Abstract
Stress cardiomyopathy (SC) typically presents as potential acute coronary syndrome (ACS) in previously healthy people. While there may be physical or mental stressors, the initial symptom is usually chest pain. This form conforms to the published Mayo diagnostic criteria, is well reported and as the presentation is initially cardiac, is considered primary SC. Increasingly we see SC develop several days into the hospitalization secondary to medical or surgical critical illness. This condition is more complex, presents atypically, is not easy to recognize and carries a much worse prognosis. Label of Secondary SC is appropriate as it manifests in sicker hospitalized patients with numerous comorbidities. We review the limited but provocative literature pertinent to SC in the critically ill and describe important clues to identify global, subclinical and probable forms of SC. We illustrate the several unique clinical features, demographic differences and propose a diagnostic algorithm to optimize cardiac care in the critically ill.
1. Introduction
Stress cardiomyopathy (SC) was first described in Japanese literature in 1990.1 The number of publications about this condition has exploded over the last 2 decades.2, 3, 4 As there are several causes and morphological variants, our understanding of SC is continuing to evolve. SC occurs much more frequently in the critically ill than the medical community recognizes.5, 6 The diagnosis of SC is challenging in the critically ill and does not conform to the published criteria. Several important critical care publications evaluating a spectrum of cardiac abnormalities over the last few decades have labelled SC variably, thus significantly limiting our current understanding. We offer a provocative broader understanding of SC gleaned from the published critical care literature and cardiac imaging studies to optimize the diagnosis of SC in the critically ill.
2. Defining SC
Originally described as Tako-tsubo cardiomyopathy (TC) and apical ballooning syndrome (ABS), we have subsequently included many morphological variants like basal and mid-ventricular forms under the umbrella of SC.7, 8 There are still no definite criteria for the diagnosis of Stress Cardiomyopathy. The pathogenesis remains unknown and thus the diagnosis is not definitive for the majority of patients.
Based on our evolving understanding, the following clinical framework appears to encompass the broad spectrum of SC: Stress cardiomyopathy (SC) is acute reduction in cardiac function oftentimes due to mental or physical stress with spontaneous complete normalization of cardiac function within days to weeks. This describes a clinical phenomenon that arises from a variety of causes and a myriad of clinical presentations but overlaps with some specific causes of cardiac dysfunction.9
The emphasis of this simple definition of SC is on regional wall motion abnormality (RWMA) and its spontaneous recovery. The clinical presentation can vary from asymptomatic to crushing substernal pain and cardiogenic shock. Electrocardiogram (ECG) may include a wide range of abnormalities from sinus tachycardia, ventricular ectopy, ischemic ST depressions and deep T inversions to ST elevation myocardial infarction (STEMI) patterns.10 Mayo criteria for SC diagnosis requires coronary angiogram to confirm absence of culprit lesions that may account for the RWMA.7, 11 Our interest is in early non-invasive diagnosis of SC in the critically ill. We have reviewed the various etiologies of cardiac dysfunction in the critically ill12 and highlighted management issues specific to the critically ill.13, 14 We routinely encounter potential SC in various ICU settings and have published our algorithm to definitively diagnose SC without catheterization.9 This requires clinical suspicion, good quality echocardiographic windows to characterize the RWMA and repeat echocardiogram in about 5–7 days to confirm normalization/improvement of cardiac function. Recently, the European society of cardiology has published a position statement where they have differentiated primary SC presenting to the ER with chest pain from secondary takotsubo syndrome that develops during the course of hospitalization for another medical, surgical, anaesthetic, obstetric, or psychiatric condition.15
3. Literature
There has been an exponential growth in the publications on SC over the last 15 years. Between 2004 and 2014 over half a dozen criteria were published to diagnose SC. The widely used 2004 Mayo criteria were based on 16 patients with ‘chest pain – potential ACS’ where SC was diagnosed only after catheterization excluded CAD.11 In 2008 Mayo expanded their criteria to include non-ABS variants and patients with SC due to neurological events.7 All the published criteria over the past decade stem from clinical experience of high volume centers describing patients presenting with cardiac symptoms initially and undergoing catheterization for potential ACS.16, 17, 18, 19 Thus, all the studies have performed catheterization to exclude ACS and base the diagnosis of SC on excluding CAD. NT-proBNP elevation appears to be more in SC and the ratio of troponin I to LV EF may help differentiate SC from ACS.20, 21 These studies were not been performed in the critically ill and thus may not carry he same diagnostic utility in this population. Higher levels of Interleukin (IL)-6, IL-10 and carbohydrate-antigen (CA)-125 are associate with a more complicated hospital course and risk for recurrence of SC. These inflammatory and cancer biomarkers may be valuable risk markers in SC.22, 23
We do not have any published diagnostic criteria for secondary SC that develops during the course of medical or surgical critical illness. Extrapolating the 2008 revised Mayo criteria to the critically ill sepsis, surgical and neurological disease populations is difficult and does not serve this population well. Our experience suggests these SC patients will be served better if the emphasis shifts to echocardiographic RWMA recognition instead of relying on catheterization for the diagnosis.9, 24 This non-invasive diagnosis requires clinical suspicion and is imperative in the critically ill given the limitations of catheterization.
3.1. InterTAK registry
The InterTAK registry is the first international multicenter effort to gather data about SC systematically.8 This registry significantly improves our understanding of SC and its variants. In nearly 44% a physical trigger was identified as potential cause of SC. SC can be fatal as shown by InterTAK, where 4% mortality was noted. For at least 3 reasons, we believe the InterTAK data does not represent the full spectrum of SC, especially the SC developing in critical care settings. Most importantly, the registry inclusion is based on fulfilling the 2008 modified Mayo criteria for SC. As this requires catheterization to exclude CAD, many critically ill SC patients might not undergo catheterization due to their comorbidities and thus fail to qualify for the registry. Secondly, presenting symptoms were uniformly cardiac −namely chest pain (75.9%), dyspnea (46.9%) and syncope (7.7%). In critical care setting, chest pain is not common and majority of SC patients are recognized due to troponin elevation, hypotension, heart failure or tachyarrhythmias. Lastly, among the 1750 SC patients in this registry, global LV dysfunction was not recognized as a variant of SC. This is due to sampling issues and the inclusion criteria requiring catheterization. We believe the 26 collaborating cardiovascular centers in the registry collected their data from cardiology- related hospitalizations for chest pain and catheterization laboratory database of potential ACS. InterTAK is the best available literature currently to understand the clinical presentation and outcomes in SC from the cardiologist viewpoint. However, InterTAK does not adequately address SC that occurs secondarily in the critically ill.
