogy of Fallot
The tetralogy of Fallot (TOF) is the most common cyanotic heart defect at a frequency of 7% to 10% in children with CHD. The tetralogy of Fallot is characterized by: 1- a VSD; 2- an overriding aorta; 3- infundibular pulmonary stenosis; and 4- right ventricular hypertrophy. However, in utero, the RV hypertrophy is almost always absent and the four-chamber cardiac view is normal. As the defect results from an anterior deviation of the conal septum, the RVOT, the overriding of the aorta, and a malalignment VSD are the clues to this diagnosis of TOP, enabled by the five-chamber view and the basal short axis view. Furthermore, a smaller pulmonary artery and a larger aorta are observed in 3VT view (Fig. 18). The cardiac anomalies more frequently associated with TOF and reliably diagnosed in fetuses are: right aortic arch (20%); AVSD (5%); additional muscular DSV; and anomalous left subclavian.44 Di George syndrome, trisomies (13, 18 and 21), omphalocele and pentalogy of Cantrell, among other conditions, may be associated with TOF.45
Fig. 18.
Tetralogy of Fallot. (a) The five-chamber view showing the overriding of the aorta and an outlet VSD. (b) The three vessels view image showing a large aorta and a small pulmonary artery. RV, right ventricle; LV, left ventricle; VSD, ventricular septal defect; PA, pulmonary artery; AO, aorta; SVC, superior vena cava.
The management and prognosis of TOF depend on details of the anatomy and physiology. TOF with pulmonary stenosis is the classical form. TOF with pulmonary atresia (TOF/PA) is an extreme form of tetralogy in which the patent pulmonary outflow may progress to pulmonary atresia during fetal life. In fetuses with TOF/PA, the pulmonary arteries are severely hypoplastic or absent and the pulmonary blood flow is dependent on collaterals or on ductus arteriosus. Longitudinal aortic view with color Doppler facilitates in showing the flow from the ductus, or from the descending aorta collaterals to the pulmonary artery. Unusually, the TOF is associated with the absence of the pulmonary valve (TOF/APV). Interestingly, the ductus arteriosus is typically absent in cases of TOF/APV, and an aneurysmal dilatation of the pulmonary artery may progress with a risk of heart failure in utero. Differently to the other forms of TOF, the four chambers in TOF/APV are abnormal with an enlarged RV and in cases with tricuspid regurgitation, a dilated RA. The pulmonary valve is usually absent and incompetent and the pulmonary arteries are enlarged. After birth, in severe cases the patients present pulmonary hypoplasia or severe airway disease due to the chronic extrinsic compression of the airways by the aneurysmal pulmonary arteries during fetal life. Airways and lungs fetal magnetic resonance imaging (MRI) may be considered as enabling the risk stratification risk for liquid ventilation and extracorporeal membrane oxygenation (ECMO) at birth.44
Classic forms of TOF tend to being well tolerated during the neonatal period and the patient may be discharged for follow-up, usually after the first week. In general, the surgical correction will be performed during the first year of life, or some cases may require a prior palliative surgical Blalock-Taussig operation. In cases of TOF/PA with pulmonary flow ductal-dependence, the prostaglandin infusion should be initiated with a sequential surgical approach soon after birth. The forms of TOF/APV may evolve with congestive heart failure and higher perinatal mortality. Pregnant women with fetal TOP with pulmonary valve agenesis should be referred for delivery in a tertiary reference center, with a pediatric cardiologist team, available cardiac surgery and ECMO.37
6.2. Transposition of the great arteries
The transposition of the great arteries (TGA) is a frequent cyanotic CHD characterized by discordant ventriculoarterial connection, in which the aortic artery arises from the RV and the pulmonary artery from the LV. It occurs in 5% to 7% of cases of CHD in childhood, however it is one of the CHD most commonly underdiagnosed in utero.46
TGA is rarely associated with chromosomal or extracardiac anomalies and in 40% of cases is associated with VSD. Serial fetal echocardiographic is recommended as the risk of associated outflow tract defects (pulmonary stenosis and aortic arch obstruction). Two major types of TGA have been described: dextro- or D-TGA and levo- or L-TGA.
