Abstract
Anti-cancer drugs such as cisplatin and doxorubicin are effectively used more than radiotherapy. Cisplatin is a chemotherapeutic drug, used for treatment of various forms of cancer. However, it has side effects such as ototoxicity and nephrotoxicity. Cisplatin-induced nephrotoxicity increases tubular damage and renal dysfunction. Consequently, we investigated the beneficial effect of cynaroside on cisplatin-induced kidney injury using HK-2 cell (human proximal tubule cell line) and an animal model. Results indicated that 10 μM cynaroside diminished cisplatin-induced apoptosis, mitochondrial dysfunction and caspase-3 activation, cisplatin-induced upregulation of caspase-3/MST-1 pathway decreased by treatment of cynaroside in HK-2 cells. To confirm the effect of cynaroside on cisplatin-induced kidney injury in vivo, we used cisplatin exposure animal model (20 mg/kg, balb/c mice, i.p., once a day for 3 days). Renal dysfunction, tubular damage and neutrophilia induced by cisplatin injection were decreased by cynaroside (10 mg/kg, i.p., once a day for 3 days). Results indicated that cynaroside decreased cisplatin-induced kidney injury in vitro and in vivo, and it could be used for improving cisplatin-induced side effects. However, further experiments are required regarding toxicity by high dose cynaroside and caspase-3/MST-1-linked signal transduction in the animal model.
Keywords: Cisplatin, Cynaroside, HK-2, Nephrotoxicity, MST-1
INTRODUCTION
Cynaroside is a flavone, a flavonoid-like compound. It is known under various names (Luteolin-7-O-glucoside, Luteoloside, Cinaroside), commonly found in Lonicera japonica Thunb. and Angelica keiskei. According to recent studies, cynaroside has absorbed in gastrointestinal tract, and it has an anti-oxidant effect, inhibiting lipid and protein oxidation (1–4). However, the protective effect of cynaroside on cisplatin-induced nephrotoxicity has not been elucidated.
Incidence of cancer mortality has increased with pollution and diet, despite development of medical standards and life (5). Cisplatin (cis-diamminedichloroplatium[II]) an anti-cancer drug, is commonly used in cancer treatment. However, despite wide use of cisplatin for cancer treatment, there are restrictions in using this anti-cancer drug because it may induce cardiotoxicity and nephrotoxicity (6). Cisplatin is eliminated from the body through filtration and secretions in kidneys, but it may cause nephrotoxicity if excessively used for cancer treatment (7–9). Thus, research on medicinal plants and active ingredients isolated from resource plants are needed for reducing negative side effects of chemotherapy.
MST (mammalian sterile 20-like kinase), a protein kinase (10), is involved in various cellular processes, such as transcriptional regulation, cell death, signal transduction, and post-translational modification (11,12). The MST protein family are common proteins expressed in almost all tissues. MST-1 was mostly expressed in kidneys. It was proteolytically activated caspase protein during apoptosis, and activated by apoptotic stimulation as well as other stress responses. MST-1 activation requires phosphorylation and caspase-3 mediated cleavage, resulting in autophosphorylation at Thr183 and Thr187 within its catalytic domain, leading to initiation of cell death including DNA fragmentation and chromatin condensation (13–15). Therefore, this study focused on the role of cynaroside under the condition of cisplatin exposure in HK-2 cell (human proximal tubule cell line). Thus, this study investigated 1) the relationship between cynaroside and caspase-3/MST-1 signal pathway in cisplatin-induced nephrotoxicity, 2) the protective effect of cynaroside on cisplatin-induced nephrotoxicity in vivo model.
MATERIALS AND METHODS
Chemicals
Cynaroside isolated from Lonicera japonica Thunb. (CAS 5373-11-5, purity: 97.5%) was purchased from Biopurify (Chengdu, Sichuan, China). Cisplatin (479306) and was purchased from Sigma Aldrich (St. Louis, MO, USA). Cisplatin fully dissolved in 0.9% NaCl, was stored at −20°C by protection from light.
Cell culture
HK-2 cells (human kidney proximal tubule cell) obtained from the Korean Cell Line Bank (KCLB, Seoul, Korea), were grown at 37°C in 5% CO2 in Roswell Park Memorial Institute medium (RPMI 1640 medium) with 10% FBS (fetal bovine serum) and 1% penicillin/streptomycin. The media was changed every other day. Passaged cells were plated to yield near-confluent cultures at the end of experiments.
Animals
Male BALB/c mice (6 weeks of age) purchased from Samtako (Osan, Korea) were separated into 3 groups (Control; n = 7, Cisplatin; n = 7, Cisplatin + cynaroside; n = 7). Mice were administrated cisplatin (20 mg/kg, i.p., once a day, 3 days). After cisplatin administration for 30 min, cisplatin + cynaroside group was administrated with cynaroside (10 mg/kg, i.p., once a day, 3 days).
Measurement of cell viability
Cell viability measured by using Celliter 96® aqueous one solution cell proliferation assay (Promega, Fitchburg, WI, USA), according to manufacturer’s instructions. Cells cultured in 96 well plate were incubated with various concentration of cynaroside (10, 20, 40, and 80 μM) or after treatment with 20 μM cisplatin for 30 min, HK-2 cells were treated with cynaroside (10, 20, 40, and 80 μM) for 24 hr. Cells were washed twice PBS (phosphate buffered salin) and treated with RPMI 1640 medium containing 20 μL MTS solution for 2 hr. Absorbance measured by using microplate reader infinite® PRO (TECAN, Mannedorf, Switzerland) at 490 nm. Relative cell viability (%) was expressed as percentage relative to the control group (non-treated group).
