Abstract
Statement of Problem:
Heat cure acrylic resin material with reduced monomer content is generally recommended for clinical usage as it leads to improved mechanical, physical, and biocompatibility properties.
Aims and Objective:
The purpose of the present study was to evaluate and compare the amount of the residual monomer in the conventional and three different groups of deep-frozen heat cure polymethylmethacrylate (PMMA) acrylic resin.
Materials and Methods:
Totally 40 Specimens of heat cure PMMA acrylic resin (DPI India) (10 conventional heat cure and 30 deep frozen) were made using two disc-shaped stainless steel molds and invested into type II dental stone using compression molding technique. Each group contained 10 specimens (n = 10). Group 1: Control group of conventional polished PMMA specimen stored in water for 24 h at +37°C (10 specimens), Group 2: Deep frozen unpolished PMMA (10 specimens), Group 3: Deep frozen polished PMMA (10 specimens), and Group 4: Deep frozen polished PMMA specimen stored in water for 24 h at +37°C (10 specimens). Amount of the residual monomer content in all the specimens was measured using high-performance liquid chromatography. Data were analyzed using One-Way Analysis of Variance and multiple comparison Tukey's post hoc test (α = 0.05).
Results:
Least residual monomer content was found in Group 4 (0.12 wt%) followed by Group 3 (0.19 wt%), Group 2 (0.23 wt%), and Group 1 (0.26 wt%). Statistically significant difference (P < 0.05) was found in residual monomer content for all the four groups tested. Post hoc test for intergroup comparison also showed a significant difference between groups.
Conclusion:
The amount of the residual monomer was found to be least in deep-frozen polished PMMA specimen stored in water for 24 h at +37°C (Group 4). Thus, it can be concluded that deep freezing, polishing, and storing in water can reduce the residual monomer content.
Keywords: Deep-frozen heat cured polymethylmethacrylate resin, heat cured polymethylmethacrylate resin, high-performance liquid chromatography (HPLC), residual monomer
INTRODUCTION
Heat cured acrylic resins, are the most commonly used materials for the fabrication of denture bases in dentistry, since its introduction by Dr. Walter Wright in 1937.[1] They are usually composed of prepolymerized polymethylmethacrylate (PMMA) powder particles, which are mixed with monomers of methyl methacrylate (MMA) and cross-linking agent such as ethylene glycol dimethacrylate.[2] The polymerization reaction (the curing process) results in the conversion of MMA into poly-MMA during which the monomer molecules are converted into polymers. During this process, not all the monomer molecules are converted, and thus, some unreacted residual monomers remain unpolymerized.[3]
Rashid et al.[4] in a review paper, on allergic effects of the residual monomer of denture base acrylic resins concluded that irrespective of the curing method used, the occurrence of unreacted residual monomer in acrylic dentures was unavoidable and posed a serious challenge for both dental surgeons and patients. Another review done by Leggat and Kedjarune[5] on the toxicity of MMA in dentistry also concluded that the dentists and dental technicians should try to opt techniques to reduce leaching of MMA from newly made dentures.
Isik and Harrison[6] evaluated deep-freezing of heat cure acrylic resin as a method for fabrication of denture bases. They concluded that freezing the heat cure acrylic material up to 1 month in the dough stage did not alter the impact strength, hardness, flexural modulus, and flash thickness. This also led to increased flexural strength and working time.
Researchers are constantly in search of an ideal denture base resin, which may have minimum residual monomer leading to less hypersensitivity, less porosity, and less surface discoloration. Less monomer content will also lead to improved mechanical and physical properties of denture base resin. Sufficient literature is still not present which has evaluated the amount of residual monomer in the deep-frozen heat cure acrylic resin. Thus, an in vitro study was conducted to evaluate and compare the amount of residual monomer content between conventional and the deep-frozen acrylic resin.
The aim of the study was to evaluate and compare the amount of the residual monomer in the conventional and three different groups of deep-frozen heat cure PMMA acrylic resin.
