Abstract
Background
African American (AA) women have higher incidence of aggressive, young-onset (<40 years) breast cancers. Young- and older-onset disease may have distinct tumor biologies and etiologies; however, studies investigating age differences among AA women have been rare and generally underpowered.
Methods
We examined tumor characteristics and breast cancer risk factors associated with premenopausal young (<40) vs. older (≥40) AA women’s breast cancer in the African American Breast Cancer Epidemiology and Risk Consortium (2,008 cases and 5,144 controls). Unconditional logistic regression models assessed heterogeneity of tumor biology and risk factor associations by age, overall and by estrogen receptor status.
Results
Premenopausal AA women <40 years had higher frequency of poorer-prognosis tumor characteristics compared to older women, including negative estrogen and progesterone receptor status, triple-negative subtype, high grade, higher stage, and larger tumors. Adiposity (i.e., waist-to-hip ratio) and family history of breast cancer were more strongly associated with young-onset disease (case-control OR=1.46, 95% CI=1.04,2.05; OR=3.10, 95% CI=2.08,4.63, respectively) compared to older-onset disease (OR=1.11, 95% CI=0.91,1.35; OR=1.57, 95% CI=1.26,1.94). Breastfeeding showed a slight inverse risk association among young women (OR=0.70, 95% CI=0.43,1.16). Oral contraceptive use was associated with increased risk regardless of age. Considering various cutpoints for young age (<40, <45, <50), age-related heterogeneity was greatest when <40 was used.
Conclusions
Among premenopausal AA women, diagnosis before age 40 is associated with more aggressive breast tumor biology and some etiologic differences.
Impact
Modifiable risk factors including breastfeeding, adiposity, and oral contraceptive use may be important targets for mitigating harms of young-onset breast cancer.
Keywords: Breast cancer, epidemiology, age, young, risk factors, tumor characteristics
INTRODUCTION
African American (AA) women have a higher relative frequency of breast cancers with advanced stage, larger size, higher grade, hormone receptor negative, and basal-like subtype compared to white women with breast cancer(1–8). Similar tumor biology is also evident in young-onset (<40 years) breast cancers, which are more common among AA women(1,9–11). Differences in risk factor profiles for young and older women may reflect distinct etiologies for breast cancers arising in young and AA women. Risk factors such as parity, age at first birth, oral contraceptive use, and obesity have been shown to differentially affect the risk of breast cancer according to age at diagnosis(12–19). These same risk factors are also differentially associated with hormone receptor positive and negative disease(16,17,20,21), which may confound observed age-related patterns. However, population-based studies examining whether risk factor associations vary by age at diagnosis among AA women are rare and have been hampered by small sample sizes, overall and by age(17,22,23). Furthermore, previous studies of young women’s breast cancer have used inconsistent definitions of young age, defining young with varying age cutpoints or confounding age and menopausal status(17–19,22–26), complicating comparisons across studies.
The present study investigated risk factors for young AA women’s breast cancer in the African American Breast Cancer Epidemiology and Risk (AMBER) Consortium, a large collaboration of breast cancer studies among AA women with extensive clinical, molecular, and epidemiologic data. We restricted our analysis to premenopausal women, as previous work has suggested that age and menopausal status may have independent roles in young women’s breast cancer(12,24). Our objectives were two-fold: first, to characterize the biology of breast cancers diagnosed among young and older premenopausal AA women in the AMBER Consortium, and second, to identify epidemiologic risk factors associated with premenopausal young- vs. older-onset breast cancers overall and by estrogen receptor (ER) status. We hypothesized that more aggressive breast tumor characteristics and distinct patterns of breast cancer risk factors would be associated with young-onset breast cancers (<40 years), and that ER status would modify observed risk factor associations by age at diagnosis.
MATERIALS AND METHODS
Study population
The African American Breast Cancer Epidemiology and Risk (AMBER) Consortium is a collaboration of four of the largest epidemiologic studies of breast cancer among African American (AA) women(27). Included are two case-control studies, the Carolina Breast Cancer Study (CBCS) (17,28) and Women’s Circle of Health Study (WCHS)(29), as well as two prospective cohort studies, the Black Women’s Health Study (BWHS)(30) and the Multiethnic Cohort Study (MEC)(31). The AMBER Consortium and participating studies have been described in detail previously(27). Briefly, the CBCS recruited breast cancer cases and controls aged 20–74 years across 24–44 North Carolina counties in three phases (Phase 1: 1993–1996, Phase II: 1996–2001, and Phase III (cases only): 2008–2013). The WCHS recruited cases and controls aged 20–75 years in New York (2002–2008) and New Jersey (2006-present). The BWHS enrolled participants aged 21–69 years from 17 continental states in 1995 with biennial follow-up to record changes to exposure history, incident disease, and mortality, and provided nested case-control data comprised of all incident breast cancer cases and up to four matched controls for each case to the Consortium(27). The MEC was not included in this analysis because participants were age 45 and older at enrollment.
The present study included premenopausal AA cases diagnosed with invasive breast cancer and matched premenopausal controls from the CBCS (701 cases, 298 controls), WCHS (569 cases, 565 controls), and BWHS (738 cases, 4,281 controls), for a total study population of 2,008 cases and 5,144 controls. All postmenopausal women (defined based on self-reported cessation of menstruation, bilateral oophorectomy, or ovary irradiation), and women with unknown menopausal status were excluded to estimate age effects independent of menopausal status. Each study and the AMBER Consortium collaboration were approved by Institutional Review Boards at participating institutions, and all participants gave written informed consent.
