Abstract
Amid revolutionary changes in toxicity assessment brought about by increasing regulation of chemicals, adverse outcome pathways (AOPs) have emerged as a useful framework to assess adverse effect of chemicals using molecular level effect, which aid in setting environmental regulation policies. AOPs are biological maps that describe mechanisms linking molecular initiating event to adverse outcomes (AOs) at an individual level. Each AOP consists of a molecular initiating event, key events, and an AO. AOPs use molecular markers to predict endpoints currently used in risk assessment, promote alternatives to animal model-based test methods, and provide scientific explanations for the effects of chemical exposures. Moreover, AOPs enhance certainty in interpreting existing and new information. The application of AOPs in chemical toxicity testing will help shift the existing paradigm of chemical management based on apical endpoints toward active application of in silico and in vitro data.
Keywords: Adverse outcome pathway, Risk assessment, Chemical management, Alternative test methods, Molecular markers
INTRODUCTION
The chemical industry has grown markedly worldwide since the 1970s, as the use of chemical products has become an integral part of everyday life. The cost of chemical production worldwide has increased from USD 171 billion in 1970 to USD 4.12 trillion in 2010 [1]. The use of chemicals and chemical products has increased so much that the number of substances marketed in volumes greater than ten tons per year easily exceeded 10 000 by 2007 [2]. Since 2007, the European Union (EU) has implemented the Registration, Evaluation, Authorisation & Restriction of Chemicals (REACH) policy based on and ‘Precautionary’ principle. As the EU’s reinforced chemical toxicity testing policies are adopted internationally, demand for toxicity testing on chemicals is growing worldwide.
Despite this growing need for toxicity assessment, the burgeoning numbers of animals killed to address this need is causing increasing ethical controversy. According to the statistical report on animals used for experimental and other scientific purposes published by the European Commission (EC) in 2013, the number of animals used in experiments during the year 2011 was well over 11.5 million, with a particularly remarkable rate of rodent use [3]. This has led to a growing interest in methods that offer alternatives to animal testing, to minimize the number of animals used. Alternative test methods are in accord with the principle of the ‘3Rs’, which British zoologist WM Russell and microbiologist RL Burch presented in “The principles of humane experimental technique” in 1959. The principle underlying the 3Rs is the goal of developing toxicity testing methods that reduce the number of animals used for toxicity testing (Reduction), refine experimental methods to minimize pain and stress applied to animals on occasions necessitating animal testing (Refinement), and replace vertebrates with lower animals thought to feel little pain compared to vertebrates, or apply in silico methods, such as quantitative structure-activity relationships (QSAR) for toxicity assessment (Replacement) [4]. Since 2013, the EU has banned animal testing for all cosmetic materials following the EU cosmetic directives (7th amendment) (76/768/EEC), and totally banned the sale of animaltested cosmetic substances or products in the European market since 2013. As a result of these trends, the demand for alternative methods of testing chemical toxicity is markedly increasing.
In response to the increasing demand, the US National Research Council published a report suggesting a new vision for 21st century toxicity research entitled “Toxicity testing in the 21th century: a vision and a strategy” in 2007. This report suggests a new approach that applies novel emerging technologies to existing toxicity assessments, and provides a vision for future toxicity assessments. It suggests replacing expensive and time intensive animal testing with analysis of toxicity pathways in human cells using automated high throughput screening, and using advanced methodologies such as toxicogenomics, bioinformatics, systems biology, and computational toxicology [5]. This new approach has resulted in revolutionary changes in the field of toxicity testing. Amid these changes, adverse outcome pathways (AOPs) have emerged as a new framework to predict apical toxic outcome using molecular level effects.
THE CONCEPT AND DEVELOPMENT OF ADVERSE OUTCOME PATHWAYS
Concept of Adverse Outcome Pathways
AOP is a framework introduced to apply molecular markers to risk assessment and regulation policies. It is a biological map describing toxicity mechanisms from molecular initiating event to apical adverse outcome (AO), as observed at the individual level. AOPs consist of a molecular initiating event (MIE), one or more key events (KEs), and an AO (Figure 1) [6].