The various objectives of the study were to evaluate the amount of the residual monomer in the conventional polished PMMA specimen kept in water for 24 h at +37°C, in the deep-frozen unpolished PMMA, deep-frozen polished PMMA, and deep frozen polished PMMA specimen kept in water for 24 h at +37°C. Further objective of the study was to compare the amount of the residual monomer of the conventional polished PMMA specimen kept in water for 24 h at +37°C with three separate groups of deep-frozen PMMA.
The suggested null hypothesis was that there exists no difference in the amount of the residual monomer content in the conventional control heat cure acrylic resin group and all the three groups of deep-frozen heat cure PMMA acrylic resin.
MATERIALS AND METHODS
The present in vitro study was conducted in the department of the Prosthodontics and Crown and Bridge. Ethical approval for the study was granted by the Institutional Ethics Committee, with Approval No. SVIEC/ON/DENT/SRP/16208. On the basis of a previous study done by Isik and Harrison[6], a One-Way Analysis of Variance (ANOVA) study, sample sizes of 10, 10, 10, and 10 were obtained from the 4 Groups whose means were to be compared. The total sample of 40 achieved a power of 80% to detect differences among the means versus the alternative of equal means using an F test with a 0.05000 significance level. The size of the variation in the means was represented by their standard deviation (SD), which was 15. The common SD within a group was assumed to be 2.4.
Totally 40 specimens of heat cure PMMA acrylic resin (DPI India) were made. The four groups were as follows
Group 1: Control (the conventional polished heat cured PMMA specimen stored in water for 24 h at +37°C) (10 specimens).
Group 2: Deep frozen unpolished heat cured PMMA (10 specimens).
Group 3: Deep frozen polished heat cured PMMA (10 specimens).
Group 4: Deep frozen polished heat cured PMMA specimen stored in water for 24 h at +37°C (10 specimens).
Method to obtain deep-frozen heat cure polymethylmethacrylate resin
Isik and Harrison[6] evaluated the effect of freezing the acrylic resin dough on the range of the properties of polymerized denture base materials frozen for 24 h, 1 week, 1 month, 3 months, and 6 months. They concluded that acrylic dough mix could be best stored up to 1 month in the freezer without any statistically significant effect on the properties. Hence, a 1-month deep-frozen PMMA was chosen for this study.
Polymer was mixed with monomer in 3:1 ratio by volume as per the manufacturer's instructions. The mixture was left to stand in a porcelain-mixing jar until it reached the dough stage. For evaluation of the dough stage of the powder/liquid mixtures, a packing plasticity test was performed using a needle penetrometer. When the mix reached the initial dough stage, it was transferred into airtight plastic containers lined with aluminum foil. The containers were transferred to Vertical Deep Freezer (Remi RGV-2000 plus) stored at −18°C [Figures 1 and 2]. After 1-month portion of the mix was removed, allowed to thaw, assessed using a needle penetrometer, and then, packed in type II dental stone mold in a dental flask which was prepared as follows.
Figure 1.

Vertical deep freezer (Remi RGV-2000 plus)
Figure 2.

Storage of Head Cured polymethylmethacrylate resin at Dough stage in Deep Freezer
Disc-shaped stainless steel mold (50 mm × 3 mm in diameter for 30 specimens that were later polished)[7] and (50 mm × 2 mm diameter for 10 specimens that were left unpolished) were used for the preparation of all the 40 specimens. All 40 specimens were made according to International Organization for Standardization ISO 1567:2000.[8] The discs were invested into Type II dental stone. For the 10 conventional heat cured control group specimens, powder/liquid ratio was maintained in proportions as recommended by the manufacturer and packed in the mold. For the other 30 deep frozen heat cured specimens, the methodology mentioned above of deep freezing was used to pack in the mold.
All 40 specimens were kept for 12 h for bench curing. Consani et al. in 2001[9] and 2004[10] proved the presence of less denture base discrepancy when the acrylic resin dough was processed in conventional curing cycle after the 12 and 24 h postpressing times. Hence, the samples were kept for 12 h of postpressing time in this study to duplicate ideal laboratory processing conditions. A long curing cycle (70°C for 9 h and 100°C for 30 min)[11] was employed to cure the specimens of both Conventional and Deep frozen PMMA.