Data collection
The collection of tumor characteristic and risk factor exposure data in the AMBER Consortium has been described previously(27,32). Briefly, each study contributed paraffin-embedded breast tumor tissue to two core research facilities (the Translational Pathology Lab (TPL) at the University of North Carolina at Chapel Hill (UNC) for the CBCS and the Roswell Park Cancer Institute for the WCHS and BWHS) where tissue microarrays (TMAs) were constructed for all available tumor specimens. Immunohistochemistry (IHC) assays were conducted on all TMAs at UNC’s TPL to define expression of estrogen and progesterone receptors (ER/PR) and human epidermal growth factor receptor 2 (HER2)(32). Positive expression was defined as ≥1% staining for ER and PR, and ≥10% staining at the 3+ level for HER2 consistent with previous work(32). Breast cancer subtype was defined as four groups based on positivity of three IHC markers: luminal A (ER+ or PR+, HER2−), luminal B (ER+ or PR+, HER2+), HER2+/ER− (ER−, PR−, HER2+), and triple-negative (ER−, PR−, HER2−). For cases with missing IHC-based tumor characteristics, ER, PR, and HER2 data were defined from medical records (representing 60% of ER, 59% of PR, and 73% of HER2 expression data). Cases with both IHC-based and clinical hormone receptor data showed high agreement for the two measures (κ statistic range=0.68–0.76, concordance range=88–91%), and IHC-based measures were preferentially selected for inclusion in analyses when available. Tumor grade was centrally reviewed by a study pathologist for 56% of cases, with grade data obtained from medical records for remaining cases (κ statistic=0.95, concordance=96% for both grade measures). Other tumor characteristics (including stage (I-IV), lymph node status (positive vs. negative), and estimated tumor size (≤2, 2–4.9, ≥5cm) were acquired from medical records.
Risk factor exposure data for cases and controls were obtained via in-home interviews by study staff (CBCS and WCHS) or mailed questionnaire (BWHS), as described previously(27). Participants were asked questions regarding their medical and family histories as well as biologic, anthropometric, reproductive, and lifestyle exposures. For CBCS and WCHS, interviewers also measured body weight, height, and waist and hip circumferences during home interviews; for the BWHS, these measures were self-reported on questionnaires by study participants(33). Questionnaire and interview data from each study were then harmonized by the AMBER Biostatistics and Data Management core to create a central database with consistent exposure definitions across studies. Breast cancer risk factors were categorized as: age at menarche (<13, ≥13 years), parity (nulliparous, 1–2, ≥3 live births), age at first live birth (<25, ≥25 years), age at last live birth (<30, ≥30 years), time since last live birth (<10, ≥10 years), lifetime duration of breastfeeding (never, <3 months, ≥3 months), oral contraceptive use (never, ever), duration (never/<1 year, 1–4 years, ≥5 years) and recency (never, <10 years, ≥10 years), and first-degree family history of breast cancer (no, yes). Body mass index (BMI) was defined as body weight/height (kg/m2) using categories from the National Heart, Lung, and Blood Institute (<25 normal/underweight, 25.0–29.9 overweight, and ≥30 obese)(34). Waist-to-hip ratio (WHR) was calculated as the ratio of waist/hip circumference (cm) and categorized in tertiles as <0.77, 0.77–0.83, and ≥0.84, consistent with previous work(12).
Statistical analysis
Case-case and case-control analyses were conducted to identify differences in the associations between tumor characteristics and epidemiologic risk factors and breast cancer by age at diagnosis (<40 vs. ≥40 years) among premenopausal AA women (age range 22–59 years). Case-case analyses of tumor characteristics associated with young- vs. older-onset disease included all cases (N=2,008), while case-control analyses of risk factors included all cases and controls except cases from Phase III of the CBCS (total N cases=1,592; N controls=5,144), as no matched controls were available for Phase III. Case-control analyses examined risk factor associations for breast cancer among young and older women overall and further stratified by ER status among young women, in which ER-positive and ER-negative cases were compared separately to all controls. Unconditional logistic regression models were used to estimate adjusted odds ratios (ORs) and 95% confidence intervals (CIs) to assess differences in tumor characteristics and breast cancer risk factors associated with breast cancer by age at diagnosis for all analyses. In case-control analyses, effect measure modification by age was evaluated using likelihood ratio tests in which the estimated log-likelihood of the adjusted model was compared to that of the same model including a multiplicative interaction term for age and the corresponding risk factor. Statistically significant modification was assessed using an α-level of 0.1. Heterogeneity in risk factor associations by ER status among young women was assessed by comparing case-case odds ratios (ORs), with ER status defined as the outcome and each risk factor as the explanatory variable. These case-case ORs represent the ratio of case-control ORs for risk factors associated with ER-positive vs. ER-negative disease, and statistical significance was defined using an α-level of 0.05. Additionally, we conducted sensitivity analyses to examine whether patterns of tumor characteristics and risk factors associated with young- vs. older-onset disease were impacted by the cutpoint used to define young age (40, 45, and 50 years). All models controlled for study, diagnosis year, geographic region, and education status to account for differences between studies. Case-control models additionally adjusted for other risk factors that were identified a priori via directed acyclic graphs as potential confounders of each risk factor association. Models for age at first live birth, time since last birth, and lifetime breastfeeding duration were restricted to parous women. Statistical significance was defined at an α-level of 0.05. All analyses were performed using SAS software, version 9.4 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Breast tumor biology varies by age at diagnosis among AA women (case-case analyses)
Among premenopausal AA women, young age (<40 years) at breast cancer diagnosis was associated with poorer-prognostic tumor characteristics compared to older age at diagnosis (≥40 years) (Table 1). Young women were significantly more likely to have higher stage and triple-negative tumors. While not significant, both luminal B and HER2+/ER− tumors were associated with younger age at diagnosis. Young-onset breast cancers were also significantly more likely to be ER and PR negative, with markedly higher grade and larger tumor size. No age associations were observed for lymph node positivity.
Table 1.
