Abstract
Background
Anxiety is common among persons with alcohol use disorder during early abstinence from alcohol. Although benzodiazepines are effective for short-term treatment of anxiety, they are rarely used beyond acute detoxification due to concerns about misuse or interactions with alcohol.
Objectives
We conducted an open-label trial to explore the effects of coadministering lorazepam and disulfiram to alcohol dependent patients with anxiety disorder symptoms. The rationale for this model is to minimize the risks of the benzodiazepine, while also potentially enhancing adherence to disulfiram.
Methods
Forty-one participants with DSM-IV alcohol dependence who also met syndromal criteria for anxiety disorder with or without co-occurring major depressive syndrome initiated treatment with lorazepam (starting dose 0.5 mg three times daily) and disulfiram (starting dose 500mg three times weekly). Participants received 16 weeks of monitored pharmacotherapy with manualized medical management.
Results
Adherence to treatment decreased steadily with time (85.4% at 4 weeks, 36.6% at 16 weeks). Participants showed significant increases in percent abstinent days during treatment and at 24 week follow-up. Large reductions in anxiety, depression, and craving were observed during treatment, and improvement remained significant at 24 weeks. Duration of adherence with disulfiram strongly predicted abstinence at 16 weeks. There was no evidence of misuse of lorazepam or dose escalation during the study.
Conclusion
Lorazepam can be safely used for short-term treatment of anxiety in combination with disulfiram treatment of alcohol use disorder. Controlled trials are necessary to determine the effects of lorazepam on anxiety, retention in treatment, and drinking in this context.
Keywords: alcohol dependence, alcohol use disorder, anxiety, disulfiram, lorazepam, benzodiazepine
INTRODUCTION
Although disulfiram has been FDA approved for treatment of alcohol dependence for over half a century, it has not consistently out-performed placebo in blinded randomized trials (1–3). In part this appears to be due to low rates of treatment adherence in many trials, and to the fact that the main mechanism by which disulfiram inhibits drinking is the expectation of the alcohol-disulfiram reaction—a mechanism by which placebo should be equally effective unless and until alcohol is sampled. Recent studies incorporating measures to enhance adherence, and in some cases using open-label design, have yielded more favorable results in general populations (4–8), adolescents (9, 10), people with alcohol dependence and co-occurring cocaine use disorder (11, 12), and dually diagnosed alcohol dependent patients with psychiatric disorders (13). A recent systematic literature review by Jorgensen et al. found that supervised treatment with disulfiram has positive effects on short-term abstinence, days until relapse, and number of drinking days when compared with placebo, no medication, or other treatments for alcohol use disorder (14). A recent meta-analysis confirmed the efficacy of disulfiram in open-label trials, with medium-sized effects relative to naltrexone (g = .77), acamprosate (g = .76), or no medication (g = .43) (15). The authors noted that open label designs are more appropriate than double-blind trials in studying the effects of disulfiram, because expectancy plays an integral role in the therapeutic effects of this medication. Disulfiram does not appear to have any consistent direct effects on anxiety or mood (16, 17).
Both primary and secondary anxiety disorders are highly prevalent in patients with alcohol use disorder (18–21). The relationships between alcohol use disorder and anxiety disorders are complex, and probably involve bidirectional causal relationships as well as common underlying factors (22, 23). High levels of anxiety are frequently observed during and soon after detoxification (24, 25). Although anxiety levels tend to decrease with duration of abstinence, trait anxiety can persist (26), and anxiety is a frequently cited cause of relapse (26–28).
Benzodiazepines, though effective for alcohol withdrawal (29), have not been studied extensively as treatment for alcohol dependence per se. There is some evidence that benzodiazepines may be helpful in controlling anxiety and craving during early abstinence, but no well-controlled trials have examined the effect of benzodiazepines on abstinence rates beyond the immediate detoxification period (30). An early uncontrolled trial suggested that benzodiazepine treatment of alcohol dependent people post-detoxification was associated with improved treatment engagement and no difference in abstinence rates (31). Although the abuse potential of benzodiazepines in patients without substance use disorders is fairly low (30, 32), the potential for misuse may be greater in substance dependent populations. Patients with history of alcohol dependence (33) or positive family history (34) have a more positive subjective response to benzodiazepines than do controls, suggesting an increased potential for abuse. A large majority of benzodiazepine dependent people have other co-occurring substance dependence diagnoses (35, 36). Although drug use disorders appear to be much more commonly comorbid than alcohol use disorders in patients with benzodiazepine dependence, misuse of benzodiazepines by people with active alcohol use disorders is fairly common (37). Another obvious concern is benzodiazepines’ additive or synergistic effects with alcohol which can lead to lethal CNS depression, although this risk may not be shared equally by all benzodiazepines (38). In 2010 alcohol was involved in 111,165 of 408,021 (27.2%) of emergency department visits related to benzodiazepines, and this combination accounted for 393 deaths (39). On the other hand, longitudinal studies provide evidence that prescription of benzodiazepines in patients with a history of alcohol use disorder is unlikely to lead to misuse of benzodiazepines (40) and does not increase the rate of relapse to alcohol dependence (41).
The combination of disulfiram and a benzodiazepine could be particularly useful during initial treatment of patients with alcohol dependence and primary or secondary anxiety disorders. People who take therapeutic doses of disulfiram are very unlikely to drink large quantities of alcohol, but poor treatment adherence limits its clinical effectiveness. By providing an effective short-term treatment of anxiety, the benzodiazepine could enhance retention and serve as a reinforcer of adherence to disulfiram. Benzodiazepines could also help with insomnia, a common problem in early abstinence (42).