The 30 specimens to be polished[7] were made of 50 mm in diameter and 3 mm thickness. This procedure was adopted because after finishing and polishing the thickness would be reduced to standardized 2 mm for all the 40 specimens. 10 specimens of unpolished group were made directly of 50 mm in diameter and 2 mm thickness. Verification of specimen diameter was done with digital Vernier caliper.
In the polished groups, 10 specimens were of conventional control (Group 1) and 20 specimens were of deep-frozen acrylic resin (Groups 3 and 4). Polishing was done with universal polishing paste composed of aluminum oxide, (Ivoclar, Germany) on soft cloth wheel for 60 s using polishing unit at 3000 rpm.[12] In the control (Group 1) and deep frozen (Group 4), the polished specimens were stored in water for 24 h at +37°C in an Incubator.[13]
Bural et al.,[11] Vallittu et al.,[13] and Bayraktar et al.,[14] had recommended the use of a 30 min terminal boiling for the polymerization process. Therefore, in this study, all the 40 specimens were made with long curing cycle and with terminal boiling of 30 min. They also recommended storage of acrylic dentures in water at +37°C for a minimum 1–2 days before denture insertion. Both these procedures reduce the residual MMA and may evade any possible cytotoxic effects. The immersion of the dentures in water for 24 h before insertion has been also recommended by Singh et al.[15] and Ebrahimi Saravi et al.[16] Keeping all these studies in mind the deep frozen and control specimens (Group 1 and 4) were kept in water at +37°C for 24 h.
Determination of residual monomer in the denture base materials using high-performance liquid chromatography
High-performance liquid chromatography (HPLC)[17] was used to evaluate the quantity of residual MMA content in all the 40 specimens of heat-cure resin. An acrylic trimming bur with slow speed on micromotor was used to obtain the powder form of specimens. Powdered sample of 50 mg from each specimen was dissolved in 1 ml of acetone, and then 10 ml of methanol was added to the solution to precipitate the polymer. The supernatant of the solution was filtered through a 0.45 μm pore Millipore filter. HPLC analysis was performed using LC2010C Shimadzu Japan system equipped with a CAPCELL PAK C18 column [Figure 3]. According to Ohyama and Imai,[18] 10 ml of the sample solution was injected and analyzed at 40°C at a flow rate of 1.0 ml min–1 with acetonitrile water (50/50). The residual content of MMA (x) in 1 g was calculated using the following formula:[17]
Figure 3.

High performance liquid chromatography
(MMA) X = (Pvz x nst x Cst x 20)/(Pst x nvz)
Where,
Pvz = Average of injected area, nst = weight of standard sample, Cst = purity of sample, Pst = Average weight of standard sample, and nvz = sample weight.
Statistical analysis
Mean and SD was calculated for each group. The data were analyzed with SPSS (Statistical Package for Social Sciences) Version 20.1 (IBM Corp. Chicago, USA) software for descriptive and analytical statistics. The parametric One-Way ANOVA test was used to check differences in mean scores between groups and pairwise comparison was done using Tukey's honestly significant difference (HSD) post hoc test.
RESULTS
The standard calibration curve for MMA is shown in Figure 4. It shows the characteristic peak at approximately 2.93 min of retentive time.
Figure 4.
