Abstract
Adverse Event
Decreased abiraterone exposure after introducing carbamazepine.
Drugs Implicated
Abiraterone acetate and carbamazepine.
The Patient
A 65‐year‐old man with metastatic castration resistant prostate cancer, was treated with abiraterone acetate and prednisolone, and received concomitant carbamazepine for treatment of facial neuropathy.
Evidence that Links the Drug to the Event
The interaction was confirmed by a decrease in abiraterone exposure >2‐fold (area‐under‐the‐curve and trough levels). After discontinuation of carbamazepine therapy, the abiraterone exposure normalized. No alternative causes were found that explain the decrease in abiraterone exposure.
Mechanism
Induction of CYP3A and potentially phase I metabolism (SULT2A1) by carbamazepine.
Implications for Therapy
Clinicians and pharmacists should be aware of this clinically relevant interaction. The national drug–drug interaction checker does not warn for this interaction, whereas both the Lexicomp® and Micromedex® advice to avoid if possible or to increase the abiraterone dose frequency to twice daily. Carbamazepine should not be combined with abiraterone to avoid underexposure and suboptimal therapy. Therapeutic drug monitoring of abiraterone is useful to guide therapy when drug–drug interactions cannot be avoided.
Keywords: abiraterone, drug‐drug interaction, prostate cancer
http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9288 is a CYP17‐inhibitor used to treat metastatic castration resistant prostate cancer(mCRPC) 1, 2. There is accumulating evidence that, in the clinically used dose, exposure correlates well with treatment outcome (2–4) and that a threshold for http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6745 Ctrough (8.4 ng ml−1) is correlated with prostate specific antigen (PSA) response during therapy 3, 4, 5, 6. In our clinic, Therapeutic Drug Monitoring (TDM) of abiraterone is performed to support treatment, for example, in case of drug–drug interactions 7. Abiraterone is metabolized by sulfotransferase2A1 (SULT2A1) and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1337 and inhibits http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1329 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1325 metabolism 8. A retrospective study found theoretical potential drug–drug interactions in 52% of the mCRPC patients; however, many theoretical interactions have not been studied thus far 9, 10. We report on an interaction between http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5339 and abiraterone acetate leading to a clinically relevant decrease in abiraterone exposure.
A 65‐year‐old man was diagnosed with de novo bone metastatic prostate cancer and progressed within 5 months under androgen‐deprivation therapy. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6809 was initiated as first‐line therapy for castration‐resistant prostate cancer, but had to be discontinued following 1 cycle due to severe treatment‐related toxicity. Combination treatment with radium‐223‐chloride and abiraterone acetate 1000 mg once daily plus http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2866 10 mg was commenced due to symptomatic clinical and radiological progression (PSA 85 μg l−1, reference value <4,5 μg l−1). Ten days after start of abiraterone acetate, the patient visited the neurologist for facial neuropathy, possibly due to bone metastatic disease of the skull base affecting the trigeminal nerve. Therefore, carbamazepine, which is known to be a strong inducer of CYP3A4, was prescribed in a dose of 200 mg twice daily. The medical oncologist noticed the potential drug–drug interaction. Following consultation with the neurologist, it was decided to continue the carbamazepine as no good alternative was available; after consulting the pharmacologist, the decision was made to evaluate the severity of the potential drug–drug interaction by monitoring the plasma concentration of abiraterone. Co‐medication that was used concurrently with abiraterone acetate and prednisolone therapy was http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5239, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1175, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7260, calcium/cholecalciferol, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6886, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6063, and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1626. No interference of the other co‐medication on abiraterone metabolism is expected. We assessed the plasma pharmacokinetics (PK) of abiraterone by sampling a PK curve 2 weeks after start of carbamazepine (PSA 98 μg l−1). This time period was considered to be appropriate to reach maximal inducing effects on metabolizing enzymes and steady‐state pharmacokinetics of abiraterone. Abiraterone was measured with our validated LC‐MS/MS method that is described in further detail in a previously published article 11. The assay ranged from 1 to 500 ng ml−1, and the limit of detection was 0.01 ng ml−1. Within‐ and between‐day precisions and accuracies were below 13.4% and within 95–102%. Carbamazepine was discontinued after one month due to side effects (anemia, leukocytopenia, and thrombocytopenia). One month after discontinuation of carbamazepine (PSA 100 μg l−1), an additional PK assessment was performed to compare abiraterone exposure alone versus abiraterone with concomitant carbamazepine. A 67% decrease in abiraterone area under the concentration (AUC) time curve was observed during treatment with carbamazepine, and Ctrough level was decreased by 61% (Figure 1). Although the patient's condition had improved, no PSA response was observed (maximum PSA decline of 5% at 2 months following treatment initiation). After 7 months of abiraterone therapy, treatment was discontinued due to radiological and biochemical progression (PSA level 640 μg l−1). Informed consent for publication of anonymous medical data was given by the patient.