Case-case ORs of tumor characteristics by age among premenopausal cases in the AMBER Consortium.
| ≥40 years (ref) (N=1,475) |
<40 years (N=533) |
|||
|---|---|---|---|---|
|
| ||||
| Tumor characteristic | N (%) | N (%) | OR (95% CI)a | |
| Mean age (±SD) | 46.2 (±4.3) | 34.7 (±3.8) | ||
| Stage | ||||
| Stage I | 450 (35.8) | 116 (24.9) | 1.0 | |
| Stage II | 572 (45.5) | 264 (56.8) | 1.81 (1.34, 2.45) | |
| Stage III/IV | 234 (18.6) | 85 (18.3) | 1.32 (0.90, 1.94) | |
| Missing | 219 | 68 | ||
| Subtype | ||||
| Luminal A | 492 (51.4) | 157 (43.5) | 1.0 | |
| Luminal B | 150 (15.7) | 49 (13.6) | 1.37 (0.86, 2.17) | |
| HER2+/ER− | 65 (6.8) | 29 (8.3) | 1.35 (0.77, 2.37) | |
| Triple-negative | 250 (26.1) | 126 (34.9) | 1.56 (1.09, 2.21) | |
| Missing | 518 | 172 | ||
| ER status | ||||
| Positive | 768 (62.1) | 227 (52.1) | 1.0 | |
| Negative | 469 (37.9) | 209 (47.9) | 1.35 (1.03, 1.77) | |
| Missing | 238 | 97 | ||
| PR status | ||||
| Positive | 699 (56.8) | 199 (46.2) | 1.0 | |
| Negative | 531 (43.2) | 232 (53.8) | 1.57 (1.20, 2.06) | |
| Missing | 245 | 102 | ||
| HER2 status | ||||
| Negative | 752 (77.5) | 286 (78.1) | 1.0 | |
| Positive | 218 (22.5) | 80 (21.9) | 1.15 (0.81, 1.65) | |
| Missing | 505 | 167 | ||
| Grade | ||||
| Low | 144 (12.7) | 33 (8.4) | 1.0 | |
| Moderate | 373 (33.0) | 117 (29.8) | 2.00 (1.11, 3.62) | |
| High | 613 (54.2) | 243 (61.8) | 2.17 (1.23, 3.85) | |
| Missing | 345 | 140 | ||
| Node status | ||||
| Negative | 486 (53.8) | 175 (51.2) | 1.0 | |
| Positive | 418 (46.2) | 167 (48.8) | 1.13 (0.87, 1.47) | |
| Missing | 571 | 191 | ||
| Tumor size | ||||
| ≤2 cm | 477 (40.2) | 131 (30.6) | 1.0 | |
| 2–4.9 cm | 487 (41.0) | 206 (48.1) | 1.70 (1.28, 2.26) | |
| ≥5 cm | 223 (18.8) | 91 (21.3) | 1.31 (0.83, 2.07) | |
| Missing | 288 | 105 | ||
Adjusted for study site, index year, geographic region, and education status.
Abbreviations: SD=standard deviation, OR=odds ratio, CI=confidence interval, ER=estrogen receptor, PR=progesterone receptor, HER2=human epidermal growth receptor 2.
Age modifies breast cancer risk factor associations among AA women (case-control analyses)
To examine whether breast cancers arising among young and older premenopausal AA women are etiologically distinct, we estimated case-control ORs for risk factor associations among premenopausal women stratified by age (Table 2). Age at diagnosis most strongly modified associations with first-degree family history of breast cancer, with a three-fold increase in risk among young women that was attenuated among older women (interaction p=0.005). Likelihood ratio tests also showed significant age modification for associations with waist-to-hip ratio (p=0.06) and breastfeeding duration (p=0.1). Higher WHR was more strongly associated with young- compared to older-onset breast cancer, while breastfeeding, regardless of duration, had a reduced though nonsignificant OR for young- but not older-onset disease. ORs for BMI were not significantly modified by age, though obese BMI (≥30 kg/m2) was more strongly associated with a reduced association among young women. Associations with parity were not modified by age, though higher parity appeared to increase the odds of disease among young but not older women. Later age at first birth was associated with older-onset but not younger-onset breast cancer, while longer time since last birth appeared to reduce odds of breast cancer for older women. Oral contraceptive use showed similar patterns of association across age groups, with ever and more recent use as well as longer use duration associated with an increased OR among young and older women. Later age at menarche was not associated with young-onset breast cancer but showed a significantly reduced OR for older-onset breast cancer. In summary, we observed differences in risk factor patterns by age among premenopausal AA women, with the strongest differences for family history, waist-to-hip ratio, and breastfeeding duration.
Table 2.
Case-control ORs of breast cancer risk factors by age among premenopausal women in the AMBER Consortium.