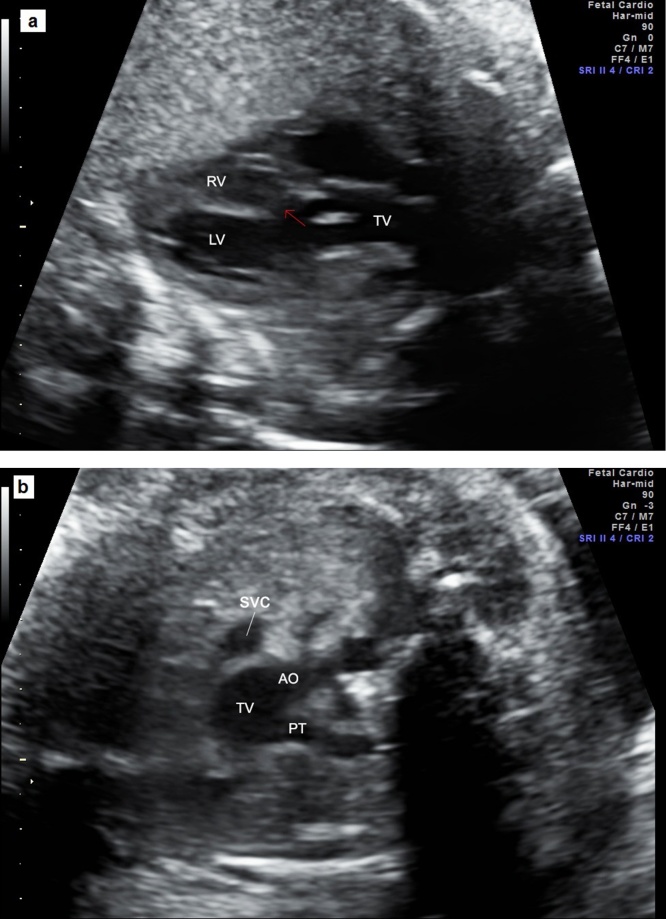
In most cases of D-TGA, the atrial situs is solitus. In D-TGA, the ventricular looping is normal (D-looped: RV is the right-sided and anterior ventricle), and the relationship of the great arteries is abnormal with mitral-pulmonary fibrous continuity. The four-chamber view is normal in fetuses with isolated D-TGA but the outflow tracts are markedly abnormal. The arteries do not cross each other (parallel arteries) and can be visualized in a single imaging plane. Instead, the five-chamber view shows the pulmonary artery arising from the LV and the aorta arising from the RV. The arteries are arranged in a different fashion in the 3VT view with a right anterior aorta and a left posterior pulmonary artery.46 Consequently, the finding of only 2 vessels (transverse aorta and SVC) in the 3VT view is frequent in cases of TGA as the pulmonary trunk is not visible in the great vessels transverse view.47
In L-TGA, the atrial situs is normal, however, the ventricles are L-looped (the RV is the left-sided and posterior ventricle). L-TGA is characterized by the combination of discordant atrioventricular connection and discordant ventriculoarterial connection. Because of the discordance at two levels, the physiology of this defect is congenitally corrected, which explains it being known as congenitally corrected transposition of the great arteries. Many cases of L-TGA are associated with VSD, Ebstein’s anomaly of left-sided tricuspid valve, complete heart block, and pulmonary setenosis/atresia. Typically, the four-chamber view is abnormal with a left-sided ventricle containing the moderator band which characterizes the morphologically RV. The aorta is located anterior and left to the pulmonary artery.46
D-TGA is an absolute indication of delivery in a tertiary referral center, with a pediatric cardiologist and cardiac surgery available. After birth, newborns with D-TGA should receive prostaglandin infusion to maintain the ductus patency and may require ballon atrial atrioseptostomy. Subsequently, patients with D-TGA usually undergo the Jatene arterial switch operation within the first one to two weeks after delivery. In cases of D-TGA with VSD and pulmonary stenosis, the Rastelli operation (conduit between LV-aorta) will be performed or, in such cases, an atrial switch operation. After delivery, patients with L-TGA may not require any intervention. However, the presence of associated cardiac abnormalities may require a subsequent surgical intervention (double-switch operation), or even a neonatal intervention.46
6.3. Double outlet right ventricular
Double-outlet right ventricular outflow tract (DORV) refers to a group of heart defects in which both great arteries arise predominantly (>50%) from the morphologically right ventricle. In DORV, there is aortic-mitral discontinuity and, in almost all cases, a VSD is present. The type of DORV, depending on the type of the VSD and the outflow tracts features: DORV type Fallot (subaortic VSD + pulmonary stenosis), Taussig-Bing anomaly (subpulmonary VSD + transposed great arteries) or DORV with and without VSD (doubly or non-committed VSD). The incidence of this CHD in newborns is 0.03–0.07 per 1000 live births.48
The prognosis will depend on the type of DORV, the complexity of associated cardiac anomalies, the extracardiac lesions and the presence of chromosomal abnormalities. Trisomies 21, 18, and 13, extracardiac abnormalities and Di George syndrome (22q11) are more common in DORV-type Fallot. Delection in the chromosomal 22q11 is associated with thymic hypoplasia or absence that can be detected at the fetal ultrasound in the 3VT. As is typical of the conotruncal anomalies, the four-chamber view is normal and all fetuses with DORV have abnormalities of the outflow tracts view. The presence of a malaligned VSD (>50%) and aortic-mitral discontinuity may be helpful to enable the differential diagnosis in the five-chamber view.47
The delivery counseling in a tertiary referral center, with pediatric cardiologist and cardiac surgery available, will depend on the type of DVSD and the presence of associated extracardiac and/or chromosomal abnormalities.