Figure 1.
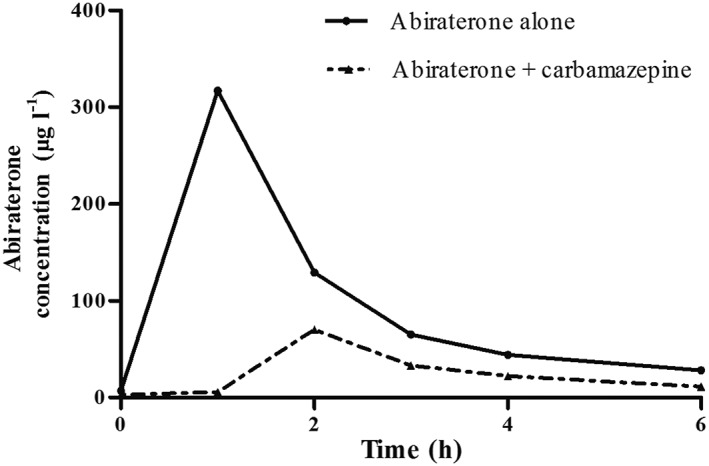
Pharmacokinetic curve for abiraterone 1000 mg once daily alone and with co‐medication carbamazepine 200 mg b.i.d. Solid line: Abiraterone alone AUC: 696 μg*h l−1; Ctrough 6.9 μg l−1. Dashed line: Abiraterone with carbamazepine AUCinf: 229 μg*h l−1; Ctrough 2.7 μg l−1. The mean exposures in the phase III trials; AUC of 993 ug*h l−1 and Ctrough was 11.1 μg l−1 7
We report a clinical meaningful drug–drug interaction between abiraterone and carbamazepine that resulted in a decreased exposure of abiraterone (67% and 61% for AUC and Ctrough, respectively). We could not identify any alternative explanation. Our case adds to the limited information that is available, since no clinical data on a drug–drug interaction between abiraterone and carbamazepine were published previously. In a drug–drug interaction study, mean abiraterone exposure (AUC) was decreased by 55% with another strong CYP3A4 inducer, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2765 10. Currently, the package labels (FDA and EMA) advice to avoid strong inducers of CYP3A4, whenever possible. If no alternatives are available, the FDA recommends an increase of abiraterone to 1000 mg twice daily (bid) dosing, however without previous clinical data to corroborate this advice (Table 1) 7, 8. Our case supports the recommendation to avoid this combination, but whether a bid dosing schedule would be sufficient to overcome this interaction remains unanswered. Such an intervention should be supported with TDM where possible, since the high inter‐ and intra‐patient variability of abiraterone exposure complicates the prediction of the effect. The effect of carbamazepine on abiraterone exposure in this case exceeds the intra‐patient variability (31%) by twofold; therefore, it is likely that the observed decrease in exposure is not merely explained by intra‐patient variability 3.
Table 1.
CYP3A4 inducers, product label advice and evidence for drug–drug interaction
| CYP3A4 inducer | Effect on abiraterone exposure | FDA label | EMA label | Evidence |
|---|---|---|---|---|
| Rifampicin | 55% decrease in exposure (AUC) | Avoid or increase dose frequency to twice daily | Avoid unless there is no alternative | Bernard et al.10 |
| Rifabutin | Unknown | Avoid or increase dose frequency to twice daily | Avoid unless there is no alternative | Not available |
| Rifapentine | unknown | Avoid or increase dose frequency to twice daily | Avoid unless there is no alternative | Not available |
| Carbamazepine | 67% decrease in exposure (AUC) | Avoid or increase dose frequency to twice daily | Avoid unless there is no alternative | This case |
| Phenytoin | unknown | Avoid or increase dose frequency to twice daily | Avoid unless there is no alternative | Not available |
| St. Johns Wort | unknown | Not mentioned in the label | Avoid unless there is no alternative | Not available |
| Phenobarbital | unknown | Avoid or increase dose frequency to twice daily | Avoid unless there is no alternative | Not available |
According to the Drug Interaction Probability Score, this interaction is probable with a score of 5 out of 9 12. The observed interaction is consistent with the known effects of carbamazepine, since CYP3A4 is one of the main metabolizing enzymes for abiraterone. Induction of both CYP3A and SULT2A1 is mediated by the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=606 (PXR) which is induced by carbamazepine 13 (Figure 2). There were insufficient data to assess the time course of the interaction; however, we anticipated that the induction by carbamazepine was maximal after 2 weeks of therapy and was resolved 1 month after stopping carbamazepine. It was not possible to re‐challenge this interaction, no other objective clinical consequences could be measured and no dosage changes of carbamazepine or abiraterone were evaluated. Unfortunately, our assay was not developed to measure the (inactive) metabolites of abiraterone. This could have provided more data on the mechanism of the interaction. Bayesian dose forecasting is a promising technique to tailor dosing as was shown previously, for example, in patients treated with immunosuppressive drugs and antibiotics 14, 15. This technique could also be useful for oral anticancer drugs. However, frequent monitoring and validation of this technique would be necessary to apply to the current drug–drug interaction case report given the intra‐patient variability of abiraterone (>30%) 16.