| <40 years (N=1,775) | ≥40 years (N=4,961) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Risk factor | Controls N (%) | Cases N (%) | OR (95% CI)a | Controls N (%) | Cases N (%) | OR (95% CI)a | p for heterogeneityb | |
| Body mass index (kg/m2) | ||||||||
| <25.0 | 466 (35.0) | 149 (35.1) | 1.0 | 955 (25.4) | 283 (24.6) | 1.0 | 0.8 | |
| 25–29.9 | 368 (27.7) | 119 (28.1) | 0.92 (0.66, 1.28) | 1,172 (31.2) | 376 (32.7) | 0.99 (0.81, 1.21) | ||
| ≥30.0 | 497 (37.3) | 156 (36.8) | 0.71 (0.51, 0.98) | 1,628 (43.4) | 491 (42.7) | 0.91 (0.75, 1.10) | ||
| Trend test | p=0.0005 | p=0.2 | ||||||
| Missing | 16 | 4 | 42 | 14 | ||||
| Waist-to-hip ratio | ||||||||
| <0.77 | 441 (37.5) | 104 (26.3) | 1.0 | 1,166 (34.3) | 1,166 (34.3) | 1.0 | 0.06 | |
| 0.77–0.83 | 341 (29.0) | 128 (32.4) | 1.14 (0.81, 1.59) | 952 (28.0) | 952 (28.0) | 1.02 (0.83, 1.25) | ||
| ≥0.84 | 394 (33.5) | 163 (41.3) | 1.46 (1.04, 2.05) | 1,285 (37.8) | 1,285 (37.8) | 1.11 (0.91, 1.35) | ||
| Trend test | p=0.003 | p=0.9 | ||||||
| Missing | 171 | 33 | 394 | 77 | ||||
| Age at menarche | ||||||||
| <13 years | 803 (59.8) | 251 (58.6) | 1.0 | 2,038 (53.9) | 654 (56.4) | 1.0 | 0.3 | |
| ≥13 years | 540 (40.2) | 177 (41.4) | 0.97 (0.76, 1.24) | 1,746 (46.1) | 506 (43.6) | 0.85 (0.74, 0.98) | ||
| Missing | 4 | 0 | 13 | 4 | ||||
| Parity | ||||||||
| Nulliparous | 516 (38.3) | 111 (26.9) | 1.0 | 872 (23.0) | 222 (19.1) | 1.0 | 0.2 | |
| 1–2 births | 644 (47.8) | 224 (52.3) | 1.19 (0.85, 1.66) | 2,081 (54.8) | 654 (56.2) | 1.09 (0.90, 1.34) | ||
| ≥3 births | 187 (13.9) | 93 (21.7) | 1.26 (0.78, 2.03) | 844 (22.2) | 288 (24.7) | 0.97 (0.75, 1.25) | ||
| Age at first live birthc | ||||||||
| <25 years | 481 (59.0) | 202 (63.9) | 1.0 | 1,633 (56.9) | 547 (58.8) | 1.0 | 0.3 | |
| ≥25 years | 334 (41.0) | 114 (36.1) | 1.03 (0.74, 1.43) | 1,238 (43.1) | 383 (41.2) | 1.18 (0.99, 1.41) | ||
| Missing | 16 | 54 | 12 | |||||
| Age at last live birthc | ||||||||
| <30 years | 483 (59.6) | 198 (62.9) | 1.0 | 1,436 (50.5) | 453 (48.9) | 1.0 | 0.1 | |
| ≥30 years | 327 (40.4) | 117 (37.1) | 0.89 (0.63, 1.25) | 1,408 (49.5) | 474 (51.1) | 1.03 (0.86, 1.23) | ||
| Missing | 21 | 2 | 81 | 15 | ||||
| Time since last birthc | ||||||||
| <10 years | 515 (63.6) | 202 (64.1) | 1.0 | 521 (18.3) | 183 (19.7) | 1.0 | 0.3 | |
| ≥10 years | 295 (36.4) | 113 (35.9) | 1.14 (0.82, 1.57) | 2,323 (81.7) | 744 (80.3) | 0.86 (0.69, 1.07) | ||
| Missing | 21 | 2 | 81 | 15 | ||||
| Breastfeeding durationc | ||||||||
| Parous, never | 388 (47.7) | 177 (56.2) | 1.0 | 1,444 (50.3) | 491 (52.7) | 1.0 | 0.1 | |
| <3 months | 114 (14.0) | 30 (9.5) | 0.70 (0.43, 1.16) | 315 (11.0) | 96 (10.3) | 1.08 (0.82, 1.42) | ||
| ≥3 months | 312 (38.3) | 108 (34.3) | 0.83 (0.58, 1.17) | 1,111 (38.7) | 344 (37.0) | 0.94 (0.78, 1.13) | ||
| Missing | 17 | 2 | 55 | 11 | ||||
| Oral contraceptive (OC) use | ||||||||
| Never | 177 (13.2) | 74 (17.3) | 1.0 | 539 (14.2) | 232 (20.0) | 1.0 | 0.7 | |
| Ever | 1,167 (86.8) | 354 (82.7) | 1.22 (0.86, 1.72) | 3,254 (85.8) | 929 (80.0) | 1.18 (0.97, 1.44) | ||
| Missing | 3 | 0 | 4 | 3 | ||||
| OC use duration | ||||||||
| Never/<1 year | 413 (30.7) | 124 (29.0) | 1.0 | 1,296 (35.2) | 410 (35.2) | 1.0 | 1.0 | |
| 1–4 years | 473 (35.1) | 135 (31.6) | 1.13 (0.82, 1.55) | 1,165 (30.7) | 332 (28.5) | 1.02 (0.85, 1.23) | ||
| ≥5 years | 460 (34.2) | 168 (39.3) | 1.42 (1.04, 1.93) | 1,334 (35.2) | 422 (36.3) | 1.22 (1.03, 1.46) | ||
| Missing | 1 | 1 | 2 | 0 | ||||
| OC use recency | ||||||||
| Never | 177 (13.2) | 74 (17.3) | 1.0 | 539 (14.2) | 232 (20.0) | 1.0 | 0.4 | |
| <10 years | 840 (62.5) | 254 (59.3) | 1.16 (0.69, 1.95) | 1,065 (28.1) | 329 (28.3) | 1.48 (1.08, 2.03) | ||
| ≥10 years | 327 (24.3) | 100 (23.4) | 0.90 (0.56, 1.47) | 2,189 (57.7) | 600 (51.7) | 1.13 (0.87, 1.45) | ||
| Missing | 3 | 0 | 4 | 3 | ||||
| Family history of breast cancer | ||||||||
| No | 1,276 (94.7) | 363 (84.8) | 1.0 | 3,472 (91.4) | 996 (85.6) | 1.0 | 0.005 | |
| Yes | 71 (5.3) | 65 (15.2) | 3.10 (2.08, 4.63) | 325 (8.6) | 168 (14.4) | 1.57 (1.26, 1.94) | ||
Adjusted for age, study site, index year, geographic location, education level, and confounders, by model. BMI: WHR, parity; WHR: BMI, parity; parity: age at first live birth; age at last live birth: parity, age at first birth; time since last birth: parity, age at first live birth, age at last live birth; breastfeeding duration: BMI, parity, age at first live birth, age at last live birth; oral contraceptive (OC) use/duration/recency: parity, age at first live birth, age at last live birth (OC use duration and recency models also adjusted for the other).