Tunel assay
Tunel assay was conducted using Dead-EndTM fluorometric TUNEL system (Promega), according to manufacturer’s instructions. HK-2 cells were seeded on cover glass in 12 well plate fixed in 10% NBF (neutralized buffered formalin) at 4°C for 20 min followed by permeabilization with 0.2% (v/v) Triton X-100 in PBS for 5 min, and washed with cold PBS for twice. Washed cells were used for Tunel assay. Paraffin sections were deparaffinized with xylene (Sigma Aldrich) and hydrated with water and diluted ethanol, and used for Tunel assay. Samples were incubated with equilibration buffer provided by kit for 5 min. After 5 min, samples were incubated with 100 μL rTdT incubation buffer at 37°C for 1 hr, and washed with 2X SSC buffer for twice. Samples were mounted with ProLing® glod antifade reagent with DAPI (Thermo, Waltham, MA, USA). Imaging was conducted on the epifluorescence microscope (Carl Zeiss, Oberkochen, Germany).
Protein extraction and Wes analysis
HK-2 cells were lysed in RIPA cell lysis buffer 2 (Enzo, Farmingdale, NY, USA) containing PierceTM protease and phosphatase inhibitor mini tablets (Thermo) for 1 hr. Cell lysate was centrifuged for 10 min at 13,000 rpm at 4°C, protein levels were quantified using Bradford procedure. Whole cell lysate (1 μg) analyzed using Wes (Protein simple, CA, USA) according to manufacturer’s instructions.
Caspase-3 activity assay
Caspase-3 activity assay conducted by using Caspase-3 colorimetric detection kit (Enzo), according to manufacturer’s instructions. HK-2 cells were lysed in RIPA cell lysis buffer 2 (Enzo) containing PierceTM protease and phosphatase inhibitor mini tablets (Thermo) for 1 hr. Cell lysate was centrifuged for 10 min at 13,000 rpm at 4°C, protein levels were quantified using Bradford procedure. Whole cell lysate (20 μg) used to caspase-3 activity assay.
Annexin-V/PI (propidium iodide) staining
Annexin-V/PI staining conducted with Annexin V-FITC apoptosis detection kit (Enzo) according to modified manufacturer’s instructions. HK-2 cells cultured in 6 well plate were washed with cold PBS and then filled with 1 mL of Accutase (Innovative Cell Technologies, CA, USA). After 2 min, plate was carefully tapped to detach cells. Resuspended cells were washed twice with cold PBS and then resuspended in a binding buffer containing FITC-conjugated annexin V and PI. After 10 min at room temperature in the dark, apoptotic cells were analyzed by CytoFLEX (Beckman Clulter, Indianapolis, IN, USA). FITC and PI double negative cells (LL; lower-left) were considered as normal cells. FITC-positive and PI-negative cells (LR; lower-right) were early apoptotic cells and FITC and PI double positive cells (UR; upper-right) were late apoptotic cells. FITC-positive and PI positive cells (UL; upper-left) were considered as necrosis. Apoptotic cell death was calculated by the sum of early apoptotic cells and late apoptotic cells (LR + UR).
JC-1 staining
HK-2 cells cultured in 6 well plates were incubated with 2 μM 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl benzimidazolylcarbocyanine iodide (JC-1; Thermo) for 30 min. Cells were washed with cold PBS and then filled with fresh cold PBS. Stained cells were observed with the epi-fluorescence microscope (Carl Zeiss) under following fluorescence (JC-1 Monomer: Ex 485 and Em 530 nm, Aggregated JC-1: EX 535 and Em 590 nm). For flow cytometry analysis, washed cells were incubated with Accutase (Innovative Cell Technologies). After 2 min, plates were carefully tapped to detach cells. Resuspended cells were washed twice with cold PBS and then resuspended in cold PBS. Flow cytometry was used to quantify the JC-1 fluorescence. Lowered mitochondrial membrane potential is indicated by a switch to a decrease in red fluorescence accompanied with an increase in green fluorescence.
Blood cell analysis
Blood cells analysis conducted using IDEXX Procyte DX gematology analyzer (IDEXX, Westbrook, ME, USA) according to manufacturer’s instructions. Whole blood was collected in BD VactutainerTM glass blood collection tube with K3 EDTA (Thermo), and used for blood cell analysis. Imaging was indicated lymphocyte (blue color), neutrophil (pink color), and monocyte (red color).
Blood chemistry analysis
Blood chemistry analysis conducted using FUJI DRI-CHEM 4000i (Fujifilm, Tokyo, Japan), according to manufacturer’s instructions. Whole blood collected in BD VacutainerTM SST tube (Thermo), incubated at room temperature for 10 min. Blood centrifuged for 10 min at 4,000 rpm at 4°C, separated serum samples were used for blood chemistry analysis (BUN, blood urine nitrogen; Cre, creatinine).