Figure 1.
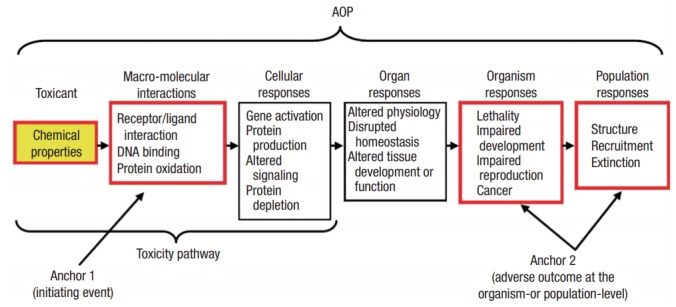
Adverse outcome pathways (AOPs) framework. From Ankley GT et al. Environ Toxicol Chem 2010;29(3):730-741 [7].
MIE is an specialized type of KE that represents the interaction between a chemical and its biological target(s) at the molecular level. It refers to an biological event which initiates a toxicity pathway (e.g., DNA recombination, protein oxidation, or receptor-ligand interaction). KEs are biological events (e.g., gene activation, altered cell chemistry, and tissue development) that occur in response to an MIE at various biological levels (cell, tissue, and organ) leading to a specific AO. The series of events are interconnected through the KE relationship (KER). AO is an outcome (e.g., death, reproductive disorders, cancer, or extinction) resulting from MIE and its associated KE(s) at the individual and/or population level. It is relevant to regulatory decision-making (human health or ecological risk assessment). Implementation of the AOP concept facilitates toxicity assessment on an entire group of chemical substances that act through the same MIE/KEs in AOP.
Development and Testing of Adverse Outcome Pathway
Development of AOPs is followed bottom-up approach in which a phenomenon measured at in chemico or in vitro level is associated with an effect observed at in vivo level. AOP can be developed by 1) linking MIE and KE to observed final AO, or 2) predicting the AO from known MIE and KE. In developing an AOP, structural information of chemicals that can initiate a pathway, or in vivo tests results, which measure the endpoints directly associated with an AO can be used. MIE must be precisely defined, as this is the basic stage and anchor point of AOP. The MIE is usually defined in the form of a receptor interaction, a protein (enzyme) interaction, or a DNA interaction. The collection of KEs between the MIE and the AO is referred to as a response matrix, and the AOP becomes more complex as the number of KEs increases. Understanding of the physiological pathway of an AOP is fundamental for identifying KEs. As information on KEs can be obtained by reviewing existing literature, literature reviews are crucial to AOP development. KE should be reliable and relevant to the given AO, and can be experimentally assessed. The AO can be defined at varying levels including cell, tissue, organ, system, individual, population, and ecosystem [6].
The top-down approach that identifies MIE starting with an AO, the bottom-up approach that links to an AO starting from a well-known MIE, and the middle-out approach that starts from the KE of an observed biological effect to find links to an AO are all possible for AOP development. AOPs can also be developed through case study that apply an AOP previously established for a thoroughly researched chemical to another chemical, analogy that apply an established AOP for a specific animal or taxon model to the another organism, and datamining that identifies KER based on high-throughput data such as omics [8].
Several principles apply to AOP development [6]:
1) AOPs are not chemical-specific. An interaction with a chemical generally occurs only in the MIE, not the KEs or the AO. So if any chemical causes a particular MIE, it can be assumed that it will lead to the AO through the AOP. In cases in which an MIE is dependent on the chemical properties of specific chemicals, the utility of an AOP can be limited.
