Abstract
Aims
Intravenous high‐dose free methylprednisolone (MP) hemisuccinate is the primary treatment for an acute relapse in relapsing–remitting multiple sclerosis. However, it is inconvenient and its side effects are undesirable. Both dose and dosing frequency can be reduced by incorporating free MP in glutathione‐PEGylated liposomes, creating a slow‐release formulation with reduced toxicity and prolonged peripheral efficacy. This first‐in‐human study was designed to assess the safety, pharmacokinetics and pharmacodynamics of glutathione‐PEGylated liposomes containing MP (2B3–201).
Methods
The first part was a double‐blind, three‐way cross over study in 18 healthy male subjects, receiving ascending doses of 2B3–201, active comparator (free MP) or placebo. Part 2 of the study was an open‐label infusion of 2B3–201 (different doses), exploring pretreatment with antihistamines and different infusion schedules in another 18 healthy male subjects, and a cross‐over study in six healthy female subjects. MP plasma concentrations, lymphocyte counts, adrenocorticotropic hormone, osteocalcin and fasting glucose were determined. Safety and tolerability profiles were assessed based on adverse events, safety measurements and central nervous system tests.
Results
The most frequent recorded AE related to 2B3–201 was an infusion related reaction (89%). 2B3–201 was shown to have a plasma half‐life between 24 and 37 h and caused a prolonged decrease in the lymphocyte count, adrenocorticotropic hormone and osteocalcin, and a rise in fasting glucose.
Conclusion
2B3–201 is considered safe, with no clinically relevant changes in central nervous system safety parameters and no serious adverse events. In addition, 2B3–201 shows a long plasma half‐life and prolonged immunosuppressive effects.
Keywords: liposomes, methylprednisolone, multiple sclerosis
What is Already Known about this Subject
Intravenous high‐dose methylprednisolone (MP) is the primary treatment for an acute relapse in relapsing–remitting multiple sclerosis. Although effective, this treatment is inconvenient and its side effects are undesirable.
Dose and dosing frequency can be reduced by incorporating MP in glutathione‐PEGylated liposome, creating a central nervous system‐targeted formulation, 2B3–201, with reduced toxicity and prolonged efficacy.
What this Study Adds
2B3–201 at doses up to 450 mg was considered safe.
2B3–201 has a slow‐release like pharmacokinetic profile of MP, with a plasma half‐life between 24 and 37 h.
2B3–201 caused a prolonged decrease in the lymphocyte count, adrenocorticotropic hormone and osteocalcin, and a mild rise in fasting glucose.
Introduction
Multiple sclerosis (MS) is one of the most prevalent neuroinflammatory diseases and the leading cause of chronic disability in young adults. In MS, central nervous system (CNS) infiltration of leucocytes leads to overt inflammation and demyelination and results in neuronal dysfunction 1 High‐dose http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7088, given 500–1000 mg daily for 3–5 consecutive days, is the primary treatment for an acute relapse in relapsing–remitting (RR) MS 2. However, it is often given intravenously and causes undesirable short‐term and long‐term side effects include insomnia, depression and agitation 3, 4.
Both dose and dosing frequency of glucocorticoids may significantly be reduced by incorporating steroids in (PEGylated) liposomes, which is expected to result in reduced systemic toxicity while maintaining peripheral efficacy 5. The additional conjugation of glutathione (GSH) to target active GSH transporters on the blood–brain barrier, has been shown to facilitate the delivery of the liposome‐encapsulated drug into the brain 6, 7.
2B3–201 is MP hemisuccinate encapsulated in GSH‐PEGylated liposomes that has been developed with the aim to enhance the sustained delivery of MP into the brain, thereby potentially augmenting CNS activity. Preclinical studies in animal models showed that 2B3–201 at therapeutic levels in animal models had fewer behavioural side effects (unpublished) and a superior efficacy compared to MP hemisuccinate 8, 9, 10. Also, plasma circulation of 2B3–201‐derived MP was significantly increased by encapsulation in GSH‐PEGylated liposomes 11. Based on these preclinical data, we expected a longer half‐life and fewer side effects of 2B3–201 in human subjects when compared to MP.
In this first‐in‐human study we aimed to assess the safety, pharmacokinetic and pharmacodynamic profile of 2B3–201 in healthy male and female subjects. Plasma concentrations of lymphocytes, osteocalcin, adrenocorticotropic hormone (ACTH) and fasting glucose were used as pharmacodynamic endpoints, as intravenous administration of prednisolone causes rapid inhibition of the hypothalamic–pituitary–adrenal axis 12, glucose homeostasis disturbances, and depletion of osteocalcin 13, 14 and lymphocytes 15. CNS effects were measured with the NeuroCart 16.
Methods
Design
Initially a randomized, double‐blind, placebo‐ and active comparator‐ controlled three‐way crossover study with three cohorts of six healthy males each was performed. Subsequently the study was extended while applying a parallel open label design with four cohorts, each containing six healthy subjects.
In cohorts 1, 2 and 3, a single dose of 150 mg, 300 mg and 450 mg 2B3–201 respectively was tested and compared to free MP and placebo (Table 1). The time interval between the occasions in the cross‐over parts was 1 week. Cohorts 4, 5 and 6 had a single dose of 300 mg (cohort 5) and 450 mg (cohorts 4 and 6) 2B3–201 tested while applying altered infusion schedules and pre‐treatment with clemastine. Cohort 7 included females and compared 450 mg 2B3–201 to 1000 mg of free MP in a double‐blind crossover design.
Table 1.