6.4. Truncus arteriosus
Truncus arteriosus is a rare condition (1.5% of CHD in newborns) in which only a single arterial arises from the ventricles and gives flow to the systemic, pulmonary and coronary circulations. In almost all cases there is a large malaligned VSD and it is strongly associated with Di George syndrome.44
In fetuses with truncus arteriosus, the four-chamber view is normal. The five-chamber view is markedly abnormal with the presence of a thickened truncal valve that overrides a large VSD. During fetal life the differentiation between TOF with PA and truncus can be a challenge, as both the 3 V and 3VT views show only two vessels. In TOF/PA, the aortic valve is large and thin and in truncus, the truncal valve is thickened. Furthermore, in truncus arteriosus, the pulmonary arteries usually have good size and the ductus arteriosus is absent. Depending on anatomy, four types of truncus are described: type I- a pulmonary trunk arises from the truncal trunk (Fig. 19); type II- pulmonary arteries arising from the truncal trunk; type III- one of the pulmonary arteries is absent with collateral circulation; and type IV-truncus arteriosus with an interrupted arch aortic.49
Fig. 19.
Fetal echocardiography showing a case of truncus arteriosus type I. (a) The five-chamber view showing the presence of a thickened truncal valve that overrides a large VSD (arrow). (b) The three vessels view showing two vessels: superior vena cava and truncal vessel (Type I: pulmonary trunk arising from the truncal vessel). TV, truncal vessel; VSD, ventricular septal defect; LV, left ventricle; RV, right ventricle; VSD, ventricular septal defect; PT, pulmonary artery trunk.
Commonly, patients with truncus arteriosus undergo surgical repair within the first few weeks after delivery, with VSD closure and usually a placement of a pulmonary conduit into the RVOT.
7. Aortic arch anomalies
7.1. Coarctation of the aorta/interrupted aortic arch
Coarctation of the aorta represents a narrowing of the aortic arch and, in general, it is located between the origin of the left subclavian artery and the ductus arteriosus (aortic isthmus). Prenatally, coarctation may be difficult to detect mainly in the mild and moderate forms, because the ductus arteriosus and is patent. Chromosomal abnormalities are present in about 30% of fetuses with coarctation of the aorta.
Prenatally, the ventricular size discrepancy with right dominance in four-chamber view should increase suspicion for the diagnosis of coarctation of the aorta. In 3VT view, the presence of abnormal size of the great arteries (PA/AO >1.5) and/or isthmus-ductus ratio (< 0.74), increase the potential presence of coarctation (Fig. 20).50 Finally, the presence of a transverse aortic arch hypoplasia and isthmus hypoplasia in the long-axis of the aortic arch is the most sensitive feature.51 Isthmus measurements and Z scores (< −2.0) for gestational age are very helpful to detect hypoplasia.52 Coarctation of aorta may develop in utero and sequential ultrasound/echocardiogram evaluation is recommended.
Fig. 20.
The three vessels view showing the small size of the transverse aorta in a case of coarctation of the aorta. SVC, superior vena cava; AO, aorta; PA, pulmonary artery.
After birth, depending on the severity of the coarctation, these patients will require catheter or surgical intervention with an excellent prognosis. However, after neonatal period repair, the frequency of reintervention for recoarctation is higher (up to 50% of cases). In cases of critical coarctation, the delivery should be planned (38–39 weeks of gestation) at hospital with a team of hemodynamics and/or cardiovascular surgery should be prepared.
Interrupted aortic arch, in almost all cases, is associated with a malaligned VSD. In such cases, the flow across the defect is reversed (left-right shunt). The discrepancy in ventricular and the arteries size is also present. In the most common form of interrupted aortic arch (type B), the ascending aorta appears to be straighter than normal and the left subclavian artery arises from the junction of the ductus arteriosus with descending aorta (Fig. 21). Furthermore, the evaluation of the thymus as 22q11 microdeletion is frequently associated with interrupted aortic arch.53
Fig. 21.
