Abstract
Background/Aims
The aim of this study was to investigate the use of non-exposure endoscopic wall-inversion surgery (NEWS) and the combination of laparoscopic and endoscopic approaches to neoplasia with non-exposure technique (CLEAN-NET) in gastric tumors.
Methods
We reviewed all cases of NEWS and CLEAN-NET performed in the department of surgery of the Royal Vinohrady Teaching Hospital.
Results
Our department performed 12 gastric tumor resections (NEWS, n=10 and CLEAN-NET, n=2) between March 2016 and February 2017. The cases chosen for these resections included predominantly submucosal tumors with no signs of dissemination or local invasion and early gastric carcinomas (T1SM1 and T1M), where tumor location made it impossible to use endoscopic submucosal dissection. R0 resection margins were confirmed in all the cases.
Conclusions
NEWS and CLEAN-NET allow en bloc non-exposed full-thickness gastric wall resection in a way that uses a “close first, cut later” approach to prevent seeding of the peritoneal cavity with tumor cells. These mini-invasive techniques combine laparoscopic and endoscopic techniques, and preserve the full function of the stomach.
Keywords: Gastrointestinal stromal tumors, Stomach, Endoscopy, Laparoscopy
INTRODUCTION
Laparoscopic wedge resections have become an accepted mini-invasive surgical technique for resecting gastrointestinal stromal tumors (GIST) of the stomach [1]. However, as these tumors are mucosally or submucosally located, they are often poorly visualized. To compensate for this, excessive tissue is often removed, resulting in deformation of the stomach and gastric stasis.
Endoscopic submucosal dissection (ESD) circumvents this problem, as it directly visualizes the tumor from within the stomach. However, it often fails to achieve negative resection margins, as it does not remove the muscular layer or serosa of the stomach. Endoscopic full-thickness resections and laparoscopic-endoscopic cooperative surgery can remove the stomach wall in its full thickness. However, in doing so, they allow communication between the stomach lumen and peritoneal cavity, which brings the risk of infection and dissemination of tumor cells into the peritoneal cavity. Thus, non-exposure laparoscopic-endoscopic cooperative surgery was developed. Non-exposure techniques do not create communication between the gastric lumen and the peritoneal cavity; thus, they prevent peritoneal seeding and infection. In addition, as they visualize the tumor from the gastric lumen, can accurately achieve negative resection margins [2].
The first of these non-exposure techniques was developed by Inoue et al. in 2012 and is called “combination of laparoscopic and endoscopic approaches to neoplasia with non-exposure technique” (CLEAN-NET) [3]. Subsequently, Mitsui et al. developed another non-exposure technique, known as “non-exposure endoscopic wall-inversion surgery” (NEWS) [4]. CLEAN-NET is performed by making a seromuscular incision laparoscopically around the tumor. The tumor is then pulled away from the stomach and resected with a stapler. The resected tissue is then extracted through a laparoscopic port [5,6]. NEWS is performed by first visualizing the lesion margins by using coagulation on both the mucosal and serosal surfaces of the stomach. This is followed by injecting a solution of saline, glycerol, and methylene blue in the submucosal layer. After this, a laparoscopic seromuscular incision around the lesion can be performed. Sutures are then made laparoscopically, inverting the lesion so that it can be resected on the mucosal side. The resected tissue specimen is then extracted perorally, using the endoscope [7-10].
MATERIALS AND METHODS
Twelve localized gastric tumor resections (NEWS, n=10 and CLEAN-NET, n=2) were performed between March 2016 and February 2017 in our department. The main indications for these procedures were predominantly submucosally located tumors with no signs of dissemination or local invasion. The tumors were diagnosed on the basis of gastroscopic, endosonographic, histological, and computed tomography (CT) examinations. Advanced tumors or tumors not endoscopically visible were excluded from the study. Patients were indicated as suitable candidates by a multidisciplinary team of oncologists, surgeons, gastroenterologists, and pathologists.
The surgical protocol was approved by the ethics commission of the University Hospital Královské Vinohrady. All the patients included in the study provided informed consent for the examination and procedure.