3.2. McMaster series
Lim et al. from McMaster University have published several studies over the last 10 years addressing cardiac injury in the critically ill.25, 26, 27, 28, 29, 30, 31 Troponin levels were followed systematically through the hospitalization and correlated with ECG changes, clinical picture and hemodynamic alterations. Along the lines of several similar but smaller studies over the last 2 decades, the McMaster experience also estimates that troponin elevation occurs in about half of all critically ill patients. Their 2010 series evaluated 103 patients and identified 49 patients with elevated troponins.28 Authors evaluated patient charts for secondary causes of troponin elevation, namely sepsis (n = 9), left ventricular hypertrophy/strain, intracranial hemorrhage/stroke, cardiac contusion/cardiopulmonary resuscitation (n = 3), cardiac infiltrative disorders, chemotherapy, myocarditis, pericarditis, cardiac surgery, congestive heart failure (n = 2), cardiomyopathy, pulmonary embolism/pulmonary hypertension, chronic obstructive pulmonary disease (n = 3) and renal failure (n = 6). Based on troponin patterns, type II MI (due to hypotension, hypovolemia, supraventricular tachycardia, severe anemia or vasospasm) was identified in 10 (38% of MI) patients. Type I MI due to plaque rupture was determined to have caused the troponin elevation in 16 (62%) patients. A limitation of the series is that the authors did not consider SC in their differential for troponin elevation. Based on the study design and comorbidities of the patients, we suspect SC accounted for a significant portion of the type II MI, resuscitation and COPD groups. As catheterization was not performed in majority of patients, it is likely that some of the troponin elevations attributed to ‘plaque rupture’ also represent SC. This series highlights the adverse prognostic implications of troponin elevation and difficulties in catheterizing critically ill patients. This series also underscores the limited recognition of SC among providers and researchers in critical care.
3.3. Critical care literature
Unlike the exponentially growing literature in the classically defined SC, the disease in the critically ill remains largely unstudied. Absence of set diagnostic criteria for SC in the critically ill is only the beginning of the problem. Symptoms are atypical and difficult to elicit. Arrhythmias and hemodynamic changes may be attributed to underlying illness, medications and metabolic derangements. Often Cardiologists are not involved early in the care or imaging of these patients. The limited applicability of cardiac catheterization in these settings leaves SC among many potential diagnoses despite suggestive enzyme elevations or wall motion abnormalities. Some of the literature regarding sepsis induced cardiomyopathy dates back 40 years and various investigators have dealt with this entity differently with regard to imaging modality and study parameters.32, 33, 34 We will retrace the available literature to glean some understanding of the scope of SC in the critical care setting.
Troponin elevation occurs in about 30–50% of critically ill patients.35, 36 While overall mortality was 27%, subgroup with troponin elevation had a much higher mortality of 51% compared to 16% among patients without troponin elevation.37 Maeder et al. reviewed the literature regarding troponin elevation in sepsis and came to similar conclusions.38 Elevated troponin levels in patients with sepsis indicate a higher severity of disease, the presence of myocardial dysfunction, and a worse prognosis. It is debatable if this troponin leak and cardiac injury is directly causative or just a marker for mortality. Echocardiographic studies of LV function in septic shock are limited and offer little insight into left ventricular RWMA39, 40 and available only for a very small number of patients.41 In a recent echocardiographic series of 106 severe sepsis or septic shock patients, 62% demonstrated cardiac dysfunction. Left ventricular diastolic dysfunction was present in 39 patients (37%), LV systolic dysfunction in 29 (27%), and RV dysfunction in 33 (31%). Reimaging within 5 days among survivors documented significant improvement in cardiac function. Wall motion abnormalities were not specifically reported in the group with LV systolic dysfunction.42 Thus we have not separated sepsis-induced cardiomyopathy in further discussion of SC in the critically ill.
Bailen et al. serially performed echocardiograms in critically ill patients without heart disease.43 They excluded sepsis and identified 33 patients with median initial left ventricular ejection fraction of 0.34 [range, 0.16–0.48]. Segmental contractility disturbances were detected initially in all patients and also normalized with time. All patients presented with electrocardiogram changes that normalized in line with the echocardiographic changes. ECG changes included ST depression in 11 patients (1–3 mm), ST elevation in 13 (1–4 mm), Q wave in 6, left bundle branch block in one, T-wave inversion in 15, a peaked T wave in 3 and a lengthening of the QTc interval in 5. Coronary angiography was performed in seven patients and was normal.
Park et al. from Seoul, South Korea defined LV apical ballooning (LVAB) as symmetric severe hypokinesia or akinesia of the left ventricular wall, except for the basal part of the left ventricle, with a <50% ejection fraction.44 They performed serial echocardiograms in 92 consecutive medical ICU patients on the day of ICU admission, and on the third and seventh days in the hospital stay. LVAB was observed in 26 patients (28%). Sepsis was the reason for hospitalization in 16 (61%) and hypoxic/hypercarbic respiratory failure accounted for the remaining 10 (39%) patients. The mean lowest ejection fraction was 33 ± 8% (range, 19–46%). Importantly at the time of this study in 2003, basal and mid ventricular variants of SC were yet undescribed and are likely to have been missed. LVAB patients had a higher frequency of sepsis, a higher prevalence of hypotension on ICU admission, more frequent use of inotropic agents, and a higher frequency of cardiomegaly and pulmonary edema. Mortality at 2 months was nearly doubled (48%) in LVAB patients compared to 29% in ICU patients without LVAB.