Summary of study characteristics
| Cohort | 1 | 2 | 3 | 4 | 5 | 6 * | 7 |
|---|---|---|---|---|---|---|---|
| 2B3‐201 dose | 150 mg | 300 mg | 450 mg | 450 mg | 300 mg | 450 mg | 450 mg |
| Population | Healthy males | Healthy males | Healthy males | Healthy males | Healthy males | Healthy males | Healthy females |
| Design | Randomized | Randomized | Randomized | Randomized | |||
| Crossover | Crossover | Crossover | Crossover | ||||
| Placebo‐controlled | Placebo‐controlled | Placebo‐controlled | |||||
| Active comparator: methylprednisolone | Active comparator: methylprednisolone** | Active comparator: methylprednisolone | Active comparator: methylprednisolone | ||||
| Double blind | Double blind | Double blind | Open‐label | Open‐label | Open‐label | Double blind | |
| Number of subjects | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
Cohort 6 had an infusion that was twice as long, data from this cohort has not been used in our pharmacokinetic and pharmacodynamic analyses.
Dose of methylprednisolone was an intravenous infusion of 300 (cohort 2 only) or 1000 mg.
An interim analysis was conducted after completion of cohorts 1, 2 and 3, at which point, safety, pharmacokinetics and pharmacodynamics results were evaluated and a decision to continue to the next cohort was made.
The study was approved by the Medical Ethics Committee of the BEBO Foundation (Assen, The Netherlands). The study was conducted according to the Dutch Act on Medical Research Involving Human Subjects (WMO) and in compliance with Good Clinical Practice (ICH‐GCP) and the Declaration of Helsinki.
Subjects
Forty‐six healthy subjects were recruited via the CHDR database and advertisements. All subjects gave written informed consent and were subsequently medically screened before entry into the study. Healthy subjects were not allowed to smoke >10 cigarettes per day and had to refrain from smoking during the study days. In the 48 h prior to the study days they were asked not to drink alcohol and to avoid xanthine‐ containing drinks. The use of medication was not allowed during the study period (except occasional use of paracetamol, up to 1 g per day). Healthy subjects with a positive Mantoux test and or recent (<1 month prior to screening) or current significant infection, were not enrolled.
Treatments
Seven study cohorts with a total of 46 subjects received an infusion with 150 mg, 300 mg or 450 mg 2B3–201, 300 mg or 1000 mg free MP, or placebo. An overview of all cohorts can be found in Table 1. Subjects in cohorts 1–3 had 3 study periods, during which they either received 2B3–201, free MP or placebo (5% dextrose). Cohorts 4 and 6 received open label infusions of 450 mg 2B3–201, and cohort 4 also assessed the pretreatment effect of 2 mg clemastine on adverse events. Subjects in cohort 5 received 300 mg 2B3–201 and were also pretreated with clemastine. In cohort 6, a longer infusion duration was assessed. In cohort 7, healthy female subjects received 450 mg 2B3–201 while being pretreated with clemastine, and 1000 mg free MP in a double blind two‐way cross‐over fashion.
Safety
Adverse events, electrocardiogram (ECG), lymphocyte count, fasting glucose, blood pressure and heart rate measurements were collected throughout the study. Twelve‐lead ECG recordings were made using Electrocardiograph Marquette 800/5500 or Dash 3000. Blood pressure and heart rate were assessed using a Nihon‐Kohden BSM‐1101 K monitor or a Colin Pressmate BP 8800 or a Dash 4000. All ECG, blood pressure and heart rate measurements were performed after subjects had been resting in a supine position for at least 5 min.
Pharmacokinetics
Whole blood samples were taken for assay of the active component MP and the encapsulated prodrug MP hemisuccinate. Blood samples were taken 0.25 h predose and 0.25, 0.5, 1, 2, 4, 6, 8, 12, 24, 26, 48 and 72 h postdose for all cohorts, and up to 288 h for cohorts 4–7. The blood was drawn in 2 mL NaF/K‐oxalate tubes, directly placed on ice and then centrifuged (2000 g, 10 min, at 2–8°C), transferred to 2 mL Sarsted tubes and stored at –80°C within 30 min after sampling. The concentrations of MP and MP hemisuccinate in human sodium fluoride / potassium oxalate plasma were determined using a validated liquid chromatography with tandem mass spectrometry (LC–MS/MS) assays by Analytical Biochemical Lab (Assen, the Netherlands). The lower limit of quantitation (LLOQ) was 1 ng ml–1 for MP. Concentrations for MP were calculated by interpolation from a calibration curve while applying a range of 1–1000 ng ml–1.
The following pharmacokinetic variables were calculated: area under the plasma concentration–time curve (AUC) from time 0 to the time of the last quantifiable concentration (AUC0‐t) and from time 0 extrapolated to infinity (AUC0‐inf); maximal observed plasma drug concentration (Cmax); time to maximum observed plasma drug concentration (tmax); half‐life (t½), volume of distribution (Vd); and clearance. For the noncompartmental analysis only MP concentrations up to 74 h were used.
Pharmacodynamics
Lymphocyte count
Time points for measurement of lymphocytes were 2 h predose (cohorts 1–3 only), 15 min predose (cohorts 4–7) and 1, 2, 4, 8, 12, 24, 48 and 72 h postdose for all cohorts, and up to 288 h postdose for cohorts 4–7. The 2 mL EDTA‐sample was directly, without preprocessing, sent to a hospital haematology and chemistry laboratory for analysis. The normal range for lymphocyte count was 1.00–3.50 × 109 l–1.