Hematoxylin & Eosin staining
Kidneys were harvested from mice, fixed in 10% NBF overnight and then paraffinized with paraffin, xylene, and diluted ethanol. Paraffinized kidney samples were cut into 4 μM using microtome (Leica, Wetzlar, Germany). Paraffin sections were deparaffinized with xylene (Sigma Aldrich) and hydrated with water and diluted ethanol, and used for hematoxylin & eosin staining.
Statistical analysis
Results were expressed as mean ± the SEM. Values are mean ± SEM of three independent experiments. For group comparisons, statistical analysis was conducted using one-way ANOVA by SPSS (SPSS Inc., IL, USA), followed by the Tukey post hoc test and Duncan’s multiple rage test, were used. A value of p < 0.05 was considered significant.
RESULTS
Cynaroside treatment reduces cisplatin-induced cell death and DNA fragmentation in HK-2 cell
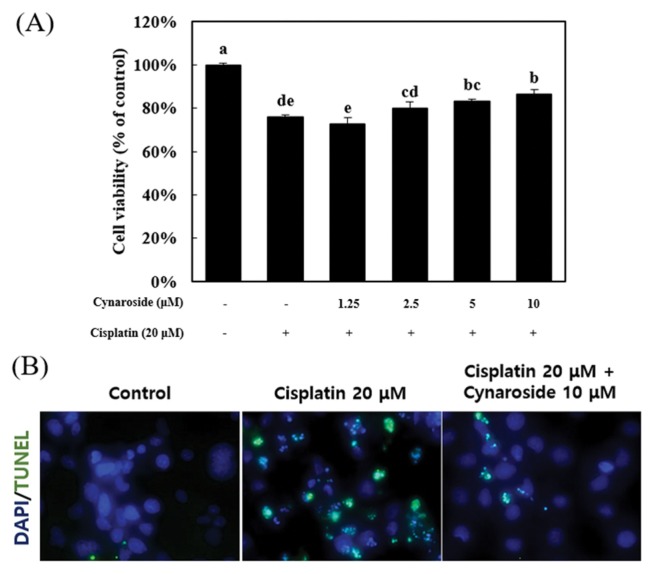
To confirm the protective effect of cynaroside in cisplatin-induced cell death, HK-2 cells (human proximal tubule cell) were pretreated with various concentrations (1.25, 2.5, 5, and 10 μM) for 30 min. After pre-treatment, cells were treated with 20 μM cisplatin for 24 hr. Cell viability was measured by MTS assay, HK-2 cell death was reduced by pretreatment of 5 and 10 μM cynaroside (Fig. 1A). As shown in Fig. 1A, cell viability significantly decreased by 20 μM cisplatin at 75.8 ± 1.0%, but recovered by 10 μM cynaroside at 86.6 ± 3.0%. Next, Tunel assay was conducted to reveal DNA fragmentation during the cell death process, visualized by Tunel assay (Fig. 1B). The Tunel positive signal increased by treatment of 20 μM cisplatin, but decreased by pre-treatment of 10 μM cynaroside. This result indicated that cisplatin-induced cell death was diminished by pre-treatment of 10 μM cynaroside in HK-2 cells.
Fig. 1.
Cynaroside prevents cisplatin-induced cell death in HK-2 cells. (A) HK-2 cells were treated with cynaroside for various concentration (1.25, 2.5, 5 and 10 μM). Cell viability was measured by MTS assay. Data represent the mean ± SEM of four independent experiments. (B) After pretreatment with 10 μM cynaroside, HK-2 cells were treated with 20 μM cisplatin for 24 hr. HK-2 cells were visualized by tunel assay. Representative images were taken from at least three independent experiments. *Means with difference letters are significantly different at p < 0.05 by Duncan’s multiple range test.
Cynaroside treatment attenuates cisplatin-induced apoptosis and mitochondrial dysfunction in HK-2 cell
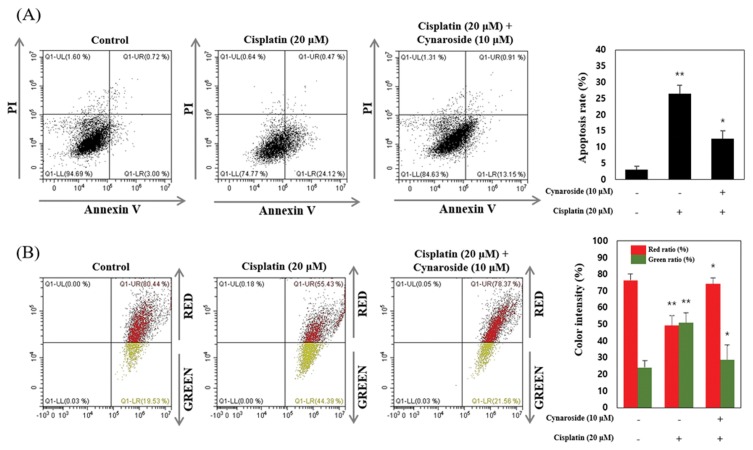
To reveal the inhibition effect of apoptosis and mitochondrial dysfunction during cell death, cells were pre-treated with 10 μM cynaroside for 30 min. After pre-treatment, cells were treated with 20 μM cisplatin for 24 hr. Apoptotic cell death was measured by Annexin V/PI staining. Under these condition, dysfunction of mitochondrial membrane potential (MMP) determined by JC-1 staining. As shown in Fig. 2A, apoptotic cell death increased by 20 μM cisplatin at 24.12 ± 2.6%. but was diminished by 10 μM cynaroside at 13.15 ± 2.4% (Fig. 2A). MMP is indicated by decrease in the red/green fluorescence intensity ratio, the green color shift is due to formation of red fluorescence J-aggregates. Results indicated that red fluorescence decreased by 20 μM cisplatin at 49.26 ± 5.8% than control group (red fluorescence; 76.41 ± 3.8%), and recovered by 10 μM cynaroside at 74.47 ± 3.5% (Fig. 2B). Collectively, apoptotic cell death and mitochondrial dysfunction were diminished by pre-treatment of cynaroside on cisplatin-induced cell death in HK-2 cell.
