Abstract
Environmental stimuli can lead to the excessive accumulation of reactive oxygen species (ROS), which is one of the risk factors for premature skin aging. Here, we investigated the protective effects of 7-MEGATM 500 (50% palmitoleic acid, 7-MEGA) against oxidative stress-induced cellular damage and its underlying therapeutic mechanisms in the HaCaT human skin keratinocyte cell line (HaCaT cells). Our results showed that treatment with 7-MEGA prior to hydrogen peroxide (H2O2)-induced damage significantly increased the viability of HaCaT cells. 7-MEGA effectively attenuated generation of H2O2-induced reactive oxygen species (ROS), and inhibited H2O2-induced inflammatory factors, such as prostaglandin E2 (PGE2), tumor necrosis factor-α (TNF-α), and interleukin-1β (IL-1β). In addition, cells treated with 7-MEGA exhibited significantly decreased expression of matrix metalloproteinase-1 (MMP-1) and increased expression of procollagen type 1 (PCOL1) and Elastin against oxidative stress by H2O2. Interestingly, these protective activities of 7-MEGA were similar in scope and of a higher magnitude than those seen with 98.5% palmitoleic acid (PA) obtained from Sigma when given at the same concentration (100 nL/mL). According to our data, 7-MEGA is able to protect HaCaT cells from H2O2-induced damage through inhibiting cellular oxidative stress and inflammation. Moreover, 7-MEGA may affect skin elasticity maintenance and improve skin wrinkles. These findings indicate that 7-MEGA may be useful as a food supplement for skin health.
Keywords: 7-MEGA, Palmitoleic acid, Anti-oxidantion, Anti-inflammation, Skin regeneration
INTRODUCTION
Skin is the primary barrier that serves to protect our body from various chemical and physical external stimuli. The skin consists of the epidermis, dermis, and subcutaneous tissue, and the epidermis is mostly composed of keratinocytes. These keratinocytes are susceptible to external stimuli, such as UV radiation, environmental toxins, and heat (1). In particular, reactive oxygen species (ROS) produced by external stimuli induce processes related to skin aging, by decreasing skin regeneration and increasing wrinkle formation (2,3).
Omega fatty acids are essential fatty acids, meaning they are produced only in small quantities in our bodies so they must be ingested primarily through foods. The representative omega fatty acids are omega-3, omega-6, omega-7 and omega-9. Among these, omega-3, which is abundant in fish and soybean oils, has been shown to possess anti-oxidative (4), anti-inflammatory (5), neuroprotective (6), and chemopreventive (7) effects. Omega-6 and -9 have been linked to obesity prevention (8) and anti-inflammation (9).
However, there has been little reported on omega-7 compared with other omega fatty acids. Omega-7, also known as palmitoleic acid (16:1, Cis-9-hexadecenoic acid), is a monounsaturated fatty acid that is found in fishes and plants, such as macadamias, cold water fish, and sea buckthorn berries (10). Previous research has shown that omega-7 can improve cardiovascular function (11) and increase insulin sensitivity (12). However, the effects of omega-7 on the health of skin have not yet been elucidated. Thus, we investigated the physiological activity of 7-MEGATM 500 (purity 50% of omega-7, 7-MEGA) in human keratinocytes, which reside in the main outermost layer of the skin. As expected, we determined that 7-MEGA exhibited anti-oxidation and anti-inflammatory effects and improved skin cell regeneration, which suggests it may be useful as a functional food supplement for promoting skin health.
MATERIALS AND METHODS
Preparation of 7-MEGATM 500
7-MEGATM 500 concentrates were made by Organic Technologies in Eastern Ohio, USA. Pollock was recovered from the Alaskan Bering Sea, and was subsequently processed, purified, and concentrated to produce 7-MEGATM 500 containing more than 500 mg/g of palmitoleic acid (Table 1). 7-MEGA was dissolved in 99.5% ethanol and stocked at −80°C. Palmitoleic acid (purity 98.5% of omega-7, PA) (Sigma-Aldrich, St. Louis, MO, USA) was also dissolved under the same conditions. Every aliquot was used not more than two times.
Table 1.
7-MEGA main compounds
| Molecular formula | Name | mg/g % | |
|---|---|---|---|
| C14:0 | Myristic | 4.4 ± 5.0 | 0.04 |
| C16:0 | Palmitic | 257.3 ± 27.1 | 25.7 |
| C16:1 n-7 | Palmitoleic | 535.6 ± 10.9 | 53.5 |
| C20:5 | Eicosapentaenoic (EPA) | 5.6 ± 6.4 | 0.06 |
Cell culture
The human keratinocyte cell line HaCaT (ATCC, Rockville, MD, USA) was maintained in Dulbecco’s Modified Eagle Medium (DMEM) (HyClone, Logan, UT, USA),containing 10% FBS (Gibco, CA, USA) and 1% antibiotics (penicillin [100 U/mL], streptomycin [100 μg/mL]) (Sigma-Aldrich) in a 37°C, 5% CO2 incubator. The medium was changed every 2~3 days. Cells were subcultured in a 100-mm culture dish (Nunc, Rochester, NY, USA) 24 hr before treatment.
