Abstract
This study was aimed at evaluating the efficacy of berberine-rich fraction (BF) as a protective and/or a therapeutic agent against inflammation and oxidative stress during male infertility. Sexually mature Sprague-Dawley male rats were divided into five groups treated with either corn oil, BF (100 mg/kg BW, orally, daily for 30 days), gossypol acetate (5 mg/kg BW, i.p.) eight times for 16 days, BF alone for 14 days then coadministered with gossypol acetate for the next 16 days (protected group), or gossypol acetate for 16 days then treated with BF for 30 days (treated group). All animals completed the experimental period (46 days) without obtaining any treatments in the gap period. Sperm parameters, oxidative index, and inflammatory markers were measured. Gossypol injection significantly decreased the semen quality and testosterone level that resulted from the elevation of testicular reactive oxygen and nitrogen species (TBARS and NO), TNF-α, TNF-α-converting enzyme, and interleukins (IL-1β, IL-6, and IL-18) by 230, 180, 12.5, 97.9, and 300%, respectively, while interleukin-12 and tissue inhibitors of metalloproteinases-3 were significantly decreased by 59 and 66%, respectively. BF (protected and treated groups) significantly improved the semen quality, oxidative stress, and inflammation associated with male infertility. It is suitable to use more advanced studies to validate these findings.
1. Introduction
Infertility is the disability of a couple to achieve pregnancy after one year without intercourse precautions [1]. Male infertility is found to contribute 45%–50% of infertility cases [2]. Africa and Central/Eastern Europe were considered to have the highest rates of infertility [3]. Many factors are known to impair male infertility, including varicocele, testicular failure, treatment with radiation, and illicit drugs [4]. Infertility produces psychological stress as a couple fails achieve the expected goal of reproduction, causing disappointment and frustration that is attributed to generalized increased oxidative stress levels [5]. Oxidative stress is a well-known causative factor involved in the etiology of male infertility [6, 7]. Oxidative stress arises when reactive oxygen species (ROS) or free radical production overwhelms the endogenous antioxidant defense of the male reproductive tract [1, 8]. ROS have destructive effects on semen quality by disrupting the integrity of sperm nuclear DNA and ATP production [1]. The spermatozoal cell membrane contains a high abundance of polyunsaturated fatty acids representing the most vulnerable target for free radical damage and lipid peroxidation and, hence, influencing the sperm viability, count, motility, and morphology [9]. Inflammation could be linked to oxidative stress, and as oxidative stress primarily occurs, it can further induce inflammation and vice versa; thus, they strengthen each other, causing destructive effects to the cells [10, 11].
Cytokines, including interleukins (ILs) and tumor necrosis factor alpha (TNF-α), are important mediators of immunity and can be contributed in numerous physiological processes in the male reproductive tract [2]. Cytokines have different effects on the semen quality and sperm function. IL-1β, IL-6, and IL-18 are proinflammatory cytokines, can be produced by specific cells in the male reproductive system (such as testicular somatic cells and Sertoli cells), are included in the inflammatory reaction, and can induce apoptosis [2], while IL-12 improves male fertility due to its immunomodulatory properties [12]. It is involved in the induction and maintenance of the immune response during both cell-mediated (helper T1) and humoral responses (helper T2) [13] and regulating antigen-presenting activity and natural killer cell activity [14]. TNF-α has destructive effects on sperm [8], and the TNF-α-converting enzyme (TACE; ADAM-17) is an enzyme involved in the proteolytic liberation of TNF-α from the pro-TNF-α molecule. Since ADAM-17 was found to be inhibited by tissue inhibitors of metalloproteinases-3 (TIMP-3); TIMP-3 are important factors involved in the regulation of the inflammatory process and the disease progression [15].
Antioxidants quench free radicals and protect gonadal cells and mature spermatozoa from ROS production and oxidative damage [9]. According to the World Health Organization (WHO), developing countries make use of herbal medicinal products for a variety problems due to their safety and the side effects of chemical drugs [16]. Berberine is an isoquinoline alkaloid that belongs to the structural class of protoberberines [17] and is present in roots, rhizomes, and stem bark of the Berberis species that belongs to the Berberidaceae family. Berberine is the most active constituent in Berberis vulgaris [18–20]. Several studies have indicated that berberine acts as a natural medicine with multiple biochemical and pharmacological activities [21, 22] including anti-inflammatory [23], antioxidant [24], antidepressant [25, 26], anticancer [27], hypoglycemic, hypolipidemic [22], and antimicrobial activities [19].
Gossypol was used as antifertility agent in male rats. Gossypol is a very toxic crystalline polyphenolic compound and is found in the highest concentration in the seeds of cotton plants [28]. Gossypol induces oxidative stress by the imbalance between antioxidants and prooxidants, resulting in the accumulation of ROS [29]. Oral gossypol acetate was found to reduce the levels of serum testosterone and luteinizing hormone in a dose- and duration-dependent manner [30]. Gossypol acts directly on testes and induces azoospermia or oligospermia [31]. Furthermore, gossypol blocked cAMP formation in sperms, which subsequently decreased sperm motility [32]. It also reduced the secretory activity of accessory sex glands [33]. Therefore, gossypol was used as an efficient male contraceptive drug [34].
The present study was aimed at assessing the therapeutic and/or protective effects of BF against the inflammation process produced during male infertility induced in rats by using gossypol acetate. The study will demonstrate its effect on biochemical blood parameters (TBARS, GSH, testosterone, cholesterol, glucose, and albumin), semen quality (sperm count, motility, morphology, α- glucosidase activity, and fructose level), and finally the inflammatory markers [testicular TBARS, NO, TNF-α, ADAM-17, TIMP-3, and interleukins (IL-1β, IL-6, IL-12, and IL-18)].
2. Materials and Methods
2.1. Plant Collection and Preparation of Ethanol Extract and Different Fractions
Barberry roots were purchased and authenticated by Professor Salma El-dareir, Botany Department, Faculty of Science, Alexandria University, Egypt. This classification was dependent on the data about the plant published in the Dargon Herbarium. Barberry roots were subjected to steam distillation, and the ethanol extract was prepared as described by [27]. The extract was lyophilized and the obtained powder (35 g, ethanolic extract) was dissolved in 1% HCl and then filtrated. The pH of the filtrate was optimized to 8 by using concentrated NH4OH. The tertiary alkaloids were extracted from the previous solution by using chloroform, and this fraction (chloroform fraction) was evaporated and lyophilized (25 g). The obtained powder was dissolved in the minimum amount of chloroform and then was subjected to a silica gel 100–200 mesh column. A berberine-rich fraction (2.5 g) was obtained by using gradient elution with CHCl3 : MeOH (9 : 1; 8 : 2) and finally methanol [35]. The presence of berberine in the fraction was identified using TLC, melting point [36], HPLC [27], and 1H-NMR [37]. The obtained powder was dissolved in polyethylene glycol (20%) to be administrated to rats.
2.2. Preparation of Gossypol Acetate
The Egyptian cottonseeds (Giza 70) were collected with the help of Professor Ali Aisa Nawar, Department of Crop Science, Faculty of Agriculture, Alexandria University, Egypt. The collected seeds were cleaned and crushed; the decorticated kernels were mashed by a meat chopper and finally extracted with peroxide-free ether by percolation. Briefly, the decorticated kernels (1 kg) were defatted by petroleum ether for at least 2 h at room temperature (RT), then filtrated by using a Bűchner funnel and dried. The dried kernels were soaked in peroxide-free ether at RT, overnight in the dark. The oil-ether extract was filtrated as mentioned above, and the extract was concentrated by using rotary evaporator (Büchi, Switzerland). Glacial acetic acid was added to the extract (1 : 1, v/v) and stirred thoroughly, and the gossypol acetate crystals were precipitated overnight. The crystals were collected and stored in refrigerator until use [38].