Fig. 2.
Cynaroside reduces cisplatin-induced apoptosis and mitochondrial dysfunction in HK-2 cells. (A–B) After pretreatment with 10 μM cynaroside, HK-2 cells were treated with 20 μM cisplatin for 24 hr. Annexin V and propidium iodide staining and JC-1 staining were analyzed by flow cytometry. Data represent the mean ± SEM of three independent experiments. Representative images were taken from at least three independent experiments. *Means with difference letters are significantly different at *p < 0.05 vs. cisplatin (20 μM), **p < 0.05 vs. control.
Cisplatin-induced caspase-3/MST-1 signal is attenuated by cynaroside treatment
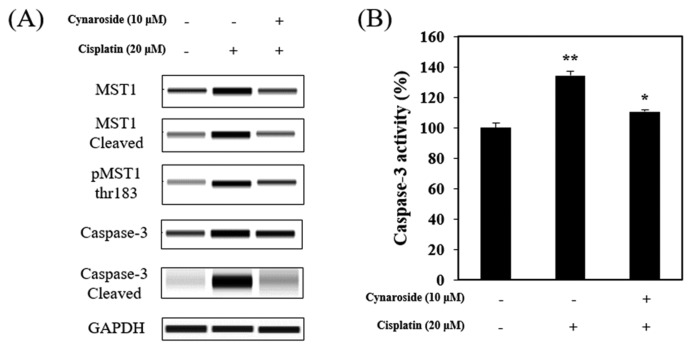
Amin et al. (13) reported that MST-1 interacted with caspase-3 protein during apoptotic cell death in mammalian cell. It has also been reported that MST-1 is mostly expressed in kidney, closely associated with apoptotic cell death (11). In this study, 20 μM cisplatin treatment increased Caspase-3/MST-1 signaling pathway, decreased by pre-treatment with 10 μM cynaroside (Fig. 3A). Caspase-3 activity increased by 20 μM cisplatin at 134.40 ± 1.5% than the control group, but was diminished with pre-treatment of 10 μM cynaroside at 110.70 ± 2.9% (Fig. 3B). In summary, cisplatin-induced apoptosis via caspase-3/MST-1 signaling pathway and caspase-3 activation was abolished by cynaroside treatment, suggesting that cynaroside may be inhibited caspase-3/MST-1 signaling during cisplatin-induced cell death in HK-2 cell.
Fig. 3.
Cynaroside reduces caspase-3/MST-1 signal pathway in HK-2 cells. (A–B) After pretreatment with 10 μM cynaroside, HK-2 cells were treated with 20 μM cisplatin for 24 hr. Cell extracts were subjected to protein analysis using wes and caspase-3 activity assay. Data represent the mean ± SEM of three independent experiments. Representative images were taken from at least three independent experiments. *Means with difference letters are significantly different at *p < 0.05 vs. cisplatin (20 μM), **p<0.05 vs. control.
Effects of cynaroside on cisplatin-induced nephrotoxicity on blood analysis
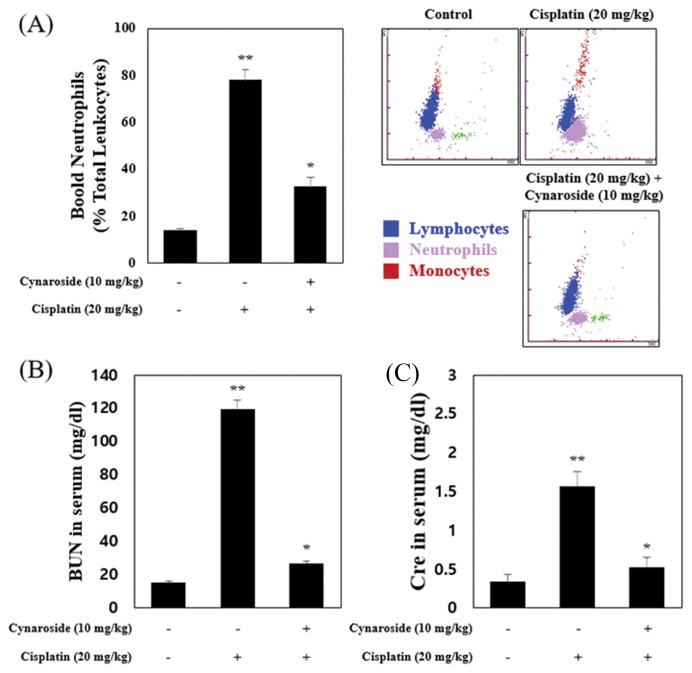
To evaluate the protective effect of cynaroside on cisplatin-induced nephrotoxicity in vivo, we used the cisplatin nephrotoxicity model (20 mg/kg, balb/c mice, i.p., once a day for 3 days). In accordance with results from HK-2 cells, cisplatin administration increased blood neutrophils (Fig. 4A). BUN (blood urine nitrogen) and Cre (creatinine) in serum increased by cisplatin administration (Fig. 4B). As shown Fig. 4A, neutrophils in whole blood increased by cisplatin administration at 77.47 ± 4.6% on white blood cells than the control group (blood neutrophil; 14.12 ± 0.7%), and recovered by cynaroside administration at 32.72 ± 3.6% on white blood cells. Serum level of BUN and Cre increased by cisplatin administration (cisplatin group; BUN, 124.90 ± 5.2 mg/dL; Cre, 1.57 ± 0.1 mg/dL), and decreased by cynaroside administration (cisplatin + cynaroside group; BUN, 26.57 ± 1.6 mg/dL; Cre, 0.52 ± 0.1 mg/dL). Collectively, serum level of BUN and Cre increased by cisplatin decreased by cyanroside administration in balb/c mice.