Non-exposure endoscopic wall-inversion surgery
Fig. 1 shows the position of the team and Fig. 2 shows the main steps in the NEWS procedure. All the patients were placed in the reverse Trendelenburg position, with their lower extremities spread widely apart. The surgeon was positioned on the left side of the patient, with the assistant between the patient’s legs. The endoscopist and endoscopic nurse were positioned on the left side of the patient’s head. In each patient we inserted a 10-mm camera port above the umbilicus, a 12-mm port in the right hypochondrium and created a 12-mmHg positive-pressure capnoperitoneum. After the endoscopist determined the locations of the tumors another two or three 5-mm ports were inserted as needed in the upper abdomen.
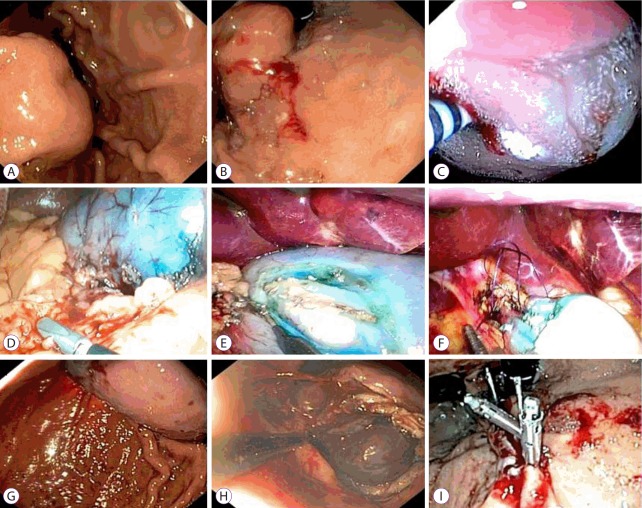
Fig. 1.
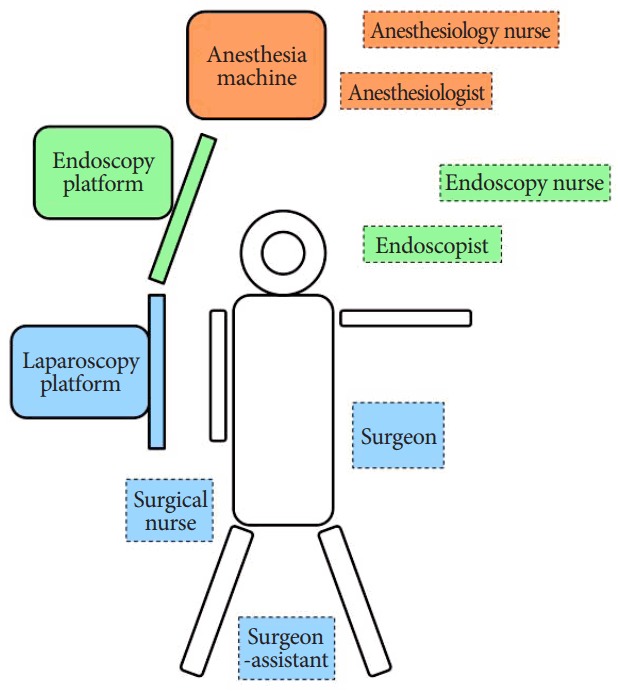
Position of the team.
Fig. 2.
Non-exposure endoscopic wall-inversion surgery. (A) Endoscopic view of a gastrointestinal stromal tumor of the stomach. (B) Marking the lesion with electrocautery. (C) Injection of methylene blue dye into the lesion. (D) Visualisation of the stained lesion on the serosal surface of the stomach. (E) A circumferential seromuscular incision around the lesion. (F) A suture placed around the lesion. (G) Inversion of the tumor into the stomach. (H) Circumferential muco-submucosal dissection. (I) Hemostatic clips applied to the stomach wall to achieve hemostasis.