Subsequently, Haghi from Germany identified SC in ICU setting in 6 (30%) of 20 consecutive patients.6 Average age was 68 years and half the patients were men. They all had classical apical ballooning that improved over days. Interestingly 5 of these 6 patients presented initially with hypotension or shock and one patient had giant T inversion on routine ECG. Catheterization was done to exclude CAD in all 6 patients.
Marcelino et al. from Portugal routinely performed transthoracic echocardiograms (TTE) on all ICU patients.45 TTE was performed by ICU physicians within the first 24 h of admission on 704 consecutive patients. Echocardiographic abnormalities were detected in 234 (33%) patients. Severe, previously unknown echocardiographic diagnoses were detected in 53 (7.5%) patients. Severe LV dysfunction was detected in 23 patients. The wall motion abnormalities were not further characterized in this study but none of these patients had myocardial ischemia. We can infer that majority of these patients indeed had SC. Atypical forms of SC may occur in younger age ranges due to acute neurological processes like acute multiple sclerosis.46 Inflammation and sympathetic over activity are likely involved in the causation of SC in the critically ill.
4. Global SC
Several case series suggest that global LV dysfunction without any focal wall motion abnormalities may be another manifestation of SC. Belcour et al. evaluated the prevalence of SC after convulsive status epilepticus in 32 (21 men) ICU patients.47 Serial echocardiograms were performed at ICU admission and after 6, 12, 24, and 48 h of hospitalization. They defined SC as a 20% decrease in left ventricular ejection fraction noted initially and this recovered by 48 h. SC was diagnosed in 18 patients (56%) with mean left ventricular ejection fraction of 45 ± 14%. Global hypokinesia occurred in 15 patients and regional wall motion abnormalities were noted only in 3 patients. Catheterization was not routinely performed but was negative in the 3 patients with regional wall motion abnormalities. This series highlights an aspect of SC that is not addressed in the large InterTAK registry, namely global SC. InterTAK does not describe global SC in any of their patients as their methodology limits enrolling secondary SC patients.
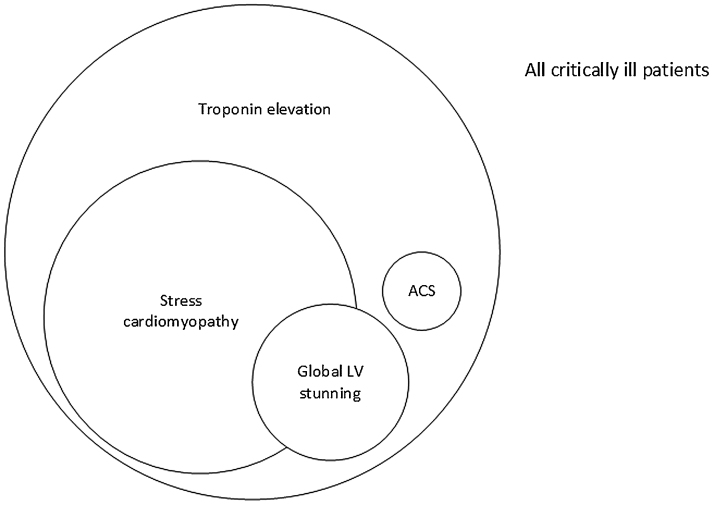
The major cardiovascular causes of reversible LV dysfunction are tachyarrhythmias, myocarditis, hypertensive emergency and following cardiac arrest- cardiopulmonary resuscitation. Metabolic derangements due to sepsis, septic shock, severe acidosis, electrolyte abnormalities and toxin exposure may result in transient global LV dysfunction.12, 48 We can argue that these ‘physical stressors’ have caused SC. On the other hand, some believe sepsis related global LV dysfunction is a specific entity.49, 50, 51 Due to the numerous etiologies and absence of peculiar wall motion patterns, this form of SC is the most difficult to recognize. Using our broad definition for SC as any stress related acute LV dysfunction that spontaneously reverses, we believe many of the above etiologies of global LV dysfunction, including septic shock, would come within the broader umbrella of SC. Fig. 1 illustrates our current understanding of the prevalence of troponin elevation, the real scope of SC and global LV stunning in the critically ill.
Fig. 1.
Stress cardiomyopathy likely accounts for the largest portion of critically ill patients with cardiac injury (troponin elevation). Many patients with global stunning likely have a form of SC as well. True ACS is rare in the critically ill. RWMA indicates regional wall motion abnormalities; and VT, ventricular tachycardia.
5. Primary vs secondary SC
A significant majority of the publications describing tako-tsubo cardiomyopathy, atypical variants as well as the InterTAK registry would fit criteria for primary SC.7, 8, 15 Community dwelling people in otherwise reasonable health, due to acute physical or mental stress, develop cardiac symptoms like angina, dyspnea or palpitation. Clinical presentation mimics ACS with ECG ischemic ST T wave changes, troponin elevation, tachyarrhythmias, pulmonary congestion and hemodynamic alterations. Cardiac catheterization is the mainstay of diagnosis and is often performed early. When the coronaries are free of disease or culprit lesions then the diagnosis of SC is made. This patient population is well recognized by the cardiology community, fulfill the Mayo diagnostic criteria and are represented in the InterTAK registry. We believe this primary SC group accounts for 2/3 of all the SC with initial cardiac symptoms typically bringing these previously healthy patients to medical attention.
Stress cardiomyopathy of the critically ill, or secondary SC, is acute cardiac dysfunction developing during the course of hospitalization with critical medical, surgical or neurological illness (Table 1). Half the patients are male and cardiac symptoms are much less evident.6, 44 Global LV dysfunction may be a common form of SC in the critically ill.47 Although ECG and troponin trends suggest myocardial injury, cardiac catheterization is not routinely performed due to bleeding concerns, renal dysfunction, hemodynamic instability or acute neurological processes. Serial echocardiography is the basis for diagnosis and helps direct supportive care, hemodynamics management and prognostication.24, 52 Echocardiography also serves as gatekeeper for selectively performing catheterization in a minority of these patients who have potential for improvement with revascularization.9
Table 1.