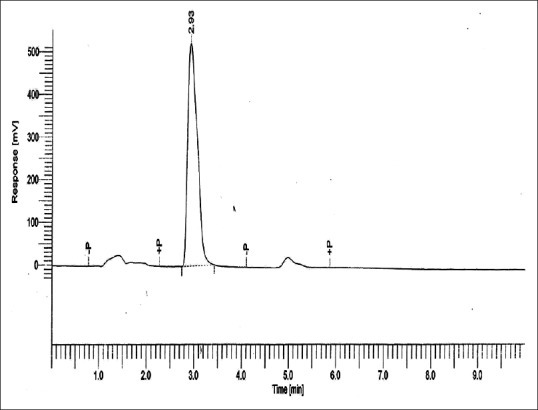
Standard calibration curve for methyl methacrylate
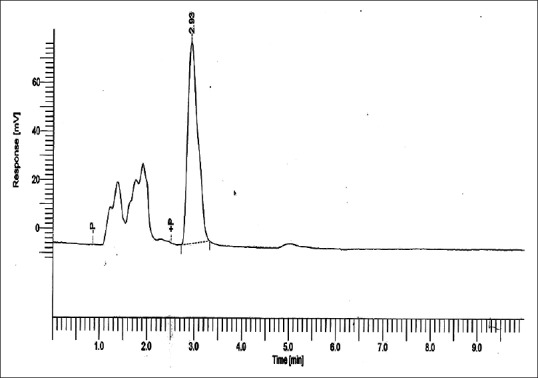
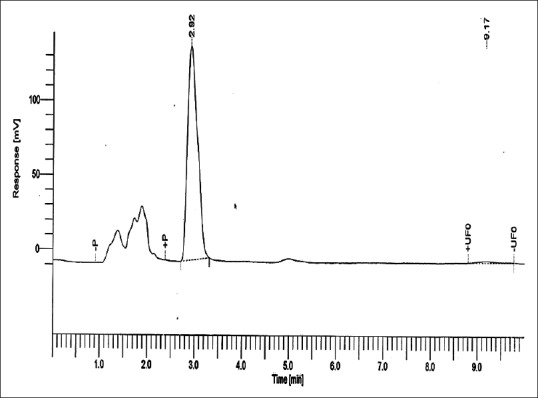
Figures 5–8 show curves for Group 1: Control (conventional polished PMMA specimen kept in water for 24 h at +37°C), Group 2: (deep frozen unpolished PMMA), Group 3: (deep frozen polished PMMA), and Group 4: (deep frozen polished PMMA specimen kept in water for 24 h at +37°C), respectively. The content of MMA was calculated from the area under the peak at approximately 2.93 min of retentive time.
Figure 5.
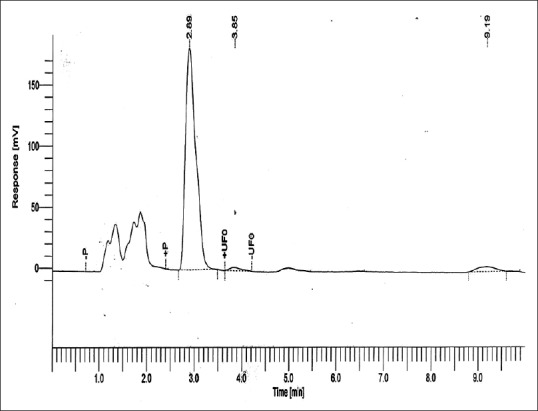
Residual methyl methacrylate calibration curve collected from 1 g of Group 1: Control (conventional polished polymethylmethacrylate specimen stored in water for 24 h at +37°C)
Figure 8.
Residual methyl methacrylate calibration curve collected from 1 g of Group 4: (deep frozen polished polymethylmethacrylate specimen stored in water for 24 h at +37°C)
Figure 6.
Residual methyl methacrylate calibration curve collected from 1 g of Group 2: (deep frozen unpolished polymethylmethacrylate)
Figure 7.
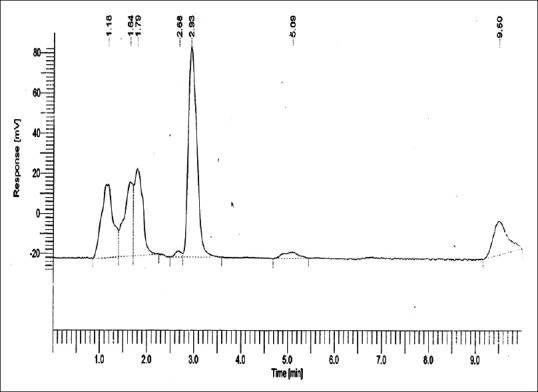
Residual methyl methacrylate calibration curve collected from 1 g of Group 3: (deep frozen polished polymethylmethacrylate)
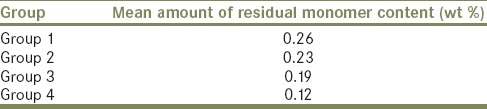
The mean residual monomer content is shown in Table 1 and graphically represented in Graph 1. Group 4 (deep-frozen polished PMMA specimen kept in water for 24 h at +37°C) showed the least residual monomer value of 0.12 wt%, followed by Group 3 (deep frozen polished PMMA) with value of 0.19 wt%, Group 2 (deep frozen unpolished PMMA) with value of 0.23 wt% and Group 1 (control group of conventional polished PMMA specimen kept in water for 24 h at +37°C) with value of 0.26 wt%.