Osteocalcin
Serum osteocalcin was measured several times per occasion: predose on day 0, 8, 24, 48 and 72 h postdose for all cohorts, and up to 288 h postdose for cohorts 4–7. Intact osteocalcin was measured in serum with ELISA 13, the normal range used was 0.4–4.0 nmol l–1.
ACTH
ACTH was measured 12 times per occasion. Samples were taken 0.25 h predose and 0.25, 0.5, 1, 2, 4, 6, 8, 12, 24, 26, 48 and 72 h postdose for all cohorts, and up to 288 h postdose for cohorts 4–7. The ACTH samples (2 ml in an EDTA‐tube) were put on ice immediately and centrifuged within 10 min. Normal range was <75 ng l–1.
Fasting glucose
As a pharmacodynamics and safety marker, measurement of fasting glucose levels was performed. Samples were taken predose, 2, 6, 12, 24 and 72 h postdose for all cohorts, and up to 288 h for cohorts 4–7. 2 ml was collected in a NaF tube; the used normal range was 3.1–6.4 mmol l–1.
Complement and IgE
To confirm if the observed infusion related reactions in cohort 1 were complement mediated and not allergic reactions, we measured, for cohorts 2–7, complement factors SC5b‐9, C3a, C4d and Bb (4 mL blood EDTA tube) and IgE (2 mL blood, EDTA tube). These samples were taken predose (depending on cohort at –20, –9 or –7 min) and 5, 30 and 120 min after start of the infusion.
CNS tests
CNS tests performed with the NeuroCart included: pharmaco‐EEG 17, 18, 19, maze learning 20, visual verbal learning test, Stroop test 21, adaptive tracking 22, VAS Bond and Lader 23 and VAS Bowdle 24 and saccadic and smooth pursuit eye movements 25.
Statistics
To compare the pharmacodynamics and pharmacokinetics between treatments the mean and standard deviation were calculated per time point by treatment. Cohorts with the same treatment are combined into one treatment group. For MP, values below LLOQ are set to 0 ng ml–1 before dosing and set to half of LLOQ (0.5 ng ml–1) after dosing. For ACTH, all values below LLOQ were set to half of LLOQ (2.5 ng l–1).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 26, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18.
Results
Demographics
A total of 46 subjects participated, of whom 41 completed the study. Five subjects retracted consent during the study, and four of them were replaced. The subjects who participated in this study were all healthy young adults; subject characteristics are listed in Table 2.
Table 2.
Subject characteristics
| Cohort | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 2B3–201 dose | 150 mg | 300 mg | 450 mg | 450 mg | 300 mg | 450 mg | 450 mg |
| Population | Healthy males | Healthy males | Healthy males | Healthy males | Healthy males | Healthy males | Healthy females |
| Age (years), mean (range) | 25 (20–30) | 24.9 (19–36) | 25.3 (20–45) | 21.3 (19–25) | 25.5 (20–35) | 19.8 (18–23) | 23 (20–30) |
| Weight (kg), mean (range) | 77.6 (66–95) | 71.3 (66–77) | 75.5 (68–88) | 81.1 (68.9–116.6) | 71.0 (59.8–85.6) | 72.1 (65.9–77.8) | 66.8 (54.4–81.2) |
| Height (cm), mean (range) | 183.9 (177–191) | 179.9 (159–189) | 183.4 (165–194) | 184.9 (178.4–199.6) | 177.3 (165.0–188.2) | 184.2 (179.2–191.5) | 171.5 (162.7–179.0) |
| BMI (kg m –2 ), mean (range) | 23.0 (20–28) | 22.1 (20–26) | 22.6 (18–26) | 23.5 (21.5–29.3) | 22.7 (18.4–25.2) | 21.2 (20.2–23.1) | 22.8 (19.1–26.9) |
| Number of subjects | 6 | 6 | 6 (1 dropout) | 6 | 6 | 5 (1 dropout) | 6 (2 dropouts) |
BMI, body mass index
Safety
No clinically relevant changes were observed in ECG, physical examination or vital signs (temperature, heart rate, systolic and diastolic blood pressure). Safety laboratory assessments for blood haematology, chemistry and urinalysis also showed no clinically meaningful abnormalities with the exception of a decrease in lymphocytes, which will be discussed in more detail in the pharmacodynamics section.
The most frequently reported adverse events related to 2B3–201 were infusion related reactions, defined as any sign or symptom experienced by the subject within 4 h after the start of the infusion 27, 28. Symptoms related to infusion that occurred within 4 h after start of the infusion, such as chest discomfort, urticaria, angioedema and back pain, were clustered 29. Infusion reaction related symptoms were reported by 41 of the 46 healthy subjects (89%). Other frequently reported adverse events were somnolence (15%), gastroesophageal reflux disease (8%), back pain (not assessed as an infusion related reaction) (8%), fatigue (8%) and dizziness (8%).
Pretreatment with 2 mg clemastine (cohorts 4, 5 and 7) at 20 min before infusion did not result in fewer infusion related reactions: all subjects in these cohorts showed symptoms of an infusion related reaction (see Table 3). Not all infusion related reactions resulted in (temporary) halt of the infusion or lowering of the infusion speed.
Table 3.
Infusion related reactions (IRRs) per cohort
| Cohort | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 2B3–201 dose | 150 mg | 300 mg | 450 mg | 450 mg | 300 mg | 450 mg | 450 mg |
| No. of IRRs | 4 | 5 | 5 | 6 | 6 | 6 | 6 |
| Infusion (temporary) stopped due to IRR symptoms | 2 | 3 | 5 | 2 | 4 | 6 | 3 |
All adverse events were mild in severity, short lasting and self‐limiting. One adverse event related to 1000 mg free MP was classified as moderate: a male subject (cohort 1) developed an acute tonsillitis with fever 3 days after the infusion. He was subsequently treated with pheneticillin and recovered fully.