With this rationale we conducted an open-label study to test the feasibility of using a combination of disulfiram and lorazepam in the treatment of patients with alcohol dependence and co-occurring anxiety disorder. Specifically, we implemented a model in which receiving lorazepam is contingent on adherence to disulfiram. The goals of the study were to determine treatment adherence and retention rates, to describe within-treatment diagnosis and treatment of anxiety and mood disorders, to determine pre-post treatment effect sizes for measures of alcohol use and anxiety, to explore predictors of drinking outcomes, and to identify any pattern of unexpected adverse events or other safety concerns.
MATERIALS AND METHODS
Overview
Participants with active alcohol dependence and primary or secondary anxiety disorder received monitored disulfiram and lorazepam, in the context of a structured Medical Management (MM) model. In weeks 9–15 lorazepam was tapered, and disulfiram was stopped at the end of week 16. Adherent participants who achieved 4 weeks abstinence and met criteria for a primary anxiety or mood disorder could receive FDA-approved non-benzodiazepine treatment, with specific options for each disorder described in the protocol. A final follow-up assessment occurred at week 28. The primary outcomes were Percent Days Abstinent (PDA) and retention in treatment. Secondary outcomes were categorical abstinence, symptoms of anxiety and depression, and alcohol craving.
Participants
Participants were recruited from an outpatient addiction treatment program and from the community through advertisements. All study procedures were reviewed and approved by the Institutional Review Board of the investigators’ institution. Participants were males and females age 18 and over with DSM-IV alcohol dependence who were able to provide voluntary informed consent, had at least 4 heavy drinking days in the past 30 days (4 or more drinks in a day for women, 5 or more drinks in a day for men), had a primary or secondary anxiety disorder ascertained by the Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID) (43), had a goal of abstinence, had at least 2 days abstinence at the time of study entry, were willing to come to the clinic 3x/week, and, if female of child-bearing potential, were willing to use approved method of contraception. Participants were excluded if they had allergy or hypersensitivity to disulfiram or lorazepam, moderate or severe alcohol withdrawal, history of withdrawal seizures or delirium tremens, exclusionary medical conditions, urine drug screen positive for opioids or barbiturates, hypersensitivity to thiuram derivatives, pregnancy, exclusionary laboratory abnormalities, the need to take excluded medication, exclusionary psychiatric conditions (schizophrenia, schizoaffective disorder, bipolar disorder; opioid dependence, benzodiazepine or other sedative hypnotic dependence), change in psychiatric medications within 4 weeks of baseline assessment, or treatment with benzodiazepines within 4 weeks of baseline assessment, except for treatment of alcohol withdrawal. Any benzodiazepines used for alcohol withdrawal were completely tapered and discontinued prior to the beginning of study treatment. Except for benzodiazepines and other sedative-hypnotics acting at gamma-aminobutyric acid (GABA) A or B receptors, participants were allowed to continue psychiatric medications provided that the medications had been prescribed at stable doses for at least 1 month.
Interventions
Disulfiram
Disulfiram was started at a dose of 500 mg every Monday, Wednesday, and Friday, with administration monitored at the clinic. Participants were able to earn take-home privileges for weekly dispensing (250 mg daily, first dose monitored at the clinic) based on abstinence and adherence for at least 4 weeks. To get take-home doses participants were required to identify a cohabiting person who could observe the participant taking disulfiram daily and report to the clinic if the participant was not adherent. Dosage could be increased to a maximum of 1000 mg three times weekly or 500 mg per day if there was evidence of drinking without alcohol-disulfiram reaction in the context of medication compliance. Breath alcohol was measured prior to dispensing. If a participant tested positive for alcohol, disulfiram and lorazepam were withheld until the next scheduled dispensing day.
Lorazepam
Lorazepam was dispensed with the disulfiram, and the first dose of lorazepam was administered at the clinic. The remaining doses were taken at home. Participants received lorazepam only if they were adherent to disulfiram, and received only enough lorazepam to cover the interval until the next time disulfiram was due to be dispensed. Dosage of lorazepam started at 0.5 mg three times daily and increased to a maximum of 2 mg three times daily based on continuing anxiety symptoms. Dosage could be decreased or discontinued if necessary due to side effects such as sedation. Lorazepam was tapered slowly over 3–7 weeks (depending on dose) to minimize rebound anxiety, which has been observed in short-term detoxification studies with oxazepam (44, 45).
Psychosocial Treatment
The MM manual used in the NIAAA COMBINE study (A Multisite Trial of Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence) (46) was adapted for this trial. As in COMBINE, 9 visits were scheduled at weeks 0, 1, 2, 4, 6, 8, 10, 12, and 16. Checklists were completed by the practitioner at each visit to document adherence to the treatment model. The MM provider completed the anxiety and depression assessments and provided clinical treatment of depressive and anxiety disorders as described below.
Concomitant medications for anxiety disorder and depression
For participants achieving 4 or more weeks of abstinence and meeting DSM-IV criteria for a primary anxiety or depressive disorder, the clinician offered the participant ancillary pharmacotherapy according to FDA-approved dosages for the diagnosed disorder or disorders.