Table 1.
Mean amount of residual monomer content (wt%) value
Graph 1.
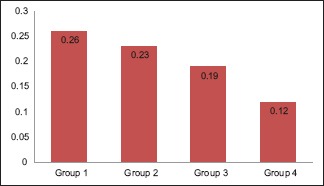
Mean amount of residual monomer content (wt%) for each group
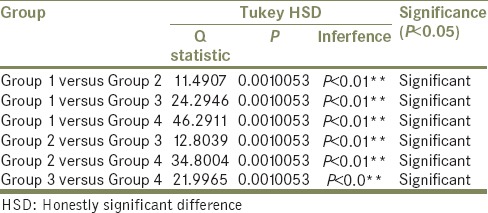
It was also observed that the residual monomer content of all the four groups was lower than that of the ISO-1567 standard for denture base material [Table 2]. The summary statistics is shown in Table 3. The results of One-Way ANOVA showed a statistically significant difference in the mean amount of residual monomer content (P < 0.05) for the four groups [Table 4]. Tukey's HSD Post hoc test [Table 5] for multiple comparisons also showed statistically significant difference (P < 0.05) in the mean residual monomer content values.
Table 2.
The result of methyl methacrylate content of denture base materials compared with ISO standard

Table 3.
Summary of descriptive statistics
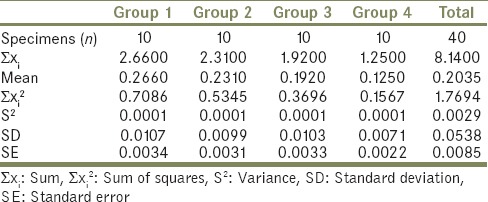
Table 4.
One-way analysis of variance result summary
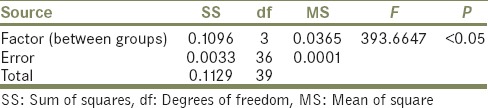
Table 5.
Tukey's honestly significant difference post hoc test results among groups
DISCUSSION
It is very important to determine the residual monomer content of the acrylic resins, as this property influences the allergy susceptibility of acrylic dentures.[19] In a rare case, it was reported that acrylic resin based on MMA can lead to type IV hypersensitivity reaction.[20] The released residual monomer is the primary cause of irritation, allergic reaction, and cell cytotoxicity.[21] According to ISO 1567, the maximum residual monomer content of denture base materials should not exceed 2.2% wt.[8] Thus to reduce the risks of possible complications and side effects of monomer exposure, novel approaches should be explored.[22]
Evidences from previous studies have proved that the long curing with terminal boiling, polishing, and storing the conventional PMMA at +37°C for at least 24 h reduces the monomer content to permissible levels. Therefore, the present study assumed that the Group 1 conventional control specimens made under ideal conditions will lead to minimum amount of residual monomer, which further needs to be compared with deep frozen PMMA of unpolished (Group 2), polished (Group 3), and polished specimens kept in water at +37°C for 24 h (Group 4).
As this study wanted to evaluate and compare the monomer content of three deep-frozen PMMA groups with conventional PMMA, it was required that basic laboratory and clinical protocols of polishing and storage in water must also be followed in deep-frozen PMMA groups same as that of conventional dentures. Thus, intentionally, the deep-frozen groups were kept as unpolished (Group 2), polished (Group 3), and polished with storage in water at +37°C for 24 h (Group 4). However, this study having three groups of deep-frozen PMMA was able to justify that if deep-frozen PMMA is also given the same sequential treatment of polishing and storage in water, it leads to the better results.