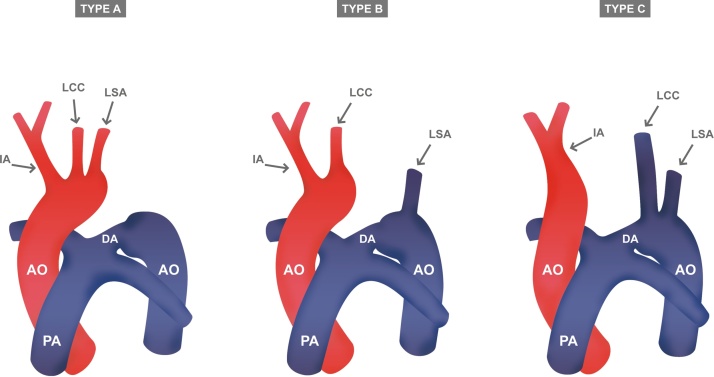
Figures of types of interrupted aortic arch classified according to the location of the interruption (a, b, and c). LCA, left carotid artery; LSA, left subclavian artery; IA, innominate artery; AO, aorta; PA, pulmonary artery; DA, ductus arteriosus.
Interrupted aortic arch represents a ductal-dependent systemic circulation requiring initiation of prostaglandin E1 infusion, immediately after delivery. These patients require surgical neonatal repair and the delivery must be planned near term at a hospital with a team of pediatric cardiologists and cardiac surgery available.
8. Vascular rings
8.1. Right aortic arch and vascular ring/double aortic arch
Right aortic arch with a left subclavian or innominate artery with a left ductus is not uncommon. The right-sided descending aorta is observed at 3 V and four-chamber views. Additionally, a gap between the pulmonary trunk and the ascending aorta and a U-shaped vascular loop enable the diagnosis on 3 V view (Fig. 22).
Fig. 22.
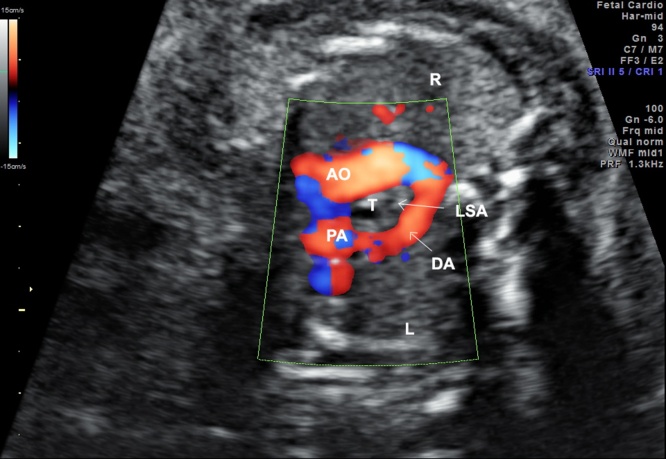
Image showing a right sided aortic arch with a left subclavian artery at three-vessel view (U-shaped). AO, aorta; PA, pulmonary artery; LSA, left subclavian artery; DA, ductus arteriosus; T, trachea; R, right; L, left.
Double aortic arch is the only form of vascular ring in which the ring consists exclusively of vessels. In double arch, usually the right arch is larger than the left-sided arch. A left ductus and a left descending aorta are present. The two aortic arches, pulmonary trunk, and ductus arteriosus form the vascular ring as a figure of numbers “6” or “9” at the 3 V view.54
9. Complex congenital heart disease
9.1. Univentricular heart
The univentricular heart or “single ventricle” is a condition in which both atria are connected to a dominant ventricle. This ventricle maintains the systemic and pulmonary circulations. The most common form of univentricular atrioventricular connection is double-inlet. The other two forms of univentricular AV connection are: single inlet (mitral or tricuspid atresia); and common inlet (single AV valve). The dominant ventricular chamber can be a morphologic LV or RV chamber or even undetermined.55 The rudimentary chamber may be underdeveloped or absent. It is a rare cardiac anomaly that occurs in 2.5% of live births with congenital heart disease.
The presence of a main ventricular chamber with an absence of the ventricular septum in the four-chamber view enables the diagnosis of univentricular heart. The existence of a “single ventricle” per se usually does not present a significant hemodynamic change in the fetus.
After birth, the hemodynamics of the univentricular heart depends on other associated anomalies. Depending on the outflow tracts the newborns are submitted to palliative surgery with PA banding or systemic-pulmonary shunt (Blalock-Taussig operation). A surgical cava-anastomose between SVC and pulmonary artery (Glenn operation) is performed at about six months of age and it is completed by directing the flow of the inferior vena cava to the pulmonary artery (Fontan operation), generally between two and four years of age.
10. Cardiac biometric
The initial evaluation of a fetus with heart failure or at risk of myocardial dysfunction includes the assessment of the presence or absence of cardiomegaly. Cardiac thoracic index (CTI) is the ratio between the cardiac and chest circumferences (normal values ≤0.5) or the ratio between the cardiac and thoracic areas (normal values ≤0.35).56 CTI is altered in conditions that involve global cardiomegaly such as tricuspid dysplasia and Ebstein’s anomaly, cardiomyopathy, or secondary to extra-cardiac causes, such as anemia, twin-to-twin transfusion, and infection.