Likelihood ratio tests assessed age-related heterogeneity in risk factor associations by comparing the estimated log-likelihood of adjusted models to that of the adjusted model including a multiplicative interaction term for age and the corresponding risk factor (e.g., BMI*age). Statistically significant heterogeneity by age was defined with α=0.1.
Among parous women
Given the difference in tumor characteristics observed between young and older-onset breast cancer in AA women, we examined whether breast cancer biology modified the etiologic patterns we observed, specifically among young women (Supplemental Table 1). Increased odds of young-onset breast cancer associated with higher WHR was limited to ER-negative disease (OR=1.64, 95% CI=0.98, 2.75), conversely, higher BMI had a stronger inverse association with ER-positive disease (OR=0.61, 95% CI=0.38, 0.98). Additionally, family history of breast cancer was positively associated with young-onset disease regardless of ER status, though the association was stronger for ER-negative disease. However, no statistically significant differences by ER status were observed for these or any other risk factor associations that we examined, suggesting that etiologic associations for young-onset breast cancer are not strongly modified by disease subtype.
Age-dependent risk factor associations are most pronounced with age 40 cutpoint
To examine whether our findings were sensitive to the cutpoint used to define young age, we repeated our analyses of tumor characteristics and risk factors associated with young-onset disease using older cutpoints of 45 and 50 years. We observed the strongest age-related heterogeneity when comparing the youngest women (<40) to women at least 40 years of age. Figure 1 shows ORs and 95% CI for risk of young-onset breast cancer defined as <40, <45, and <50 in our cohort for the three risk factors showing the strongest heterogeneity by age: breastfeeding history (ever/never), waist-to-hip ratio (highest/lowest tertile), and family history of breast cancer (yes/no). For all three factors, the associations for young-onset breast cancer were attenuated when defining young women as <45 or <50 at diagnosis.
Figure 1. Impact of age cutpoints on risk factor analyses.
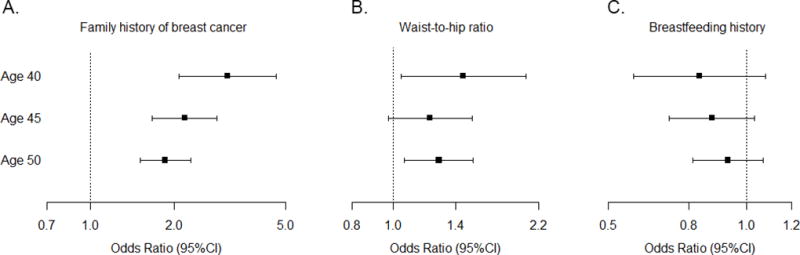
Case-control ORs for associations between family history of breast cancer (yes/no; panel A), waist-to-hip ratio (highest/lowest tertile; panel B), and breastfeeding history (ever/never; panel C) and premenopausal young-onset breast cancer. Cutpoints defining “young” varied at <40, <45, or <50 years of age. Error bars represent 95% CIs.
DISCUSSION
Using data from one of the largest and most comprehensive study of breast cancer biology and epidemiology among AA women to date, the AMBER Consortium, we observed substantial differences in tumor characteristics and some evidence for etiologic heterogeneity of premenopausal young- and older-onset breast cancers. The etiologic associations that vary by age appear not to be driven by differences in ER status, since few associations among young women were modified by ER status. Furthermore, age-dependent heterogeneity of risk factor associations with breast cancer were greatest when comparing the youngest women (<40) to older (≥40) premenopausal women.
The age-related patterns of tumor characteristics we observed are consistent with previous findings(1–8,10–12,35), and our work supports the growing hypothesis that breast cancers diagnosed among young women <40 years are biologically distinct from those diagnosed in older women. It is well-established that AA women are more likely to be diagnosed with breast cancer under 40 years of age compared with white women(1,9–11), highlighting the importance of identifying prevention strategies for young women’s breast cancer, particularly for AA women.
Some risk factors for young-onset breast tumors are potentially modifiable. In our study, breastfeeding had a slightly reduced OR for breast cancer in young women while higher WHR was associated with an increased odds of young-onset disease. Both risk factors showed the strongest associations among ER-negative tumors. In contrast, higher BMI showed an inverse association with young-onset disease that was strongest among ER-positive cancers, consistent with previous work(14–17,33,36). The observed differences between BMI and WHR underscore these factors as distinct measures of body fatness and suggest that abdominal adiposity, as represented by WHR, is an important factor contributing to young-onset disease(37,38). Few studies have examined etiologic differences according to age and breast cancer subtype in populations of AA women. Millikan et al.(17) and Bertrand et al.(25) reported that a lack of breastfeeding and higher WHR were significantly positively associated with young-onset and basal-like (or ER-negative) breast cancers among AA women in the CBCS and BWHS, respectively. Other studies in predominately white populations have observed similar associations(12,24,26), suggesting that interventions to improve breastfeeding rates and reduce abdominal adiposity may benefit young women of all races. Given that AA women tend to breastfeed at lower rates and for shorter durations than white women(39) and are more likely to have ER-negative disease, breastfeeding-related interventions may be particularly relevant for reducing risk of young-onset disease among AA women. Additionally, oral contraceptive use ≥5 years was associated with significantly increased ORs regardless of age, with a stronger association among young women that did not vary according to ER status. Others have shown similarly increased risk with longer and more recent oral contraceptive use for young and AA women(18,23,40–42), highlighting that reduced oral contraceptive use may mitigate breast cancer risk within this demographic.