Free radical scavenging activity assay
The free radical scavenging activity of garlic extracts on 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals was determined using the method described by Blois (13). Ascorbic acid (vitamin C, Vc) was used as a positive control. Vc and PAwas dissolved in 99.5% ethanol to 1 mg/mL. 7-MEGA concentrates were used at the same volume as PAin 99.5% ethanol. Then, 10 μL of 7-MEGA concentrate was mixed with 90 μL of DPPH solution (126 μg/mL). After incubation for 10 min at room temperature in the dark, the absorbance at 517 nm was measured using a plate reader (BioTek, Winooski, VT, USA). The free radical scavenging activity of the sample was calculated by the following formula:
DPPH free radical scavenging activity (%) = (Control group OD – Sample addition group OD/Control group OD) × 100.
Cell viability assay
Cell viability was determined using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kit (Sigma-Aldrich). HaCaT cells were plated at 2 × 104 cells/well on a 48-well plate (Nunc). After 24 hr incubation, cells were treated with either 7-MEGA (10~100 nL/mL) or PA (100 nL/mL) for 24 hr. We then investigated whether 1 hr of pretreatment with 7-MEGA (10~100 nL/mL) or PA (100 nL/mL) affected the cell viability of HaCaT cells treated with 1 mM H2O2 for 24 hr. After the incubation period, 10 μL of the MTT solution (500 μg/mL) was added to each well and cells were incubated for 2 hr in a 37°C, 5% CO2 incubator. The absorbance was determined at 540 nm using a microplate reader (BioTek).
Oxidative stress assay
The level of intracellular ROS was quantified by fluorescence using dichlorofluorescin diacetate (DCF-DA; Invitrogen, Carlsbad, CA, USA). HaCaT cells were first plated in a 48-well plate. We then investigated whether 1 hr of pretreatment with 7-MEGA (10~100 nL/mL) or PA (100 nL/mL) affected ROS generation in HaCaT cells treated with 1 mM H2O2 for 5 min. After washing with PBS, cells were stained with 10 μM DCF-DA in PBS for 20 min in the dark at 37°C. Fluorescence was recorded with an excitation wavelength of 525 nm.
Superoxide dismutase and glutathione assays
Superoxide dismutase (SOD) was measured using a SOD Assay Kit (Cayman Chemical, Ann Arbor, MI, USA). HaCaT cells were seeded in a 100-mm culture dishand cultured in a 37°C, 5% CO2 incubator for 24 hr. Then, cells were treated with various concentrations of 7-MEGA and 1 mM H2O2 in a 37°C, 5% CO2 incubator for 24 hr. After that, lysis buffer was added to the cells, which were subsequently homogenized and centrifuged. Then, 10 μL of sample was mixed with 200 μL radical detector and 20 μL xanthine oxidase at room temperature. After 30 min, absorbance was measured at 450 nm using a microplate reader (BioTek). Glutathione (GSH) was measured using a GSH assay kit (Cayman Chemical). HaCaT cells were seeded in a 100-mm culture dish and maintained in a 37°C, 5% CO2 incubator for 24 hr. After that, cells were treated with various concentrations of 7-MEGA and 1 mM H2O2 in a 37°C, 5% CO2 incubator for 24 hr. After that, lysis buffer was added to the cells, which were homogenized and centrifuged. Then, 50 μL of sample was mixed with 150 μL reaction buffer (which included MES buffer, cofactor mixture, enzyme mix, and DTNB mixture buffer in the dark and at room temperature. After 30 min, absorbance was measured at 410 nm using a microplate reader (BioTek).
Western blotting
After the cells were treated in the same manner as the method for measuring cell viability, total protein from HaCaT cell lysates were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis using 12% gels. Protein bands were then transferred to PVDF membranes (BioRad, Hercules, CA, USA), which were blocked with 5% skim milk in PBS then incubated with a 1:1000 v/v dilution of primary antibodies against COX-2, PGE2, PCOL1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), β-actin (Cell Signaling, Danvers, MA, USA), MMP-1 and Elastin (Abcam, Cambridge, MA, USA) in PBS with 1% skim milk overnight at 4°C. The blots were then incubated with peroxidase-conjugated goat anti-rabbit IgG (PGE2, β-actin, MMP-1, Elastin) and were then incubated with peroxidase-conjugated goat anti-mouseIgG (COX-2, PCOL1) (1:10,000 v/v, Millipore, CA, USA) for 1 hr. The immunoreactions were visualized with SuperSignal West Dura Extended Duration Substrate (Thermo Scientific, CA, USA) on a ChemiImager analyzer system (Alpha Innotech, San Leandro, CA, USA).
Inflammatory cytokine analysis
After the cells were treated in the same manner as the method for measuring cell viability, the concentrations of IL-1β and TNF-α in samples of supernatant were determined with ELISA kits (Abcam). In a 96-well plate, 100 μL samples were plated in cell culture medium at room temperature for 150 min. Then, 100 μL biotinylated IL-1β and TNF-α detection antibodies were added and cells were incubated at room temperature for 1 hr. Then, 100 μL HRP-streptavidin solution was added and cells were further incubated at room temperature for 45 min. Next, 100 μL TMB 1-Step Substrate reagent was added and cells were incubated at room temperature for an additional 30 min. The plate was washed between each step. For the final step, 50 μL stop solution was added to the wells without washing and absorbance was measured at 450 nm using a microplate reader (BioTek).