The locations of the tumors were established using a flexible endoscope, and the lesion margins, including a safety rim, were marked using coagulation (DualKnife, KD-650L; Olympus Medical Systems, Tokyo, Japan). Following this, the extent of the tissue to be resected was marked on the serosal surface of the stomach. This was performed laparoscopically using a coagulation hook under guidance from the endoscope by applying pressure with the end of a DualKnife against the internal surface of the gastric wall. ESD was then performed, with the injection of saline, glycerol, and methylene blue solution in the coagulation marks peripherally around the tumor lesion. The serosa and muscle layer, including a safety margin, were dissected laparoscopically using the coagulation hook cutting settings. At this point, the lesions were located purely on the gastric mucosa itself and were then inverted into the gastric lumen. The stomach was then desufflated, and the gastric wall defect closed with uninterrupted sutures by using a Vicryl 3/0 thread in a single layer. The endoscopist then completed the resection using the ESD technique by dissecting the mucosa (muco-submucosa) externally from the coagulation marks, employing the DualKnife and IT Knife (KD-611L; Olympus Medical Systems). After investigating the suture integrity, the detached lesions were extracted perorally with a loop or a net (Roth Net polyp retriever; US Endoscopy, Mentor, OH, USA).
Combination of laparoscopic and endoscopic approaches to neoplasia with non-exposure technique
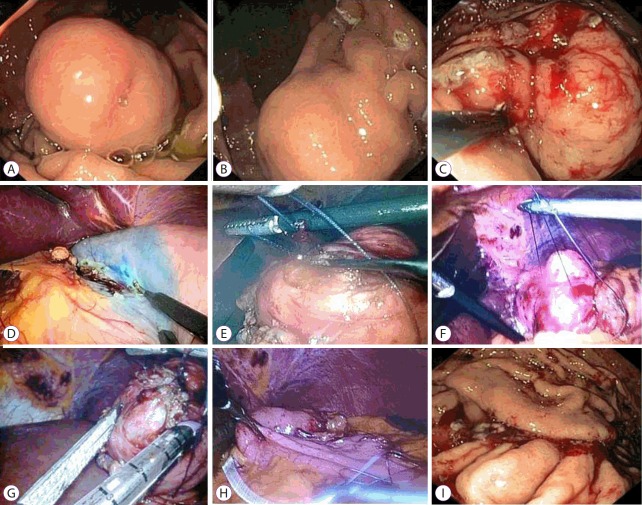
Fig. 3 shows the main steps in the CLEAN-NET procedure. The CLEAN-NET procedures were performed in the same way as the NEWS procedures until the lesions were marked on the serosal side of the stomach. After this, two sutures were laparoscopically placed around (but not directly into) the tumors, which were used to lift the tumors away from the gastric wall. An Endo GIA™ (Medtronic, Minneapolis, MN, USA) was then used to resect the lesions. When necessary the stapler suture on the serosal side was additionally secured using uninterrupted seromuscular sutures. The resected lesions were then placed into a bag and extracted through the largest incision (the 12-mm port in the right hypochondrium).
Fig. 3.
Combination of laparoscopic and endoscopic approaches to neoplasia with non-exposure technique. (A) Endoscopic view of a gastrointestinal stromal tumor of the stomach. (B) Marking the lesion with electrocautery. (C) Injection of methylene blue dye into the lesion. (D) A circumferential seromuscular incision. (E) Sutures placed around the lesion. (F) The tumor with the surrounding submucosa elevated away from the stomach wall. (G) Resection of the tumor with a stapler. (H) Suture of the stomach wall on the serosal surface. (I) Suture of the stomach wall on the mucosal surface.
A single Redon drain was placed into the abdominal cavity at the end of each procedure and a final endoscopic inspection of suture sufficiency and resection line hemostasis was performed. After this, the laparoscopic ports were extracted and the capnoperitoneum was terminated. A nasogastric tube was placed into the patients’ stomachs and left for 24 hours after the operation, and, in accordance with the patient’s tolerance levels, sipping was initiated on the first postoperative day. All patients received proton pump inhibitors for the entire duration of the perioperative period.
RESULTS
Patient characteristics
The patients’ characteristics are summarized in Table 1. The study cohort consisted of seven female and five male patients. The mean age of the patients was 67 years (range, 53–83 years), and the mean body mass index was 27.9 kg/m2. Seven patients (58%) were asymptomatic. Symptoms included anemia and abdominal pain.
Table 1.