Several exposures associated with young women’s breast cancer are not targetable for prevention. Family history of breast cancer showed the greatest heterogeneity according to age in our study, with a markedly higher OR among young women and a moderately elevated OR for older women. Family history often serves as a surrogate for genetic susceptibility for breast cancer, and other work has shown that women diagnosed with breast cancer at an early age have a greater frequency of genetic mutations related to tumorigenesis(43,44). However, an individual’s family history is variable over time and changes with age; older women are more likely to have a positive family history than young women given that breast cancer risk increases with age. Thus, the attenuated risk associations that we observed among older women may be explained by a stronger contribution of environment (relative to germline genetics) in family history of older women. This also underscores that a positive family history in a young woman is a strong marker of familial/genetic risk.
Reproductive exposures have most consistently shown differential patterns with breast cancer risk by age, as young women are more proximal to reproductive years than older women. In contrast to other studies, we did not observe the expected dual risk associations for parity, in which higher parity is associated with increased risk among young women but reduced risk for older women(13,45,46). We observed suggestions of this relationship in that parity was associated with increased risk of breast cancer among young women, though no associations with parity were statistically significant. However, other associations between reproductive factors and breast cancer were consistent with previous work, showing younger age at first live birth and longer time since last birth as protective for older-onset but not young-onset breast cancer (12,17,21,24).
Prior epidemiologic studies of young women’s breast cancer have used inconsistent cutpoints to classify young women, ranging from 35–50 years of age(12,17–19,22–26). Many studies have also included limited representation of young women, as women <40 years of age represent less than 7% of all breast cancers diagnosed in the United States(47). As such, conclusions regarding whether young- and older-onset breast cancers have distinct etiologies have been mixed, and different studies have yielded varied directions and magnitudes of associations for many risk factors. However, reproductive (particularly parity, breastfeeding history, and age at first birth) and body size exposures have consistently shown the strongest differences in patterns of association for young and older women. We identified that varying the age cutpoint in our study population from 40 to 50 years resulted in attenuated effect estimates with increased age for many risk factor associations. Additionally, dichotomizing our cohort at age 40 enabled a comparison of younger and older premenopausal women, as we previously showed that age and menopausal status are best considered as separate factors in studies of young women’s breast cancer(12). Taken together, our findings suggest that age-dependent heterogeneity in risk factor associations are most pronounced when classifying young women as <40 years.
Our results should be interpreted in light of some limitations. In our study, we did not evaluate underlying genetic or epigenetic factors that may differ according to age, and these factors may have contributed to our observed differences in breast tumor biology and risk associations with family history among young and older women. Additionally, differences in breast cancer screening rates and/or adherence between young and older AA women may have influenced some tumor characteristics among young women, although screening data were unavailable in the Consortium. Breast cancers detected via screening tend to have more favorable tumor characteristics than self- or clinically-detected tumors(48,49). However, interval cancers, or those diagnosed between regular screening intervals, are more likely to be aggressive and may be present regardless of screening(50). While screening differences may contribute to differences in observed tumor characteristics, screening is unlikely to have influenced the etiologic associations we described by age and ER status.
In summary, we found strong evidence that breast cancers diagnosed among young AA women have tumor characteristics suggestive of poorer prognosis, underscoring the need for greater understanding of the etiology of young-onset disease. In one of the largest epidemiologic studies of young AA women’s breast cancer to date, our findings suggest that potentially modifiable risk factors, such as breastfeeding and adiposity, are associated with young-onset breast cancer, in addition to other non-modifiable factors such as family and reproductive history.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the support of all AMBER staff and participants.
Financial support: National Cancer Institute (NCI) at the National Institutes of Health (NIH) [NCI P01-CA151135 to C.B. Ambrosone, J.R. Palmer, A.F. Olshan.; the NCI Specialized Program of Research Excellence (SPORE) in Breast Cancer at UNC (NIH/NCI P50-CA58223) to A.F. Olshan, M.A. Troester; NIH U01 CA179715 to A.F. Olshan, M.A. Troester; a Lineberger Comprehensive Cancer Center (LCCC) core grant (NIH/NCI P30-CA16086); NCI P30-CA016086 to the Translational Pathology Laboratory; R01-CA058420 to L.A. Rosenberg; UM1-CA164974 to J.R. Palmer, L.A. Rosenberg; R01-CA098663 to J.R. Palmer; R01-CA100598 to C.B. Ambrosone; R01-CA185623 to E.V. Bandera, C.C. Hong; and the University of North Carolina LCCC Cancer Control and Education Program training grant (NIH/NCI 5R25CA057726-25) to L. Chollet-Hinton], the Department of Defense Breast Cancer Research Program, the University Cancer Research Fund of North Carolina and Susan G. Komen for the Cure Foundation to A.F. Olshan, M.A. Troester, and the Breast Cancer Research Foundation to C.B. Ambrosone.