Figure 2.
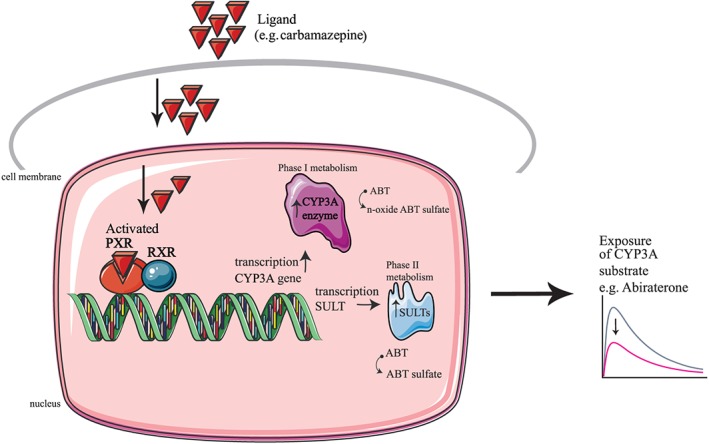
Schematic and simplified illustration of drug–drug interaction mediated by the pregnane X receptor. The nuclear receptor PXR is activated by the ligand (e.g., carbamazepine) and forms a heterodimer with the nuclear receptor RXR. The activated PXR complex induces expression of the CYP3A enzyme (mainly in the enterocytes and hepatocytes), and this affects the metabolism of the substrate of CYP3A (abiraterone) resulting in a decreased exposure. CYP3A, cytochrome P450; PXR, pregnane X receptor RXR; retinoid X receptor; ABT, abiraterone; SULT, sulfotransferase
Furthermore, this case represents a further example of the lack of information on an important drug–drug interaction. Consistent with our recently published article in the journal, no uniform recommendation on the management was found for three widely used drug interaction databases (Micromedex®, Lexicomp®, and the drug Dutch database) 17.
In conclusion, this case demonstrates a clinically relevant drug–drug interaction, and awareness of clinicians is necessary. The combination is best avoided pending data on adequate dose adjustments. If there is no alternative to use, then dose adjustments should be accompanied by measurement of plasma concentrations to prevent underexposure to abiraterone.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 18, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 19, 20.
Competing Interests
N.P.v.E. reports grants from Janssen Cilag, grants from Astellas, grants from Novartis, grants from Gilead, grants from Boehringer Ingelheim, grants from Astrazenica, outside the submitted work; M.J.v.d.D. reports research funding from Bayer outside the submitted work; All remaining authors have declared no conflicts of interest.
We are grateful to Lindsey te Brake and Roeland Wasmann for creative and technical revisions of the artwork.
Benoist, G. E. , van der Doelen, M. J. , ter Heine, R. , van Erp, N. P. , and Mehra, N. (2018) A clinically relevant decrease in abiraterone exposure associated with carbamazepine use in a patient with castration‐resistant metastatic prostate cancer. Br J Clin Pharmacol, 84: 1064–1067. doi: 10.1111/bcp.13532.