2) AOPs consist of modules. In order to apply an AOP to risk assessment, it should be clear, easy to understand, and easy to apply, and its applicability should be flexible and wide-ranging. KEs and KERs are biological phenomena. Thus, they may be in multiple AOPs rather than just one. KEs and KERs needs to be established which facilitates combination of suitable KEs and KERs in AOP development in order to develop AOPs in an efficient manner.
3) In practice, an AOP network is a functional unit. Even though an individual AOP is the simplest unit, chemicals which yield a single MIE are rare. Therefore, AOP networks that link various AOPs that share the same KEs and KERs should be considered, to better predict a chemical’s overall toxic effect.
AOPs developed based on these principles undergo assessment that evaluates empirical evidence to establish its reliability. AOP validation is a process that analyzes qualitative and quantitative relationships of AOPs, including dose-response relationships, the strength of association between KEs and AO, and between AO and MIE, the biological plausibility of empirical evidence, and uncertainties. In the end, the reliability of an AOP is established by mechanical understanding of biological responses and general understanding of the nature of the interaction between a chemical and the biological systems it contacts [6].
INTERNATIONAL EFFORT FOR ADVERSE OUTCOME PATHWAY DEVELOPMENT
History of Adverse Outcome Pathway Development
AOP framework were established at the Pellston workshop (an expert workshop organized by the Society of Environmental Toxicology and Chemistry [SETAC]) in Oregon, 2009. The AOP concept introduced in the workshop aimed to lead conventional ecological risk assessment to the 21st century toxicity testing methods [9]. The AOP framework has been developed even further since the workshop. It has gained recognition in multiple fields of science as a powerful approach to chemical risk assessment. The Organization for Economic Cooperation and Development (OECD) later initiated an international AOP development program, and the first OECD guidelines for AOP development and evaluation were published in 2013 [6].
AOPs have been further developed for application to general toxicology and chemical regulations at a workshop hosted in Somma, Italy in 2014. The concept of the AOP, originally introduced to the ecotoxicity field, was expanded to cover human toxicity. Evaluation of network and mixture of AOP were also discussed for the application to risk assessment. The importance of a shared role by government, industry, and academia was declared with respect to AOP development and application. This declaration strongly promoted the application of AOPs [10].
AOP development continues to achieve remarkable progress through various expert workshops including one that discussed the potential application of omics data to AOPs for environmental risk assessment in 2014, and the 2017 SETAC Pellston workshop, which presented the development of AOPs using the horizon scanning approach.
Organization for Economic Cooperation and Development-led Adverse Outcome Pathway Development
Since the 2012 publication of the first OECD report ‘Adverse Outcome Pathway (AOP) for Skin Sensitisation Initiated by Covalent Binding to Proteins,’ the OECD has been leading AOP R&D, and continues to publish articles promoting AOP development (Table 1). The draft of the first OECD guidelines for AOP development and evaluation was published in 2013. These guidelines offer basic knowledge of AOPs, including information required for examining and documenting AOPs, and instructions for AOP evaluation. The OECD published a report supplementing existing guidelines, which reflected feedback on the initial publication, and included a final AOP assessed by the OECD in 2016. According to the OECD AOP development programme workplan updated in March 2018, 61 currently registered projects are in progress. These include 56 AOP development projects, 1 project creating guidelines for AOP development and evaluation, 3 projects developing a knowledge management tool, and 1 other project [11].
Table 1.