Assessments
Research diagnosis
The SCID for DSM-IV was used at study entry to make diagnoses of substance use disorders, anxiety syndromes, and major depressive syndrome, and to rule out exclusionary disorders such as schizophrenia and bipolar disorder. Diagnoses were made solely on the basis of whether syndromal criteria were met, without attempting to distinguish between primary and secondary disorders.
Substance use and related measures
A substance use calendar (Timeline Follow-back, (47)) was completed at each visit, recording each day of use for alcohol and other commonly abused substances since the last visit (and 90 days back from baseline). PDA was calculated as 100 times the number of abstinent days in the assessment period divided by the total number of days in the period. Participant with PDA = 100 were considered to be categorically abstinent at that time point. Breath alcohol data were collected each time medication was dispensed. Urine drug screens were obtained at each visit, including tests for cocaine, methamphetamine, opiates, and cannabinoids. Consequences of drinking were assessed at baseline using the Drinkers Inventory of Consequences (DrInC) (48). Craving was assessed at each visit using the Penn Alcohol Craving Scale (PACS) (49).
Alcohol withdrawal
The Clinical Institute Withdrawal Scale—Alcohol, revised (CIWA-Ar, (50)) was used to assess alcohol withdrawal at screening, baseline, and each dispensing of medication during the first week of treatment.
Anxiety and depression
The Hamilton Anxiety Rating Scale (Ham A)(51) and the Hamilton Depression Rating Scale (Ham D, 17-item version) (52) were completed at each of the 9 visits by the clinician.
Clinical diagnosis
The mood and anxiety disorder sections of the Mini-International Neuropsychiatric Interview (MINI) (53, 54) were completed by the clinician to determine clinical mood and anxiety disorder diagnoses whenever the following three conditions were met: 1) the participant had been abstinent for 4 weeks or more; 2) the Ham A score was greater than 14 or the Ham D score was greater than 9; and 3) a primary mood or anxiety disorder had not yet been diagnosed.
Adherence
Treatment adherence was quantified using dispensing records, participant self-report, collateral reports, pill counts, and MM session attendance. In the case of disulfiram, administration was directly observed except in those participants receiving weekly take-home medication. Adherence with disulfiram and lorazepam that was taken home was queried by participant self-report and verified by counting of remaining medication in returned pill bottles and, in the case of disulfiram, direct observation by a cohabiting person.
Medical and safety assessments
Screening assessment included medical history, physical exam, urinalysis, screening blood tests including liver function tests (LFTs), electrocardiogram, and pregnancy test for women of childbearing potential. LFTs were repeated at weeks 2, 4, 8, 12, and 16. Concomitant medications were recorded at each visit. Adverse events were elicited using the Systematic Assessment for Treatment Emergent Events (SAFTEE) (55), with nomenclature developed for the specific adverse events expected with disulfiram and lorazepam.
Statistical analysis
T-tests (categorical measures) and bivariate correlations (continuous measures) were computed to investigate whether baseline characteristics predicted duration of adherence to disulfiram treatment. T-tests (normally distributed continuous measures), Mann-Whitney U tests (non-normally distributed continuous or ordinal measures), and Chi-square tests (categorical measures) were used to determine whether baseline characteristics were associated with availability of follow-up data at 16 weeks. The significance of pre-post change was computed for each assessment time point relative to baseline using paired t-tests for Ham D scores and Wilcoxon signed ranks tests for Ham A scores, PACS scores, and PDA as the latter measures were not normally distributed. Categorical abstinence rates were contrasted with baseline values using McNemar tests. Hedges g adjusted for small sample bias (56) was calculated to assess the magnitude of changes in craving, depression, anxiety, and PDA. Logistic regression was used to evaluate relationships of various baseline characteristics and within-treatment measures to drinking status at the end of treatment (categorical abstinence at 16 weeks). These candidate predictors were each tested in separate models including only the predictor and a constant, and we did not attempt to construct more complicated models post-hoc.
RESULTS
Participants
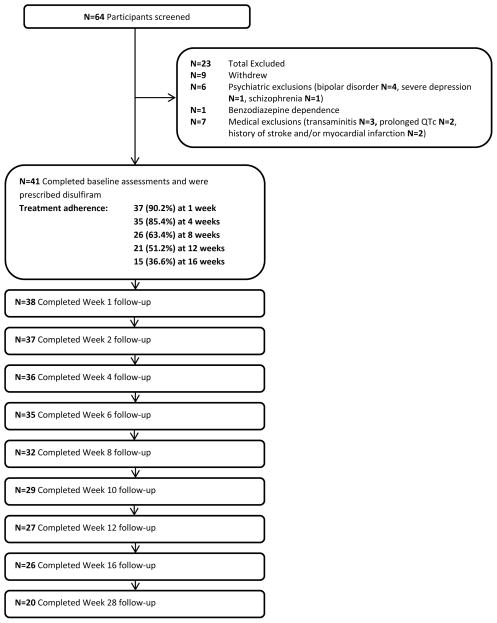
Sixty-five participants provided informed consent. Twenty-four participants failed screening or dropped out prior to treatment, as summarized in Figure 1. Treatment was initiated for 41 participants (intent-to-treat sample), one of whom dropped out before receiving study medication. Baseline characteristics of participants are summarized in Table 1. In addition to having one or more anxiety disorders, 24 participants (58.5%) had a current major depressive syndrome. Ten participants were on stable doses of antidepressants at baseline.
Figure 1.
Participant flow: recruitment, treatment exposure, and retention
Table 1.