Abnormal size of cardiac chambers could be an early indicator of CHD. In fetuses with cardiac asymmetry, left and right ventricular widths and lengths should be evaluated. The RV and LV widths are measured in a four-chamber cardiac view when the atrioventricular valves are closed and before the onset of cardiac systole (at the end of diastole). The maximal RV and LV widths are measured just below the atrioventricular valve from inner to inner edged of each ventricle.57 The RV to LV width ratio can be calculated and has been used to screen cardiac anomalies. Before 25 weeks of gestation, the RV dominance should raise the suspicion of coarctation of the aorta.50, 51 However, during the third trimester, a slighter dominance of RV may be physiological (normal: RV/LV <1,5).58 The maximal lengths of the ventricles are measured from the AV valve to the apex of each ventricle. LV hypertrophy and changes in cardiac geometry may occur, characterized by more a globular heart and can be assessed using the LV sphericity index (the ratio between the longitudinal and transverse diameters of LV = 0.5).59
Indeed, the z-score system expresses the ventricular chambers and vessels measurements as a number of standard deviations for gestational age or by adding fetal anthropometric values (femur lengh/biparietal diameter). The Z-scores are very helpful tool to screen RV or LV hypoplasia and outflow tract anomalies.57, 60
11. Conclusion
Considering that CHD is the most important cause of infant mortality due to birth defects, the fetal diagnosis of cardiac defects is the point of care to improve the outcome in critical CHD, especially where the circulation depends on patency of the ductus. In this scenario, cardiac screening in fetuses remains a challenge that involves a team of professionals. In the modern era, ultrasonography has shown important advances and CHD is expected be diagnosed in detail during fetal life, which may improve the prognosis for most cases of CHD. Furthermore, it is important to know the importance of the cardiovascular system in fetal well-being. Therefore, in this study, important aspects of cardiac sonography that may be applied in clinical practice, have been covered in order improve the diagnosis of a cardiac defect enabling the management in utero, planning delivery, and identifying the CHD which may progress in utero cardiac. In conclusion, many studies and guidelines have been published and further studies should be done in order to provide the tools to enable the diagnosis of CHD by ultrasound examiners.
References
- 1.Hoffman J.L., Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 2.Moons P., Sluysmans T., De Wolf D. Congenital heart disease in 111 225 births in Belgium: birth prevalence, treatment and survival in the 21 st century. Acta Paediatr. 2009;98:472–477. doi: 10.1111/j.1651-2227.2008.01152.x. [DOI] [PubMed] [Google Scholar]
- 3.Gilboa S.M., Salemi J.L., Nembhard W.N., Fixler D.E., Correa A. Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation. 2010;122:2254–2263. doi: 10.1161/CIRCULATIONAHA.110.947002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oster M, Lee K, Honein M, Colarusso T, Shin M, Correa A. Facts about Critical Congenital Heart Defects, [Online], cited 2016-12-26, Available from: URL: http://www.cdc.gov/ncbddd/heartdefects/cchd-facts.html.
- 5.Kovalchin J.P., Silverman N.H. The impact of fetal echocardiography. Pediatr Cardiol. 2004;25:299–306. doi: 10.1007/s00246-003-0593-1. [DOI] [PubMed] [Google Scholar]
- 6.Linask K.K. The heart-placenta axis in the first month of pregnancy: induction and prevention of cardiovascular birth defects. J Pregnancy. 2013;2013:320413. doi: 10.1155/2013/320413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoo S.J., Lee Y.H., Kim E.S. Three-vessel view of the fetal upper mediastinum: an easy means of detecting abnormalities of the ventricular outflow tracts and great arteries during obstetric screening. Ultrasound Obstet Gynecol. 1997;9:173–182. doi: 10.1046/j.1469-0705.1997.09030173.x. [DOI] [PubMed] [Google Scholar]
- 8.Yagel S., Weissman A., Rotstein Z. Congenital heart defects: natural course and in utero development. Circulation. 1997;96:550–555. doi: 10.1161/01.cir.96.2.550. [DOI] [PubMed] [Google Scholar]