Statistical analysis
All experiments were performed at least in triplicate, and data were expressed as means ± SEMs. Statistical significance was determined using Student’s t-test and ANOVA to assess differences between two groups. Differences with p-values < 0.05 were considered statistically significant.
RESULTS AND DISCUSSION
Cytotoxicity and DPPH radical scavenging action 7-MEGA in HaCaT cells
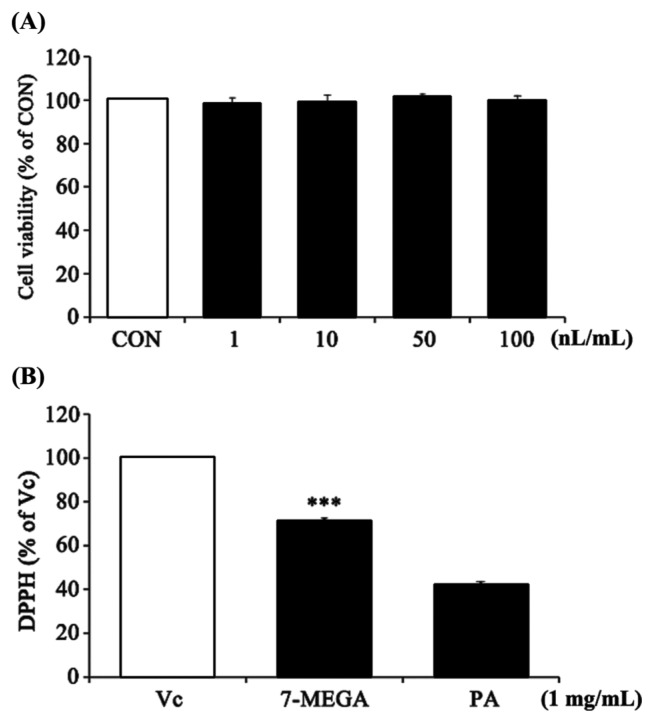
To assess the cytotoxicity of 7-MEGA in HaCaT cellsand potential anti-oxidant effects of 7-MEGA, MTT and DPPH free radical scavenging activity assays were conducted. HaCaT cells were treated with 7-MEGA at a concentration of 1~100 nL/mL for 24 hr. There were no visible cytotoxic effects of 7-MEGA until a concentration of 100 nL/mL was used, when compared with untreated control cells (Fig. 1A). At a concentration of 1 mg/mL, 7-MEGA showed DPPH free radical scavenging activity at 71 ± 1.3% of that of Vitamin C, and about 30% higher than that of PA (Fig. 1B). Thus, 7-MEGA showed no toxicity until a concentration of 100 nL/mL in HaCaT cells, and showed potential as an anti-oxidant material.
Fig. 1.
Cytotoxicity and DPPH radical scavenging ability of 7-MEGA in HaCaT cells. (A) Viability of HaCaT cells after treatment with increasing concentrations of 7-MEGA (1~100 nL/mL) for 24 hr. (B) DPPH radical scavenging ability of 7-MEGA. Data are expressed as the mean ± SEM of three independent experiments, ***p < 0.001 vs. PA.
Effect of 7-MEGA on cell viability in HaCaT cells under oxidative stress
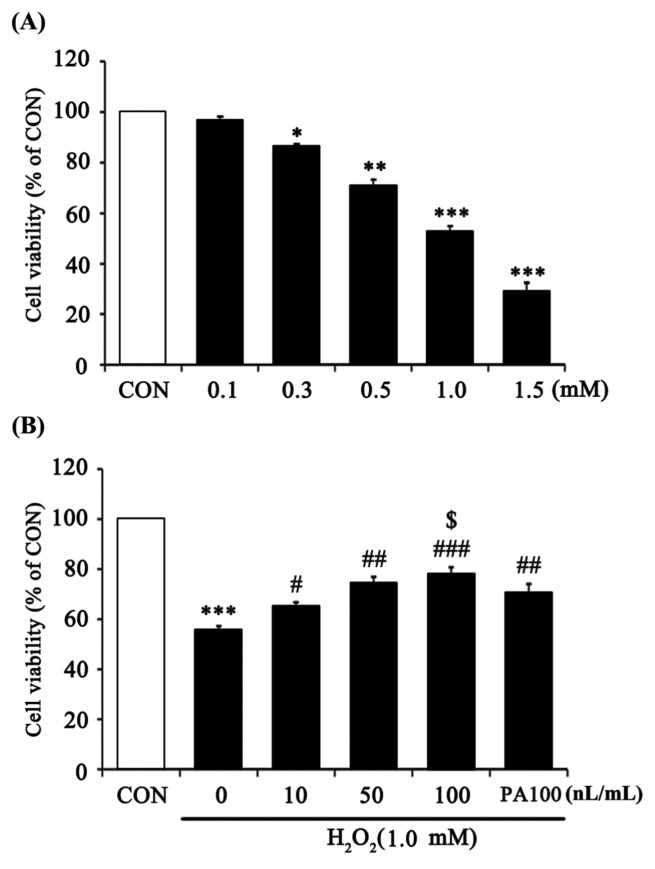
To quantify the oxidative stress effects of H2O2, HaCaT cells were treated with H2O2 at various concentrations (0.1~1.5 mM) for 24 hr (Fig. 2A). Cell viability decreased 60% after 24 hr of treatment with 1.0 mM H2O2. Thus, 1.0 mM H2O2 was used in all subsequent experiments. Pretreatment with 7-MEGA for 1 hr prior to incubation with 1 mM H2O2 for 24 hr resulted in significantly increased viability compared with cells incubated with H2O2 alone. Especially, compared to using the same concentration (100 nL/mL) of PA, cells treated with 7-MEGA showed a higher cell survival rate. This result shows that 7-MEGA had a higher protective effect against cell damage from oxidative stress when compared to PA (Fig. 2B).