Baseline Characteristics of the Intent-to-Treat Sample (N = 41)
| Demographics | |
| Gender (% Male) | 39% (n = 16) |
| Age in years | 41.66 (SD = 13.07) |
| Ethnicity | |
| Non-Hispanic White | 48.8% (n = 20) |
| Hispanic | 39.0% (n = 16) |
| Other | 12.2% (n = 5) |
| Mean years of education | 13.63 (SD = 2.62) |
| Employment Status | |
| Unemployed | 63.4% (n = 26) |
| Employed full time | 19.5% (n = 8) |
| Employed part-time | 9.8% (n = 4) |
| Retired | 7.3% (n = 3) |
| Household income | $21,991 (SD = $26,732) |
| Substance Use/Consequences | |
| Proportion Abstinent Days | .32 (SD = .31) |
| Drinks per Drinking Day | 16.12 (SD = 10.97) |
| Peak Blood Alcohol Content | .34 (SD = .24) |
| Urine drug screen positive | |
| Cannabinoids | 19.5% (n = 8) |
| Cocaine | 2.4% (n = 1) |
| Methamphetamine | 2.4% (n = 1) |
| Current cannabis dependence | 7.3% (n = 3) |
| Current stimulant dependenc | 2.4% (n = 1) |
| DrInCa total Score | 40.07 (SD = 7.58) |
| Current psychiatric (SCIDb) diagnoses | |
| Major depressive syndrome | 58.5% (n = 24) |
| Panic attacks | 82.9% (n = 34) |
| Social phobia | 34.1% (n = 14) |
| Specific phobia | 14.6% (n = 6) |
| PTSD | 39.0% (n =16) |
| Generalized anxiety | 100% (n = 41) |
| Hamilton Anxiety Score | 21.7 (SD = 9.4) |
| Hamilton Depression score | 19.8 (SD = 6.3) |
Drinkers Inventory of Consequences
Structured Clinical Interview for DSM-IV
Treatment adherence and follow-up
Treatment retention and assessment follow-up rates are shown in Figure 1. Only one participant required a disulfiram dose increase to 1000 mg Monday, Wednesday, and Friday. Eleven participants received weekly take-home privileges at some point during treatment. Participants remained on disulfiram for a mean of 70.9 SD 41.0 days, calculated as one plus the number of days between the first and last doses taken. Participants remained on Lorazepam for a mean of 66.2 SD 36.5 days. During the period that medications were taken, participant were adherent to disulfiram a mean of 93.7 SD 16.4 percent of days. For lorazepam they were fully adherent 91.7 SD 16.7 percent of days, and were partially adherent (taking some but not all of the medication prescribed) 2.4 SD 4.7 percent of days. Demographic characteristics such as gender, ethnicity, marital status, employment status, education, age, and household income did not predict treatment drop-out. Baseline craving scores were correlated with duration of treatment adherence, with greater craving predicting shorter duration of treatment (r = −.397, n = 41, p = .010). Week 4 craving scores and change in craving from baseline to week 4 did not predict retention in treatment. Baseline mood and anxiety disorder diagnoses did not predict duration of retention in treatment with the exception of panic attacks, with greater duration of adherence in those with current panic attacks at baseline (n = 34) than those without (n = 7) (mean days on disulfiram 76.9 SD 37.1 vs. 41.3 SD 48.7, t = 2.20, p = .034). Ham A and Ham D scores at baseline and 4 weeks were not significantly correlated with retention in treatment, nor were change in Ham A or Ham D scores from baseline to 4 weeks. Treatment retention was also not correlated with measures of baseline drinking intensity and consequences. Availability of outcome data at 16 weeks was unrelated to any demographic factor except for academic degree, with greater academic attainment among those who provided data at 16 weeks (t(37.96) = 2.170, p =.036). Baseline mood and anxiety disorder diagnoses did not predict availability of 16 week data, nor did baseline measures of drinking intensity and consequences, Ham A score, or Ham D score.
Within-treatment psychiatric diagnoses and treatment
Lorazepam doses remained low for most study participants. Although the maximum dose of lorazepam allowed in the study was 2 mg three times daily, no patient ever requested or received this dose. One patient received a maximum dose of 1.5 mg three times daily, 12 received 1 mg three times daily, and 27 never exceeded the starting dose of 0.5 mg three times daily. Three participants requested a decrease in the lorazepam dose to 0.5 mg once or twice daily, due to sedation. The mean maximum daily dose of lorazepam was 2.02 (SD 0.80) and the mean daily dose at week 8 was 1.85 (SD 0.89). There was no evidence of misuse of lorazepam or illicit benzodiazepines by any patient in the course of the study.
During the course of the study, participants who had achieved 1 month of abstinence and continued to have significant mood or anxiety symptoms (Ham D > 9 or Ham A > 14) were assessed by a clinician using the MINI. Of the 20 patients so assessed, 19 had at least one anxiety or mood disorder diagnosis. Fourteen had at least 1 anxiety disorder diagnosis, with specific diagnoses including panic disorder (n = 5), agoraphobia (n = 7), social phobia (n = 9), obsessive-compulsive disorder (n = 1), PTSD (n = 4), and generalized anxiety disorder (n = 5). Eighteen of 20 patients met criteria for a depressive disorder, with 15 meeting criteria for major depression and 3 meeting criteria for dysthymia without major depression. Of the 19 participants meeting diagnostic criteria for an anxiety disorder or a depressive disorder, 16 received new or additional treatment per the study protocol, all of them starting between 4 and 10 weeks after initiating treatment. Three declined additional treatment. Of the 16 treated, 12 were treated for both mood and anxiety disorders, three were treated for major depression alone, and one was treated for an anxiety disorder alone.