2.3. Animal Experimental Design
Thirty albino sexually mature Sprague-Dawley male rats, about 10–12 weeks of age (100–130 g body weight), were purchased from the experimental animal house of the Faculty of Science, Cairo University, and housed in the animal house of the physiology department of the Faculty of Medicine, Alexandria University. The animals were grouped (six rats/cage), under standard laboratory conditions with water and food provided ad libitum. All animal experiments were performed following the ethical standards according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (Institute of Laboratory Animal Resources 1996) in the Faculty of Medicine, Alexandria University, Egypt.
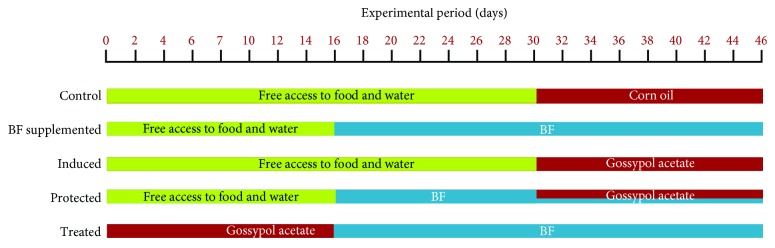
The healthy experimental animals were equally divided into five groups (Figure 1). Group 1 (control) received corn oil (0.5 ml, intraperitoneally) eight times for 16 days. Group 2 (BF supplemented) received BF (100 mg/kg BW, orally by gavage) daily for 30 days. Group 3 (induced) received gossypol acetate (5 mg/kg BW, intraperitoneally, dissolved in corn oil) eight times for 16 days. Group 4 (protected) was administered BF alone for 2 weeks and then was coadministered with gossypol acetate for the next 16 days. Group 5 (treated) received gossypol acetate for 16 days and then was treated with BF for 30 days. The doses of BF and gossypol acetate were as mentioned in groups 2 and 3, respectively (Figure 1). Experimental animals in groups 1, 2, 3, and 4 were allowed free access to water and food without any treatments until the 16th day for groups 2 and 4 for and until the 30th day for groups 1 and 3.
Figure 1.
An illustration of the experimental design groups. Corn oil (0.5 ml, intraperitoneally) eight times for 16 days, BF (100 mg/kg BW, orally by gavage) daily for 30 days, and gossypol acetate (5 mg/kg BW, intraperitoneally, dissolved in corn oil) eight times for 16 days. Green colour indicates free access to food and water, red colour indicates the administration of corn oil or gossypol acetate, and blue colour indicates treatment with BF.
At the end of the experiment (after 46 days), the rats were fasted for eight hours and then the blood was collected from the eye canthus to measure the blood glucose level. Rats were allowed to complete fasting overnight and then decapitated to collect the blood and testes. Sera were isolated and stored at −20°C.
2.4. Preparation of Testicular and Epididymal Tissues
After decapitation, one testis was removed and most of the parenchyma (2/3) was weighed. A 10% (w/v) homogenate of testis tissues in 0.1 M phosphate buffer saline (PBS), pH 7.4, was prepared by using a mortar in an ice bath and centrifuged at 10,000g for 20 min at 4°C. The supernatant was collected and stored for further biochemical investigations. The second testis and one epididymis were postfixed overnight in 10% neutral buffered formalin for histopathological study. The second epididymis was isolated, washed, crushed in 2 ml Ham's F-10 medium (0.5% bovine serum albumin, BSA), and incubated at 37°C for the estimation of the spermatozoal quality by automated examination by MiraLab's Computer Aided Semen Analysis System (CASA, WLJY 9000, Beijing Weili New Century Science and Tech. Dev. Co. Ltd., China) [39]. Methylene blue and eosin red stains were used, respectively, for studying the sperm morphology.
2.5. Biochemical Assays
Serum-reduced glutathione (GSH) and TBARS as well as testicular TBARS and nitric oxide levels were determined according to Jollow et al. [40], Tappel and Zalkin [41], and Menaka et al. [42], respectively. Testosterone level was determined in serum by using an ELISA commercial kit [43], and the levels of glucose [44], cholesterol [45], and albumin [46] were also measured in the serum by using commercial kits (Biosystems S.A., Spain).
2.6. Semen Parameters
Sperm count, motility, and morphology index were assessed using CASA. Alpha-glucosidase activity and fructose level are important parameters related to semen quality. Alpha-glucosidase activity was measured by using the method of Han and Srinivasan [47], in which the specific activity (IU/mg) of the enzyme was defined as micromoles (μmol) of p-nitrophenol released per min per milligram (mg) of protein. The semen fructose level was determined according to Foreman et al. [48].
2.7. Determination of Sperm Inflammatory Markers
Testicular IL-1β, IL-6, IL-12, and IL-18 were estimated by using enzyme-linked immunosorbent assay (ELISA) kits (Koma Biotech–Korea), and testicular TNF-α, ADAM-17, and TIMP-3 were determined by Sun Red (England) ELISA kits. Precoated wells with the captured antibodies were washed four times with the washing buffer. Standard or samples (100 μl) were added to each well in duplicate, covered, and incubated at RT for 2 h. The plates were then washed four times and 100 μl of the diluted detection antibody was added per well, covered, and incubated at RT for 2 h. 100 μl of streptavidin-HRP was added to each well and incubated for 30 min at RT for a proper colour development. The plates were washed and 100 μl of the substrate solution (3,3′,5,5′-tetramethylbenzidine, TMB) was added to each well and incubated for 30 min at RT. The reaction was terminated by adding 100 μl of the stop solution (H2SO4, 5%) to each well, and the colour developed was read at 450 nm on a plate reader (Sanofi Diagnostics Pasteur, France).
2.8. Histopathological Changes
The testes and epididymides of the control and experimental groups were removed, postfixed overnight in 10% neutral buffered formalin, dehydrated in ascending grades of alcohol (70%, 80%, 95%, and absolute alcohol), and cleaned by immersion in xylene followed by impregnation in melted paraffin wax for 1–2 h. Sections 5 μm thick were cut by using a rotary microtome. Finally, the sections were stained with conventional hematoxylin and eosin (H&E) stain for examination under a light microscope of any histopathological changes. The histopathological study was carried out in the Histopathology Department, Faculty of Medicine, Alexandria University.
2.9. Statistical Analysis
Data was analysed by one-way analysis of variance (ANOVA) using the Primer of Biostatistics (Version 5) software program. The significance of means ± SD was detected between groups by a post hoc test (Tukey) at p < 0.05.