Fig. 2.
Protective effect of 7-MEGA on cell viability against oxidative stress induced by H2O2 in HaCaT cells. Viability of HaCaT cells after treatment with increasing concentrations of H2O2 (0.1~1.5 mM) for 24 hr. (B) Viability of HaCaT cells after treatment with 1.0mM H2O2 for 24 hr after pretreatment with 7-MEGA (10~100 nL/mL) for 1 hr. Data are expressed as the mean ± SEM of three independent experiments, *p<0.05, **p < 0.01, ***p < 0.001 vs CON, #p<0.05, ##p< 0.01, ###p <0.001 vs. H2O2, $p< 0.05 vs. PA100.
Anti-oxidantive effect of 7-MEGA in HaCaT cells under oxidative stress
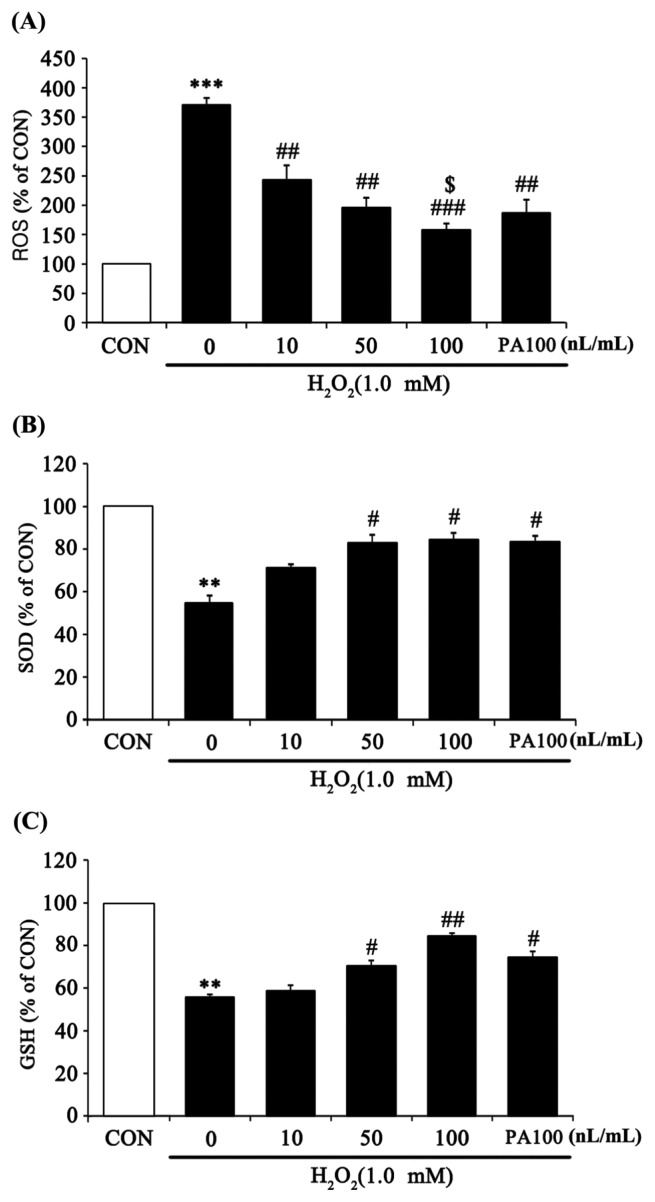
To confirm the anti-oxidant capacity of 7-MEGA to eliminate oxidative stress, ROS, SOD, and GSH were measured in HaCaT cells treated with various concentrations of 7-MEGA and 1.0 mM H2O2. ROS are a highly active intermediate product of oxygen molecules that are incompletely reduced during respiration (3). When reacting with surrounding biomolecules, such as lipids, nucleic acids and proteins, ROS interrupt normal cellular function, thus inhibiting skin regeneration and promoting skin aging (14). From the experimental results, ROS production significantly increased after H2O2 treatment compared with untreated controls. However, ROS production in 7-MEGA and H2O2 co-treated cells significantly decreased in a dose-dependent manner compared with cells treated with H2O2 alone. Especially, compared to cells treated with the same concentration (100 nL/mL) of PA, those treated with 7-MEGA showed a significant decrease in ROS production (Fig. 3A). SOD is an enzyme that catalyzes the process of defending cells from oxidative toxicity by catalyzing a disproportionation reaction that converts excess oxidizing ions into oxygen and hydrogen peroxide in the first step of anti-oxidation (15). GSH is an enzyme that prevents peroxidative damage in organisms by catalyzing the reaction which produces oxidized glutathione and water from H2O2 and reduced glutathione (16). In this study, SOD and GSH production in cells treated with H2O2 alone were significantly decreased compared to untreated control cells. On the other hand, cells co-treated with 7-MEGA and H2O2 showed a significant increase in the production of these two enzymes in a dose-dependent manner compared with cells treated with H2O2 alone. When compared to cells treated with the same concentration (100 nL/mL) of PA, cells treated with 7-MEGA didn’t show significantly increased SOD or GSH production (Fig. 3B, 3C).