Treatment outcomes
Table 2 shows results for PDA and categorical abstinence at baseline and each follow-up time point, with observed cases only and with imputation of the baseline value for missing cases. PDA data were highly non-normally distributed. PDA increased significantly relative to baseline for all follow-up time points (p < .0005 for all time points except week 28, at which point p = .004, Wilcoxon signed rank test). Outcomes for categorical abstinence followed a similar pattern, with significant improvement at all time points (p < .0005 for all time points except week 28, at which point p = .016, McNemar test). Positive breath alcohol readings were uncommon, with only 4 positive readings observed during the study following initiation of treatment: 1 out of 22 completed at 16 weeks and 3 out of 19 at 28 weeks. Rates of positive urine drug screens did not change significantly during study participation.
Table 2.
Drinking outcomes: (observed cases and baseline imputed)
| Percent Days Abstinent | ||||||
|---|---|---|---|---|---|---|
| Observed cases | Baseline value imputed | |||||
| N | Mean | SD | N | Mean | SD | |
| Baseline | 41 | 31.55 | .30.617 | 41 | 31.55 | 30.617 |
| Week 1 | 38 | 87.45 | .29.925 | 41 | 82.11 | 35.074 |
| Week 2 | 37 | 100.00 | .00.000 | 41 | 91.30 | 27.436 |
| Week 4 | 36 | 95.72 | .17.054 | 41 | 86.00 | 31.637 |
| Week 6 | 35 | 99.78 | .01.300 | 41 | 87.94 | 29.907 |
| Week 8 | 32 | 97.72 | .08.598 | 41 | 80.23 | 35.593 |
| Week 10 | 29 | 96.55 | .18.570 | 41 | 77.36 | 37.493 |
| Week 12 | 27 | 89.68 | .28.731 | 41 | 68.28 | 41.551 |
| Week 16 | 26 | 87.00 | .29.596 | 41 | 65.14 | 41.004 |
| Week 28 | 19 | 62.16 | .40.820 | 41 | 47.96 | 37.993 |
| Proportion of participants categorically abstinent | ||||
|---|---|---|---|---|
| Observed cases | Baseline value imputed | |||
| N | Proportion | N | Proportion | |
| Baseline | 41 | .0000 | 41 | .0000 |
| Week 1 | 38 | .7368 | 41 | .6829 |
| Week 2 | 37 | 1.0000 | 41 | .9024 |
| Week 4 | 36 | .8611 | 41 | .7561 |
| Week 6 | 35 | .9714 | 41 | .8293 |
| Week 8 | 32 | .9063 | 41 | .7073 |
| Week 10 | 29 | .9655 | 41 | .6829 |
| Week 12 | 27 | .8519 | 41 | .5610 |
| Week 16 | 26 | .6923 | 41 | .4390 |
| Week 28 | 19 | .3684 | 41 | .1707 |
Table 3 shows descriptive outcomes for anxiety (Ham A), depression (Ham D), and alcohol craving (PACS). Significant improvement was observed for all three measures at each follow-up time point (For the Ham A scores, p < .0005 (Wilcoxon signed rank tests) for all time points except week 28, at which point p = .009; for Ham D scores, p < .0005 (paired t-tests) for all time points except week 28, at which point p = .003; for PACS scores, p < .0005 (Wilcoxon signed rank tests) for all time points except week 28, at which point p = .014).
Table 3.
Hamilton A, Hamilton D, and Penn Alcohol Craving Scale Scores
| Hamilton A | Hamilton D | Penn Alcohol Craving Scale | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| Baseline | 41 | 21.7317 | 9.42874 | 41 | 19.7805 | 6.25904 | 41 | 19.5610 | 8.04689 |
| Week 1 | 35 | 11.9714 | 7.46161 | 35 | 11.8286 | 6.45567 | 35 | 10.2571 | 7.18799 |
| Week 2 | 36 | 11.4722 | 9.90955 | 35 | 11.0571 | 6.70357 | 36 | 9.6389 | 7.14804 |
| Week 4 | 34 | 11.6471 | 9.53743 | 34 | 10.7353 | 7.39905 | 34 | 9.5294 | 7.59679 |
| Week 6 | 33 | 10.7576 | 10.07481 | 33 | 10.3636 | 7.70478 | 33 | 7.6061 | 7.20217 |
| Week 8 | 27 | 10.7407 | 10.83652 | 26 | 10.2308 | 7.94636 | 27 | 6.5185 | 6.94135 |
| Week 10 | 24 | 10.6250 | 10.52869 | 24 | 9.2083 | 7.81292 | 24 | 5.2500 | 5.31814 |
| Week 12 | 23 | 7.9565 | 9.36375 | 23 | 6.8261 | 7.75566 | 23 | 5.1739 | 7.09451 |
| Week 16 | 22 | 8.8636 | 10.45760 | 22 | 7.7727 | 8.14705 | 22 | 6.5909 | 8.11617 |
| Week 28 | 20 | 14.5500 | 11.85205 | 20 | 13.2500 | 8.65037 | 20 | 11.1000 | 10.2233 |
Magnitude of Observed Changes
Relative to baseline values, the estimated pre-post effect sizes at the end of treatment (16 weeks) were moderate to very large for the five measures tested: craving (g = 1.28), depression (g = 1.22), anxiety, (g = 1.84), PDA (g = 1.16), and PDA with imputation of the baseline value (g = .76). At 24 weeks the observed reductions were in the moderate range: craving (g = .61), depression (g = .72), anxiety (g = .59), PDA (g = .80), and PDA with imputation (g = .48).