References
- 1.Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol. 2009;36:237–249. doi: 10.1053/j.seminoncol.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 3.Anderson WF, Rosenberg PS, Menashe I, Mitani A, Pfeiffer RM. Age-related crossover in breast cancer incidence rates between black and white ethnic groups. J Natl Cancer Inst. 2008;100:1804–1814. doi: 10.1093/jnci/djn411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunningham JE, Montero AJ, Garrett-Mayer E, Berkel HJ, Ely B. Racial differences in the incidence of breast cancer subtypes defined by combined histologic grade and hormone receptor status. Cancer Causes Control. 2010;21:399–409. doi: 10.1007/s10552-009-9472-2. [DOI] [PubMed] [Google Scholar]
- 5.Clarke CA, Keegan TH, Yang J, Press DJ, Kurian AW, Patel AH, et al. Age-specific incidence of breast cancer subtypes: understanding the black-white crossover. J Natl Cancer Inst. 2012;104:1094–1101. doi: 10.1093/jnci/djs264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson WF, Chen BE, Brinton LA, Devesa SS. Qualitative age interactions (or effect modification) suggest different cancer pathways for early-onset and late-onset breast cancers. Cancer Causes Control. 2007;18:1187–1198. doi: 10.1007/s10552-007-9057-x. [DOI] [PubMed] [Google Scholar]
- 7.Elledge RM, Clark GM, Chamness GC, Osborne CK. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J Natl Cancer Inst. 1994;86:705–712. doi: 10.1093/jnci/86.9.705. [DOI] [PubMed] [Google Scholar]
- 8.Furberg H, Millikan R, Dressler L, Newman B, Geradts J. Tumor characteristics in African American and white women. Breast Cancer Res Treat. 2001;68:33–43. doi: 10.1023/a:1017994726207. [DOI] [PubMed] [Google Scholar]
- 9.Winchester DP, Osteen RT, Menck HR. The National Cancer Data Base report on breast carcinoma characteristics and outcome in relation to age. Cancer. 1996;78:1838–1843. doi: 10.1002/(sici)1097-0142(19961015)78:8<1838::aid-cncr27>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 10.Keegan TH, DeRouen MC, Press DJ, Kurian AW, Clarke CA. Occurrence of breast cancer subtypes in adolescent and young adult women. Breast Cancer Res. 2012;14:R55. doi: 10.1186/bcr3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 12.Chollet-Hinton L, Anders CK, Tse CK, Bell MB, Yang YC, Carey LA, et al. Breast cancer biologic and etiologic heterogeneity by young age and menopausal status in the Carolina Breast Cancer Study: a case-control study. Breast Cancer Res. 2016;18:79. doi: 10.1186/s13058-016-0736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pathak DR, Speizer FE, Willett WC, Rosner B, Lipnick RJ. Parity and breast cancer risk: possible effect on age at diagnosis. Int J Cancer. 1986;37:21–25. doi: 10.1002/ijc.2910370105. [DOI] [PubMed] [Google Scholar]
- 14.Ursin G, Longnecker MP, Haile RW, Greenland S. A meta-analysis of body mass index and risk of premenopausal breast cancer. Epidemiology. 1995;6:137–141. doi: 10.1097/00001648-199503000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Brinton LA, Swanson CA. Height and weight at various ages and risk of breast cancer. Ann Epidemiol. 1992;2:597–609. doi: 10.1016/1047-2797(92)90004-a. [DOI] [PubMed] [Google Scholar]
- 16.Yang XR, Chang-Claude J, Goode EL, Couch FJ, Nevanlinna H, Milne RL, et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103:250–263. doi: 10.1093/jnci/djq526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, et al. Epidemiology of basal-like breast cancer. Breast cancer research and treatment. 2008;109:123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Althuis MD, Brogan DD, Coates RJ, Daling JR, Gammon MD, Malone KE, et al. Breast cancers among very young premenopausal women (United States) Cancer Causes Control. 2003;14:151–160. doi: 10.1023/a:1023006000760. [DOI] [PubMed] [Google Scholar]
- 19.White E, Malone KE, Weiss NS, Daling JR. Breast cancer among young U.S. women in relation to oral contraceptive use. Journal of the National Cancer Institute. 1994;86:505–514. doi: 10.1093/jnci/86.7.505. [DOI] [PubMed] [Google Scholar]
- 20.Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004;13:1558–1568. [PubMed] [Google Scholar]
- 21.Palmer JR, Viscidi E, Troester MA, Hong CC, Schedin P, Bethea TN, et al. Parity, lactation, and breast cancer subtypes in African American women: results from the AMBER Consortium. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall IJ, Moorman PG, Millikan RC, Newman B. Comparative analysis of breast cancer risk factors among African-American women and White women. Am J Epidemiol. 2005;161:40–51. doi: 10.1093/aje/kwh331. [DOI] [PubMed] [Google Scholar]
- 23.Mayberry RM. Age-specific patterns of association between breast cancer and risk factors in black women, ages 20 to 39 and 40 to 54. Ann Epidemiol. 1994;4:205–213. doi: 10.1016/1047-2797(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 24.Warner ET, Colditz GA, Palmer JR, Partridge AH, Rosner BA, Tamimi RM. Reproductive factors and risk of premenopausal breast cancer by age at diagnosis: are there differences before and after age 40? Breast Cancer Res Treat. 2013;142:165–175. doi: 10.1007/s10549-013-2721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertrand KA, Bethea TN, Adams-Campbell LL, Rosenberg L, Palmer JR. Differential patterns of risk factors for early-onset breast cancer by ER status in African American women. Cancer Epidemiol Biomarkers Prev. 2016 doi: 10.1158/1055-9965.EPI-16-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li CI, Beaber EF, Tang MT, Porter PL, Daling JR, Malone KE. Reproductive factors and risk of estrogen receptor positive, triple-negative, and HER2-neu overexpressing breast cancer among women 20–44 years of age. Breast Cancer Res Treat. 2013;137:579–587. doi: 10.1007/s10549-012-2365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer JR, Ambrosone CB, Olshan AF. A collaborative study of the etiology of breast cancer subtypes in African American women: the AMBER consortium. Cancer Causes Control. 2014;25:309–319. doi: 10.1007/s10552-013-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newman B, Moorman PG, Millikan R, Qaqish BF, Geradts J, Aldrich TE, et al. The Carolina Breast Cancer Study: integrating population-based epidemiology and molecular biology. Breast Cancer Res Treat. 1995;35:51–60. doi: 10.1007/BF00694745. [DOI] [PubMed] [Google Scholar]