Clinical features and demographic highlights distinguishing SC in the critically ill (secondary SC) from widely recognized SC presenting from the community (primary SC). LV indicates left ventricle; and ACS, acute coronary syndrome.
| Primary SC | Secondary SC | |
|---|---|---|
| Age range | 60–75 | 40–80 |
| Male: Female ratio | 1:9 | 1:1–1:3 |
| Prevalence | 2–6% of ‘ACS’ patients | 7–30% of critically ill |
| Chest pain | 75% | <20% |
| Physicians typically involved in care | ER >90% | Intensivists 90% |
| Cardiologists >90% | Neurologists 40% | |
| Anesthesiologists 30% | ||
| Cardiologists 20% | ||
| Presentation | From home, previously ‘healthy’ | Currently in-hospital with medical, surgical or neurological critical illness |
| Clinical picture | Acute angina, dyspnea, ischemic ECG changes – similar to ACS | Wide spectrum: troponin elevation, arrhythmia, hypotension, CHF or “ACS” |
| Diagnosis of SC suspected | At admission- based on negative catheterization or echocardiography | Typically after days of stay in ICU during echo for troponin elevation or ECG changes |
| Echocardiographic regional wall motion abnormality patterns | Apical ballooning 81.7% | Apical ballooning 50% |
| Mid ventricular 14.6% | Mid ventricular 10% | |
| Basal variant 2.2% | Basal variant 5% | |
| Focal type 1.5% | Focal type 5% | |
| Global hypokinesia 0% | Global hypokinesia 10–40% | |
| Cardiac catheterization | 90–100% | 10–20% |
| Shock | 9.9% | 30–69% |
| Hospital mortality | 4.1% | 35–50% |
The RETAKO registry recently reported their SC experience with 328 patients. They have divided their cohort based on potential triggers into 2 groups: primary SC if no triggers or an important psychic stress and secondary SC if patients had physical factors like asthma, surgery,trauma, etc.53 Since this is based on the modified Mayo criteria, the RETAKO registry, just like the InterTAK series, fails to capture the true spectrum of SC in the critically ill. We have presented several studies in critically ill patients that focus on troponin elevation and echocardiographic abnormalities. Intensivists recognize troponin as an adverse prognostic marker.35, 36 Routine bedside echocardiograms are increasingly performed to direct hemodynamic management.54, 55, 56 With a better understanding of secondary SC, we may recognize this entity sooner, helping the care of the critically ill.
6. Subclinical SC
SC is clinically diagnosed when patients present with persistent cardiac symptoms and testing reveals significant LV dysfunction. Acute physical or emotional stress appears to account for 3/4th of patients and disproportionally high endogenous catecholamine levels is postulated as the basis for the transient cardiac stunning.57 We, like some investigators, believe that clinical SC is one end of the spectrum with overt symptoms, echocardiographic abnormalities and hemodynamic changes.17 Lesser physical or emotional stress is likely to elicit a smaller magnitude catecholamine response. Clinical symptoms may be transient and ignored. ECG manifestations may be less dramatic or nonspecific for ischemia; arrhythmias could be attributed to electrolyte abnormalities or comorbidities; and troponin elevation can be related to a long list of conditions like sepsis, intracranial hemorrhage/stroke, cardiac contusion/cardiopulmonary resuscitation, congestive heart failure, chronic obstructive pulmonary disease or renal failure. The regional wall motion abnormalities may at times recover rapidly with complete normalization of LV function within 36 h.58 Critically ill patients without RWMA or LV dysfunction, may have milder forms of SC manifesting only as cardiac symptoms, ECG changes or troponin elevation. Several type II myocardial infarction patients, where troponin elevation is attributed to ‘supply-demand mismatch’, may indeed have subclinical SC. Basal hypercontractility may be an hyper- acute feature due to volume depletion and may preceded RWMA.59
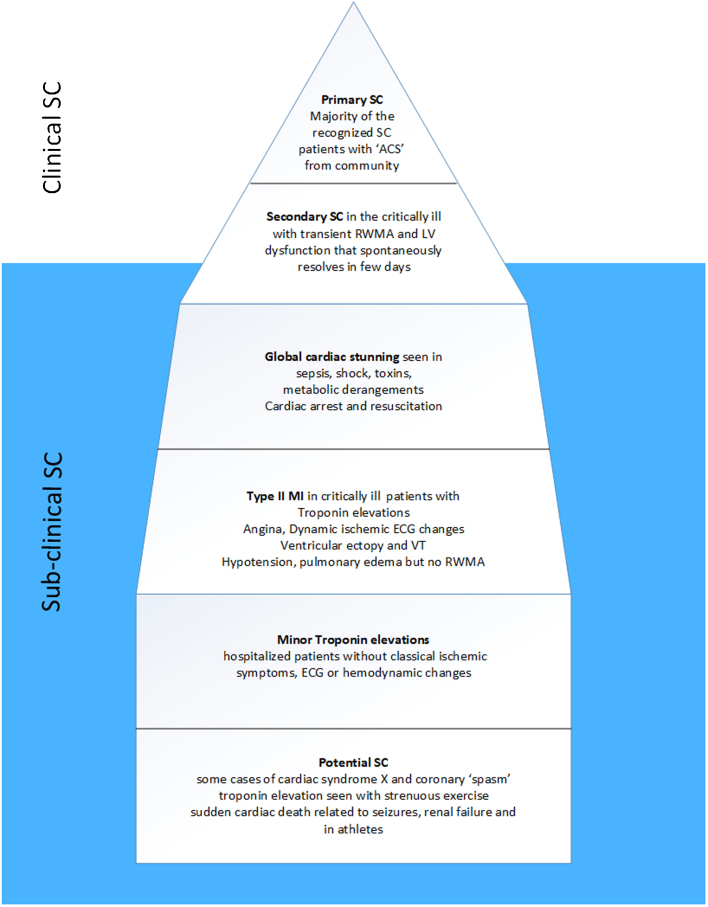
We suspect for every primary SC patient diagnosed, at least 1–2 other patients may have subclinical SC. In the critically ill, subclinical secondary SC is likely more prevalent. Due to the increasingly complex critically ill patients, prolonged survival with comorbidities, hemodynamic alterations and high inotrope infusions the heart is subject to multifold stressors. Even daily ‘routine’ bedside echocardiograms may fail to detect RWMA if cardiac abnormalities are very transient lasting only hours or even minutes.58 Again, subclinical SC patients may just not develop RWMA and thus echocardiography may not be the ideal imaging tool in this setting. Since troponin levels remain elevated for several days after any cardiac injury, this may serve as the best ‘screening tool’ for subclinical SC. Use of point of care ultrasound devices in critical care environments is increasing and likely to identify more SC. A recent review highlights the importance of diagnosing SC with assessment of LV function, wall motion and outflow obstruction.60 In the secondary SC population, we postulate for every clinically diagnosed patient, another 3–5 patients remain subclinical and undiagnosed. In Fig. 2 we have illustrated the common clinical scenarios where SC is currently diagnosed (the tip of the iceberg) and situations where SC may contribute to cardiac issues but is not recognized as such. Subclinical SC likely accounts for a significant proportion of critically ill patients manifesting transient symptoms, tachyarrhythmia, ECG abnormalities, heart failure, troponin elevation and hypotension.