Predictors of drinking outcome
To examine candidate predictors of treatment outcome (categorical abstinence at 16 weeks), logistic regressions were first run with baseline imputation, i.e., participants who had dropped out were assumed to have relapsed. A baseline diagnosis of major depressive syndrome significantly predicted end-of-treatment abstinence (β = 1.493, Wald = 4.856, p = .028), but panic attacks, social phobia, and PTSD were not significant predictors. The baseline craving score significantly predicted end-of-treatment outcome, with lower craving increasing the probability of abstinence at 16 weeks (β = −.104, Wald = 5.044, p = .025). Week 4 craving was not significantly related to abstinence at end of treatment, nor was change in craving from baseline to 4 weeks. Length of adherence to disulfiram strongly predicted abstinence at end of treatment (β =.081, Wald = 11.294, p = .001), as did categorical retention in treatment at 12 weeks (β =4.391, Wald = 14.164, p < .0005). Age, gender, income, employment, consequences of drinking, being prescribed psychiatric medications at baseline, being prescribed new psychiatric medications during the study, baseline or week 4 Ham A score, baseline or week 4 Ham D score, change in Ham A score or Ham D score from baseline to 4 weeks, baseline percent drinking days, and baseline drinks per drinking day did not significantly predict being present and abstinent at end of treatment.
We repeated the logistic regressions for the significant predictors using only actual drinking data for the 26 participants assessed at week 16. Baseline major depressive syndrome remained a significant predictor or abstinence (β = 2.398, Wald = 4.177, p = .041). Baseline craving was no longer a significant predictor of abstinence (β =−.104, Wald = 2.600, p = .107). However, length of adherence remained a significant predictor (β = .061, Wald = 6.461, p = .011), as did categorical 12-week adherence (β = 3.344, Wald = 7.024, p = .008). None of these effects remained significant at week 28.
Adverse events
There were no treatment-related serious adverse events. Three participants discontinued disulfiram due to elevated transaminases during the trial (2 at 6 weeks and one at 8 weeks). The maximum observed aspartate transaminase (AST) was 222 units per liter, and the maximum observed alanine transaminase (ALT) was 314 units per liter. Mean transaminases values changed modestly during the study. The only nominally significant change was a decrease in AST from baseline to week 2 (26.7 SD 10.8 vs. 23.1 SD 9.8, t(35)=2.079, p =.045). Bilirubin was significantly decreased from baseline at 2, 6, and 16 weeks (Week 2: 0.62 SD 0.37 vs. 0.48 SD 0.21, t(35)=2.267, p =.030; week 6: 0.61 SD 0.39 vs. 0.51 SD 0.27, t(31)=2.145, p =.040; week 16: 0.69 SD 0.45 vs. 0.44 SD 0.20, t(18)=3.216, p =.005). Twelve participants reported alcohol-disulfiram reactions on a total of 15 occasions during the study. None of these was rated as severe. Five were of moderate severity, and the rest were minimal or mild.
DISCUSSION
In this single-group, open-label trial, alcohol dependent patients with co-occurring anxiety disorder tolerated the combination of disulfiram and lorazepam well. Drinking outcomes were very good during treatment, particularly for those who were retained in treatment. Mood, anxiety, and craving symptoms also decreased markedly during treatment. Drinking, mood, anxiety, and craving remained significantly improved from baseline during the 12 weeks after the end of treatment, although less markedly so than at the end of treatment.
Adherence to treatment (85.4% at 4 weeks, 36.6% at the 16 weeks) was not particularly good relative to past disulfiram trials with supervised administration (1, 14), suggesting that lorazepam did not act as a strong reinforcer of adherence with disulfiram. This interpretation is supported by the fact that there was no evidence of misuse of lorazepam or dose escalation during the study. While this is reassuring with respect to the abuse potential of lorazepam in this context, it also suggests that other methods will be necessary incentivize disulfiram adherence if high rates are to be achieved.
There were few meaningful baseline predictors of drinking outcome, with major depressive syndrome at baseline significantly predicting abstinence at 16 weeks with or without imputation of outcomes, and lower craving predicting abstinence at end of treatment only when missing participants were assumed to be drinking. However, duration of disulfiram/lorazepam treatment strongly predicted abstinence at 16 weeks. These results suggest that the combination treatment was effective in this study sample. However, it is also possible that the participants who were adherent with treatment were predisposed to do well regardless of treatment.
In spite of marked improvement in mood and anxiety symptoms in the sample as a whole, almost half of the sample (19 out of 41 participants) was diagnosed with a primary mood or anxiety disorder in the course of treatment, and most of these participants (16/19) received additional treatment through the study, consisting of an antidepressant in all but one case. There results demonstrate the feasibility of using standardized screening (Hamilton scales) and diagnosis (MINI) to identify persisting mood and anxiety disorders during early abstinence in the course of alcoholism treatment.