- 9.International Society of Ultrasound in Obstetrics and Gynecology, Carvalho J.S. ISUOG Practice Guidelines (updated): sonographic screening examination of the fetal heart. Ultrasound Obstet Gynecol. 2013;41:348–359. doi: 10.1002/uog.12403. [DOI] [PubMed] [Google Scholar]
- 10.Donofrio M.T., Moon-Grady A.J., Hornberger L.K. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014;129:2183–2242. doi: 10.1161/01.cir.0000437597.44550.5d. [DOI] [PubMed] [Google Scholar]
- 11.Comstock C.H. Normal fetal heart axis and position. Obstet Gynecol. 1987;70:255–259. [PubMed] [Google Scholar]
- 12.Chaoui R., Kalache K.D., Heling K.S., Tennstedt C., Bommer C., Körner H. Absent or hypoplastic thymus on ultrasound: a marker for deletion 22q11: 2 in fetal cardiac defects. Ultrasound Obstet Gynecol. 2002;20:546–552. doi: 10.1046/j.1469-0705.2002.00864.x. [DOI] [PubMed] [Google Scholar]
- 13.Bataeva R., Bellsham-revell H., Zidere V., Allan L.D. Reliability of fetal thymus measurement in prediction of 22q11.2 deletion: a retrospective study using four-dimensional spatiotemporal image correlation volumes. Ultrasound Obstet Gynecol. 2013;41:172–176. doi: 10.1002/uog.11194. [DOI] [PubMed] [Google Scholar]
- 14.Bronshtein M., Gover A., Zimmer E.Z. Sonographic definition of the fetal situs. Obstet Gynecol. 2002;99:1129–1130. doi: 10.1016/s0029-7844(02)02017-3. [DOI] [PubMed] [Google Scholar]
- 15.Huhta J.C., Smallhorn J.F., Macartney F.J. Two dimensional echocardiographic diagnosis of situs. Br Heart J. 1982;48:97–108. doi: 10.1136/hrt.48.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valsangiacomo E.R., Hornberger L.K., Barrea C., Smallhorn J.F., Partial Yoo S.J. total anomalous pulmonary venous connection in the fetus: two-dimensional and Doppler echocardiographic findings. Ultrasound Obstet Gynecol. 2003;22:257–263. doi: 10.1002/uog.214. [DOI] [PubMed] [Google Scholar]
- 17.Galindo A., Gutiérrez-Larraya F., Escribano D., Arbues J., Velasco J.M. Clinical significance of persistent left superior vena cava diagnosed in fetal life. Ultrasound Obstet Gynecol. 2007;30:152–161. doi: 10.1002/uog.4045. [DOI] [PubMed] [Google Scholar]
- 18.Van der Linde D., Konings E.E., Slager M.A. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:2241–2247. doi: 10.1016/j.jacc.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 19.Stallmeyer B., Fenge H., Nowak-Göttl U., Schulze-Bahr E. Mutational spectrum in the cardiac transcription factor gene NKX2. 5 (CSX) associated with congenital heart disease. Clin Genet. 2010;78:533–540. doi: 10.1111/j.1399-0004.2010.01422.x. [DOI] [PubMed] [Google Scholar]
- 20.Webb G., Gatzoulis M.A. Atrial septal defects in the adult. Circulation. 2006;114:1645–1653. doi: 10.1161/CIRCULATIONAHA.105.592055. [DOI] [PubMed] [Google Scholar]
- 21.Wilson A.D., Rao P.S., Aeschlimann S. Normal fetal foramen flap and transatrial Doppler velocity pattern. J Am Soc Echocardiogr. 1990;3:491–494. doi: 10.1016/s0894-7317(14)80366-0. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy K., Ho S., Anderson R. Defining the morphologic phenotypes of atrial septal defects and interatrial communications. Images Paediatr Cardiol. 2003;5:1–24. [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson R.H., Lenox C.C., Zuberbuhler J.R. The morphology of ventricular septal defects. Perspect Pediatr Pathol. 1984;8:235–268. [PubMed] [Google Scholar]
- 24.Lenz F., Machlitt A., Hartung J., Bollmann R., Chaoui R. Fetal pulmonary venous flow pattern is determined by left atrial pressure: report of two cases of left heart hypoplasia, one with patent and the other with closed interatrial communication. Ultrasound Obstet Gynecol. 2002;19:392–395. doi: 10.1046/j.1469-0705.2002.00684.x. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman J.I. Incidence of congenital heart disease: II. Prenatal incidence. Pediatr Cardiol. 1995;16:155–165. doi: 10.1007/BF00794186. [DOI] [PubMed] [Google Scholar]