Fig. 3.
Effect of 7-MEGA on reactive oxygen species (ROS) generation and anti-oxidative activity (SOD, GSH) in H2O2-treated HaCaT cells. (A) HaCaT cells were pretreated with 7-MEGA (10~100 nL/mL) for 1 hr, then oxidative stress was induced using H2O2 (1.0 mM) for 5min. ROS generation was evaluated by DCF-DA. (B–C) HaCaT cells were pretreated with 7-MEGA (10~100 nL/mL) for 1 hr, then oxidative stress was induced using H2O2 (1.0mM) for 24 hr. SOD and GSH expression were measured in cell lysates by ELISA. Data are expressed as the mean± SEM of three independent experiments, **p< 0.01, ***p< 0.001 vs. CON, #p<0.05, ##p<0.01, ###p< 0.001 vs. H2O2, $p< 0.05 vs. PA100.
Anti-inflammatory effect of 7-MEGA in HaCaT cells under oxidative stress
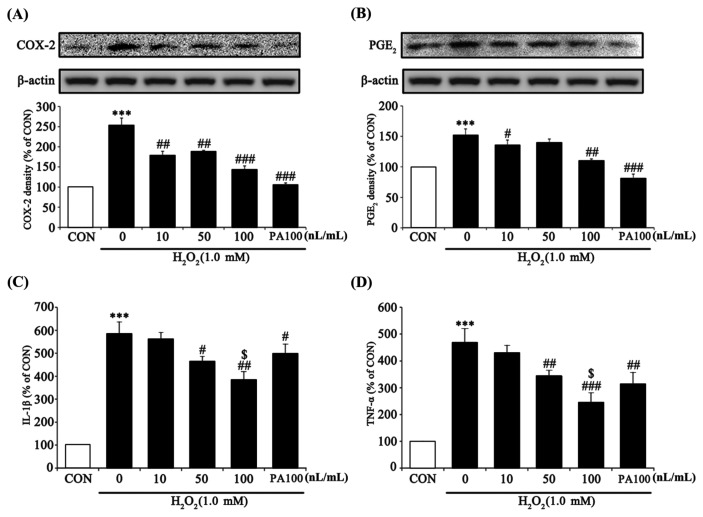
To assess the anti-inflammation effect of 7-MEGA, pro-inflammatory factors were measured in HaCaT cells treated with various concentrations of 7-MEGA and 1.0 mM H2O2. An inflammatory reaction occurs when there is too much active oxygen in the body (17,18). TNF-α and IL-1β are representative inflammatory cytokines, and act in the early stage of the inflammatory reaction. These cytokines are produced by activation of COX-2. COX-2 is an enzyme that catalyzes the production of PGE2 and plays a major role in controlling the inflammatory reaction, cell proliferation and necrosis, and cytokine generation (19,20). In this experiment, COX-2 and PGE2 expression in cells treated with H2O2 alone were significantly increased, whereas cells co-treated with 7-MEGA and H2O2 exhibited significantly decreased expression of these factors in a dose-dependent manner compared with the cells treated with H2O2 alone. When compared to cells treated with the same concentration (100 nL/mL) of PA, cells treated with 7-MEGA didn’t show significantly different COX-2 and PGE2 expression (Fig. 4A, 4B). IL-1β and TNF-α generation in cells treated with H2O2 alone were significantly increased, whereas cells co-treated with 7-MEGA and H2O2 shoed significantly decreased expression of these two pro-inflammatory cytokines in a dose-dependent manner compared to cells treated with H2O2 alone. Especially, when compared to cells treated with the same concentration (100 nL/mL) of PA, cells treated with 7-MEGA showed significantly decreased IL-1β and TNF-α generation (Fig. 4C, 4D). Together, these results demonstrate that 7-MEGA induced strong anti-inflammatory activity in HaCaT cells.
Fig. 4.