AOP-related documents published by the OECD
| Category | Year | Title |
|---|---|---|
| Series on testing and assessment | 2012 | The AOP for skin sensitization initiated by covalent binding to proteins (series on Testing and Assessment No. 168) |
| 2013 | Guidance document on developing and assessing AOPs (series on Testing and Assessment No. 184) | |
| Series on AOPs | 2016 | Users' handbook supplement to the guidance document for developing and assessing AOPs (OECD series on AOPs No. 1) |
| 2016 | AOP on protein alkylation leading to liver fibrosis (OECD series on AOPs No. 2) | |
| 2016 | AOP on alkylation of DNA in male pre-meiotic germ cells leading to heritable mutations (OECD series on AOPs No. 3) | |
| 2016 | AOP on aromatase inhibition leading to reproductive dysfunction (in fish) (OECD series on AOPs No. 4) | |
| 2016 | AOP on chronic binding of antagonist to N-methyl-D-aspartate receptors during brain development induces impairment of learning and memory abilities (OECD series on AOPs No. 5) | |
| 2016 | AOP on binding of agonists to ionotropic glutamate receptors in adult brain leading to excitotoxicity that mediates neuro nal cell death, contributing to learning and memory impairment (OECD series on AOPs No. 6) |
AOP, adverse outcome pathway; OECD, Organization for Economic Cooperation and Development.
Adverse Outcome Pathway-Knowledge Base
Among the areas of AOP R&D led by the OECD, one of the most important activities is the establishment of an AOP knowledge base (AOP-KB). The OECD established the AOP-KB in collaboration with the EU Reference Laboratory for alternatives to animal testing and the US Environmental Protection Agency (EPA) in 2014 to further promote development of AOPs by facilitating the collection of existing knowledge. The AOP-KB is a platform that collects effect data in toxicity studies, and combines this data to produce quantitative relationships between KEs. The ultimate goal of establishing the AOP-KB is to achieve a comprehensive collection of resources available for dissemination of AOPs meeting international standards.
The AOP-KB (https://aopkb.oecd.org/) consists of AOP Wiki, Effectopedia, Intermediate Effects Database and AOP Xplorer (Figure 2), and offers a search function for AOPs registered on e.AOP.Portal (https://aopkb.oecd.org/search.ashx). Currently, AOP Wiki is officially in operation. The beta version of Effectopedia has been made public. The Intermediate Effects Database and AOP Xplorer are in an early phase of development.
Figure 2.
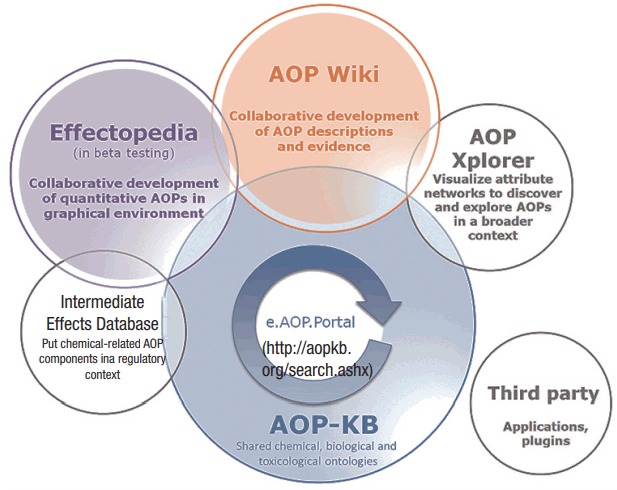
AOP-KB (https://aopkb.oecd.org/). AOP, adverse outcome pathway; KB, knowledge base.
AOP Wiki (https://aopwiki.org/) was launched in 2014 through the collaboration of the OECD, the EU, and the US EPA. AOP Wiki is a module that encourages collaboration between researchers, and maximizes efficacy of collaborative AOP development efforts. It is a web-based open source tool for consistent and effective collection of AOP information needed for risk assessment. It allows users to easily bring existing knowledge or published research information to an AOP using the familiar Wiki-based interface, and facilitates free exchange of opinion on registered AOPs.