3. Results
3.1. Characterization of Berberine-Rich Fraction
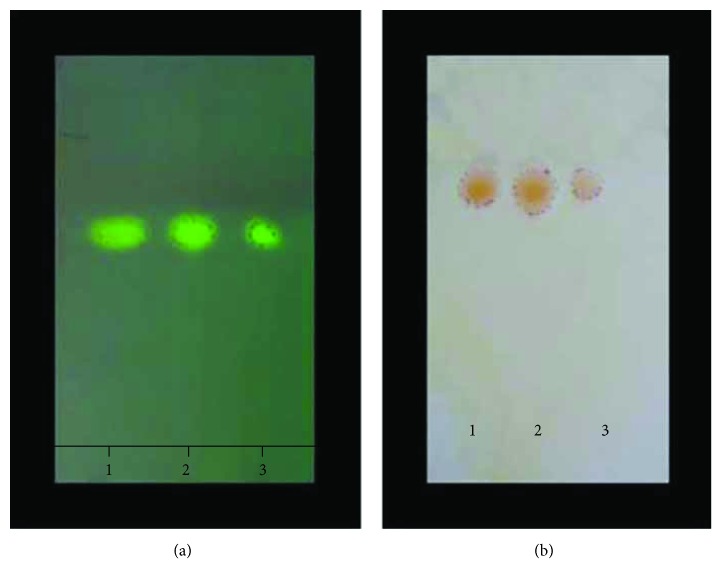
Table 1 shows the berberine concentration in different prepared samples, and BF had the highest concentration (0.89 mg/mg extract). The melting point of berberine chloride was 190°C and that of the berberine base was 165°C. Both the chloroform fraction and BF had the same berberine spot equal distance as shown in TLC results in Figure 2. Table 2 presents the 1H-NMR (DMSO/TMS) showing δ: 3.17 (2H, t, H 5), 4.03 (3H, s, H 10-OCH3), 4.05 (3H, s, H 9-OCH3), 4.89 (2H, t, J = 5.35 Hz, H 6), 6.14 (2H, s, 3-OCH2O), 7.06 (1H, s, H 4), 7.77 (1H, s, H 1), 7.96 (1H, d, H 12), 8.17 (1H, d, J = 8.4 Hz, H 11), 8.90 (1H, s, H 13), and 9.85 (1H, s, H 8) as our team previously published [36].
Table 1.
The berberine concentration in different prepared samples.
| Extract | Berberine concentration (mg/mg extract) |
|---|---|
| Ethanolic extract | 0.6 |
| Chloroform fraction | 0.73 |
| Berberine-rich fraction | 0.89 |
Figure 2.
TLC identification of standard berberine chloride (spot 1), chloroform fraction (spot 2), and isolated berberine base (Spot 3).
Table 2.
1H-NMR (DMSO/TMS) chemical shifts of isolated berberine in ppm [36].
| Proton | δ (ppm) |
|---|---|
| H 5 | 3.17 (2H, t) |
| H 10-OCH3 | 4.03 (3H, s) |
| H 9-OCH3 | 4.05 (3H, s) |
| H 6 | 4.89 (2H, t, J = 5.35 Hz) |
| H 2,3-OCH2O | 6.14 (2H, s) |
| H 4 | 7.06 (1H, s) |
| H 1 | 7.77 (1H, s) |
| H 12 | 7.96 (1H, d, J = 8.4 Hz) |
| H 11 | 8.17 (1H, d, J = 8.4 Hz) |
| H 13 | 8.90 (1H, s) |
| H 8 | 9.85 (1H, s) |
3.2. Effect of Berberine-Rich Fraction on Blood Parameters
Serum GSH, TBARS, testosterone, cholesterol, glucose, and albumin levels were measured to assess the effect of BF alone or in combination with gossypol on rats' fertility. The administration of BF to healthy rats showed no significant change in the levels of GSH, testosterone, or albumin compared to the normal control group. While it significantly decreased the levels of TBARS, cholesterol, and glucose compared with those of the control group as shown in Table 3, gossypol acetate-injected rats (induced group) had a significantly increased TBARS level and decreased GSH, testosterone, cholesterol, glucose, and albumin levels compared to the control group. BF coadministration with gossypol acetate (protected group) significantly decreased the TBARS level and increased GSH, testosterone, cholesterol, glucose, and albumin levels compared to the induced group, at p < 0.05. The treatment with BF (treated group) normalized the TBARS level and significantly enhanced the GSH, testosterone, cholesterol, glucose, and albumin levels, approaching the control levels (Table 3).
Table 3.
Effect of berberine-rich fraction on blood parameters of different experimental groups.
| Groups | TBARS (nmol/ml) | GSH (mg/ml) | Testosterone (ng/ml) | Cholesterol (mg/dl) | Glucose (mg/dl) | Albumin (g/dl) |
|---|---|---|---|---|---|---|
| Control | 0.49 ± 0.01 | 0.38 ± 0.01 | 3.7 ± 0.21 | 110 ± 5.2 | 95 ± 2.4 | 3.5 ± 0.2 |
| BF supplemented | 0.44 ± 0.02∗ | 0.37 ± 0.02 | 3.8 ± 0.15 | 90 ± 1.6∗ | 80 ± 3.1∗ | 3.4 ± 0.1 |
| Induced | 0.58 ± 0.03∗ | 0.29 ± 0.01∗ | 0.36 ± 0.23∗ | 65 ± 2.3∗ | 65 ± 2.1∗ | 2.9 ± 0.04∗ |
| Protected | 0.52 ± 0.03# | 0.32 ± 0.06# | 3.2 ± 0.34# | 75 ± 1.9∗# | 75 ± 2.8∗# | 3.3 ± 0.05 # |
| Treated | 0.49 ± 0.05# | 0.30 ± 0.08# | 3.4 ± 0.16# | 85 ± 3.9∗# | 82 ± 2.6∗# | 3.1 ± 0.2# |
Values represent the mean ± SD of 6 rats. ∗p ≤ 0.05 versus control. #p ≤ 0.05 versus induced group. ANOVA (one-way) followed by Tukey's test.
3.3. Effect of Berberine-Rich Fraction on Semen Quality
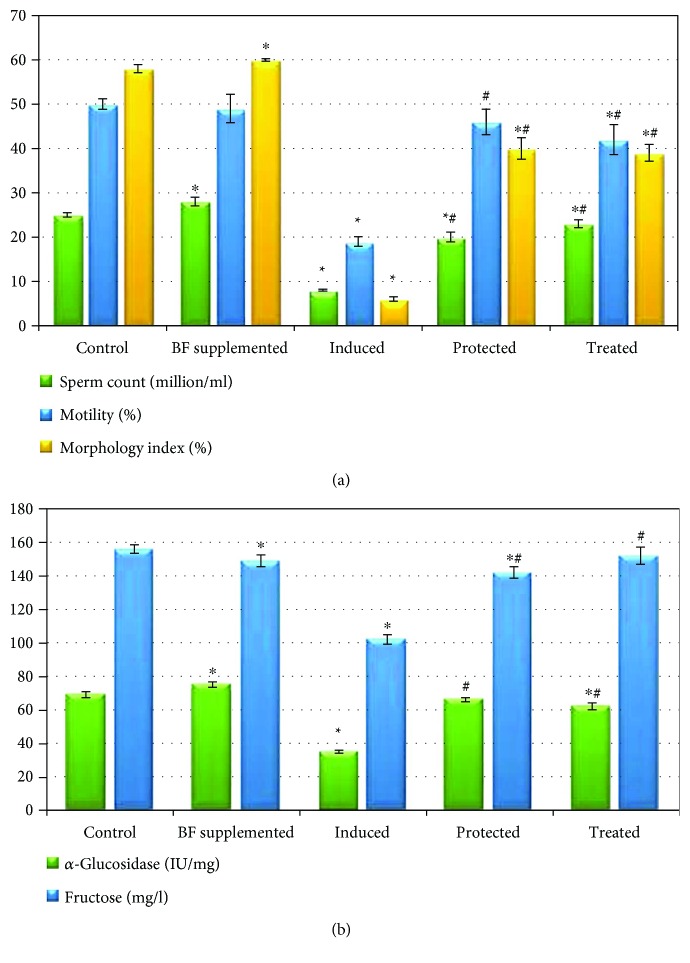
The BF-supplemented group showed normal sperm motility and had significantly improved sperm count and morphology compared with the control group. In addition, BF administration significantly increased the α-glucosidase activity compared to that of the control group. The male infertility-induced group showed a highly significant decrease in the sperm count, inhibited sperm motility, and a marked change in the sperm morphology to irregular shapes compared to the control group. Gossypol acetate injection also significantly inhibited the α-glucosidase activity and significantly decreased the semen fructose level compared with the control group. The BF-protected and BF-treated groups had significantly improved sperm count, motility, and morphology as well as significantly increased α-glucosidase activity and semen fructose level compared to the induced group (p < 0.05), with their values approaching the values of the control group, as shown in Figure 3.