Effect of 7-MEGA on the protein expression of pro-inflammatory markers (TNF-α, IL-1β), COX-2, and PGE2 in H2O2-treated HaCaT cells. HaCaT cells were pretreated with 7-MEGA (10~100 nL/mL) for 1 hr, then oxidative stress was induced using H2O2 (1.0 mM) for 24 hr. (A–B) Whole cell lysates were subjected to Western blot analysis to evaluate COX-2 and PGE2 expression. (C–D) IL-1β and TNF-α were measured in the culture supernatant by ELISA. Data are expressed as the mean ± SEM of three independent experiments, ***p< 0.001 vs. CON, #p<0.05, ##p< 0.01, ###p< 0.001, vs. H2O2, $p< 0.05 vs. PA100.
Skin regenerative effect of 7-MEGA in HaCaT cells under oxidative stress
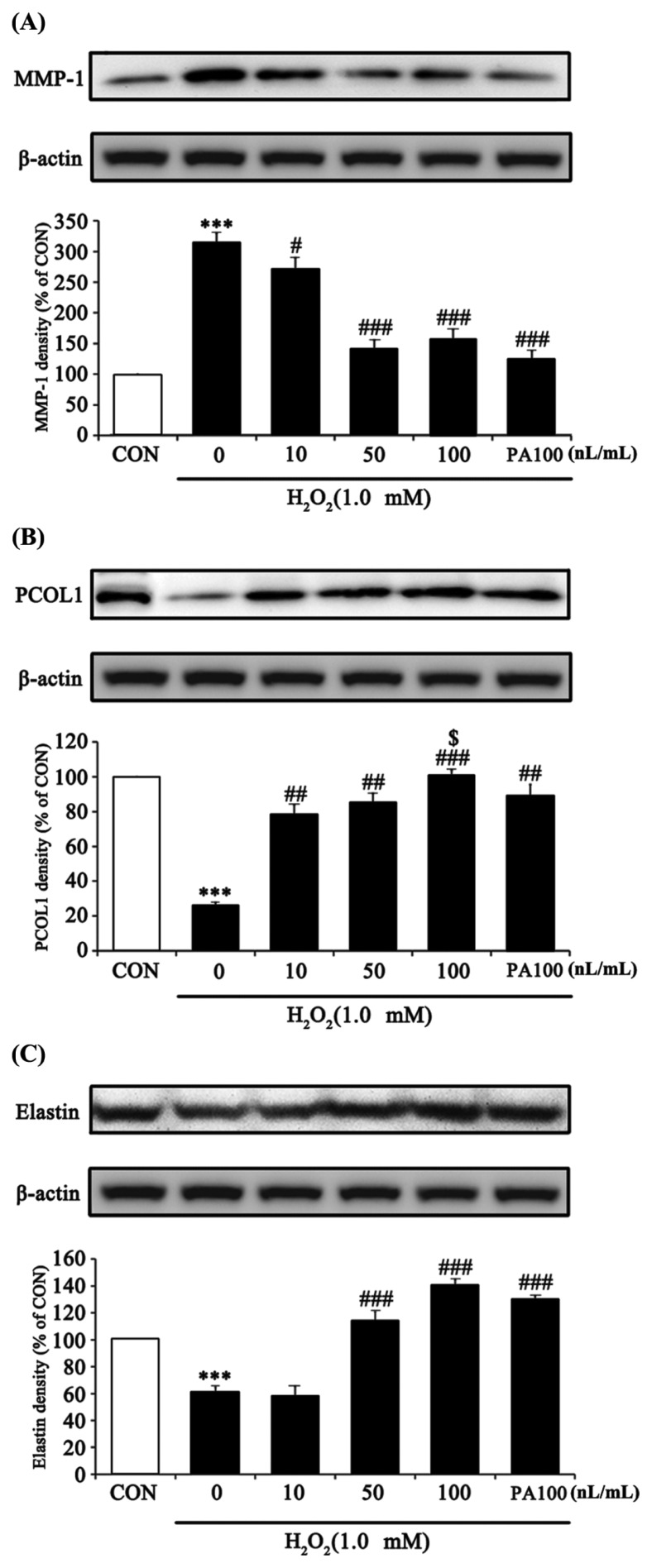
To evaluate the skin regeneration effect of 7-MEGA, MMP-1, PCOL1 and Elastin were measured in HaCaT cells treated with various concentrations of 7-MEGA and 1.0 mM H2O2. Collagen is one of the main constituents of connective tissue, and a balance between the activity of PCOL1, a synthesizing enzyme, and MMP-1, a catabolic enzyme, is maintained in skin cells (21,22). An imbalance in the activity of both of these enzymes leads to a decrease in collagen and elastin production, followed by the formation of wrinkles and a reduction in skin regeneration (23–27). In this experiment, the protein expression of skin regeneration factors was measured to examine how 7-MEGA impacts the H2O2-induced oxidative stress response. According to the results, MMP-1 expression in cells treated with H2O2 alone was significantly increased, whereas co-treatment with 7-MEGA and H2O2 significantly decreased the expression of MMP-1 in a dose-dependent manner when compared to treatment with H2O2 alone. When compared to cells treated with the same concentration (100 nL/mL) of PA, cells treated with 7-MEGA didn’t exhibit significantly decreased MMP-1 expression (Fig. 5A). PCOL1 and Elastin expression in cells treated with H2O2 alone were significantly decreased, whereas in cells co-treated with 7-MEGA and H2O2 there was significantly increased expression of these markers in a dose-dependent manner compared with the cells treated with H2O2 alone. Especially, when compared to cells treated with the same concentration (100 nL/mL) of PA, cells treated with 7-MEGA showed significantly increased PCOL1 expression. However, there was no significant difference in Elastin expression between these two cell populations (Fig. 5B, 5C). The ability of MMP-1 to regulate collagen synthesis was reduced by oxidative stress induced by H2O2. Pretreatment with 7-MEGA inhibited collagen degradation caused by MMP-1 downregulation, increased collagen synthesis by increasing PCOL1 expression, and increased skin elasticity through an increase in Elastin. Thus, 7-MEGA can be used to improve the functionality of skin as a protective barrier, as well as to improve wrinkles and prevent skin aging.