Limitations of this study relate primarily to the lack of a control condition, the relatively small sample size, lack of biological measures of alcohol use, and the heterogeneity of the sample, including co-occurring major depressive syndrome and co-occurring use of cannabis in a substantial proportion of participants. Due to these considerations, it is not possible to draw any conclusions as to the efficacy of the treatments that were initiated during the study. Because of the design of the study it is not possible to isolate the effect of the benzodiazepine treatment on drinking from that of the disulfiram, or the effect of benzodiazepine treatment on anxiety from that of decreased drinking. The significant amount of missing data is also a limitation, but this issue is mitigated by the fact that results are similar when baseline values are imputed for missing data, a relatively pessimistic assumption.
CONCLUSIONS
We conclude that lorazepam can be safely used for short-term treatment of anxiety in the context of disulfiram treatment of alcohol use disorder. Randomized trials will be necessary to determine the relative benefit of this combination. Additional psychosocial interventions such as contingency management may be useful to enhance treatment adherence in future trials.
Acknowledgments
This study was supported by NIAAA grant K24AA016555. We also wish to recognize the contributions of Lindsay Worth, Craig Pacheco, Robert Kushner, and Roberta Chavez, who coordinated the study and collected data.
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest.
References
- 1.Suh JJ, Pettinati HM, Kampman KM, O’Brien CP. The status of disulfiram: a half of a century later. J Clin Psychopharmacol. 2006;26(3):290–302. doi: 10.1097/01.jcp.0000222512.25649.08. [DOI] [PubMed] [Google Scholar]
- 2.Ulrichsen J, Nielsen MK, Ulrichsen M. Disulfiram in severe alcoholism--an open controlled study. Nordic journal of psychiatry. 2010;64(6):356–62. doi: 10.3109/08039481003686180. [DOI] [PubMed] [Google Scholar]
- 3.Yoshimura A, Kimura M, Nakayama H, Matsui T, Okudaira F, Akazawa S, et al. Efficacy of disulfiram for the treatment of alcohol dependence assessed with a multicenter randomized controlled trial. Alcohol Clin Exp Res. 2014;38(2):572–8. doi: 10.1111/acer.12278. [DOI] [PubMed] [Google Scholar]
- 4.De Sousa A, De Sousa A. An open randomized study comparing disulfiram and acamprosate in the treatment of alcohol dependence. Alcohol Alcohol. 2005;40(6):545–8. doi: 10.1093/alcalc/agh187. [DOI] [PubMed] [Google Scholar]
- 5.De Sousa A, De Sousa A. A one-year pragmatic trial of naltrexone vs disulfiram in the treatment of alcohol dependence. Alcohol Alcohol. 2004;39(6):528–31. doi: 10.1093/alcalc/agh104. [DOI] [PubMed] [Google Scholar]
- 6.Chick J, Gough K, Falkowski W, Kershaw P, Hore B, Mehta B, et al. Disulfiram treatment of alcoholism. Br J Psychiatry. 1992;161:84–9. doi: 10.1192/bjp.161.1.84. [DOI] [PubMed] [Google Scholar]
- 7.Laaksonen E, Koski-Jannes A, Salaspuro M, Ahtinen H, Alho H. A randomized, multicentre, open-label, comparative trial of disulfiram, naltrexone and acamprosate in the treatment of alcohol dependence. Alcohol Alcohol. 2008;43(1):53–61. doi: 10.1093/alcalc/agm136. [DOI] [PubMed] [Google Scholar]
- 8.De Sousa AA, De Sousa J, Kapoor H. An open randomized trial comparing disulfiram and topiramate in the treatment of alcohol dependence. J Subst Abuse Treat. 2008;34(4):460–3. doi: 10.1016/j.jsat.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Niederhofer H, Staffen W. Comparison of disulfiram and placebo in treatment of alcohol dependence of adolescents. Drug Alcohol Rev. 2003;22(3):295–7. doi: 10.1080/0959523031000154436. [DOI] [PubMed] [Google Scholar]
- 10.De Sousa A, De Sousa A. An open randomized trial comparing disulfiram and naltrexone in adolescents with alcohol dependence. Journal of Substance Use. 2008;13(6):382–8. [Google Scholar]
- 11.Carroll KM, Nich C, Ball SA, McCance E, Rounsavile BJ. Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction. 1998;93(5):713–27. doi: 10.1046/j.1360-0443.1998.9357137.x. [DOI] [PubMed] [Google Scholar]
- 12.Carroll KM, Nich C, Ball SA, McCance E, Frankforter TL, Rounsaville BJ. One-year follow-up of disulfiram and psychotherapy for cocaine-alcohol users: sustained effects of treatment. Addiction. 2000;95(9):1335–49. doi: 10.1046/j.1360-0443.2000.95913355.x. [DOI] [PubMed] [Google Scholar]
- 13.Petrakis IL, Poling J, Levinson C, Nich C, Carroll K, Rounsaville B. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biol Psychiatry. 2005;57(10):1128–37. doi: 10.1016/j.biopsych.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Jorgensen CH, Pedersen B, Tonnesen H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcohol Clin Exp Res. 2011;35(10):1749–58. doi: 10.1111/j.1530-0277.2011.01523.x. [DOI] [PubMed] [Google Scholar]