Figure 3.
Effects of berberine-rich fraction on sperm parameters. Graph (a) shows sperm count, motility, and morphology index. Graph (b) shows α-glucosidase activity and fructose level. Values represent the mean ± SD of 6 rats. ∗p ≤ 0.05 versus control group. #p ≤ 0.05 versus induced group. One-way ANOVA followed by Tukey's test was used.
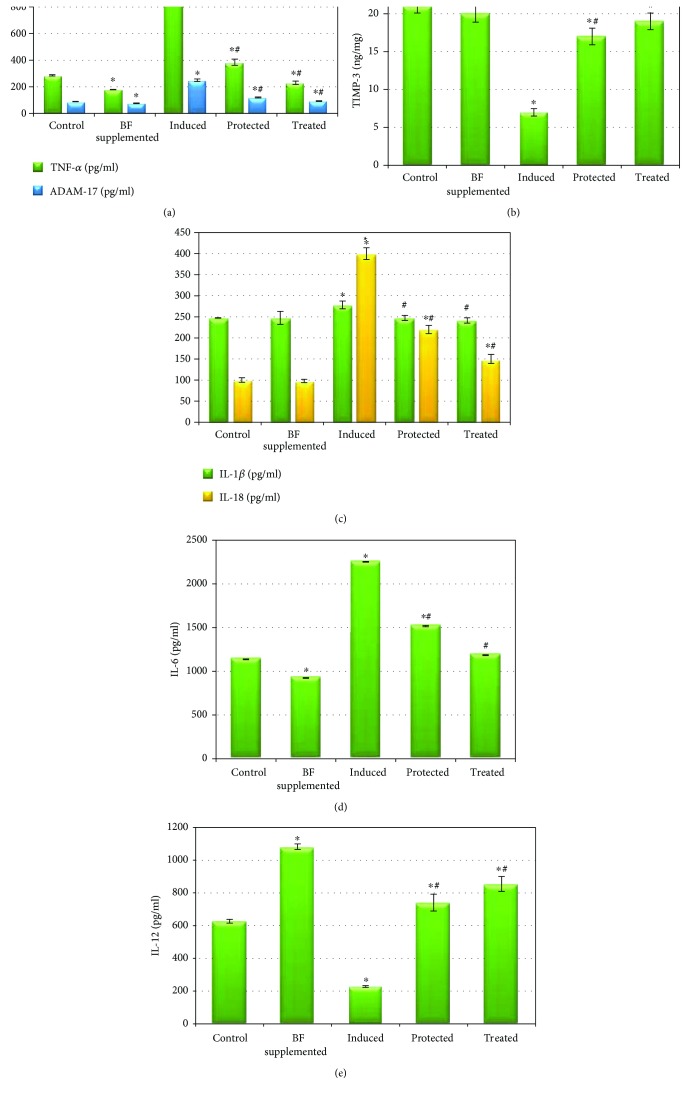
3.4. Effect of Berberine-Rich Fraction on the Testicular Inflammatory Markers
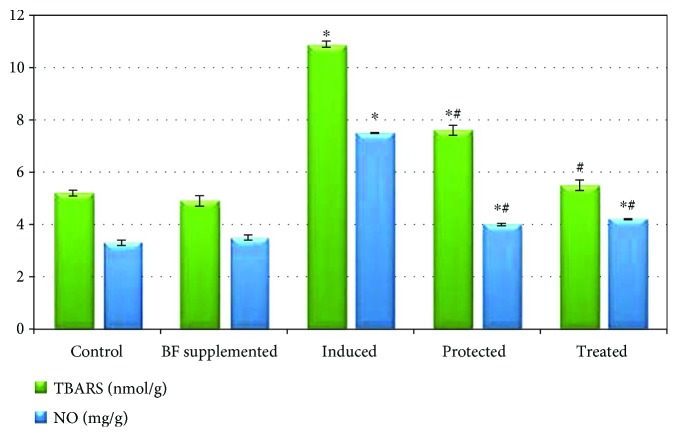
Figures 4 and 5 demonstrate that the administration of BF to healthy rats resulted in a nonsignificant change in the levels of testicular TBARS, NO, TIMP-3, and interleukins (IL-1β and IL-18) compared to the normal control group, at p < 0.05. It also significantly decreased TNF-α, ADAM-17, and IL-6 while it increased IL-12 compared to that of the control group (at p < 0.05). Gossypol-induced male infertility markedly and significantly increased the levels of testicular TBARS, NO, TNF-α, ADAM-17, and interleukins (IL-1β, IL-6, and IL-18) while significantly decreasing the levels of both TIMP-3 and IL-12 compared with those of the control group. A significant decrease in the levels of testicular TBARS, NO, ADAM-17, TNF-α, and interleukins (IL-18, IL-6, and IL-1β) as well as a significant increase in the levels of TIMP-3 and IL-12 was observed in the BF-protected and BF-treated groups compared to the gossypol-induced group. In addition, the administration of BF after gossypol injection (treated group) normalized the levels of testicular TIMP-3, TBARS, and interleukins (IL-1β and IL-6), approaching the values of the control group.
Figure 4.
Effect of berberine-rich fraction on sperm prooxidants. Values represent the mean ± SD of 6 rats. ∗p ≤ 0.05 versus control group. #p ≤ 0.05 versus induced group. One-way ANOVA followed by Tukey's test was used.
Figure 5.
Effect of berberine-rich fraction on sperm inflammatory markers. Values represent the mean ± SD of 6 rats. ∗p ≤ 0.05 versus control group. #p ≤ 0.05 versus induced group. One-way ANOVA followed by Tukey's test was used.