Fig. 2.
Stress cardiomyopathy in the critically ill may manifest with cardiac dysfunction and wall motion abnormalities (secondary SC). However, a larger subset likely have subclinical SC with cardiac stunning, ECG changes, hypotension or isolated troponin elevation. In the community many transient cardiac conditions may represent subclinical SC as well. RWMA indicates regional wall motion abnormalities.
7. ‘Probable SC’ and benefits of this label
Our early research focused on dynamic LV outflow obstruction (LVOTO) as potential etiology for SC.10, 61 We believe protocol driven initiation of inotropes and pressor agents in post-surgical and septic shock patients as well as insufficient volume replacement resulted in smaller LV cavity size and hypercontractility.14, 62 We have published our echocardiography based diagnostic algorithm from our critical care experience.9 Based on the clinical picture (signs, symptoms, ECG changes, troponin trends) and characteristic RWMA on echocardiography, without requiring catheterization, we diagnose ‘probable SC’. The diagnosis of SC is confirmed in 3–7 days with a repeat echocardiogram confirming normalization (or significant improvement) in cardiac function.
The early clinical suspicion and echo based labelling as ‘probable SC’ has several distinctive advantages in the critically ill:
-
1.
Anticoagulants and dual antiplatelet agent loading may be avoided thus reducing bleeding risk
-
2.
Focus shifts to optimizing hemodynamics and volume status
-
3.
Minimizing and early discontinuation of pressor agents and inotropes
-
4.
Reduced need for invasive testing and cardiac catheterization thereby reducing procedural complications and delays
-
5.
Prognostication and level of care planning
The one downside of this ‘probable SC’ diagnosis is the risk of misclassifying a true ACS patient as SC and thereby missing the optimal time window to revascularize culprit coronary lesions. ACS with coronary occlusion is rare in the critically ill. There is only a limited role for catheterization/revascularization due to advanced organ system dysfunction, bleeding, infection or poor survival expectancy.63, 64 However, coronary angiography or ischemia evaluation with suitable stress testing should be planned electively after tiding over the critical illness.
8. Algorithm for diagnosis/management
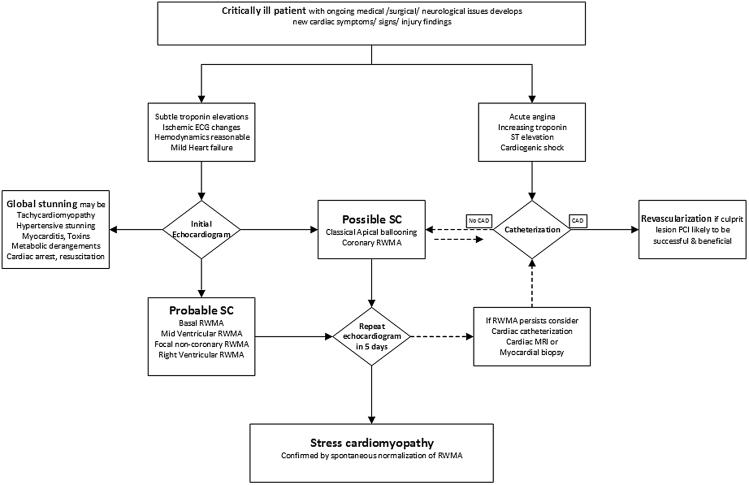
There remains a substantial overlap of clinical presentations, troponin trends, dynamic ECG changes and even echocardiographic features between ACS and SC. The traditional apical ballooning variant of SC may actually be most difficult to differentiate from ACS with LAD occlusion.10 The focal RWMA variant may also be challenging to differentiate from ACS if it involves a coronary distribution. The mid ventricular, basal variant, right ventricular and global SC forms are more readily identified as probable SC as they do not conform to any particular ACS manifestation. Fig. 3 is an algorithm to triage the critically ill patients and reduce use of higher risk cardiac catheterization/revascularization. Unless the clinical suspicion is very high for acute coronary occlusion, we may not require emergent catheterization. Majority of the critically ill would benefit from early bedside echocardiogram to carefully evaluate LV function and quantify RWMA patterns. Many of the global LV dysfunction patients may also have SC but it is imperative to exclude specific correctable etiologies first.12 Cardiac MRI can offer early clarity by detecting ischemia and infraction thus guiding therapy.65 SC can be definitively diagnosed usually in 3–7 days with repeat echocardiogram confirming normalization (or significant improvement) in the RWMA and LV function.
Fig. 3.
Diagnostic algorithm in the critically ill with cardiac injury. Except for the rare patient with concern for true ACS, catheterization plays a limited role. Echocardiography is the mainstay for diagnosis of secondary SC.