Fig. 5.
Effect of 7-MEGA on MMP-1, procollagen type 1, Elastin protein expression in H2O2-treated HaCaT cells. HaCaT cells were pretreated with 7-MEGA (10~100 nL/mL) for 1 hr, then oxidative stress was induced using H2O2 (1.0 mM) for 24 hr. (A–C) Whole cell lysates were subjected to Western blot analysis to evaluate MMP-1, PCOL1 and Elastin expression. Data are expressed as the mean ± SEM of three independent experiments, ***p< 0.001 vs. CON, #p< 0.05, ##p<0.01, ###p< 0.001, vs. H2O2, $p< 0.05 vs. PA100.
In conclusion, our results suggest that 7-MEGA has anti-inflammatory effects in HaCaT cells, where it promotes collagen regeneration in the presence of H2O2-induced cytotoxicity. In addition, present study was showed that 7-MEGA has higher DPPH free radical scavenging activity and protective effect against cell damage from oxidative stress than PA. We could have expected this result that 7-MEGA, which contains various substances including omega-7, is more effective than omega-7 as a single substance. Our results provide strong evidence for 7-MEGA as a functional food for promoting skin health to prevent aging.
ACKNOWLEDGMENTS
The research was supported by Vitech Co., Ltd.
Abbreviation
- 7-MEGA
7-MEGATM 500
- COX-2
cyclooxygenase-2
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- H2O2
hydrogen peroxide
- IL-1β
interleukin-1β
- MMP-1
matrix metalloprotease-1
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PA
palmitoleic acid
- PCOL1
pro-collagen type 1
- PGE2
prostaglandin E2
- ROS
reaction oxygen species
- TNF-α
tumor necrosis factor-α.
REFERENCES
- 1.Fisher GJ. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B, Gutteridge JM. The importance of free radicals and catalytic metal ions in human diseases. Mol Aspects Med. 1985;8:89–193. doi: 10.1016/0098-2997(85)90001-9. [DOI] [PubMed] [Google Scholar]
- 3.Ozben T. Oxidative stress and apoptosis: impact on cancer therapy. J Pharm Sci. 2007;96:2181–2196. doi: 10.1002/jps.20874. [DOI] [PubMed] [Google Scholar]
- 4.Otton R, Marin DP, Bolin AP, de Cássia Santos Macedo R, Campoio TR, Fineto C, Jr, Guerra BA, Leite JR, Barros MP, Mattei R. Combined fish oil and astaxanthin supplementation modulates rat lymphocyte function. Eur J Nutr. 2012;51:707–718. doi: 10.1007/s00394-011-0250-z. [DOI] [PubMed] [Google Scholar]
- 5.Calder PC. Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol Nutr Food Res. 2008;52:885–897. doi: 10.1002/mnfr.200700289. [DOI] [PubMed] [Google Scholar]
- 6.Bazan NG. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr Opin Clin Nutr Metab Care. 2007;10:136–141. doi: 10.1097/MCO.0b013e32802b7030. [DOI] [PubMed] [Google Scholar]
- 7.Park JM, Kwon SH, Han YM, Hahm KB, Kim EH. Omega-3 polyunsaturated Fatty acids as potential chemopreventive agent for gastrointestinal cancer. J Cancer Prev. 2013;18:201–208. doi: 10.15430/JCP.2013.18.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whigham LD, Watras AC, Schoeller DA. Efficacy of conjugated linoleic acid for reducing fat mass: a meta-analysis in humans. Am J Clin Nutr. 2007;85:1203–1211. doi: 10.1093/ajcn/85.5.1203. [DOI] [PubMed] [Google Scholar]
- 9.Finucane OM, Lyons CL, Murphy AM, Reynolds CM, Klinger R, Healy NP, Cooke AA, Coll RC, McAllan L, Nilaweera KN, O’Reilly ME, Tierney AC, Morine MJ, Alcala-Diaz JF, Lopez-Miranda J, O’Connor DP, O’Neill LA, McGillicuddy FC, Roche HM. Monounsaturated fatty acid-enriched high-fat diets impede adipose NLRP3 inflammasome-mediated IL-1β secretion and insulin resistance despite obesity. Diabetes. 2015;64:2116–2128. doi: 10.2337/db14-1098. [DOI] [PubMed] [Google Scholar]
- 10.Maguire LS, O’Sullivan SM, Galvin K, O’Connor TP, O’Brien NM. Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. Int J Food Sci Nutr. 2004;55:171–178. doi: 10.1080/09637480410001725175. [DOI] [PubMed] [Google Scholar]