- 26.Tuuli M.G., Dicke J.M., Stamilio D.M. Prevalence and likelihood ratios for aneuploidy in fetuses diagnosed prenatally with isolated congenital cardiac defects. Am J Obstet Gynecol. 2009;201(390):e1–e5. doi: 10.1016/j.ajog.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 27.Gómez O., Martínez J.M., Olivella A. Isolated ventricular septal defects in the era of advanced fetal echocardiography: risk of chromosomal anomalies and spontaneous closure rate from diagnosis to age of 1 year. Ultrasound Obstet Gynecol. 2014;43:65–71. doi: 10.1002/uog.12527. [DOI] [PubMed] [Google Scholar]
- 28.Birk E., Silverman N.H. Intracardiac shunt malformations. In: Yagel N.H., Silverman N.H., Gembruch U., editors. Fetal Cardiology. Informa Healthcare USA, Inc; New York, NY: 2009. pp. 285–290. [Google Scholar]
- 29.Rastelli G.C., Kirklin J.W., Titus J.L. Anatomic observations on complete form of common atrioventricular canal with special reference to atrioventricular valves. Mayo Clinic Proc. 1966;41:296. [PubMed] [Google Scholar]
- 30.Berger T.J., Blackstone E.H., Kirklin J.W. Survival and probability of cure without and with surgery in complete atrioventricular canal. Ann Thorac Surg. 1979;27:104–111. doi: 10.1016/s0003-4975(10)63249-3. [DOI] [PubMed] [Google Scholar]
- 31.ter Heide H., Thomson J.D., Wharton G.A., Gibbs J.L. Poor sensitivity of routine fetal anomaly ultrasound screening for antenatal detection of atrioventricular septal defect. Heart. 2004;90:916–917. doi: 10.1136/hrt.2003.018895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao P.S. A unified classification for tricuspid atresia. Am Heart J. 1980;99:799–804. doi: 10.1016/0002-8703(80)90632-8. [DOI] [PubMed] [Google Scholar]
- 33.Tegnander E., Williams W., Johansen O.J., Blaas H.G., Eik-Nes S.H. Prenatal detection of heart defects in a non-selected population of 30,149 fetuses–detection rates and outcome. Ultrasound Obstet Gynecol. 2006;27:252–265. doi: 10.1002/uog.2710. [DOI] [PubMed] [Google Scholar]
- 34.Sharland G.K., Chita S.K., Allan L.D. Tricuspid valve dysplasia or displacement in intrauterine life. J Am Coll Cardiol. 1991;117:810–816. doi: 10.1016/0735-1097(91)90877-c. [DOI] [PubMed] [Google Scholar]
- 35.Attenhofer Jost C.H., Connolly H.M., Dearani J.A., Edwards W.D., Gordon K. Ebstein’s anomaly. Circulation. 2007;115:277–285. doi: 10.1161/CIRCULATIONAHA.106.619338. [DOI] [PubMed] [Google Scholar]
- 36.Freud L.R., Escobar-Diaz M.C., Kalish B.T. Outcomes and predictors of perinatal mortality in fetuses with ebstein anomaly or tricuspid valve dysplasia in the current era: a multicenter study. Circulation. 2015;132:481–489. doi: 10.1161/CIRCULATIONAHA.115.015839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donofrio M.T., Levy R.J., Schuette J.J. Specialized delivery room planning for fetuses with critical congenital heart disease. Am J Cardiol. 2012;111:737–747. doi: 10.1016/j.amjcard.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 38.Danielson G.K., Maloney J.D., Devloo R.A. Surgical repair of Ebstein's anomaly. Mayo Clin Proc. 1979;54:185–192. [PubMed] [Google Scholar]
- 39.da Silva J.P., Baumgratz J.F., da Fonseca L. The cone reconstruction of the tricuspid valve in Ebstein's anomaly. The operation: early and midterm results. J Thorac Cardiovasc Surg. 2007;133:215–223. doi: 10.1016/j.jtcvs.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 40.Sharland G.K., Chita S.K., Fagg N.L. Left ventricular dysfunction in the fetus: relation to aortic valve anomalies and endocardial fibroelastosis. Br Heart J. 1991;66:419–424. doi: 10.1136/hrt.66.6.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hornberger L.K., Sanders S.P., Rein A.J., Spevak P.J., Parness I.A., Colan S.D. Left heart obstructive lesions and left ventricular growth in the midtrimester fetus. Circulation. 1995;92:1531–1538. doi: 10.1161/01.cir.92.6.1531. [DOI] [PubMed] [Google Scholar]
- 42.Chaturvedi R.R., Ryan G., Seed M., van Arsdell G., Jaeggi E.T. Fetal stenting of the atrial septum: technique and initial results in cardiac lesions with left atrial hypertension. Int J Cardiol. 2013;168:2029–2036. doi: 10.1016/j.ijcard.2013.01.173. [DOI] [PubMed] [Google Scholar]
- 43.Frommelt M.A. Challenges and controversies in fetal diagnosis and treatment: hypoplastic left heart syndrome. Clin Perinatol. 2014;41:787–798. doi: 10.1016/j.clp.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Yoo S.J., Golding F., Jaeggi E. Ventricular outflow tract anomalies: so-called conotruncal anomalies. In: Yagel S., Silverman N.H., Gembruch U., editors. Fetal Cardiology. Informa Healthcare USA, Inc; New York, NY: 2009. pp. 305–328. [Google Scholar]