9. Conclusions
Stress cardiomyopathy in the critically ill patients is under-recognized. It has been reported and studied based on ECG, echocardiography and troponin abnormalities. The widely used Mayo criteria and the growing literature from InterTAK registry address previously healthy patients presenting from the community with acute coronary syndrome features. We have highlighted the clinical presentation and diagnostic features unique to the SC seen in the hospitalized sick population. Recognizing secondary stress cardiomyopathy as a unique clinical entity would help investigators gather much needed evidence to optimize the cardiac care of the critically ill.
References
- 1.Sato H.T.H., Uchida T., Dote K. Tako-tsubo-like left ventricular dysfunction due to multivessel coronary spasm. In: Kodama K., Haze K., Hori M., editors. Clinical Aspect of Myocardial Injury: From Ischemia to Heart Failure. Kagakuhyoronsha Publishing; Tokyo: 1990. pp. 56–64. [Google Scholar]
- 2.Medeiros K., O’Connor M.J., Baicu C.F. Systolic and diastolic mechanics in stress cardiomyopathy. Circulation. 2014;129(16):1659–1667. doi: 10.1161/CIRCULATIONAHA.113.002781. [DOI] [PubMed] [Google Scholar]
- 3.Scantlebury D.C., Abhiram P. Diagnosis of takotsubo cardiomyopathy—mayo clinic criteria. Circulation. 2014;78:2129–2139. doi: 10.1253/circj.cj-14-0859. [DOI] [PubMed] [Google Scholar]
- 4.Tsuchihashi K., Ueshima K., Uchida T. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol. 2001;38(1):11–18. doi: 10.1016/s0735-1097(01)01316-x. [DOI] [PubMed] [Google Scholar]
- 5.Champion S., Belcour D., Vandroux D. Stress (Tako-tsubo) cardiomyopathy in critically-ill patients. Eur Heart J Acute Cardiovasc Care. 2015;4(2):189–196. doi: 10.1177/2048872614547686. [DOI] [PubMed] [Google Scholar]
- 6.Haghi D., Fluechter S., Suselbeck T. Takotsubo cardiomyopathy (acute left ventricular apical ballooning syndrome) occurring in the intensive care unit. Intensive Care Med. 2006;32(7):1069–1074. doi: 10.1007/s00134-006-0111-z. [DOI] [PubMed] [Google Scholar]
- 7.Prasad A., Lerman A., Rihal C.S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155(3):408–417. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Templin C., Ghadri J.R., Diekmann J. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. New Engl J Med. 2015;373(10):929–938. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 9.Chockalingam A., Xie G.Y., Dellsperger K.C. Echocardiography in stress cardiomyopathy and acute LVOT obstruction. Int J Cardiovasc Imaging. 2010;26(5):527–535. doi: 10.1007/s10554-010-9590-7. [DOI] [PubMed] [Google Scholar]
- 10.Chockalingam A., Tejwani L., Aggarwal K. Dynamic left ventricular outflow tract obstruction in acute myocardial infarction with shock: cause, effect, and coincidence. Circulation. 2007;116(5):e110–3. doi: 10.1161/CIRCULATIONAHA.107.711697. [DOI] [PubMed] [Google Scholar]
- 11.Bybee K.A., Kara T., Prasad A. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004;141(11):858–865. doi: 10.7326/0003-4819-141-11-200412070-00010. [DOI] [PubMed] [Google Scholar]
- 12.Chockalingam A., Mehra A., Dorairajan S. Acute left ventricular dysfunction in the critically ill. Chest. 2010;138(1):198–207. doi: 10.1378/chest.09-1996. [DOI] [PubMed] [Google Scholar]
- 13.Junor C., Delcour K., Chockalingam A. Therapeutic challenges in combined apical ballooning syndrome and acute pulmonary decompensation. Am J Ther. 2010;17(4):e126–30. doi: 10.1097/MJT.0b013e3181ba3320. [DOI] [PubMed] [Google Scholar]
- 14.Chockalingam A., Dorairajan S., Bhalla M. Unexplained hypotension: the spectrum of dynamic left ventricular outflow tract obstruction in critical care settings. Crit Care Med. 2009;37(2):729–734. doi: 10.1097/CCM.0b013e3181958710. [DOI] [PubMed] [Google Scholar]
- 15.Lyon A.R., Bossone E., Schneider B. Current state of knowledge on Takotsubo syndrome: a position statement from the taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2016;18(1):8–27. doi: 10.1002/ejhf.424. [DOI] [PubMed] [Google Scholar]
- 16.Parodi G., Citro R., Bellandi B. Revised clinical diagnostic criteria for Tako-tsubo syndrome: the Tako-tsubo Italian Network proposal. Int J Cardiol. 2014;172(1):282–283. doi: 10.1016/j.ijcard.2013.12.239. [DOI] [PubMed] [Google Scholar]
- 17.Madias J.E. Why the current diagnostic criteria of Takotsubo syndrome are outmoded: a proposal for new criteria. Int J Cardiol. 2014;174(3):468–470. doi: 10.1016/j.ijcard.2014.04.241. [DOI] [PubMed] [Google Scholar]
- 18.Wittstein I.S. Stress cardiomyopathy: a syndrome of catecholamine-mediated myocardial stunning? Cell Mol Neurobiol. 2012;32(5):847–857. doi: 10.1007/s10571-012-9804-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redfors B., Shao Y., Omerovic E. Stress-induced cardiomyopathy (Takotsubo)—broken heart and mind? Vasc Health Risk Manage. 2013;9:149–154. doi: 10.2147/VHRM.S40163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madhavan M., Borlaug B.A., Lerman A. Stress hormone and circulating biomarker profile of apical ballooning syndrome (takotsubo cardiomyopathy): insights into the clinical significance of B-type natriuretic peptide and troponin levels. Heart. 2009;95(17):1436–1441. doi: 10.1136/hrt.2009.170399. [DOI] [PubMed] [Google Scholar]