Complement and IgE measurements showed that 2B3–201 caused a parallel rise of C3a and Bb and no increase in C4d and IgE levels were observed (Figure 1).
Figure 1.
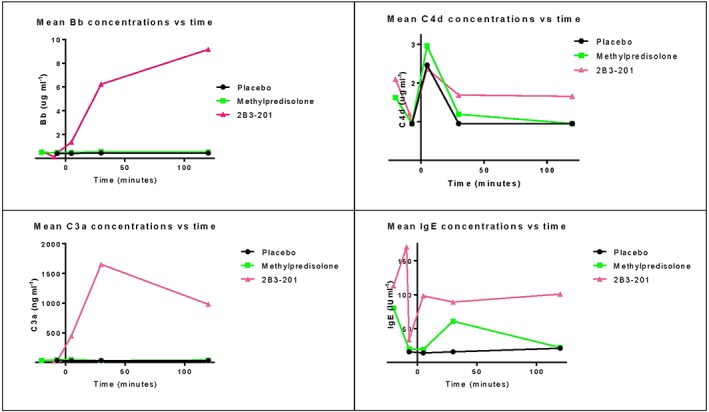
Mean values of Bb, C4d, C3a and IgE concentrations for 2B3–201, methylprednisolone and placebo
Pharmacokinetics
A concentration–time graph for MP plasma concentration at different dose levels of 2B3–201 derived MP and free MP is shown in Figure 2. Pharmacokinetic parameters per cohort are listed in Table 4. Plasma concentrations of 2B3–201 derived MP were measured up to 7 days (300 and 450 mg), for 300 mg and 1000 mg free MP concentrations were measurable until 2 days after infusion.
Figure 2.
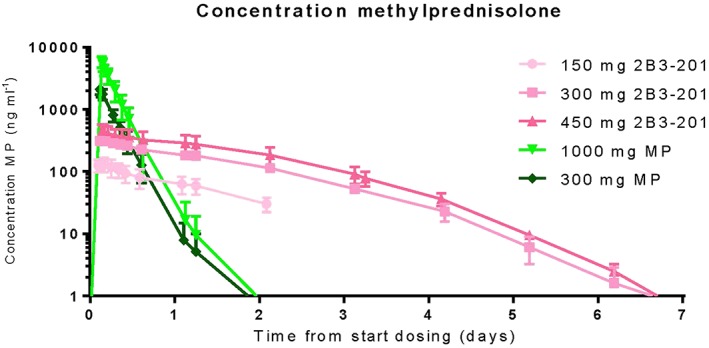
Serum methylprednisolone (MP) concentrations for 150, 300 and 450 mg 2B3–201, and 300 and 1000 mg MP. The concentrations of 150 mg 2B3–201 have only been measured for 50 h
Table 4.
Pharmacokinetic parameters of methylprednisolone, calculated from 0–72 h
| Dose (mg) | Cohort | Compound | Cmax (ng ml –1 ) (SD) | Tmax (h) (SD) | AUC_0_inf (ng h l –1 ) (SD) | Ka (h –1 ) (SD) | T ½ (h) (SD) |
|---|---|---|---|---|---|---|---|
| 150 | 1 (n = 6) | 2B3–201 | 138 (37.1) | 4.56 (1.3) | 4370 (1000) | 0.029 (0.0045) | 24.4 (3.9) |
| 300 | 2 (n = 6) | 2B3–201 | 375 (96.6) | 4.48 (1.4) | 13 400 (3220) | 0.028 (0.0032) | 25 (2.5) |
| 300 | 5 (n = 6) | 2B3–201 | 282 (35) | 4.19 (0.9) | 14 800 (4060) | 0.025 (0.0086) | 31 (9.5) |
| 450 | 3 (n = 6) | 2B3–201 | 501 (144) | 4.85 (0.75) | 17 900 (2690) | 0.024 (0.0029) | 29.2 (3.5) |
| 450 | 4 (n = 6) | 2B3–201 | 360 (57.5) | 5.12 (1.4) | 17 400 (4020) | 0.024 (0.0034) | 28.9 (4.3) |
| 450 | 7 (n = 6) | 2B3–201 | 545 (99.7) | 5.90 (2.9) | 31 800 (15500) | 0.0250 (0.010) | 37.0 (29) |
| 300 | 2 (n = 6) | Free MP | 2140 (371) | 2.67 (0.20) | 11 900 (2490) | 0.258 (0.039) | 2.74 (0.44) |
| 1000 | 1 (n = 6) | Free MP | 5930 (580) | 2.16 (0.37) | 27 800 (7370) | 0.286 (0.041) | 2.47 (0.38) |
| 1000 | 3 (n = 6) | Free MP | 5120(1120) | 3.95 (0.029) | 28 800 (10800) | 0.286 (0.032) | 2.45 (0.30) |
| 1000 | 7 (n = 6) | Free MP | 7290 (1740) | 4.20 (0.26) | 42 900 (13400) | 0.208 (0.082) | 3.81 (1.6) |
SD, standard deviation
2B3–201 derived MP had a maximum plasma concentration of 545 mg ml–1 (450 mg 2B3–201), which contrasts with the maximum plasma concentration of 7290 ng ml–1 for free MP (1000 mg). The Tmax was 5.9 h for 2B3–201 derived MP while free MP had a Tmax of 2.16–4.2 h. Plasma half‐life for 2B3–201 derived MP was between 24 and 37 h. Free MP had a half‐life of 2.2–4 hr. A t‐test showed a significant difference in AUC and Cmax (P values of respectively 0.003 and 0.006) between males and females and in weight (P value = 0.03), but not in BMI. Observed differences were tested for correlation with weight and BMI with a Spearman correlation. Correlation was found for weight, with values of –0.335 (weight and Cmax, P value = 0.03) and –0.39 (weight and AUC, P value = 0.01), but not for body mass index (BMI), with values of 0.11 (BMI and Cmax) and 0.052 (BMI and AUC). Concentrations and pharmacokinetic parameters for MP hemisuccinate are not reported (data on file).