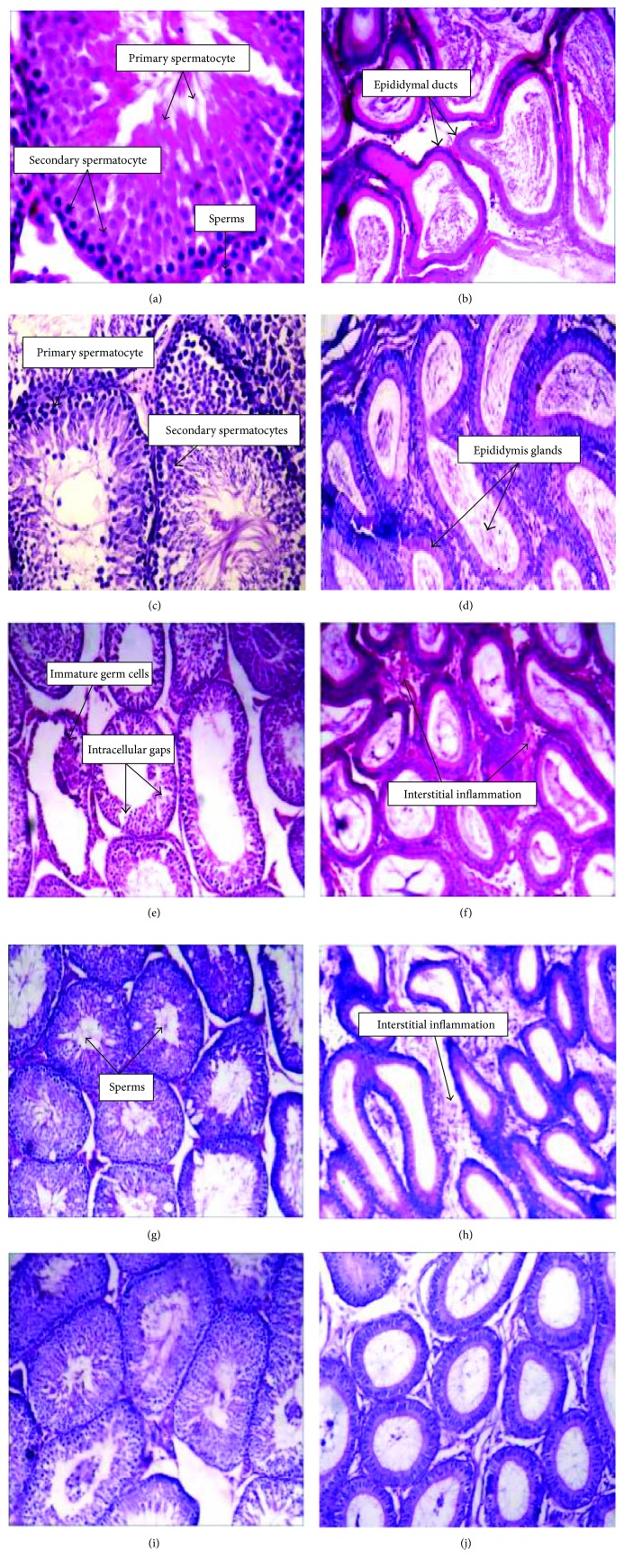
3.5. Histological Changes in Testicular and Epididymal Tissues of Different Experimental Groups
Figures 6(a)–6(d) show that the control healthy and BF-supplemented rats had normal and well-organized seminiferous tubules, and they show all stages of spermatogenesis till the stage of sperm formation. They also have normal epididymal ducts. On the other hand, the testicular tissue of the gossypol-treated group (Figure 6(e)) shows an accumulation of immature germ cells in the lumen and defects in spermatogenesis and sperm formation. Furthermore, it shows an increase in intracellular gaps due to disruption in cell-cell contacts in the seminiferous epithelium compared to the control one. Moreover, the epididymal section (Figure 6(f)) revealed leukocytic infiltration, congestion, and edema. The BF-protected group shows almost near-normal spermatogenesis with sperm formation in the testicular sections (Figure 6(g)) and mild interstitial inflammation and edema in the epididymal sections (Figure 6(h)). The testicular section of the BF-treated group (Figure 6(i)) revealed a marked improvement in spermatogenesis in all stages till sperm formation compared with those observed in the control one, but little interstitial inflammation was observed in the epididymal section (Figure 6(j).
Figure 6.
The histological examination of both testicular and epididymal tissues of different treated groups compared to the control one. (Magnification of a and c: ×40; magnification of b, d, e, f, g, h, i, and j: ×10). Testicular (a) and epididymal tissues (b) of control group. Testicular (c) and epididymal tissues (d) of berberine-rich fraction (100 mg/kg orally) supplemented group. Testicular (e) and epididymal tissues (f) of gossypol-induced group (5 mg/kg, 8 times). Testicular (g) and epididymal tissues (h) of berberine-rich fraction (100 mg/kg orally) protected group. Testicular (i) and epididymal tissues (j) of berberine-rich fraction (100 mg/kg orally) treated group.
4. Discussion
Male infertility is considered to be one of the most critical health problems that are expected to increase [49]. Multiple lifestyle and environmental factors contributed to the etiology of male infertility including cigarette smoking, alcohol, heavy metals, pesticides, radiation, and illicit drugs; these factors are growing in number and are widely spread [50, 51]. In this study, BF (89%) was shown to be a leading therapy for this problem by controlling the inflammatory markers that are produced in the case of male infertility and that result in a destructive damage to sperm and reduction in the semen quality.
Gossypol is a toxic phenolic compound extracted from cottonseeds [28, 38]. Deleterious effects of gossypol on fertility have been widely reported in the literature [29, 52]. The intraperitoneal injection of gossypol acetate significantly reduced the semen quality as evidenced by the decrease in the sperm count and motility and changed the sperm morphology as well as inhibited the α-glucosidase activity compared with those of the control group. Alpha-glucosidase activity is widely used as an indicative marker for sperm count as alpha-glucosidase is produced by the epididymis, so a low level of α-glucosidase indicates epididymal obstruction [53]. Gossypol administration significantly reduced the enzyme activity in all segments of the epididymis [54]. The major antifertility effect of gossypol is to inhibit sperm production and motility; this inhibitory action has been attributed to a dramatic drop in the production of mitochondrial ATP [29]. Furthermore, gossypol induces oxidative stress by promoting the formation of ROS and lipid peroxides, which negatively affected plasma membrane permeability, ATPase activity, and glucose transport in sperms [55, 56]. In the present study, gossypol injection significantly elevated serum and testicular TBARS and NO levels as well as depleted the antioxidant capacity (GSH) and serum glucose level, resulting in the damage of sperm membrane. These results are in concert with the reports from El-Sharaky et al. [38] and Santana et al. [29] that demonstrated the reproductive damage caused by gossypol-induced oxidative stress in rats and that from Chen et al. [52] that reported a lowered serum glucose level after gossypol treatment.
Free radical overproduction enhances proinflammatory gene expression and is associated with inflammatory reactions. On the other hand, inflammatory cells increased the generation of ROS, leading to exaggerated oxidative stress that impairs sperm function [8, 11]. The proinflammatory cytokines TNF-α, IL-1α and IL-1β may have certain physiological functions in the male genital tract. However, the elevated levels of these cytokines compared with the normal one, as seen during the inflammation process, are very harmful to sperm production [8]. Increasing the generation of ROS induces ADAM-17 expression and results in TNF-α proteolytic cleavage, which in turn increases ROS production, induces oxidative stress, promotes lipid peroxidation, causes destructive damage sperm cell membrane, decreases sperm motility, and induces apoptosis [57]. TIMP-3 is an inhibitor for ADAM-17 and negatively controls its activity [15]. Thus, the balance between ADAM-17 and TIMP-3 expression can control the inflammation process. In addition, IL-18 was found to stimulate the cytotoxic activity of T cells and natural killer cells and stimulates the production of IL-6 and TNF-α [58]. On the other hand, Naz and Evans [12] suggested a significant correlation between IL-12 levels and fertility as these improve the count and normal morphology of sperm in the semen. Therefore, male infertility may be attributed to its derangement. The destruction of testicular tissues, in the case of infertility, lowered the production of IL-12 and negatively affected the sperm count and motility [12]. In agreement with the earlier findings, gossypol acetate injection significantly increased the levels of testicular ADAM-17, TNF-α, and interleukins (IL-1β, IL-6, and IL-18), while it decreased TIMP-3 and IL-12 levels compared with those of the control group. TNF-α affects the androgenic receptor controlling testosterone activity; it decreases the production of testosterone and reduces the sperm function [2].