- 21.Novo G., Giambanco S., Bonomo V. Troponin I/ejection fraction ratio: a new index to differentiate takotsubo cardiomyopathy from myocardial infarction. Int J Cardiol. 2015;180:255–257. doi: 10.1016/j.ijcard.2014.11.186. [DOI] [PubMed] [Google Scholar]
- 22.Santoro F., Tarantino N., Ferraretti A. Serum interleukin 6 and 10 levels in takotsubo cardiomyopathy: increased admission levels may predict adverse events at follow-up. Atherosclerosis. 2016;254:28–34. doi: 10.1016/j.atherosclerosis.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Santoro F., Ferraretti A., Musaico F. Carbohydrate-antigen-125 levels predict hospital stay duration and adverse events at long-term follow-up in takotsubo cardiomyopathy. Intern Emerg Med. 2016;11(5):687–694. doi: 10.1007/s11739-016-1393-y. [DOI] [PubMed] [Google Scholar]
- 24.Citro R., Piscione F., Parodi G. Role of echocardiography in takotsubo cardiomyopathy. Heart Fail Clin. 2013;9(2):157–166. doi: 10.1016/j.hfc.2012.12.014. viii. [DOI] [PubMed] [Google Scholar]
- 25.Lim W., Qushmaq I., Cook D.J. Elevated troponin and myocardial infarction in the intensive care unit: a prospective study. Crit Care (London, England) 2005;9(6):R636–44. doi: 10.1186/cc3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim W., Holinski P., Devereaux P.J. Detecting myocardial infarction in critical illness using screening troponin measurements and ECG recordings. Crit Care (London, England) 2008;12(2):R36. doi: 10.1186/cc6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim W., Tkaczyk A., Holinski P. The diagnosis of myocardial infarction in critically ill patients: an agreement study. J Crit Care. 2009;24(3):447–452. doi: 10.1016/j.jcrc.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Lim W., Whitlock R., Khera V. Etiology of troponin elevation in critically ill patients. J Crit Care. 2010;25(2):322–328. doi: 10.1016/j.jcrc.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Lim W., Qushmaq I., Devereaux P.J. Elevated cardiac troponin measurements in critically ill patients. Arch Intern Med. 2006;166(22):2446–2454. doi: 10.1001/archinte.166.22.2446. [DOI] [PubMed] [Google Scholar]
- 30.Lim W., Cook D.J., Griffith L.E. Elevated cardiac troponin levels in critically ill patients: prevalence, incidence, and outcomes. Am J Crit Care. 2006;15(3):280–288. quiz 9. [PubMed] [Google Scholar]
- 31.Lim W., Qushmaq I., Cook D.J. Reliability of electrocardiogram interpretation in critically ill patients. Crit Care Med. 2006;34(5):1338–1343. doi: 10.1097/01.CCM.0000214679.23957.90. [DOI] [PubMed] [Google Scholar]
- 32.Weisel R.D., Vito L., Dennis R.C. Myocardial depression during sepsis. Am J Surg. 1977;133(4):512–521. doi: 10.1016/0002-9610(77)90141-6. [DOI] [PubMed] [Google Scholar]
- 33.Parker M.M., Shelhamer J.H., Bacharach S.L. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984;100(4):483–490. doi: 10.7326/0003-4819-100-4-483. [DOI] [PubMed] [Google Scholar]
- 34.Flynn A., Chokkalingam Mani B., Mather P.J. Sepsis-induced cardiomyopathy: a review of pathophysiologic mechanisms. Heart Fail Rev. 2010;15(6):605–611. doi: 10.1007/s10741-010-9176-4. [DOI] [PubMed] [Google Scholar]
- 35.Relos R.P., Hasinoff I.K., Beilman G.J. Moderately elevated serum troponin concentrations are associated with increased morbidity and mortality rates in surgical intensive care unit patients. Crit Care Med. 2003;31(11):2598–2603. doi: 10.1097/01.CCM.0000089931.09635.D2. [DOI] [PubMed] [Google Scholar]
- 36.Babuin L., Vasile V.C., Rio Perez J.A. Elevated cardiac troponin is an independent risk factor for short- and long-term mortality in medical intensive care unit patients. Crit Care Med. 2008;36(3):759–765. doi: 10.1097/CCM.0B013E318164E2E4. [DOI] [PubMed] [Google Scholar]
- 37.Quenot J.P., Le Teuff G., Quantin C. Myocardial injury in critically ill patients: relation to increased cardiac troponin I and hospital mortality. Chest. 2005;128(4):2758–2764. doi: 10.1378/chest.128.4.2758. [DOI] [PubMed] [Google Scholar]
- 38.Maeder M., Fehr T., Rickli H. Sepsis-associated myocardial dysfunction: diagnostic and prognostic impact of cardiac troponins and natriuretic peptides. Chest. 2006;129(5):1349–1366. doi: 10.1378/chest.129.5.1349. [DOI] [PubMed] [Google Scholar]
- 39.Jardin F., Fourme T., Page B. Persistent preload defect in severe sepsis despite fluid loading: a longitudinal echocardiographic study in patients with septic shock. Chest. 1999;116(5):1354–1359. doi: 10.1378/chest.116.5.1354. [DOI] [PubMed] [Google Scholar]
- 40.Poelaert J., Declerck C., Vogelaers D. Left ventricular systolic and diastolic function in septic shock. Intensive Care Med. 1997;23(5):553–560. doi: 10.1007/s001340050372. [DOI] [PubMed] [Google Scholar]
- 41.Jardin F., Brun-Ney D., Auvert B. Sepsis-related cardiogenic shock. Crit Care Med. 1990;18(10):1055–1060. doi: 10.1097/00003246-199010000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Pulido J.N., Afessa B., Masaki M. Clinical spectrum, frequency, and significance of myocardial dysfunction in severe sepsis and septic shock. Mayo Clin Proc. 2012;87(7):620–628. doi: 10.1016/j.mayocp.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruiz Bailen M., Aguayo de Hoyos E., Lopez Martnez A. Reversible myocardial dysfunction, a possible complication in critically ill patients without heart disease. J Crit Care. 2003;18(4):245–252. doi: 10.1016/j.jcrc.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Park J.H., Kang S.J., Song J.K. Left ventricular apical ballooning due to severe physical stress in patients admitted to the medical ICU. Chest. 2005;128(1):296–302. doi: 10.1378/chest.128.1.296. [DOI] [PubMed] [Google Scholar]