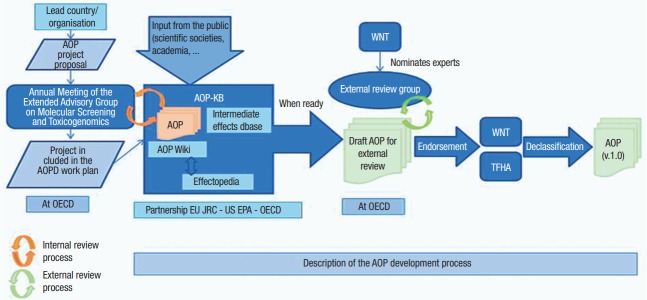
The AOP development program by the OECD is managed by OECD Extended Advisory Group on Molecular Screening and Toxicogenomics (EAGMST), and AOP Wiki is managed by the OECD EAGMST and Society for the Advancement of Adverse Outcome Pathways (SAAOP). The SAAOP is a mediating organization that promotes consistency between AOPs registered on AOP Wiki and the AOP instructions and guidelines published by the OECD. Once an AOP is registered on AOP Wiki, the AOP is supplemented through review by the SAAOP and EAGMST. The AOP finally approved by the EAGMST undergoes review by the OECD’s Working Group of National Coordinators of the Test Guidelines Programme and Task Force for Hazard Assessment (Figure 3) [12].
Figure 3.
OECD AOP development process [12]. OECD, Organization for Economic Cooperation and Development; AOPD, Adverse Outcome Pathway Development; EU, European Union; JRC, Joint Research Centre; EPA, Environmental Protection Agency; AOP, adverse outcome pathway; KB, knowledge base; WNT, Working Group of the National Coordinators of the Test Guidelines Programme; TFHA, Task Force on Hazard Assessment.
As of March, 2018, 212 AOPs were registered on AOP Wiki: 27 AOPs were under evaluation, and 185 were under development. The information on AOP Wiki is updated in real time. Of the 212 registered AOPs, 76 are on ecotoxicity, 135 are on human toxicity, and one is listed as “other”. Recently, AOP development has focused on the human toxicity (Table 2).
Table 2.
Status of AOPs in AOP Wiki list1
| Status | n | OECD program |
|---|---|---|
| AOPs ready for commenting | ||
| TFHA/WNT endorsed | 6 | Included |
| EAGMST approved | 3 | Included |
| EAGMST under review | 18 | Included |
| AOPs under development | ||
| EAGMST | 27 | Included |
| SAAOP | 149 | Not included |
| Recent AOP | 9 | Not included |
AOP, adverse outcome pathway; TFHA, Task Force for Hazard Assessment; WNT, Working Group of the National Coordinators of the Test Guidelines Programme; EAGMST, Extended Advisory Group on Molecular Screening and Toxicogenomics; SAAOP, Society for the Advancement of Adverse Outcome Pathways.
From: https://aopwiki.org/ [updated in March 2018].
Effectopedia (https://www.effectopedia.org) is a tool for public knowledge collection and application for the development of quantitative AOPs. It was designed by the International QSAR Foundation in 2006 to overcome the technical barrier of QSAR concerning in vivo risk prediction. The OECD is currently developing it with the support of the EC. A total of 20 AOPs are currently registered. Users developing new AOPs can easily add all components including chemicals, MIE, KEs, KERs, and AO. Relationships between components can be quantitatively analyzed by entering empirical data on each component. Effectopedia, a graphic editor that visualizes all causes and effects within AOPs, could serve as a platform to develop and model quantitative AOP [13].
POTENTIAL OF ADVERSE OUTCOME PATHWAY IN CHEMICAL MANAGEMENT
At varying biological levels, accurately described AOPs provide mechanical information applicable to versatile purposes. When a specific KE is scientifically verified, an AOP stimulates development of in vitro and ex vivo methods to analyze the event in a direct manner, thus contributing to the OECD Test Guideline Programme. Linking such methods will eventually lead to development of a testing method applicable to regulatory purposes. AOPs enable toxicity assessment on an entire chemical group acting through the same MIE. It can be used to develop strategies to obtain maximum useful information by establishing chemical categories and structure-activity relationships with minimum experiments. AOPs including nonspecies-specific KEs can be applied to a wide range of taxa (vertebrates and invertebrates). Thus, chemical risk assessment for a variety of species based on a given toxicity mechanism will soon be possible [14].