Fig. 4.
Cynaroside reduces BUN, Cre and neutrophils in blood on cisplatin animal models. (A–B) Mice were separated 3 group (Control; n = 7, Cisplatin (20mg/kg); n = 7, Cisplatin (20mg/kg) + Cynaroside (10mg/kg); n = 7). Mice were administered with vehicle or cisplatin (20mg/kg). Blood neutrophils were analyzed by IDEXX procyte (A). BUN (B) and Cre (C) in serum are analyzed by FUGI DRI-CHEM 4000i. Data represent the mean ± SEM of three independent experiments. *Means with difference letters are significantly different at *p < 0.05 vs. cisplatin (20 μM), **p < 0.05 vs. control.
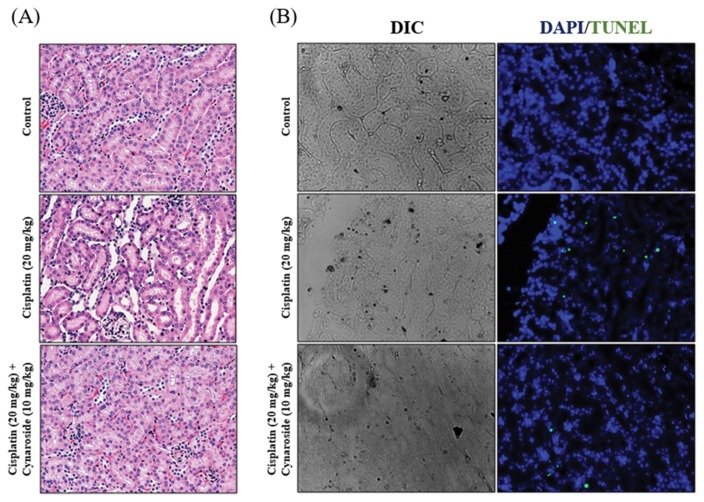
Cynaroside reduces cisplatin-induced nephrotoxicity in vivo model
Comparing the histological feature in different groups, the renal cortex of mice revealed a tubular histological structure using H&E staining (Fig. 5A). In cisplatin administrated mice (cisplatin group), histological examination revealed loss of brush border, and desquamation of epithelial cells in renal tubular epithelium. In contrast, degenerative changes such as loss of brush border markedly recovered by cynaroside administration (cisplatin + cynaroside group) in renal tissue. Cisplatin administration increased Tunel positive signal in renal tissue, in contrast, administration of cynaroside in cisplatin-injected mice reduced Tunel positive signal (Fig. 5B).
Fig. 5.
Cynaroside reduces cisplatin-induced nephrotoxicity on animal model. (A–B) Mice were separated 3 group (Control; n = 7, Cisplatin (20mg/kg); n = 7, Cisplatin (20mg/kg) + Cynaroside (10mg/kg); n = 7). Mice were administered with vehicle or cisplatin (20mg/kg). Representative images were stained by hematoxylin and eosin (H&E) staining. Representative images are from at least three independent experiments.
DISCUSSION
Pathogenesis of nephrotoxicity by cisplatin was associated with cell death and inflammation (16–18). Apoptotic cell death is an important process in cisplatin-induced nephrotoxicity, MST-1 involved in cell death of cancer cell line such as U2OS, HepG2, and HCT116 (19,20). Caspase-3 cleavage MST-1 occurs during apoptosis in mammalian cell (21–23). In recent years, use of natural medicines has developed to reduced cisplatin-induced nephrotoxicity (24–26). In this study, we demonstrated that cynaroside has a renoprotective effect on cisplatin-induced nephrotoxicity in mice kidneys, by attenuating degenerative changes such as less of brush border, desquamation of epithelial cells, and elevated BUN, Cre, and neutrophils in blood. Findings revealed that cisplatin-induced cell death in HK-2 cells was ameliorated by cynaroside treatment. Based on results, it was hypothesized that cynaroside treatment may be improved in cisplatin-induced nephrotoxicity. This hypothesis is supported by the following evidence: 1) Induction of caspase-3/MST-1 signaling, DNA fragmentation, and mitochondrial dysfunction during apoptotic cell death was attenuated by cynaroside treatment in vitro, 2) Cisplatin-induced nephrotoxicity improved by cynaroside administration on in vivo.