- 45.Marcelino P.A., Marum S.M., Fernandes A.P. Routine transthoracic echocardiography in a general Intensive Care Unit: an 18 month survey in 704 patients. Eur J Intern Med. 2009;20(3):e37–e42. doi: 10.1016/j.ejim.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 46.Santoro F., Carapelle E., Cieza Ortiz S.I. Potential links between neurological disease and Tako-Tsubo cardiomyopathy: a literature review. Int J Cardiol. 2013;168(2):688–691. doi: 10.1016/j.ijcard.2013.03.093. [DOI] [PubMed] [Google Scholar]
- 47.Belcour D., Jabot J., Grard B. Prevalence and risk factors of stress cardiomyopathy after convulsive status epilepticus in ICU patients. Criti Care Med. 2015;43(10):2164–2170. doi: 10.1097/CCM.0000000000001191. [DOI] [PubMed] [Google Scholar]
- 48.Ruiz Bailen M. Reversible myocardial dysfunction in critically ill, noncardiac patients: a review. Crit Care Med. 2002;30(6):1280–1290. doi: 10.1097/00003246-200206000-00020. [DOI] [PubMed] [Google Scholar]
- 49.Grocott-Mason R.M., Shah A.M. Cardiac dysfunction in sepsis: new theories and clinical implications. Intensive Care Med. 1998;24(4):286–295. doi: 10.1007/s001340050570. [DOI] [PubMed] [Google Scholar]
- 50.Krishnagopalan S., Kumar A., Parrillo J.E. Myocardial dysfunction in the patient with sepsis. Curr Opin Crit Care. 2002;8(5):376–388. doi: 10.1097/00075198-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Court O., Kumar A., Parrillo J.E. Clinical review: myocardial depression in sepsis and septic shock. Crit Care (London, England) 2002;6(6):500–508. doi: 10.1186/cc1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manzanal A., Ruiz L., Madrazo J. Inverted takotsubo cardiomyopathy and the fundamental diagnostic role of echocardiography. Texas Heart Inst J. 2013;40(1):56–59. [PMC free article] [PubMed] [Google Scholar]
- 53.Nunez-Gil I.J., Almendro-Delia M., Andres M. Secondary forms of takotsubo cardiomyopathy: a whole different prognosis. Eur Heart J Acute Cardiovasc Care. 2016;5(4):308–316. doi: 10.1177/2048872615589512. [DOI] [PubMed] [Google Scholar]
- 54.Marum S., Price S. The use of echocardiography in the critically ill; the role of FADE (Fast Assessment Diagnostic Echocardiography) training. Curr Cardiol Rev. 2011;7(3):197–200. doi: 10.2174/157340311798220449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vignon P. Hemodynamic assessment of critically ill patients using echocardiography Doppler. Curr Opin Crit Care. 2005;11(3):227–234. doi: 10.1097/01.ccx.0000159946.89658.51. [DOI] [PubMed] [Google Scholar]
- 56.Noritomi D.T., Vieira M.L., Mohovic T. Echocardiography for hemodynamic evaluation in the intensive care unit. Shock (Augusta, Ga) 2010;34(Suppl. 1):59–62. doi: 10.1097/SHK.0b013e3181e7e8ed. [DOI] [PubMed] [Google Scholar]
- 57.Wittstein I.S., Thiemann D.R., Lima J.A. Neurohumoral features of myocardial stunning due to sudden emotional stress. New Engl J Med. 2005;352(6):539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 58.Eitel I., Lucke C., Behrendt F. Full recovery of takotsubo cardiomyopathy (apical ballooning) in two days. Int J Cardiol. 2010;143(3):e51–3. doi: 10.1016/j.ijcard.2008.12.044. [DOI] [PubMed] [Google Scholar]
- 59.Ieva R., Santoro F., Ferraretti A. Hyper-acute precipitating mechanism of Tako-Tsubo cardiomyopathy: in the beginning was basal hyperkinesis? Int J Cardiol. 2013;167(3):e55–7. doi: 10.1016/j.ijcard.2013.03.138. [DOI] [PubMed] [Google Scholar]
- 60.Ha Y.R., Toh H.C. Clinically integrated multi-organ point-of-care ultrasound for undifferentiated respiratory difficulty, chest pain, or shock: a critical analytic review. J Intensive Care. 2016;4:54. doi: 10.1186/s40560-016-0172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chockalingam A., Dellsperger K.C. Isolated dynamic left ventricular outflow tract obstruction can cause hypotension that rapidly responds to intravenous beta blockade. Am J Ther. 2011;18(5):e172–6. doi: 10.1097/MJT.0b013e3181cea0dd. [DOI] [PubMed] [Google Scholar]
- 62.Rivers E., Nguyen B., Havstad S. Early goal-directed therapy in the treatment of severe sepsis and septic shock. New Engl J Med. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 63.Rennyson S.L., Hunt J., Haley M.W. Electrocardiographic ST-segment elevation myocardial infarction in critically ill patients: an observational cohort analysis. Crit Care Med. 2010;38(12):2304–2309. doi: 10.1097/CCM.0b013e3181fa02cd. [DOI] [PubMed] [Google Scholar]
- 64.Krenn L., Delle Karth G. Myocardial infarction in critically ill patients: a diagnostic challenge. Crit Care Med. 2010;38(12):2412–2413. doi: 10.1097/CCM.0b013e3181fd6769. [DOI] [PubMed] [Google Scholar]
- 65.Kohan A.A., Levy Yeyati E., De Stefano L. Usefulness of MRI in takotsubo cardiomyopathy: a review of the literature. Cardiovasc Diagn Ther. 2014;4(2):138–146. doi: 10.3978/j.issn.2223-3652.2013.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]