Testosterone, as a steroid hormone, is essential for spermatogenesis [5]. Gossypol was found to induce the regression of Leydig cells, resulting in a significant decrease in the level of serum testosterone and reduced libido [56]. Furthermore, testosterone regulates the formation of seminal fructose, as it controls the activity and function of the accessory glands that are responsible for fructose secretion. Fructose is an important source of energy for sperms and is required for sperm motility [59]. Thus, the decreased level of semen fructose is due to gossypol-induced depletion in the testosterone level. In addition, gossypol decreased the serum level of cholesterol, which is consistent with the studies by Nwoha and Aire [60] and Obeidy et al. [61], who reported that gossypol altered the serum lipoprotein metabolism.
Antioxidants can contribute to the protection of cells and tissues against the deleterious effects of free radicals [9]. The toxicological effect of gossypol was reversed by the treatment of animals with berberine-rich fraction. Berberine (BBR) is one of the most potent ingredients in Berberis vulgaris, characterized by a diversity of pharmacological effects [17, 22] including antioxidant and anti-inflammatory properties in a variety of tissues including kidney, liver, pancreas, and adipose tissue. BBR administration reduced the oxidative stress markers (TBARS) and evaluated the antioxidant enzymes (GSH, GPx, and SOD) in diabetic animals [24]. In the present study, the oral administration of BF successfully reversed most of the hazardous effects of gossypol on semen characteristics. BF significantly enhanced the sperm count, motility, and morphology as well as improved α-glucosidase activity and increased the semen fructose level. BF has antioxidant properties, as confirmed by the reduction of TBARS and NO levels as well as the elevation of the reduced level of glutathione, and can be considered one of the most potent antioxidant agents, protecting the cell against ROS destructive damage [62]. These results confirmed the potent antioxidant capacity of BF as mentioned previously [27, 63, 64].
The oral administration of BF either with or after gossypol acetate injection represented an ameliorative effect against the analysed inflammatory markers. Previously, BBR showed a potent anti-inflammatory effect by suppressing the production of inflammatory mediators such as TNF-α, COX-2, IL-1β, IL-6, NO, and inducible nitric oxide synthase (iNOS) as well as by the inhibition of arachidonic acid metabolism [23, 24, 65, 66]. In addition, BBR was found to inhibit the activator protein-1 (AP-1, a key transcription factor in inflammation) [67] and inhibit DNA synthesis in active lymphocytes, resulting in the inhibition of lymphocyte transformation [68].
The obtained biochemical results were confirmed by the histological study where gossypol intraperitoneal injection resulted in a marked histological alterations in the testicular and epididymal tissues including depressed spermatogenesis, degenerative germ cells, and vacuoles (in testicular section) as well as leukocytic infiltration and edema (in epididymal section), which are in agreement with the El-Sharaky et al. [38]. A normal histological structure of a rat's testis and epididymis was observed in both control and BF-supplemented animals. Almost near-normal spermatogenesis with sperm formation in the testicular sections and little mild interstitial inflammation in the epididymal sections were observed in BF-protected and BF-treated groups.
In conclusion, the mechanism of gossypol-induced toxicity on rats' testes contributed to the induction of oxidative stress and inflammatory responses, leading to cell membrane damage and reduced sperm count, motility, and morphology. The administration of BF to animals was shown to be effective in preventing oxidative damage and inflammation induced by gossypol. Thus, the use of BF can be suggested as a palliative measure in animals subjected to poisoning by gossypol. However, further studies must be done to confirm the anti-inflammatory effect of BF by using different experimental models. Moreover, the concentration of active metabolite (berberine) must be measured in the testicular tissue and the pharmacokinetics of BF should be investigated in rats.
Acknowledgments
This research was supported by the Alexandria University Research Fund under the Research Enhancement Program (ALEX REP 2011-2012).
Conflicts of Interest
The authors declare that the research was conducted without any conflict of interest for each author, and the authors alone are responsible for the content and writing of the paper.
References
- 1.Bisht S., Faiq M., Tolahunase M., Dada R. Oxidative stress and male infertility. Nature Reviews Urology. 2017;14(8):470–485. doi: 10.1038/nrurol.2017.69. [DOI] [PubMed] [Google Scholar]
- 2.Havrylyuk A., Chopyak V., Boyko Y., Kril I., Kurpisz M. Cytokines in the blood and semen of infertile patients. Central European Journal of Immunology. 2015;40(3):337–344. doi: 10.5114/ceji.2015.54596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal A., Mulgund A., Hamada A., Chyatte M. R. A unique view on male infertility around the globe. Reproductive Biology and Endocrinology. 2015;13(1):p. 37. doi: 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiser H. J., Sandlow J., Köhler T. S. Causes of male infertility. In: Parekattil S. J., Agarwal A., editors. Male Infertility: Contemporary Clinical Approaches, Andrology, ART & Antioxidants. New York, NY, USA: Springer New York; 2012. pp. 3–14. [DOI] [Google Scholar]
- 5.Oremosu A. A., Akang E. N. Impact of alcohol on male reproductive hormones, oxidative stress and semen parameters in Sprague–Dawley rats. Middle East Fertility Society Journal. 2015;20(2):114–118. doi: 10.1016/j.mefs.2014.07.001. [DOI] [Google Scholar]
- 6.Adewoyin M., Ibrahim M., Roszaman R., et al. Male infertility: the effect of natural antioxidants and phytocompounds on seminal oxidative stress. Diseases. 2017;5(4):p. 9. doi: 10.3390/diseases5010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal A., Bui A. D. Oxidation-reduction potential as a new marker for oxidative stress: correlation to male infertility. Investigative and Clinical Urology. 2017;58(6):385–399. doi: 10.4111/icu.2017.58.6.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azenabor A., Ekun A. O., Akinloye O. Impact of inflammation on male reproductive tract. Journal of Reproduction & Infertility. 2015;16(3):123–129. [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal A., Virk G., Ong C., du Plessis S. S. Effect of oxidative stress on male reproduction. The World Journal of Men’s Health. 2014;32(1):1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkar O., Bahrainwala J., Chandrasekaran S., Kothari S., Mathur P. P., Agarwal A. Impact of inflammation on male fertility. Frontiers in Bioscience. 2011;E3:89–95. doi: 10.2741/e223. [DOI] [PubMed] [Google Scholar]
- 11.Biswas S. K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxidative Medicine and Cellular Longevity. 2016;2016:9. doi: 10.1155/2016/5698931.5698931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naz R. K., Evans L. Presence and modulation of interleukin-12 in seminal plasma of fertile and infertile men. Journal of Andrology. 1998;19(3):302–307. doi: 10.1002/j.1939-4640.1998.tb02009.x. [DOI] [PubMed] [Google Scholar]