Initially, we anticipated that cisplatin-induced DNA fragmentation during cell death was ameliorated by cynaroside treatment on HK-2 cells. DNA fragmentation was involved in cisplatin-induced nephrotoxicity, and was implicated in cisplatin administration in vivo (27,28). Cisplatin treatment in HK-2 cells induced DNA fragmentation, prevented by cynaroside treatment (Fig. 1B), and increased DNA fragmentation induced by cisplatin administration was diminished by cynaroside administration on mice (Fig. 5B). Apoptotic DNA fragmentation occurs during programmed cell death (apoptosis), and is a hallmark of apoptosis (29,30). Thus, cynaroside diminished cisplatin-induced DNA fragmentation during cell death. Next, we observed that apoptotic cell death rates (apopotosis + cell death on FACS analysis) and mitochondrial dysfunction were induced by cisplatin treatment on HK-2 cells. According to recent studies, mitochondrial dysfunction increased during apoptosis process (31). Mitochondrial membrane permeabilization may constitute apoptosis. Decrease in MMP (mitochondrial membrane potential) is a universal feature of apoptosis (32,33). This study confirmed measurement of apoptotic cell death (Fig. 2A) and MMP (Fig. 2B), rates of apoptotic cell death increased by cisplatin treatment, but is ameliorated by cynaroside pre-treatment (Fig. 2A). Decreased mitochondrial membrane potential induced by cisplatin recovered with pre-treatment of cynaroside on HK-2 cells (Fig. 2B), suggesting that cynaroside may be involved in mitochondria and apoptosis process inhibiting cisplatin-induced mitochondrial dysfunction and apoptotic cell death. As previously mentioned, MST-1 is involved in cell death, and its activation requires autophosphorylation at Thr183 and Thr187 and caspase-3 mediated cleavage leading to initiation of DNA fragmentation and chromatin condensation (11–13). Thus, we observed caspase-3/MST-1 signaling pathway under cisplatin-induced cell death in HK-2 cells, cisplatin treatment increased protein expression and cleaved form of MST-1, caspase-3, and autophosphorylation of MST-1 at Thr183 and Thr187, but which are diminished with pre-treatment of cynaroside (Fig. 3A). Caspase-3 activation induced by cisplatin treatment decreased by cyanroside (Fig. 3B). Yuna, et al. reported that cisplatin-induced cell death in HCT116 a human colorectal cancer cell line, it promoted by apoptosis via MST-1 in a p53-dependent manner (19). Results indicated that, MST-1 and caspase-3 induced by cisplatin treatment were involved in cisplatin-induced cell death in HK-2 cells. According to many reports, cisplatin-induced nephrotoxicity was associated with inflammation such as neutrophil infiltration and secretion of cytokines (16,34), and cisplatin administration increased creatinine (Cre) and blood urea nitrogen (BUN) in serum (35). Thus, we observed neutrophil, Cre, and BUN in blood. Neutrophil, BUN, and Cre in blood increased by cisplatin administration, and decreased by cyanroside administration (Fig. 5A, 5B).
Cisplatin administration increased tubular damage and DNA fragmentation in kidneys, ameliorated by cynaroside administration. Park et al. (36) reported that cynaroside inhibited lipopolysaccharide-stimulated inflammatory response through NF-κB. Cynaroside has anti-cancer effect on human liver cancer cells (37), and has protective effect against doxorubicin-induced injury through PTEN/Akt and ERK pathway in H9c2 cells a rat myoblast (38). Evidence supports these results, and suggests that cynaroside may be recovered cisplatin-induced nephrotoxicity via inhibiting caspase-3/MST-1 signaling pathway. In conclusion, this study found that cynaroside decreased cisplatin-induced cell death though inhibiting caspase-3/MST-1 signaling pathway in HK-2 cells, and was attenuated cisplation-induced nephrotoxicity and the elevated neutrophil, BUN, and Cre in blood. Collectively, findings suggest that cynarorside could be used to for improving cisplatininduced side effects. However, further experiments are required regarding toxicity by high dose cynaroside and caspase-3/MST-1 signaling pathway in vivo.
ACKNOWLDGMENTS
This study was supported by the Korean Medicinal Herbbased Business of the Korean Traditional Resource, Ministry of Health and Welfare, Republic of Korea.