CONCLUSION
The concept of the AOP was developed as a framework to apply molecular markers to the risk assessment of chemical substances. Application of AOPs to chemical management will increase the use of nonanimal alternative test methods in risk assessment. This will be a milestone that shifts the current paradigm of existing chemical management based on apical endpoints towards active use of in silico and in vitro data. Ultimately, it is expected to bring a paradigm shift leading to more effective chemical management.
Acknowledgments
This study was funded by the Korean Ministry of Environment through ‘Environmental Health R&D Program’ (2017001370001).
Footnotes
The authors have no conflicts of interest associated with the material presented in this paper.
Supplementary Material
Supplementary Material: Korean version is available at http://www.e-eht.org/
REFERENCES
- 1.United Nations Environment Program Global chemicals outlook: towards sound management of chemicals. 2013 [cited 2018 Jan 15]. Available from: https://sustainabledevelopment.un.org/index.php?page=view&type=400&nr=1966&menu=35.
- 2.Innovest Strategic Value Advisors Overview of the chemicals industry: overview for coming clean. 2007 [cited 2018 Jan 15]. Available from: http://www.precaution.org/lib/07/innovest.pdf.
- 3.European Commission Seventh report on the statistics on the number of animals used for experimental and other scientific purposes in the member states of the European Union. [cited 2018 Jan 15]. Available from: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52013DC0859.
- 4.Liebsch B, Grune B, Seiler A, Butzke D, Oelgeschläger M, Pirow R, et al. Alternatives to animal testing: current status and future perspectives. Arch Toxicol. 2011;85(8):841–858. doi: 10.1007/s00204-011-0718-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibb S. Toxicity testing in the 21st century: a vision and a strategy. Reprod Toxicol. 2008;25(1):136–138. doi: 10.1016/j.reprotox.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Organization for Economic Cooperation and Development . Guidance document on developing and assessing adverse outcome pathways. Paris: Organization for Economic Cooperation and Development; 2013. pp. 9–20. [Google Scholar]
- 7.Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, et al. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 2010;29(3):730–741. doi: 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- 8.Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, et al. Adverse outcome pathway (AOP) development I: strategies and principles. Toxicol Sci. 2014;142(2):312–320. doi: 10.1093/toxsci/kfu199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villeneuve DL, Garcia-Reyero N. Vision & strategy: predictive ecotoxicology in the 21st century. Environ Toxicol Chem. 2011;30(1):1–8. doi: 10.1002/etc.396. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Reyero N. Are adverse outcome pathways here to stay? Environ Sci Technol. 2015;49(1):3–9. doi: 10.1021/es504976d. [DOI] [PubMed] [Google Scholar]
- 11.Organisation for Economic Cooperation and Development The adverse outcome pathways development programme workplan. [cited 2017 Jun 20]. Available from: http://www.oecd.org/chemicalsafety/testing/projects-adverse-outcome-pathways.htm.
- 12.Organisation for Economic Cooperation and Development Adverse outcome pathways, molecular screening and toxicogenomics molecular screening and toxicogenomics. [cited 2017 Jun 20]. Available from: http://www.oecd.org/chemicalsafety/testing/adverse-outcome-pathways-molecular-screening-and-toxicogenomics.htm.
- 13.Aladjov H. Effectopedia: open research platform for AOP knowledge integration and use. 2015 [cited 2018 Jan 15]. Available from: http://www.opentox.net/events/opentoxeuro-2015/session7/effectopedia-knowledge-integration.
- 14.Kramer VJ, Etterson MA, Hecker M, Murphy CA, Roesijadi G, Spade DJ, et al. Adverse outcome pathways and ecological risk assessment: bridging to population-level effects. Environ Toxicol Chem. 2011;30(1):64–76. doi: 10.1002/etc.375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material: Korean version is available at http://www.e-eht.org/