- 13.Gazvani M. R., Bates M., Vince G., Christmas S., Lewis-Jones D. I., Kingsland C. Follicular fluid concentrations of interleukin-12 and interleukin-8 in IVF cycles. Fertility and Sterility. 2000;74(5):953–958. doi: 10.1016/S0015-0282(00)01538-7. [DOI] [PubMed] [Google Scholar]
- 14.Fairbanks F., Abrão M. S., Podgaec S., Dias J. A., Jr, de Oliveira R. M., Rizzo L. V. Interleukin-12 but not interleukin-18 is associated with severe endometriosis. Fertility and Sterility. 2009;91(2):320–324. doi: 10.1016/j.fertnstert.2007.11.060. [DOI] [PubMed] [Google Scholar]
- 15.Cesaro A., Abakar-Mahamat A., Brest P., et al. Differential expression and regulation of ADAM17 and TIMP3 in acute inflamed intestinal epithelia. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2009;296(6):G1332–G1343. doi: 10.1152/ajpgi.90641.2008. [DOI] [PubMed] [Google Scholar]
- 16.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Frontiers in Pharmacology. 2014;4:p. 177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imenshahidi M., Hosseinzadeh H. Berberis vulgaris and berberine: an update review. Phytotherapy Research. 2016;30(11):1745–1764. doi: 10.1002/ptr.5693. [DOI] [PubMed] [Google Scholar]
- 18.Rahimi-Madiseh M., Lorigoini Z., Zamani-Gharaghoshi H., Rafieian-Kopaei M. Berberis vulgaris: specifications and traditional uses. Iran J Basic Med Sci. 2017;20(5):569–587. doi: 10.22038/IJBMS.2017.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Battu S. K., Repka M. A., Maddineni S., Chittiboyina A. G., Avery M. A., Majumdar S. Physicochemical characterization of berberine chloride: a perspective in the development of a solution dosage form for oral delivery. AAPS PharmSciTech. 2010;11(3):1466–1475. doi: 10.1208/s12249-010-9520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S., Lim H. J., Park J. H., Lee K. S., Jang Y., Park H. Y. Berberine-induced LDLR up-regulation involves JNK pathway. Biochemical and Biophysical Research Communications. 2007;362(4):853–857. doi: 10.1016/j.bbrc.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 21.Chai Y. S., Hu J., Lei F., et al. Effect of berberine on cell cycle arrest and cell survival during cerebral ischemia and reperfusion and correlations with p53/cyclin D1 and PI3K/Akt. European Journal of Pharmacology. 2013;708(1–3):44–55. doi: 10.1016/j.ejphar.2013.02.041. [DOI] [PubMed] [Google Scholar]
- 22.Pang B., Zhao L. H., Zhou Q., et al. Application of berberine on treating type 2 diabetes mellitus. International Journal of Endocrinology. 2015;2015:12. doi: 10.1155/2015/905749.905749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuo C. L., Chi C. W., Liu T. Y. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Letters. 2004;203(2):127–137. doi: 10.1016/j.canlet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Li Z., Geng Y. N., Jiang J. D., Kong W. J. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evidence-based Complementary and Alternative Medicine. 2014;2014:12. doi: 10.1155/2014/289264.289264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng W. H., Lo K. L., Lee Y. H., Hung T. H., Lin Y. C. Berberine produces antidepressant-like effects in the forced swim test and in the tail suspension test in mice. Life Sciences. 2007;81(11):933–938. doi: 10.1016/j.lfs.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Kulkarni S. K., Dhir A. On the mechanism of antidepressant-like action of berberine chloride. European Journal of Pharmacology. 2008;589(1–3):163–172. doi: 10.1016/j.ejphar.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 27.Abd El-Wahab A. E., Ghareeb D. A., Sarhan E. E., Abu-Serie M. M., El Demellawy M. A. In vitro biological assessment of Berberis vulgaris and its active constituent, berberine: antioxidants, anti-acetylcholinesterase, anti-diabetic and anticancer effects. BMC Complementary and Alternative Medicine. 2013;13(1):p. 218. doi: 10.1186/1472-6882-13-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gadelha I. C. N., Fonseca N. B. S., Oloris S. C. S., Melo M. M., Soto-Blanco B. Gossypol toxicity from cottonseed products. The Scientific World Journal. 2014;2014:11. doi: 10.1155/2014/231635.231635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santana A. T., Guelfi M., Medeiros H. C. D., Tavares M. A., Bizerra P. F. V., Mingatto F. E. Mechanisms involved in reproductive damage caused by gossypol in rats and protective effects of vitamin E. Biological Research. 2015;48(1):p. 43. doi: 10.1186/s40659-015-0026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hadley M. A., Lin Y. C., Dym M. Effects of gossypol on the reproductive system of male rats. Journal of Andrology. 1981;2(4):190–199. doi: 10.1002/j.1939-4640.1981.tb00615.x. [DOI] [Google Scholar]
- 31.Xue S. P. Gossypol contraception and mechanism of action. In: Lobl T. J., Hafez E. S. E., editors. Male Fertility and Its Regulation. Dordrecht, Netherlands: Springer Netherlands; 1985. [DOI] [Google Scholar]
- 32.Zavos P. M., Zarmakoupis-Zavos P. N. The inhibitory effects of gossypol on human sperm motility characteristics: possible modes of reversibility of those effects. The Tohoku Journal of Experimental Medicine. 1996;179(3):167–175. doi: 10.1620/tjem.179.167. [DOI] [PubMed] [Google Scholar]
- 33.Nair I. N., Bhiwgade D. A. Effect of gossypol on pituitary reproductive axis: ultrastructural and biochemical studies. Indian Journal of Experimental Biology. 1990;28(8):724–732. [PubMed] [Google Scholar]
- 34.Liu Y. X. Control of spermatogenesis in primate and prospect of male contraception. Archives of Andrology. 2005;51(2):77–92. doi: 10.1080/01485010490485768. [DOI] [PubMed] [Google Scholar]
- 35.Pradhan D., Prativa B., Suri K. A. Isolation of berberine from Berberis vulgaris Linn. and standardization of aqueous extract by RP-HPLC. International Journal of Herbal Medicine. 2013;1(2):106–111. [Google Scholar]
- 36.Abd El-Salam M., Mekky H., El-Naggar E. M. B., Ghareeb D., El-Demellawy M., El Fiky F. Biological activities of Berberis vulgaris constituents: cytotoxicity, antihepatitis C virus and anti-acetylcholine esterase. Journal of Pharmaceutical Biology. 2016;6(2):89–97. [Google Scholar]
- 37.Abd El-Salam M., Mekkya H., El-Naggar E. M. B., Ghareeb D., El-Demellawy M., El-Fiky F. Hepatoprotective properties and biotransformation of berberine and berberrubine by cell suspension cultures of Dodonaea viscosa and Ocimum basilicum. South African Journal of Botany. 2015;97:191–195. doi: 10.1016/j.sajb.2015.01.005. [DOI] [Google Scholar]
- 38.El-Sharaky A. S., Newairy A. A., Elguindy N. M., Elwafa A. A. Spermatotoxicity, biochemical changes and histological alteration induced by gossypol in testicular and hepatic tissues of male rats. Food and Chemical Toxicology. 2010;48(12):3354–3361. doi: 10.1016/j.fct.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Yeung C., Pérez-Sánchez F., Soler C., Poser D., Kliesch S., Cooper T. G. Maturation of human spermatozoa (from selected epididymides of prostatic carcinoma patients) with respect to their morphology and ability to undergo the acrosome reaction. Human Reproduction Update. 1997;3(3):205–213. doi: 10.1093/humupd/3.3.205. [DOI] [PubMed] [Google Scholar]