REFERENCES
- 1.Park JC, Park JG, Kim HJ, Hur JM, Lee JH, Sung NJ, Chung SK, Choi JW. Effects of extract from Angelica keiskei and its component, cynaroside, on the hepatic bromobenzene-metabolizing enzyme system in rats. Phytother Res. 2002;16:S24–S27. doi: 10.1002/ptr.783. [DOI] [PubMed] [Google Scholar]
- 2.Sun X, Sun SG, Wang M, Xiao J, Sun XB. Protective effects of cynaroside against H2O2-induced apoptosis in H9c2 cardiomyoblasts. J Cell Biochem. 2011;112:2019–2029. doi: 10.1002/jcb.23121. [DOI] [PubMed] [Google Scholar]
- 3.Wen HU, Ting G, Jiang WJ, Dong GL, Chen DW, Yang SL, Li HR. Effects of ultrahigh pressure extraction on yield and antioxidant activity of chlorogenic acid and cynaroside extracted from flowe bubs of Lonicera japonica. Chin J Nat Med. 2015;13:445–453. doi: 10.1016/S1875-5364(15)30038-8. [DOI] [PubMed] [Google Scholar]
- 4.Lin LC, Pai YF, Tsai TH. Isolation of luteolin and luteolin-7-O-glucoside from Dendranthema morifolium Ramat Tzvel and pharmacokinetics in rat. J Agric Food Chem. 2015;9:7700–7706. doi: 10.1021/jf505848z. [DOI] [PubMed] [Google Scholar]
- 5.Kang KJ, Lee JH. Characteristics of gastric cancer in Korea-with an emphasis on the increase of the early gastric cancer (EGC) J Korean Med Assoc. 2010;53:283–289. doi: 10.5124/jkma.2010.53.4.283. [DOI] [Google Scholar]
- 6.Kang KP, Kim DH, Jung YJ, Lee AS, Lee S, Lee SY, Jang KY, Sung MJ, Park SK, Kim W. Alpha-lipoic acid attenuates cisplatin-induced acute kidney injury in mice by suppressing renal inflammation. Nephrol Dial Transplant. 2009;24:3012–3020. doi: 10.1093/ndt/gfp242. [DOI] [PubMed] [Google Scholar]
- 7.Kolb R, Ghazi M, Barfuss D. Inhibition of basolateral transport and cellular accumulation of cDDP and N-acetyl-L-cysteine-cDDP by TEA and PAH in the renal proximal tubule. Cancer Chemother Pharmacol. 2003;51:132–138. doi: 10.1007/s00280-002-0537-0. [DOI] [PubMed] [Google Scholar]
- 8.Ronald PM, Raghu KT, Ganesan R, William BR. Mechanisms of cisplatin nephrotoxicity. Toxins (Basel) 2010;2:2490–2518. doi: 10.3390/toxins2112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao X, Panichpisal K, Kurtzman M, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci. 2007;334:115–154. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- 10.Ling P, Lu TJ, Yuan CJ, Lai MD. Biosignaling of mammalian Ste20-related kinases. Cell Signal. 2008;20:1237–1247. doi: 10.1016/j.cellsig.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 11.Qin F, Tian J, Zhou D, Chen L. Mst1 and Mst2 kinases: regulations and disease. Cell Biosci. 2013;3:31. doi: 10.1186/2045-3701-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng Z, Moroishi T, Guan KL. Mechanisms of hippo pathway regulation. Genes Dev. 2016;30:1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amin A, Federico P, Zahra A, Supreet K, Vrushali K, Ting Y, Thomas F, Wufan T, Jose O, Francois P, Julie KC, Kathrin M. MST-1 is a novel regulator of apoptosis in pancreatic beta-cells. Nat Med. 2014;20:385–397. doi: 10.1038/nm.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caretha LC, Jonathan C. Cloning and characterization of a human protein kinase with homology to Ste20. J Biol Chem. 1995;270:21695–21700. doi: 10.1074/jbc.270.37.21695. [DOI] [PubMed] [Google Scholar]
- 15.Parham M, Inti Z, Kristi B, Luigi T, Luigi T, Jeremy RJ, Alessandro L. Prognostic significance of mammalian sterile20-like kinase 1 in colorectal cancer. Mod Pathol. 2007;20:331–338. doi: 10.1038/modpathol.3800740. [DOI] [PubMed] [Google Scholar]
- 16.Faunel S, Lewis EC, Reznikov L, Koke TS, Somerset H, Oh DJ, Li L, Klein CL, Dinarello CA, Edelstein CL. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J Phamacol Exp Ther. 2007;322:8–15. doi: 10.1124/jpet.107.119792. [DOI] [PubMed] [Google Scholar]
- 17.Ozkok A, Edelstein CL. Pathophysiology of cisplatin-induced acute kidney injury. Biomed Res Int. 2014;2014:967826. doi: 10.1155/2014/967826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh GS, Kim HJ, Shen AH, Lee SB, Khadka D, Pandit A, So HS. Cisplatin-induced kidney dysfunction and perspectives on improving treatment strategies. Electrolyte Blood Press. 2014;12:55–65. doi: 10.5049/EBP.2014.12.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuna F, Xie Q, Wu J, Bai Y, Mao B, Dong Y, Bi W, Ji G, Tao W, Wang Y, Yuan Z. MST-1 promotes apoptosis through regulating sirt1-dependent p53 deacetylation. J Biol Chem. 2011;286:6940–6945. doi: 10.1074/jbc.M110.182543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu C, Liu C, Huang W, Tu S, Wan F. Effect of mst1 overexpression on the growth of human hepatocellular carcinoma HepG2 cells and the sensitivity to cisplatin in vitro. Acta Biochim Biophys Sin (Shanghai) 2013;45:268–279. doi: 10.1093/abbs/gmt006. [DOI] [PubMed] [Google Scholar]