- 29.Ambrosone CB, Zirpoli G, Ruszczyk M, Shankar J, Hong CC, McIlwain D, et al. Parity and breastfeeding among African-American women: differential effects on breast cancer risk by estrogen receptor status in the Women's Circle of Health Study. Cancer Causes Control. 2014;25:259–265. doi: 10.1007/s10552-013-0323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenberg L, Adams-Campbell L, Palmer JR. The Black Women's Health Study: a follow-up study for causes and preventions of illness. J Am Med Womens Assoc. 1995;50:56–58. [PubMed] [Google Scholar]
- 31.Kolonel LN, Henderson BE, Hankin JH, Nomura AM, Wilkens LR, Pike MC, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151:346–357. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allott EH, Cohen SM, Geradts J, Sun X, Khoury T, Bshara W, et al. Performance of Three-Biomarker Immunohistochemistry for Intrinsic Breast Cancer Subtyping in the AMBER Consortium. Cancer Epidemiol Biomarkers Prev. 2016;25:470–478. doi: 10.1158/1055-9965.EPI-15-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bandera EV, Chandran U, Hong CC, Troester MA, Bethea TN, Adams-Campbell LL, et al. Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER Consortium. Breast Cancer Res Treat. 2015;150:655–666. doi: 10.1007/s10549-015-3353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Heart, Lung, and Blood Institute (NHLBI) Expert Panel on the identification, evaluation, and treatment of overweight and obesity in adults. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 35.Richardson JL, Langholz B, Bernstein L, Burciaga C, Danley K, Ross RK. Stage and delay in breast cancer diagnosis by race, socioeconomic status, age and year. Br J Cancer. 1992;65:922–926. doi: 10.1038/bjc.1992.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunter DJ, Willett WC. Diet, body size, and breast cancer. Epidemiol Rev. 1993;15:110–132. doi: 10.1093/oxfordjournals.epirev.a036096. [DOI] [PubMed] [Google Scholar]
- 37.Connolly BS, Barnett C, Vogt KN, Li T, Stone J, Boyd NF. A meta-analysis of published literature on waist-to-hip ratio and risk of breast cancer. Nutr Cancer. 2002;44:127–138. doi: 10.1207/S15327914NC4402_02. [DOI] [PubMed] [Google Scholar]
- 38.Huang Z, Willett WC, Colditz GA, Hunter DJ, Manson JE, Rosner B, et al. Waist circumference, waist:hip ratio, and risk of breast cancer in the Nurses' Health Study. Am J Epidemiol. 1999;150:1316–1324. doi: 10.1093/oxfordjournals.aje.a009963. [DOI] [PubMed] [Google Scholar]
- 39.Li R, Darling N, Maurice E, Barker L, Grummer-Strawn LM. Breastfeeding rates in the United States by characteristics of the child, mother, or family: the 2002 National Immunization Survey. Pediatrics. 2005;115:e31–37. doi: 10.1542/peds.2004-0481. [DOI] [PubMed] [Google Scholar]
- 40.Dolle JM, Daling JR, White E, Brinton LA, Doody DR, Porter PL, et al. Risk factors for triple-negative breast cancer in women under the age of 45 years. Cancer Epidemiol Biomarkers Prev. 2009;18:1157–1166. doi: 10.1158/1055-9965.EPI-08-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bethea TN, Rosenberg L, Hong CC, Troester MA, Lunetta KL, Bandera EV, et al. A case-control analysis of oral contraceptive use and breast cancer subtypes in the African American Breast Cancer Epidemiology and Risk Consortium. Breast Cancer Res. 2015;17:22. doi: 10.1186/s13058-015-0535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brinton LA, Daling JR, Liff JM, Schoenberg JB, Malone KE, Stanford JL, et al. Oral contraceptives and breast cancer risk among younger women. Journal of the National Cancer Institute. 1995;87:827–835. doi: 10.1093/jnci/87.11.827. [DOI] [PubMed] [Google Scholar]
- 43.Colditz GA, Kaphingst KA, Hankinson SE, Rosner B. Family history and risk of breast cancer: nurses' health study. Breast Cancer Res Treat. 2012;133:1097–1104. doi: 10.1007/s10549-012-1985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collaborative Group on Hormonal Factors in Breast C. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet. 2001;358:1389–1399. doi: 10.1016/S0140-6736(01)06524-2. [DOI] [PubMed] [Google Scholar]
- 45.Borges VF, Schedin PJ. Pregnancy-associated breast cancer: an entity needing refinement of the definition. Cancer. 2012;118:3226–3228. doi: 10.1002/cncr.26643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lyons TR, Schedin PJ, Borges VF. Pregnancy and breast cancer: when they collide. J Mammary Gland Biol Neoplasia. 2009;14:87–98. doi: 10.1007/s10911-009-9119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Desantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 48.Ruddy KJ, Gelber S, Tamimi RM, Schapira L, Come SE, Meyer ME, et al. Breast cancer presentation and diagnostic delays in young women. Cancer. 2014;120:20–25. doi: 10.1002/cncr.28287. [DOI] [PubMed] [Google Scholar]
- 49.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 50.Gilliland FD, Joste N, Stauber PM, Hunt WC, Rosenberg R, Redlich G, et al. Biologic characteristics of interval and screen-detected breast cancers. J Natl Cancer Inst. 2000;92:743–749. doi: 10.1093/jnci/92.9.743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