- 40.Jollow D. J., Mitchell J. R., Zampaglione N., Gillette J. R. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11(3):151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 41.Tappel A. L., Zalkin H. Inhibition of lipide peroxidation in mitochondria by vitamin E. Archives of Biochemistry and Biophysics. 1959;80(2):333–336. doi: 10.1016/0003-9861(59)90259-0. [DOI] [Google Scholar]
- 42.Menaka K., Ramesh A., Thomas B., Kumari N. S. Estimation of nitric oxide as an inflammatory marker in periodontitis. Journal of Indian Society of Periodontology. 2009;13(2):75–78. doi: 10.4103/0972-124X.55842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen A., Bookstein J. J., Meldrum D. R. Diagnosis of a testosterone-secreting adrenal adenoma by selective venous catheterization. Fertility and Sterility. 1991;55(6):1202–1203. doi: 10.1016/S0015-0282(16)54378-7. [DOI] [PubMed] [Google Scholar]
- 44.Hjelm M., Verdier C. H. Determination of serum glucose by glucose oxidase method. Scandinavian Journal of Clinical and Laboratory Investigation. 1963;15:415–428. doi: 10.3109/00365516309079764. [DOI] [PubMed] [Google Scholar]
- 45.Watson D. A simple method for the determination of serum cholesterol. Clinica Chimica Acta. 1960;5(5):637–643. doi: 10.1016/0009-8981(60)90004-8. [DOI] [PubMed] [Google Scholar]
- 46.Doumas B. T., Ard Watson W., Biggs H. G. Albumin standards and the measurement of serum albumin with bromcresol green. Clinica Chimica Acta. 1997;258(1):21–30. doi: 10.1016/S0009-8981(96)06447-9. [DOI] [PubMed] [Google Scholar]
- 47.Han Y. W., Srinivasan V. R. Purification and characterization of β-glucosidase of Alcaligenes faecalis. Journal of Bacteriology. 1969;100(3):1355–1363. doi: 10.1128/jb.100.3.1355-1363.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foreman D., Gaylor L., Evans E., Trella C. A modification of the Roe procedure for determination of fructose in tissues with increased specificity. Analytical Biochemistry. 1973;56(2):584–590. doi: 10.1016/0003-2697(73)90225-X. [DOI] [PubMed] [Google Scholar]
- 49.Pandiyan N., Khan S. D. A clinical approach to male infertility. In: Gunasekaran K., Pandiyan N., editors. Male Infertility: A Clinical Approach. New Delhi, India: Springer India; 2017. [DOI] [Google Scholar]
- 50.Sohrabvand F., Jafari M., Shariat M., Haghollahi F., Lotfi M. Frequency and epidemiologic aspects of male infertility. Acta Medica Iranica. 2015;53(4):231–235. [PubMed] [Google Scholar]
- 51.Sharma R., Biedenharn K. R., Fedor J. M., Agarwal A. Lifestyle factors and reproductive health: taking control of your fertility. Reproductive Biology and Endocrinology. 2013;11(1):p. 66. doi: 10.1186/1477-7827-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen G., Wang R., Chen H., Wu L., Ge R. S., Wang Y. Gossypol ameliorates liver fibrosis in diabetic rats induced by high-fat diet and streptozocin. Life Sciences. 2016;149:58–64. doi: 10.1016/j.lfs.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 53.Krause W., Bohring C. Why do we determine α-glucosidase activity in human semen during infertility work-up? Andrologia. 1999;31(5):289–294. doi: 10.1046/j.1439-0272.1999.00285.x. [DOI] [PubMed] [Google Scholar]
- 54.Kalla N. R., Kaur S., Ujwal N., Mehta U., Joos H., Frick J. Alpha-glucosidase activity in the rat epididymis under different physiological conditions. International Journal of Andrology. 1997;20(2):92–95. doi: 10.1046/j.1365-2605.1997.t01-1-00039.x. [DOI] [PubMed] [Google Scholar]
- 55.Wang J., Jin L., Li X., et al. Gossypol induces apoptosis in ovarian cancer cells through oxidative stress. Molecular BioSystems. 2013;9(6):1489–1497. doi: 10.1039/c3mb25461e. [DOI] [PubMed] [Google Scholar]
- 56.El-Mokadem M. Y., Taha T. A., Samak M. A., Yassen A. M. Alleviation of reproductive toxicity of gossypol using selenium supplementation in rams. Journal of Animal Science. 2012;90(9):3274–3285. doi: 10.2527/jas.2011-4545. [DOI] [PubMed] [Google Scholar]
- 57.Ghareeb D. A., Sarhan E. M. E. Role of oxidative stress in male fertility and idiopathic infertility: causes and treatment. Journal of Diagnostic Techniques and Biomedical Analysis. 2014;2:p. 1. doi: 10.4172/2469-5653.1000107. [DOI] [Google Scholar]
- 58.Feldmann M., Saklatvala J. Proinflammatory cytokines. In: Oppen-heim J. J., Feldman M., editors. Cytokine Reference. New York, NY, USA: Academic Press; 2001. pp. 291–305. [Google Scholar]
- 59.Al-Daghistani H. I., Hamad A.-W. R., Abdel-Dayem M., Al-Swaifi M., Abu Zaid M. Evaluation of serum testosterone, progesterone, seminal antisperm antibody, and fructose levels among Jordanian males with a history of infertility. Biochemistry Research International. 2010;2010:8. doi: 10.1155/2010/409640.409640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nwoha P. U., Aire T. A. Reduced level of serum cholesterol in low protein-fed Wistar rats administered gossypol and chloroquine. Contraception. 1995;52(4):261–265. doi: 10.1016/0010-7824(95)00186-E. [DOI] [PubMed] [Google Scholar]
- 61.Obeidy A., Fities I., Sheriff D. The effect of gossypol on serum lipoproteins of adult rats. Hormone and Metabolic Research. 1989;21(12):649–651. doi: 10.1055/s-2007-1009311. [DOI] [PubMed] [Google Scholar]
- 62.Radak Z., Zhao Z., Koltai E., Ohno H., Atalay M. Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling. Antioxidants & Redox Signaling. 2013;18(10):1208–1246. doi: 10.1089/ars.2011.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laamech J., el-Hilaly J., Fetoui H., et al. Berberis vulgaris L. effects on oxidative stress and liver injury in lead-intoxicated mice. Journal of Complementary and Integrative Medicine. 2017;14(1) doi: 10.1515/jcim-2015-0079. [DOI] [PubMed] [Google Scholar]
- 64.Pongkittiphan V., Chavasiri W., Supabphol R. Antioxidant effect of berberine and its phenolic derivatives against human fibrosarcoma cells. Asian Pacific Journal of Cancer Prevention. 2015;16(13):5371–5376. doi: 10.7314/APJCP.2015.16.13.5371. [DOI] [PubMed] [Google Scholar]
- 65.Pan L. R., Tang Q., Fu Q., Hu B. R., Xiang J. Z., Qian J. Q. Roles of nitric oxide in protective effect of berberine in ethanol-induced gastric ulcer mice. Acta Pharmacologica Sinica. 2005;26(11):1334–1338. doi: 10.1111/j.1745-7254.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- 66.Zhu F., Wu F., Ma Y., et al. Decrease in the production of beta-amyloid by berberine inhibition of the expression of beta-secretase in HEK293 cells. BMC Neuroscience. 2011;12(1):125–125. doi: 10.1186/1471-2202-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fukuda K., Hibiya Y., Mutoh M., Koshiji M., Akao S., Fujiwara H. Inhibition of activator protein 1 activity by berberine in human hepatoma cells. Planta Medica. 1999;65(4):381–383. doi: 10.1055/s-2006-960795. [DOI] [PubMed] [Google Scholar]
- 68.Ckless K., Schlottfeldt J. L., Pasqual M., Moyna P., Henriques J. A. P., Wajner M. Inhibition of in-vitro lymphocyte transformation by the isoquinoline alkaloid berberine. The Journal of Pharmacy and Pharmacology. 1995;47(12A):1029–1031. doi: 10.1111/j.2042-7158.1995.tb03291.x. [DOI] [PubMed] [Google Scholar]