Pharmacodynamics
Lymphocytes (Figure 3a)
Figure 3.
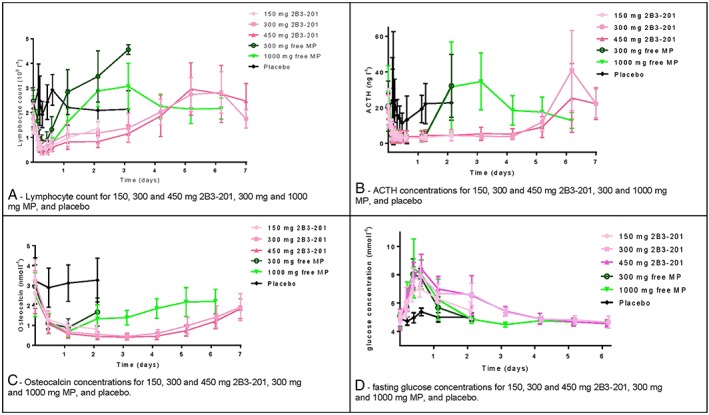
Pharmacodynamic measurements: graphs of lymphocyte count (A), adrenocorticotropic hormone concentrations (B), Osteocalcin concentrations (C) and fasting glucose concentrations (D) for different 2B3–201 doses, 300 and 1000 mg free methylprednisolone, and placebo
The effects of 2B3–201 derived MP, free MP and placebo on lymphocytes are shown in Figure 3a. Administration of 2B3–201 and free MP resulted in a maximal decrease in lymphocyte count 6–12 h after dosing. The decrease in lymphocyte count, persisted for 2 days after dosing after 150 mg 2B3–201 administration, for 3 days after dosing with 300 and 450 mg 2B3–201. Infusion of 300 mg and 1000 mg free MP resulted in a maximal decrease for 24 h. Seven days after dosing, lymphocyte values for all active groups had returned to baseline.
ACTH (Figure 3b)
ACTH concentrations were below the lower limit of quantification for almost all subjects 3 h after administration of active study medication. The decrease of ACTH was sustained for 3 days (150 mg) and 4 days (300 and 450 mg) in the 2B3–201 dosing groups, whereas for free MP ACTH plasma levels were no longer decreased after the first day after dosing, demonstrated a slight compensatory increase on days 2 and 3, and had returned to baseline values from day 4 onwards.
Osteocalcin (Figure 3c)
All the active treatment groups showed a decrease in osteocalcin concentrations in the first 24 h after dosing. In the 1000 mg MP dosing group, osteocalcin concentrations started to rise again after 24 h. For the 2B3–201 dosing groups, the decrease in osteocalcin concentrations persisted for at least 4 days.
Fasting glucose (Figure 3d)
An increase in fasting glucose was visible for all active treatment groups. Peak concentrations were measured 12 h after dosing for free MP cohorts, and 15 h after dosing for 2B3–201 cohorts. Fasting glucose concentrations were below 6 mmol l–1 (the upper limit of subjects in fasting condition) after 2 days for cohorts with free MP and 150 mg 2B3–201. For subjects who received 300 mg and 450 mg 2B3–201, fasting blood glucose had returned to levels below 6 mmol l–1 after 4 days.
CNS tests
No relevant changes in the effects on CNS between 2B3–201 and free MP could be observed.
Discussion
This first‐in‐human study with 2B3–201, a formulation of MP‐encapsulated GSH‐PEG liposomes, showed prolonged MP concentrations in serum, and as a consequence a sustained decrease in the levels of lymphocytes, osteocalcin and ACTH and increased fasting glucose over a longer period of time.
Based on pharmacokinetic properties, 2B3–201 acts like a slow release product. The estimated terminal half‐life of 2B3–201 derived MP is ten times longer than free MP. Also, the Cmax is lower for 2B3–201 (360–545 ng ml–1) than for free MP (5120–7290 ng ml–1). Based on graphical inspection, it is likely that the pharmacokinetics of 2B3–201 derived MP is characterized by first order kinetics. The observed pharmacokinetic profile of free MP corresponded with literature 30, 31 and information in the Summary of Product Characteristics.
Pharmacokinetics of 450 mg 2B3–201 in women were different from 450 mg 2B3–201 in men: the Cmax and AUC were higher, and the half‐life was longer (Table 4). This can be explained by relative lower weight of women resulting in a higher concentration of MP in serum, and a longer residence time of 2B3–201 compared to men, as the clearance is comparable (men: 0.09–0.11 l h–1, women: 0.09 l h–1).
A limitation of the cross‐over part of the study was the time interval between the cohorts. In cohort 3, dosing of 450 mg of 2B3–201 resulted for two subjects in low concentrations of MP study in predose samples at the start of subsequent occasions. However, we believe that this did not influence the major outcome as pharmacodynamic parameters lymphocytes, ACTH and fasting glucose were back to baseline in <7 days after the infusion. Also, the other four subjects in cohort 3 did not have measurable predose pharmacokinetic results.