Patient Characteristics and Histopathological and Surgical Details
| Patient no. | Age (yr) | Sex | BMI (kg/m2) | Symptoms | Tumor type | Location | Diameter (mm) | Technique | Operation time (min) | Intraoperative complications | Postoperative complications | Length of hospitalisation (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 73 | M | 30.8 | Asymptomatic | Ectopic pancreatic tissue | Anterior wall of antrum | 29 | NEWS | 80 | - | - | 6 |
| 2 | 73 | M | 26.6 | Asymptomatic | Lipoma | Prepyloric region | 70 | NEWS | 115 | - | - | 6 |
| 3 | 53 | M | 38.7 | Abdominal pain | Vanek's tumor | Prepyloric region | 29 | NEWS | 120 | Resection line bleeding | Infected liver hematoma | 6 |
| 4 | 60 | M | 27.4 | Anemia | Neuroendocrine tumor | Anterior wall of body | 30 | NEWS | 90 | - | Resection line bleeding | 6 |
| 5 | 54 | F | 31.4 | Asymptomatic | EGC | Posterior wall, upper body | 26 | NEWS | 70 | - | - | 5 |
| 6 | 60 | F | 39.1 | Abdominal pain | EGC | Posterior wall, middle body | 28 | NEWS | 105 | - | - | 7 |
| 7 | 68 | F | 34.2 | Anemia | GIST | Posterior wall, lower body | 45 | CLEAN-NET | 180 | - | - | 10 |
| 8 | 71 | M | 26.9 | Asymptomatic | GIST | Subcardial region | 22 | NEWS | 95 | - | - | 7 |
| 9 | 70 | F | 25.3 | Asymptomatic | GIST | Posterior wall, middle body | 35 | NEWS | 115 | - | - | 8 |
| 10 | 62 | F | 17.9 | Asymptomatic | GIST | Posterior wall, fundus | 30 | NEWS | 100 | - | - | 7 |
| 11 | 80 | F | 26.6 | Asymptomatic | GIST | Subcardial region | 28 | NEWS | 105 | - | - | 10 |
| 12 | 83 | F | 30.9 | Anemia | GIST | Posterior wall, upper body | 30 | CLEAN-NET | 120 | - | - | 4 |
BMI, body mass index; NEWS, non-exposure endoscopic wall-inversion surgery; EGC, early gastric cancer; GIST, gastrointestinal stromal tumors; CLEAN-NET, combination of laparoscopic and endoscopic approaches to neoplasia with non-exposure technique.
Histopathological findings
Histopathological findings are summarized in Table 1. Histological examination revealed six GISTs, two early gastric cancers, one inflammatory fibroid polyp (Vanek’s tumor), one case of ectopic pancreatic tissue, one lipoma, and one neuroendocrine tumor. All the lesions were successfully resected en bloc, and histopathological examination confirmed R0 resection margins in all the cases.
All GISTs were spindle cell subtypes. All measured between 2 and 5 cm in diameter (stage T2) and had mitotic indexes of <5 per 50 high-power fields (grade 1). Thus, according to the Miettinen criteria, the risk of recurrence in all the cases was 1.9%.
Both early gastric cancers met the extended criteria for endoscopic resection according to the European Society of Gastrointestinal Endoscopy guidelines [11]. Both were well-differentiated (grade 1) adenocarcinomas. The lesion in patient 5 was 26 mm in diameter and was located entirely in the mucosa. It was staged as T1M (T1a). The lesion in patient 6 was 28 mm in diameter and was predominantly located in the mucosa, with submucosal invasion of <50 μm in depth. It was staged as T1SM1 (T1b1). Lymph node dissection was performed in both cases using LigaSure (Covidien Medtronic, Minneapolis, MN, USA). Ten lymph nodes were removed in patient 5, and seven lymph nodes were removed in patient 6; metastases were not present in any of the removed lymph nodes.
Surgical details
The surgical details are summarized in Table 1. The mean operating time was 108 minutes for the entire cohort, 99 minutes (range, 70–120) for the NEWS procedures, and 150 minutes (range, 120–180 minutes) for the CLEAN-NET procedures.
No major intraoperative complications occurred. One patient had intraoperative bleeding from the resection line on the mucosal side, which was treated endoscopically with the application of hemoclips and Coagrasper forceps (Olympus Medical Systems). This was the longest NEWS procedure; the operation time was 120 minutes, and blood loss was 220 mL. No other intraoperative complications occurred, and in all the other cases, blood loss was <50 mL.