This study very well illustrates the statistically significant difference among all the four groups. Thus, it may be inferred that residual monomer content in all three deep frozen groups was low from the conventional control (Group 1). The probable reason for Group 3 (polished deep frozen) specimens to show less residual monomer content than Group 2 (unpolished deep frozen) can be attributed to the effect of finishing and polishing the specimens. This would have led to increase in the temperature on the PMMA surface, which might have reduced the monomer content of deep-frozen specimen even lower. Vallittu[23] in his in vitro study on auto-polymerized PMMA concluded that polishing of the denture reduced the residual MMA release into water but did not reduce the actual residual monomer content within the specimens. However, our study could have shown reduced monomer content with all polished samples possibly due to the usage of heat-polymerized PMMA, deep-freezing the acrylic, differences in the sample fabrication methods and finishing and polishing methodology.
HPLC is quite an established method to determine the amount of residual monomer.[17,23] The deep-frozen acrylic-based resins may be a feasible option with added advantage of reduced monomer content. According to Isik and Harrison,[6] increased working time is desirable in conditions when multiple denture packing is required. Deep freezing of heat cure PMMA leads to economy of both material and time without adversely affecting the properties of polymerizing material. The authors also hypothesized that deep freezing increased the interpenetrating polymer network and hence improved the properties of polymerized resin. The low monomer content found in the three different deep-frozen groups in our study could be very well attributed to the above-proven hypothesis.
Since statistically significant difference existed between all the groups (P < 0.05), the null hypothesis was rejected. Although all the groups showed mean residual monomer content lesser than ISO-1567 standard, Group 4 showed the least residual monomer content.
Clinical relevance of the study is that deep-frozen PMMA may be considered as an economical, feasible option to be used for prostheses with an additional benefit of less allergic susceptibility due to its low monomer content than conventional PMMA. Future in vitro, in vivo, and clinical trails along with evaluation of other parameters may be conducted.
The limitations with the deep-frozen acrylic system can be that it needs prior storage of 1 month and a special deep freezer set up. However, considering the results of the least residual monomer content, this limitation can be easily taken care of. Other limitation of the present study is that the temperature of the all the three polished groups was not measured during polishing. This can be addressed in future studies.
CONCLUSION
Within the limitation of the present in vitro study, it can be concluded that the least residual monomer content was found in deep-frozen polished PMMA specimen stored in water for 24 h at +37°C (Group 4). Other two groups of deep-frozen unpolished (Group 2) and polished (Group 3) specimens also demonstrated comparatively less residual monomer than the conventional heat cured acrylic control group (Group 1). Thus, it can be concluded that deep freezing, polishing, and storing in water can reduce the residual monomer content in heat cured PMMA.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Dr. A R Jani Hon. Director of Sophisticated Instrumentation Centre for Applied Research & Testing (SICART), Anand, Gujarat.
REFERENCES
- 1.Peyton FA. History of resins in dentistry. Dent Clin North Am. 1975;19:211–22. [PubMed] [Google Scholar]
- 2.Çelebi N, Yüzügüllü B, Canay Ş, Yücel Ü. Effect of polymerization methods on the residual monomer level of acrylic resin denture base polymers. Polym Adv Technol. 2008;19:201–6. [Google Scholar]
- 3.Vallittu PK, Ruyter IE, Buykuilmaz S. Effect of polymerization temperature and time on the residual monomer content of denture base polymers. Eur J Oral Sci. 1998;106:588–93. doi: 10.1046/j.0909-8836.1998.eos106109.x. [DOI] [PubMed] [Google Scholar]