As a consequence of prolonged plasma concentrations of 2B3–201 derived MP, a pronounced decrease in lymphocytes was observed for all dose levels for 3 days, an effect that lasted markedly longer than in the free MP groups (1 day). Similar prolonged pharmacodynamic effects were observed for the decreases in concentrations of osteocalcin and ACTH, as well as a rise in fasting blood glucose. All these effects were present over a longer period after dosing 2B3–201 in comparison to free MP. Even though we observed prolonged effects of 2B3–201, we could not observe significant differences in effects on CNS functioning between 2B3–201 and free MP.
Treatment with 2B3–201 led to the occurrence of mild infusion related reactions in 89% of all subjects. Increased levels of complement concentrations were found in all subjects after receiving 2B3–201 300 mg and 450 mg, although not all subjects reported symptoms related to an infusion reaction (Table 3). From this study, we can conclude that complement is activated due to administration of 2B3–201, resulting in a rise in C3a (Figure 1). With a simultaneous rise of complement factor Bb (specific for the alternative pathway, Figure 1), and a lack of rise in C4d (Figure 1) concentration (specific for classical pathway), we can conclude that 2B3–201 activated the alternative complement activation pathway. IgE concentrations (Figure 1) were not increased, indicating no anaphylactic reaction was initiated. These results correspond well with what is known as complement activation related pseudo allergy (CARPA). The relationship between liposomal drug delivery and CARPA is well known, and the observed symptoms in this study match those previously described by others 32, 33, 34.
There were a couple of adjustments described that may decrease the development of a CARPA reaction. First adjustment is to start the infusion with a low infusion rate 32. We lowered the infusion rate during cohort 1. Also, lowering the infusion speed when symptoms occur, and re‐challenging subjects has also reported to be effective 29. The same could be observed in our study: the infusions of only three subjects were eventually permanently halted as a result of an infusion related reaction. All other subjects received the complete infusion.
Another study reported that a low concentration of liposomes in the infusion fluid also led to fewer infusion related reactions 35, which was implemented in cohort 1. In cohorts 2–7 the concentration of the liposomes was, however, still relatively high, as compared to the adjusted concentration in cohort 1 without the observed infusion related reactions. The effect of pretreatment with clemastine has been discussed in literature 29, 35, and although this had not been effective in all studies, it was decided to administer 2 mg clemastine 20 min before start of the infusion in cohorts 4, 5 and 7. In our study design, subjects received 2B3–201 once, so a reported decrease of infusion related reaction with multiple dosing 35 of the same compound was not addressed.
Our current actions did not lead to a decrease in the number of reported infusion related reactions, although we did observe a reduced need to change the infusion speed because of infusion related reactions. Reducing the concentration of the liposomes at the start of the infusion may offer a solution in the future.
Methylprednisolone is first choice in medication for acute relapses in MS 36, 37. In certain European countries, usually 3 consecutive days of infusions are given 38. Based on study results in 2015 which revealed that use of oral administration of MP was noninferior to intravenous MP 39, the National Institute for Health and Care Excellence (UK) adapted their guideline accordingly 40. Nevertheless, use of intravenous MP remains part of clinical practice especially for patients who suffer from severe relapses and those who do not respond to oral treatment. With these practices in mind, use of 2B3–201 as a 1‐day intravenous treatment may be a good alternative.
Moreover, to reduce the burden of 3–5 days of hospital visits, and healthcare costs related to the days of admissions in other countries, a single infusion of 2B3–201 could be beneficial for patients and reduce side effects caused by high doses of MP. In the current study, 2B3–201 derived MP was measurable and active for 7 days after infusion, resulting in a sustained decrease of lymphocyte count, ACTH and osteocalcin, and an increase in fasting glucose. Now, studies with single administrations in patients with RRMS and a relapse measuring clinical improvement and comparing single administrations of 2B3–201 to 3 day treatments with regular MP are warranted to demonstrate this further.
Even though the infusion related reactions were all mild and self‐limiting, these reactions caused by 2B3–201 in the current setting were frequent and intense. MP treatment in MS reduces symptoms of the MS relapse on the short term, but for most patients it does not influence the disease progression in the long term 37. It is important that the side effect profile is acceptable for the patient. The observed infusion related reactions, if not resolved, may therefore limit the future widespread use of 2B3–201 as a standard therapy for the treatment of relapses in patients with RRMS.
Competing Interests
K.M.S.K., R.G.J.A.Z., E.S.K. and G.J.G. declare no conflict of interest. The study reported in this manuscript was sponsored by 2‐BBB medicines BV, Leiden, the Netherlands. W.G. and I.S. were former employees of 2‐BBB medicines BV, P.J.G. is the CEO of 2‐BBB medicines BV.
Contributors
K.M.S.K. acquired and interpreted the data, drafted the paper and co‐designed the study. R.G.J.A.Z. acquired and interpreted the data, reviewed the paper and co‐designed the study. I.S. interpreted the data and reviewed the paper. W.G. interpreted the data, reviewed the paper and co‐designed the study. P.J.G. interpreted the data, critically reviewed the paper and designed the study. E.S.K. performed statistical analyses and co‐designed the study. G.J.G. acquired and interpreted the data, critically reviewed the paper and designed the study.