Postoperative complications occurred in two patients. In patient 4, a case of early postoperative bleeding from the resection line on the mucosal surface occurred. This was detected on the first postoperative day by the appearance of blood coming out of the nasogastric tube. Vital signs remained unchanged, and a fall in hemoglobin concentration did not occur. This patient was not receiving anticoagulant medication at the time of the bleeding. Urgent gastroscopy was performed on the same day, and the lesion was treated with argon plasma coagulation and hemoclips. Patient 3 developed a late, infected subcapsular hematoma of the left lobe of the liver, which was treated with CT-controlled percutaneous drainage. The mean postoperative period of hospitalization was 6.8 days (range, 4–10 days) for the entire cohort, 6.8 days (range, 5–10 days) for the NEWS procedures, and 6 days (range, 4–10 days) for the CLEAN-NET procedures.
Anastomotic insufficiency, late bleeding, perforation, delayed gastric emptying, and early superficial surgical site infections did not occur in any of the patients. Three months after the procedures, the patients underwent gastroscopy, which showed no signs of relapse or other pathology at the resection line. Following the procedure, all the patients remained asymptomatic with no need for dietary modifications or other changes.
DISCUSSION
Our study presents a cohort of patients who underwent operation at the University Hospital Královské Vinohrady between March 2016 to February 2017. In our experience, the most challenging part of these procedures is marking the lesion margins on the serosal surface of the stomach. This can be problematic especially after significant stomach desufflation, which makes the serosal markings difficult to identify. Such cases require deeper coagulation by the DualKnife.
The seromuscular laparoscopic incision is fairly easily performed with the use of the coagulation hook’s cutting setting, which proved to be most useful for this technique. As the dye injected in the submucosal space disperses into the gastric wall, it does not accurately delineate the tumor margins and thus makes the laparoscopic seromuscular incision more difficult to perform. The first few procedures revealed that it is rather difficult to correctly identify the necessary depth of the seromuscular incision, which needs to be sufficiently deep and extend all the way under the muscularis mucosae to allow for a simple inversion of the lesion into the gastric lumen. If resection line bleeding occurs or if lymph node sampling is required, we recommend using the LigaSure sealer.
Both CLEAN-NET and NEWS have their own advantages and disadvantages. The choice of technique is based on the size, location, and direction of tumor growth. Lesions with diameters >4 cm are difficult to remove endoscopically; thus, CLEAN-NET is preferred in these cases. However, if only one dimension is larger than 4 cm, it can still be easily removed endoscopically as long as the other two dimensions are <4 cm and the largest dimension is passed along the long axis of the lumen of the esophagus. Tumors on the posterior wall are difficult to resect endoscopically. On the contrary, NEWS is preferable for tumors in the subcardial part of the stomach and the pyloric region, where a laparoscopic approach is limited and technically demanding.
Endophytic tumors are better visualized using the endoscope and thus more easily removed using NEWS, whereas exophytic tumors are best visualized using the laparoscope and removed using CLEAN-NET. A further consideration to take into account when choosing the most suitable operative technique is cost; CLEAN-NET is considerably more expensive, as it necessitates the use of laparoscopically placed staples to resect the lesion. See Table 2 for a summary of the advantages and disadvantages of NEWS and CLEAN-NET.
Table 2.
Comparison of CLEAN-NET and NEWS
| CLEAN-NET | NEWS | |
|---|---|---|
| Size | >4 cm | <4 cm |
| Location | Posterior wall | Pyloric and subcardial regions |
| Accuracy of resection | Lower | Higher |
| Cost | More expensive | Cheaper |
| Direction of tumor growth | Exophytic | Endophytic |
| Operation time | Longer | Shorter |
CLEAN-NET, combination of laparoscopic and endoscopic approaches to neoplasia with non-exposure technique; NEWS, non-exposure endoscopic wall-inversion surgery.
Although our data supports the fact that these mini-invasive techniques are relatively safe, precise, and technologically achievable, a much larger patient cohort will be needed to fully estimate the long-term safety of these procedures. However, the already published works clearly show that these techniques are effective and preserve the full function of the involved organs, which, without doubt, will be a significant future contribution to patients’ well-being.