- 21.Ura S, Masuyama M, Graves JD, Gotoh Y. MST1-JNK promotes apoptosis via caspase-dependent and independent pathways. Genes Cells. 2001;6:519–530. doi: 10.1046/j.1365-2443.2001.00439.x. [DOI] [PubMed] [Google Scholar]
- 22.Maejima Y, Kyoi S, Zhai P, Liu T, Li H, Lvessa A, Sciarretta S, Del Re DP, Zablocki DK, Hsu CP, Lim DS, Isobe M, Sadoshima J. Mst1 inhibits autophagy by promoting the interaction between Beclin1 and Bcl-2. Nat Med. 2013;19:1478–1488. doi: 10.1038/nm.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo X, Li Z, Yan Q, Li X, Tao D, Wang J, Leng Y, Gardner K, Judge S, Li Q, Hu J, Gong J. The human WW45 protein enhances MST-1 mediated apoptosis in vivo. Int J Mol Med. 2010;23:357–362. [PMC free article] [PubMed] [Google Scholar]
- 24.Hosseinian S, Rad AK, Hadjzadeh MAR, Roshan NM, Havakhah S, Shafiee S. The protective effect of Nigella sativa against cisplatin-induced nephrotoxicity in rats. Avicenna J Phytomed. 2016;6:44–54. [PMC free article] [PubMed] [Google Scholar]
- 25.Bami E, Ozakpinar OB, Ozdemir-Kumral ZN, Koroglu K, Ercan F, Cirakli Z, Sekerler T, Izzettin FV, Sancar M, Okuyan B. Protective effect of ferulic acid on cisplatin induced nephrotoxicity in rats. Environ Toxicol Pharmacol. 2017;54:105–111. doi: 10.1016/j.etap.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 26.Lou XY, Cheng JL, Zhang B. Therapeutic effect and mechanism of breviscapine on cisplatin-induced nephrotoxicity in mice. Asian Pac J Trop Med. 2015;8:873–877. doi: 10.1016/j.apjtm.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Yin X, Apostolov EO, Shah SV, Wang X, Bogdanov KV, Buzder T, Stewart AG, Basnakian AG. Induction of renal endonuclease G by cisplatin is reduced in DNase I-deficient mice. J Am Soc Nephrol. 2007;18:2544–2553. doi: 10.1681/ASN.2006080896. [DOI] [PubMed] [Google Scholar]
- 28.Basnakian AG, Apostolov EO, Yin X, Napirei M, Mannherz HG, Shah SV. Cisplatin nephrotoxicity is mediated by deoxyribonuclease I. J Am Soc Nephrol. 2005;16:697–702. doi: 10.1681/ASN.2004060494. [DOI] [PubMed] [Google Scholar]
- 29.Hua ZJ, Xu M. DNA fragmentation in apoptosis. Cell Res. 2000;10:205–211. doi: 10.1038/sj.cr.7290049. [DOI] [PubMed] [Google Scholar]
- 30.Khodarev NN, Sokolova IA, Vaughan AT. Mechanisms of induction of apoptotic DNA fragmentation. Int J Radiat Biol. 1998;73:455–467. doi: 10.1080/095530098141997. [DOI] [PubMed] [Google Scholar]
- 31.Jang YJ, Won JH, Back MJ, Fu Z, Jang JM, Ha HC, Hong SB, Chang M, Kim DK. Paraquat induces apoptosis through a mitochondria-dependent pathway in RAW 264.7 cells. Biomol Ther (Seoul) 2017;23:407–413. doi: 10.4062/biomolther.2015.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 33.Wadia JS, Chalmers-Redman RME, Ju WJH, Carlile GW, Philips JL, Fraser AD, Tatton WG. Mitochondrial membrane potential and nuclear changes in apoptosis acused by serum and nerve growth factor withdrawal: time course and modification by (−)-deprenyl. J Neurosci. 1998;18:932–947. doi: 10.1523/JNEUROSCI.18-03-00932.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tadagavadi R, Reeves WB. Neutrophils in cisplatin AKI-mediator or marker? Kidney Int. 2017;92:11–13. doi: 10.1016/j.kint.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 35.Ma P, Zhang S, Su G, Qiu G, Wu Z. Protective effects of icariin on cisplatin-induced acute renal injury in mice. Am J Transl Res. 2015;7:2105–2114. [PMC free article] [PubMed] [Google Scholar]
- 36.Park CM, Song YS. Luteolin and luteolin-7-O-glucoside inhibit lipopolysaccharide-induced inflammatory responses through modulation of NF-κB/AP-1/PI3K-Akt signaling cascades in RAW 264.7 cells. Nutr Res Pract. 2013;7:423–429. doi: 10.4162/nrp.2013.7.6.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hwang YJ, Lee EJ, Kim HR, Hwang KA. Molecular mechanisms of luteolin-7-O-glucoside-induced growth inhibition on human liver cancer cells: G2/M cell cycle arrest and caspase-independent apoptotic signaling pathways. BMB Rep. 2013;46:611–616. doi: 10.5483/BMBRep.2013.46.12.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao H, Shang Z, Wang P, Li S, Zhang Q, Tian H, Ren D, Han X. Protection of luteolin-7-O-glucoside against doxorubicin-induced injury through PTEN/Akt and ERK pathway in H9c2 cells. Cardiovasc Toxicol. 2016;16:101–110. doi: 10.1007/s12012-015-9317-z. [DOI] [PubMed] [Google Scholar]