Kanhai, K. M. S. , Zuiker, R. G. J. A. , Stavrakaki, I. , Gladdines, W. , Gaillard, P. J. , Klaassen, E. S. , and Groeneveld, G. J. (2018) Glutathione‐PEGylated liposomal methylprednisolone in comparison to free methylprednisolone: slow release characteristics and prolonged lymphocyte depression in a first‐in‐human study. Br J Clin Pharmacol, 84: 1020–1028. doi: 10.1111/bcp.13525.
References
- 1. Compston A, Coles A. Multiple sclerosis. Lancet 2008; 372: 1502–1517. [DOI] [PubMed] [Google Scholar]
- 2. van Winsen L, Polman C, Dijkstra C, Tilders F, Uitdehaag B. Suppressive effect of glucocorticoids on TNF‐alpha production is associated with their clinical effect in multiple sclerosis. Mult Scler 2010; 16: 500–502. [DOI] [PubMed] [Google Scholar]
- 3. Jongen PJ, Stavrakaki I, Voet B, Hoogervorst E, van Munster E, Linssen WH, et al Patient‐reported adverse effects of high‐dose intravenous methylprednisolone treatment: a prospective web‐based multi‐center study in multiple sclerosis patients with a relapse. J Neurol 2016; 263: 1641–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tischner D, Reichardt H. Glucocorticoids in the control of neuroinflammation. Mol Cell Endocrinol 2007; 275: 62–70. [DOI] [PubMed] [Google Scholar]
- 5. Linker R, Weller C, Luhder F, Mohr A, Schmidt J, Knauth, et al Liposomal glucocorticosteroids in treatment of chronic autoimmune demyelination: long‐term protective effects and enhanced efficacy of methylprednisolone formulations. Exp Neurol 2008; 211: 397–406. [DOI] [PubMed] [Google Scholar]
- 6. Gaillard P, Visser C, Appeldoorn C, Rip J. Enhanced brain drug delivery: safely crossing the blood–brain barrier. Drug Discov Today Technol 2012; 9: e155–e160. [DOI] [PubMed] [Google Scholar]
- 7. Kannan R, Chakrabarti R, Tang D, Kim K, Kaplowitz N. GSH transport in human cerebrovascular endothelial cells and human astrocytes: evidence for luminal localization of Na+‐dependent GSH transport in HCEC. Brain Res 2000; 852: 374–382. [DOI] [PubMed] [Google Scholar]
- 8. Lee DH, Rotger C, Appeldoorn CC, Reijerkerk A, Gladdines W, Gaillard PJ, et al Glutathione PEGylated liposomal methylprednisolone (2B3‐201) attenuates CNS inflammation and degeneration in murine myelin oligodendrocyte glycoprotein induced experimental autoimmune encephalomyelitis. J Neuroimmunol 2014; 274: 96–101. [DOI] [PubMed] [Google Scholar]
- 9. Evans MC, Gaillard PJ, de Boer M, Appeldoorn C, Dorland R, Sibson NR, et al CNS‐targeted glucocorticoid reduces pathology in mouse model of amyotrophic lateral sclerosis. Acta Neuropathol Commun 2017; 2: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reijerkerk A, Appeldoorn CC, Rip J, de Boer M, Gaillard PJ. Systemic treatment with glutathione PEGylated liposomal methylprednisolone (2B3‐201) improves therapeutic efficacy in a model of ocular inflammation. Invest Ophthalmol Vis Sci 2014; 55: 2788–2794. [DOI] [PubMed] [Google Scholar]
- 11. Gaillard P, Appeldoorn C, Rip J, Dorland R, van der Pol S, Kooij G, et al Enhanced brain delivery of liposomal methylprednisolone improved therapeutic efficacy in a model of neuroinflammation. J Control Release 2012; 164: 364–369. [DOI] [PubMed] [Google Scholar]
- 12. Russel G, Henley D, Leendertz J, Douthwaite J, Wood S, Stevens A, et al Rapid glucocorticoid receptor‐mediated inhibition of hypothalamic–pituitary–adrenal ultradian activity in healthy males. J Neurosci 2010; 30: 6106–6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heuck C, Wolthers O. A placebo‐controlled study of three osteocalcin assays for assessment of prednisolone‐induced suppression of bone turnover. J Endocrinol 1998; 159: 127–131. [DOI] [PubMed] [Google Scholar]
- 14. Kuroki Y, Kaji H, Kawano S, Kanda F, Takai Y, Kajikawa M, et al Short‐term effects of glucocorticoid therapy on biochemical markers of bone metabolism in Japanese patients: a prospective study. J Bone Miner Metab 2008; 26: 271–278. [DOI] [PubMed] [Google Scholar]
- 15. Tornatore K, Venuto R, Logue G, Davis P. CD4+ and CD8+ lymphocyte and cortisol response patterns in elderly and young males after methylprednisolone exposure. J Med 1998; 29: 159–183. [PubMed] [Google Scholar]
- 16. Groeneveld GJ, Hay JL, Van Gerven JM. Measuring blood–brain barrier penetration using the NeuroCart, a CNS test battery. Drug Discov Today Technol 2016; 20: 27–34. [DOI] [PubMed] [Google Scholar]
- 17. Cohen AF, Ashby L, Crowley D, Land G, Peck AW, Miller AA. Lamotrigine (BW430C), a potential anticonvulsant. Effects on the central nervous system in comparison with phenytoin and diazepam. Br J Clin Pharmacol 1985; 20: 619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Steveninck AL, Mandema JW, Tuk B, Van Dijk JG, Schoemaker HC, Danhof M, et al A comparison of the concentration‐effect relationships of midazolam for EEG‐derived parameters and saccadic peak velocity. Br J Clin Pharmacol 1993; 36: 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wauquier A. Aging and changes in phasic events during sleep. Physiol Behav 1993; 54: 803–806. [DOI] [PubMed] [Google Scholar]