- 45.Galindo A., Mendoza A., Arbues J., Grañeras A., Escribano D., Nieto O. Conotruncal anomalies in fetal life: accuracy of diagnosis, associated defects and outcome. Eur J Obstet Gynecol Reprod Biol. 2009;146:55–60. doi: 10.1016/j.ejogrb.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 46.Paladini D., Volpe P., Marasini M. Diagnosis, characterization and outcome of congenitally corrected transposition of the great arteries in the fetus: a multicenter series of 30 cases. Ultrasound Obstet Gynecol. 2006;27:281–285. doi: 10.1002/uog.2715. [DOI] [PubMed] [Google Scholar]
- 47.Yoo S.J., Lee Y.H., Kim E.S. Three-vessel view of the fetal upper mediastinum: an easy means of detecting abnormalities of the ventricular outflow tracts and great arteries during obstetric screening. Ultrasound Obstet Gynecol. 1997;9:173–182. doi: 10.1046/j.1469-0705.1997.09030173.x. [DOI] [PubMed] [Google Scholar]
- 48.Obler D., Juraszek A.L., Smoot L.B., Natowicz M.R. Double outlet right ventricle: aetiologies and associations. J Med Genet. 2008;45:481–497. doi: 10.1136/jmg.2008.057984. [DOI] [PubMed] [Google Scholar]
- 49.Van Praagh R., Van Praagh S. The anatomy of common aorticopulmonary trunk (truncus arteriosus communis) and its embryologic implications. A study of 57 necropsy cases. Am J Cardiol. 1965;16:406–425. doi: 10.1016/0002-9149(65)90732-0. [DOI] [PubMed] [Google Scholar]
- 50.Slodki M., Rychik J., Moszura T., Janiak K., Respondek-Liberska M. Measurement of the great vessels in the mediastinum could help distinguish true from false-positive coarctation of the aorta in the third trimester. J Ultrasound Med. 2009;28:1313–1317. doi: 10.7863/jum.2009.28.10.1313. [DOI] [PubMed] [Google Scholar]
- 51.Hornberger L.K., Sahn D.J., Kleinman C.S., Copel J., Silverman N.H. Antenatal diagnosis of coarctation of the aorta: a multicenter experience. J Am Coll Cardiol. 1994;23:417–423. doi: 10.1016/0735-1097(94)90429-4. [DOI] [PubMed] [Google Scholar]
- 52.Matsui H., Mellander M., Roughton M., Jicinska H., Gardiner H.M. Morphological and physiological predictors of fetal aortic coarctation. Circulation. 2008;118:1793–1801. doi: 10.1161/CIRCULATIONAHA.108.787598. [DOI] [PubMed] [Google Scholar]
- 53.Volpe P., Marasini M., Caruso G. 22q11 deletions in fetuses with malformations of the outflow tracts or interruption of the aortic arch: impact of additional ultrasound signs. Prenat Diagn. 2003;23:752–757. doi: 10.1002/pd.682. [DOI] [PubMed] [Google Scholar]
- 54.Galindo A., Nieto O., Nieto M.T. Prenatal diagnosis of right aortic arch: associated findings, pregnancy outcome, and clinical significance of vascular rings. Prenat Diagn. 2009;29:975–981. doi: 10.1002/pd.2327. [DOI] [PubMed] [Google Scholar]
- 55.Muñoz-Castellaños L., Espinola-Zavaleta N., Keirns C. Anatomoechocardiographic correlation double inlet left ventricle. J Am Soc Echocardiogr. 2005;18:237–243. doi: 10.1016/j.echo.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 56.Huhta J.C. Guidelines for the evaluation of heart failure in the fetus with or without hydrops. Pediatr Cardiol. 2004;25:274–286. doi: 10.1007/s00246-003-0591-3. [DOI] [PubMed] [Google Scholar]
- 57.Schneider C., McCrindle B.W., Carvalho J.S., Hornberger L.K., McCarthy K.P., Daubeney P.E. Development of Z-scores for fetal cardiac dimensions from echocardiography. Ultrasound Obstet Gynecol. 2005;26:599–605. doi: 10.1002/uog.2597. [DOI] [PubMed] [Google Scholar]
- 58.Quarello E., Stos B., Fermont L. Prenatal diagnosis of aorta coarctations. Gynecol Obstet Fertil. 2011;39:442–453. doi: 10.1016/j.gyobfe.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 59.Crispi F., Bijnens B., Figueras F. Gratacós Fetal growth restriction results in remodeled and less efficient hearts in children. Circulation. 2010;121:2427–2436. doi: 10.1161/CIRCULATIONAHA.110.937995. [DOI] [PubMed] [Google Scholar]
- 60.Pasquini L., Mellander M., Seale A. Z-scores of the fetal aortic isthmus and duct: an aid to assessing arch hypoplasia. Ultrasound Obstet Gynecol. 2007;29:628–633. doi: 10.1002/uog.4021. [DOI] [PubMed] [Google Scholar]