- 4.Rashid H, Sheikh Z, Vohra F. Allergic effects of the residual monomer used in denture base acrylic resins. Eur J Dent. 2015;9:614–9. doi: 10.4103/1305-7456.172621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leggat PA, Kedjarune U. Toxicity of methyl methacrylate in dentistry. Int Dent J. 2003;53:126–31. doi: 10.1111/j.1875-595x.2003.tb00736.x. [DOI] [PubMed] [Google Scholar]
- 6.Isik G, Harrison A. Effect of deep-freezing on some properties of acrylic resin. Acta Odontol Scand. 2005;63:158–62. doi: 10.1080/00016350510019775. [DOI] [PubMed] [Google Scholar]
- 7.Pfeiffer P, Rosenbauer EU. Residual methyl methacrylate monomer, water sorption, and water solubility of hypoallergenic denture base materials. J Prosthet Dent. 2004;92:72–8. doi: 10.1016/j.prosdent.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 8.ISO 1567. Dentistry-Denture Base Polymers. Berlin: Beuth; 2000. [Google Scholar]
- 9.Consani RL, Domitti SS, Correr Sobrinho L, Sinhoreti MA. Effect of acrylic resin post-pressing time on dimensional change of total denture base. Pesqui Odontol Bras. 2001;15:112–8. doi: 10.1590/s1517-74912001000200006. [DOI] [PubMed] [Google Scholar]
- 10.Consani RL, Domitti SS, Mesquita MF, Consani S. Effect of packing types on the dimensional accuracy of denture base resin cured by the conventional cycle in relation to post-pressing times. Braz Dent J. 2004;15:63–7. doi: 10.1590/s0103-64402004000100012. [DOI] [PubMed] [Google Scholar]
- 11.Bural C, Aktaş E, Deniz G, Ünlüçerçi Y, Bayraktar G. Effect of leaching residual methyl methacrylate concentrations on in vitro cytotoxicity of heat polymerized denture base acrylic resin processed with different polymerization cycles. J Appl Oral Sci. 2011;19:306–12. doi: 10.1590/S1678-77572011005000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao DC, Kalavathy N, Mohammad HS, Hariprasad A, Kumar CR. Evaluation of the surface roughness of three heat-cured acrylic denture base resins with different conventional lathe polishing techniques: A comparative study. J Indian Prosthodont Soc. 2015;15:374–80. doi: 10.4103/0972-4052.164910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vallittu PK, Miettinen V, Alakuijala P. Residual monomer content and its release into water from denture base materials. Dent Mater. 1995;11:338–42. doi: 10.1016/0109-5641(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 14.Bayraktar G, Guvener B, Bural C, Uresin Y. Influence of polymerization method, curing process, and length of time of storage in water on the residual methyl methacrylate content in dental acrylic resins. J Biomed Mater Res B Appl Biomater. 2006;76:340–5. doi: 10.1002/jbm.b.30377. [DOI] [PubMed] [Google Scholar]
- 15.Singh RD, Gautam R, Siddhartha R, Singh BP, Chand P, Sharma VP, et al. High performance liquid chromatographic determination of residual monomer released from heat-cured acrylic resin. An in vivo study. J Prosthodont. 2013;22:358–61. doi: 10.1111/jopr.12004. [DOI] [PubMed] [Google Scholar]
- 16.Ebrahimi Saravi M, Vojdani M, Bahrani F. Evaluation of cellular toxicity of three denture base acrylic resins. J Dent (Tehran) 2012;9:180–8. [PMC free article] [PubMed] [Google Scholar]
- 17.Mohamed SH, Al-Jadi A, Ajaal T. Using of HPLC analysis for evaluation of residual monomer content in denture base material and their effect on mechanical properties. J Phys Sci. 2008;19:127–35. [Google Scholar]
- 18.Ohyama A, Imai Y. Differential scanning calorimetric study of acrylic resin powders used in dentistry. J Dent Mater. 2000;19:346–51. doi: 10.4012/dmj.19.346. [DOI] [PubMed] [Google Scholar]
- 19.Crag RG, Brein WI, Power JM. Dental Material Properties and Manipulation. 7th ed. St. Louis: The CV Mosby Co; 2000. pp. 257–82. [Google Scholar]
- 20.Bolla SC, Gantha NS, Basha SR. Allergic reaction to an acrylic denture – A rare case report. J Res Adv Dent. 2014;3:185–8. [Google Scholar]
- 21.Crag RG. Restorative Dental Material. 11th ed. St. Louis: The CV. Mosby Co; 2002. pp. 636–66. [Google Scholar]
- 22.Gosavi SS, Gosavi SY, Alla RK. Local and systemic effects of unpolymerised monomers. J Dent Res. 2010;7:82–7. [PMC free article] [PubMed] [Google Scholar]
- 23.Vallittu PK. The effect of surface treatment of denture acrylic resin on the residual monomer content and its release into water. Acta Odontol Scand. 1996;54:188–92. doi: 10.3109/00016359609003522. [DOI] [PubMed] [Google Scholar]