References
- 1. de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364: 1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013; 368: 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stuyckens K, Saad F, Xu XS, Ryan CJ, Smith MR, Griffin TW, et al Population pharmacokinetic analysis of abiraterone in chemotherapy‐naive and docetaxel‐treated patients with metastatic castration‐resistant prostate cancer. Clin Pharmacokinet 2014; 53: 1149–1160. [DOI] [PubMed] [Google Scholar]
- 4. Xu XS, Ryan CJ, Stuyckens K, Smith MR, Saad F, Griffin TW, et al Modeling the relationship between exposure to abiraterone and prostate‐specific antigen dynamics in patients with metastatic castration‐resistant prostate cancer. Clin Pharmacokinet 2017; 56: 55–63. [DOI] [PubMed] [Google Scholar]
- 5. Xu XS, Ryan CJ, Stuyckens K, Smith MR, Saad F, Griffin TW, et al Correlation between prostate‐specific antigen kinetics and overall survival in abiraterone acetate‐treated castration‐resistant prostate cancer patients. Clin Cancer Res 2015; 21: 3170–3177. [DOI] [PubMed] [Google Scholar]
- 6. Carton E, Noe G, Huillard O, Golmard L, Giroux J, Cessot A, et al Relation between plasma trough concentration of abiraterone and prostate‐specific antigen response in metastatic castration‐resistant prostate cancer patients. Eur J Cancer 2017; 72: 54–61. [DOI] [PubMed] [Google Scholar]
- 7. EMA . European Public Assessment Report (EPAR) Zytiga (abiraterone acetate) 2011. [updated 20/11/2017. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002321/WC500112858.pdf.
- 8. FDA. US Food and Drug Administration . Prescribing information Zytiga (abiraterone acetate) tablets [Prescribing Information]. 2011. [updated April 2017. Available from: https://www.zytiga.com/shared/product/zytiga/zytiga-prescribing-information.pdf.
- 9. Bonnet C, Boudou‐Rouquette P, Azoulay‐Rutman E, Huillard O, Golmard JL, Carton E, et al Potential drug‐drug interactions with abiraterone in metastatic castration‐resistant prostate cancer patients: a prevalence study in France. Cancer Chemother Pharmacol 2017; 79: 1051–1055. [DOI] [PubMed] [Google Scholar]
- 10. Bernard A, Vaccaro N, Acharya M, Jiao J, Monbaliu J, De Vries R, et al Impact on abiraterone pharmacokinetics and safety: open‐label drug‐drug interaction studies with ketoconazole and rifampicin. Clin Pharmacol Drug Dev 2015; 4: 63–73. [DOI] [PubMed] [Google Scholar]
- 11. Benoist GE, van der Meulen E, Lubberman FJE, Gerritsen WR, Smilde TJ, Schalken JA, et al Analytical challenges in quantifying abiraterone with LC‐MS/MS in human plasma. Biomed Chromatogr 2017; 31: e3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horn JR, Hansten PD, Chan LN. Proposal for a new tool to evaluate drug interaction cases. Ann Pharmacother 2007; 41: 674–680. [DOI] [PubMed] [Google Scholar]
- 13. Oscarson M, Zanger UM, Rifki OF, Klein K, Eichelbaum M, Meyer UA. Transcriptional profiling of genes induced in the livers of patients treated with carbamazepine. Clin Pharmacol Ther 2006; 80: 440–456. [DOI] [PubMed] [Google Scholar]
- 14. Brooks E, Tett SE, Isbel NM, Staatz CE. Population pharmacokinetic modelling and Bayesian estimation of tacrolimus exposure: is this clinically useful for dosage prediction yet? Clin Pharmacokinet 2016; 55: 1295–1335. [DOI] [PubMed] [Google Scholar]
- 15. Burgard M, Sandaradura I, van Hal SJ, Stacey S, Hennig S. Evaluation of tobramycin exposure predictions in three Bayesian forecasting programmes compared with current clinical practice in children and adults with cystic fibrosis. Clin Pharmacokinet 2017. https://doi.org/10.1007/s40262-017-0610-9. [DOI] [PubMed] [Google Scholar]
- 16. FDA. US Food and Drug Administration . Clinical pharmacology and biopharmaceutics review Zytiga (Abiraterone Acetate). 2010:1‐86.
- 17. Benoist GE, van Oort IM, Smeenk S, Javad A, Somford DM, Burger DM, et al Drug‐drug interaction potential in men treated with enzalutamide: mind the gap. Br J Clin Pharmacol 2018; 84: 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 2018; 46: D1091–D106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E, et al The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 2017; 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alexander SPH, Cidlowski JA, Kelly E, Marrion NV, Peters JA, Faccenda E, et al The Concise Guide to PHARMACOLOGY 2017/18: Nuclear hormone receptors. Br J Pharmacol 2017; 174: S208–S224. [DOI] [PMC free article] [PubMed] [Google Scholar]