- 11.Anderson MM, Hazen SL, Hsu FF, Heinecke JW. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxyamino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha,beta-unsaturated aldehydes by phagocytes at sites of inflammation. J Clin Invest. 1997;99:424–432. doi: 10.1172/JCI119176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Souza CO, Teixeira AAS, Lima EA, Batatinha HAP, Gomes LM, Carvalho-Silva M, Mota IT, Streck EL, Hirabara SM, Rosa Neto JC. Palmitoleic acid (n-7) attenuates the immunometabolic disturbances caused by a high-fat diet independently of PPARalpha. Mediators Inflamm. 2014;2014:582197. doi: 10.1155/2014/582197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furuno K, Akasako T, Sugihara N. The contribution of the pyrogallol moiety to the superoxide radical scavenging activity of flavonoids. Biol Pharm Bull. 2002;25:19–23. doi: 10.1248/bpb.25.19. [DOI] [PubMed] [Google Scholar]
- 14.Babior BM. Phagocytes and oxidative stress. Am J Med. 2000;109:33–44. doi: 10.1016/S0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 15.Slater TF. Free-radical mechanisms in tissue injury. Biochem J. 1984;222:1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shanker G, Syversen T, Aschner JL, Aschner M. Modulatory effect of glutathione status and antioxidants on methylmercury-induced free radical formation in primary cultures of cerebral astrocytes. Brain Res Mol Brain Res. 2005;137:11–22. doi: 10.1016/j.molbrainres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Wu Q, Li H, Qiu J, Feng H. Betulin protects mice from bacterial pneumonia and acute lung injury. Microb Pathog. 2014;75:21–28. doi: 10.1016/j.micpath.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Choi WS, Shin PG, Lee JH, Kim GD. The regulatory effect of veratric acid on NO production in LPS-stimulated RAW264.7 macrophage cells. Cell Immunol. 2012;280:164–170. doi: 10.1016/j.cellimm.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Raz A, Wyche A, Siegel N, Needleman P. Regulation of fibroblast cyclooxygenase synthesis by interleukin-1. J Biol Chem. 1988;263:3022–3028. [PubMed] [Google Scholar]
- 20.Feldman M, Taylor P, Paleolog E, Brennan FM, Maini RN. Anti-TNF alpha therapy is useful in rheumatoid arthritis and Crohn’s disease: analysis of the mechanism of action predicts utility in other diseases. Transplant Proc. 1998;30:4126–4127. doi: 10.1016/S0041-1345(98)01365-7. [DOI] [PubMed] [Google Scholar]
- 21.Stetler-Stevenson WG, Yu AE. Proteases in invasion: matrix metalloproteinases. Semin Cancer Biol. 2001;11:143–152. doi: 10.1006/scbi.2000.0365. [DOI] [PubMed] [Google Scholar]
- 22.Vincenti MP, White LA, Schroen DJ, Benbow U, Brinckerhoff CE. Regulating expression of the gene for matrix metalloproteinase-1 (collagenase): mechanisms that control enzyme activity, transcription, and mRNA stability. Crit Rev Eukaryot Gene Expr. 1996;6:391–411. doi: 10.1615/CritRevEukarGeneExpr.v6.i4.40. [DOI] [PubMed] [Google Scholar]
- 23.Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ, Fisher GJ. Matrix-degrading metalloproteinases in photoaging. J Investig Dermatol Symp Proc. 2009;14:20–24. doi: 10.1038/jidsymp.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pentland AP, Shapiro SD, Welgus HG. Agonist-induced expression of tissue inhibitor of metalloproteinases and metalloproteinases by human macrophages is regulated by endogenous prostaglandin E2 synthesis. J Invest Dermatol. 1995;104:52–57. doi: 10.1111/1523-1747.ep12613488. [DOI] [PubMed] [Google Scholar]
- 25.Mauviel A, Halcin C, Vasiloudes P, Parks WC, Kurkinen M, Uitto J. Uncoordinate regulation of collagenase, stromelysin, and tissue inhibitor of metalloproteinases genes by prostaglandin E2: selective enhancement of collagenase gene expression in human dermal fibroblasts in culture. J Cell Biochem. 1994;54:465–472. doi: 10.1002/jcb.240540413. [DOI] [PubMed] [Google Scholar]
- 26.Talwar HS, Griffiths CE, Fisher GJ, Hamilton TA, Voorhees JJ. Reduced type I and type III procollagens in photodamaged adult human skin. J Invest Dermatol. 1995;105:285–290. doi: 10.1111/1523-1747.ep12318471. [DOI] [PubMed] [Google Scholar]
- 27.Xu Q, Hou W, Zheng Y, Liu C, Gong Z, Lu C, Lai W, Maibach HI. Ultraviolet A-induced cathepsin K expression is mediated via MAPK/AP-1 pathway in human dermal fibroblasts. PLoS ONE. 2014;9:e102732. doi: 10.1371/journal.pone.0102732. [DOI] [PMC free article] [PubMed] [Google Scholar]