A significant benefit of these techniques is their ability to produce full-thickness resection while minimizing the risk of cancer cell dissemination. It is also becoming clear that apart from submucosal lesions, these procedures can be a suitable modality for the treatment of early gastric carcinomas with low risk of lymph node involvement. However, the recommended follow-up method for patients where R0 resection was not achieved or where lymph node sampling is positive remains unclear. Where our patient cohort was concerned, this question need not be addressed. However, in the future, most definitely, recommended steps (e.g., radicalization of the procedure or application of adjuvant chemotherapy) need to be formulated. These decisions will surely be made on the basis of a multidisciplinary team, similarly to the decisions about indications for the procedure.
These new combined laparoscopic-endoscopic non-exposure techniques can bring significant benefits to patients with GIST or early gastric carcinomas, fully preserving the functions of the afflicted organs. This study should be followed up by larger case-controlled comparative studies to prove the benefit of these procedures.
Acknowledgments
This work was supported by the Research Project PROGRES-Q28, awarded by Charles University in Prague.
Footnotes
Conflicts of Interest:The authors have no financial conflicts of interest.
REFERENCES
- 1.Demetri GD, von Mehren M, Antonescu CR, et al. NCCN task force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8 Suppl 2:S1–S41. doi: 10.6004/jnccn.2010.0116. quiz S42-S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maehata T, Goto O, Takeuchi H, Kitagawa Y, Yahagi N. Cutting edge of endoscopic full-thickness resection for gastric tumor. World J Gastrointest Endosc. 2015;7:1208–1215. doi: 10.4253/wjge.v7.i16.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inoue H, Ikeda H, Hosoya T, et al. Endoscopic mucosal resection, endoscopic submucosal dissection, and beyond: full-layer resection for gastric cancer with nonexposure technique (CLEAN-NET) Surg Oncol Clin N Am. 2012;21:129–140. doi: 10.1016/j.soc.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Mitsui T, Niimi K, Yamashita H, et al. Non-exposed endoscopic wall-inversion surgery as a novel partial gastrectomy technique. Gastric Cancer. 2014;17:594–599. doi: 10.1007/s10120-013-0291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nabeshima K, Tomioku M, Nakamura K, Yasuda S. Combination of laparoscopic and endoscopic approaches to neoplasia with non-exposure technique (CLEAN-NET) for GIST with ulceration. Tokai J Exp Clin Med. 2015;40:115–119. [PubMed] [Google Scholar]
- 6.Hiki N, Nunobe S, Matsuda T, Hirasawa T, Yamamoto Y, Yamaguchi T. Laparoscopic endoscopic cooperative surgery. Dig Endosc. 2015;27:197–204. doi: 10.1111/den.12404. [DOI] [PubMed] [Google Scholar]
- 7.Kim DW, Kim JS, Kim BW, Jung JY, Kim GJ, Kim JJ. Non-exposed endoscopic wall-inversion surgery for gastrointestinal stromal tumor of the stomach: first case report in Korea. Clin Endosc. 2016;49:475–478. doi: 10.5946/ce.2016.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morinushi T, Oyama T, Takahashi A, et al. A case of non-exposed endoscopic wall-inversion surgery (news) for early gastric cancer with ulcer scar located at fornix. Endoscopic Forum for Digestive Disease. 2014;30:21–26. [Google Scholar]
- 9.Goto O, Mitsui T, Fujishiro M, et al. New method of endoscopic full-thickness resection: a pilot study of non-exposed endoscopic wall-inversion surgery in an ex vivo porcine model. Gastric Cancer. 2011;14:183–187. doi: 10.1007/s10120-011-0014-8. [DOI] [PubMed] [Google Scholar]
- 10.Goto O, Takeuchi H, Kawakubo H, et al. First case of non-exposed endoscopic wall-inversion surgery with sentinel node basin dissection for early gastric cancer. Gastric Cancer. 2015;18:434–439. doi: 10.1007/s10120-014-0406-7. [DOI] [PubMed] [Google Scholar]
- 11.Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy. 2015;47:829–854. doi: 10.1055/s-0034-1392882. [DOI] [PubMed] [Google Scholar]