- 20. Milner B. Visually‐guided maze learning in man: effects of bilateral hippocampal, bilateral frontal, and unilateral cerebral lesions. Neuropsychologia 1965; 3: 318–338. [Google Scholar]
- 21. Laeng B, Lag T, Brennen T. Reduced Stroop interference for opponent colors may be due to input factors: evidence from individual differences and a neural network simulation. J Exp Psychol Hum Percept Perform 2005; 31: 438–452. [DOI] [PubMed] [Google Scholar]
- 22. Borland RG, Nicholson AN. Visual motor co‐ordination and dynamic visual acuity. Br J Clin Pharmacol 1994; 18 (Suppl 1): 69s–72s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bond AJ, James DC, Lader MH. Sedative effects on physiological and psychological measures in anxious patients. Psychol Med 1974; 4: 374–380. [DOI] [PubMed] [Google Scholar]
- 24. Bowdle TA, Radant AD, Cowley DS, Kharasch ED, Strassman RJ, Roy‐Byrne PP. Psychedelic effects of ketamine in healthy volunteers: relationship to steady‐state plasma concentrations. Anesthesiology 1998; 88: 82–88. [DOI] [PubMed] [Google Scholar]
- 25. de Haas SL, de Visser SJ, van der Post JP, de Smet M, Schoemaker RC, Rijnbeek B, et al Pharmacodynamic and pharmacokinetic effects of TPA023, a GABA(A) alpha(2,3) subtype‐selective agonist, compared to lorazepam and placebo in healthy volunteers. J Psychopharmacol 2007; 21: 374–383. [DOI] [PubMed] [Google Scholar]
- 26. Harding S, Sharman J, Faccenda E, Southan C, Pawson A, Ireland S, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sampson H, Munoz‐Furlong A, Campbell R, Adkinson N, Bock S, Branum A, et al Second symposium on the definition and management of anaphylaxis: summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med 2006; 47: 373–380. [DOI] [PubMed] [Google Scholar]
- 28. Scarlet C. Anaphylaxis. J Infus Nurs 2006; 29: 39–44. [DOI] [PubMed] [Google Scholar]
- 29. Kang S, Saif M. Infusion‐related and hypersensitivity reactions of monoclonal antibodies used to treat colorectal cancer – identification, prevention, and management. J Support Oncol 2007; 5: 451–457. [PubMed] [Google Scholar]
- 30. Al‐Habet S, Rogers H. Methylprednisolone pharmacokinetics after intravenous and oral administration. Br J Clin Pharmacol 1989; 27: 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mollmann H, Rohdewald P, Barth J, Verho M, Derendorf H. Pharmacokinetics and dose linearity testing of methylprednisolone phosphate. Biopharm Drug Dispos 1989; 10: 453–464. [DOI] [PubMed] [Google Scholar]
- 32. Chanan‐Khan A, Szebeni J, Savay S, Liebes L, Rafique N, Alving C, et al Complement activation following first exposure to pegylated liposomal doxorubicin (Doxil): possible role in hypersensitivity reactions. Ann Oncol 2003; 14: 1430–1437. [DOI] [PubMed] [Google Scholar]
- 33. Roden M, Nelson L, Knudsen T, Jarosinski P, Starling J, Shiflett S, et al Triad of acute infusion‐related reactions associated with liposomal amphotericin B: analysis of clinical and epidemiological characteristics. Clin Infect Dis 2003; 36: 1213–1220. [DOI] [PubMed] [Google Scholar]
- 34. Szebeni J. Complement activation‐related pseudoallergy: a new class of drug‐induced acute immune toxicity. Toxicology 2005; 216: 106–121. [DOI] [PubMed] [Google Scholar]
- 35. Szebeni J, Muggia F, Gabizon A, Barenholz Y. Activation of complement by therapeutic liposomes and other lipid excipient‐based therapeutic products: prediction and prevention. Adv Drug Deliv Rev 2011; 63: 1020–1030. [DOI] [PubMed] [Google Scholar]
- 36. Nos C, Sastre‐Garriga J, Borras C, Rio J, Tintore M, Mantalban X. Clinical impact of intravenous methylprednisolone in attacks of multiple sclerosis. Mult Scler 2004; 10: 413–416. [DOI] [PubMed] [Google Scholar]
- 37. Sellebjerg F, Barnes D, Midgard R, Montalban X, Rieckmann P, Selmaj K, et al EFNS guideline on treatment of multiple sclerosis relapses: report of an EFNS task force on treatment of multiple sclerosis relapses. Eur J Neurol 2005; 12: 939–946. [DOI] [PubMed] [Google Scholar]
- 38. Nederlandse Vereniging voor Neurologie (Dutch Neurology Association): database with guidelines. Available at https://richtlijnendatabase.nl/nvn (last accessed 27 February 2018).
- 39. Le Page E, Veillard D, Laplaud D, Hamonic S, Wardi R, Lebrun C, et al Oral versus intravenous high‐dose methylprednisolone for treatment of relapses in patients with multiple sclerosis (COPOUSEP): a randomised, controlled, double‐blind, non‐inferiority trial. Lancet 2015; 386: 974–981. [DOI] [PubMed] [Google Scholar]
- 40. NICE guideline on treatment of acute exacerbation in MS. Available at https://www.nice.org.uk/guidance/CG186/chapter/1-Recommendations#relapse-and-exacerbation (last accessed 27 February 2018).