- 15.Skinner MD, Lahmek P, Pham H, Aubin HJ. Disulfiram efficacy in the treatment of alcohol dependence: a meta-analysis. PLoS One. 2014;9(2):e87366. doi: 10.1371/journal.pone.0087366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snyder S, Keeler M. Acute effects of disulfiram on anxiety levels of chronic alcoholics. Int Pharmacopsychiatry. 1981;16(1):49–56. doi: 10.1159/000468474. [DOI] [PubMed] [Google Scholar]
- 17.Goyer PF, Brown GL, Minichiello MD, Major LF. Mood-altering effects of disulfiram in alcoholics. J Stud Alcohol. 1984;45(3):209–13. doi: 10.15288/jsa.1984.45.209. [DOI] [PubMed] [Google Scholar]
- 18.Smith JP, Book SW. Comorbidity of generalized anxiety disorder and alcohol use disorders among individuals seeking outpatient substance abuse treatment. Addict Behav. 2010;35(1):42–5. doi: 10.1016/j.addbeh.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakken K, Landheim AS, Vaglum P. Substance-dependent patients with and without social anxiety disorder: occurrence and clinical differences. A study of a consecutive sample of alcohol-dependent and poly-substance-dependent patients treated in two counties in Norway. Drug Alcohol Depend. 2005;80(3):321–8. doi: 10.1016/j.drugalcdep.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61(8):807–16. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- 21.Schuckit MA, Tipp JE, Bucholz KK, Nurnberger JI, Jr, Hesselbrock VM, Crowe RR, et al. The life-time rates of three major mood disorders and four major anxiety disorders in alcoholics and controls. Addiction. 1997;92(10):1289–304. [PubMed] [Google Scholar]
- 22.Merikangas KR, Stevens DE, Fenton B, Stolar M, O’Malley S, Woods SW, et al. Co-morbidity and familial aggregation of alcoholism and anxiety disorders. Psychol Med. 1998;28(4):773–88. doi: 10.1017/s0033291798006941. [DOI] [PubMed] [Google Scholar]
- 23.Swendsen JD, Merikangas KR, Canino GJ, Kessler RC, Rubio-Stipec M, Angst J. The comorbidity of alcoholism with anxiety and depressive disorders in four geographic communities. Compr Psychiatry. 1998;39(4):176–84. doi: 10.1016/s0010-440x(98)90058-x. [DOI] [PubMed] [Google Scholar]
- 24.Trevisan LA, Boutros N, Petrakis IL, Krystal JH. Complications of alcohol withdrawal: pathophysiological insights. Alcohol health and research world. 1998;22(1):61–6. [PMC free article] [PubMed] [Google Scholar]
- 25.Bakhla AK, Khess CR, Verma V, Hembram M, Praharaj SK, Soren S. Factor Structure of CIWA-Ar in Alcohol Withdrawal. Journal of addiction. 2014;2014:745839. doi: 10.1155/2014/745839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Driessen M, Meier S, Hill A, Wetterling T, Lange W, Junghanns K. The course of anxiety, depression and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol Alcohol. 2001;36(3):249–55. doi: 10.1093/alcalc/36.3.249. [DOI] [PubMed] [Google Scholar]
- 27.Kushner MG, Abrams K, Thuras P, Hanson KL, Brekke M, Sletten S. Follow-up study of anxiety disorder and alcohol dependence in comorbid alcoholism treatment patients. Alcohol Clin Exp Res. 2005;29(8):1432–43. doi: 10.1097/01.alc.0000175072.17623.f8. [DOI] [PubMed] [Google Scholar]
- 28.Wolitzky-Taylor K, Operskalski JT, Ries R, Craske MG, Roy-Byrne P. Understanding and treating comorbid anxiety disorders in substance users: review and future directions. J Addict Med. 2011;5(4):233–47. doi: 10.1097/ADM.0b013e31823276d7. [DOI] [PubMed] [Google Scholar]
- 29.Mayo-Smith MF. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. Jama. 1997;278(2):144–51. doi: 10.1001/jama.278.2.144. [DOI] [PubMed] [Google Scholar]
- 30.Posternak MA, Mueller TI. Assessing the risks and benefits of benzodiazepines for anxiety disorders in patients with a history of substance abuse or dependence. Am J Addict. 2001;10(1):48–68. doi: 10.1080/105504901750160484. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg CM. Drug maintenance in the outpatient treatment of chronic alcoholism. Arch Gen Psychiatry. 1974;30(3):373–7. doi: 10.1001/archpsyc.1974.01760090079013. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien CP. Benzodiazepine use, abuse, and dependence. J Clin Psychiatry. 2005;66(Suppl 2):28–33. [PubMed] [Google Scholar]
- 33.Ciraulo DA, Barnhill JG, Ciraulo AM, Sarid-Segal O, Knapp C, Greenblatt DJ, et al. Alterations in pharmacodynamics of anxiolytics in abstinent alcoholic men: subjective responses, abuse liability, and electroencephalographic effects of alprazolam, diazepam, and buspirone. J Clin Pharmacol. 1997;37(1):64–73. doi: 10.1177/009127009703700111. [DOI] [PubMed] [Google Scholar]
- 34.Ciraulo DA, Barnhill JG, Ciraulo AM, Greenblatt DJ, Shader RI. Parental alcoholism as a risk factor in benzodiazepine abuse: a pilot study. Am J Psychiatry. 1989;146(10):1333–5. doi: 10.1176/ajp.146.10.1333. [DOI] [PubMed] [Google Scholar]
- 35.Busto UE, Romach MK, Sellers EM. Multiple drug use and psychiatric comorbidity in patients admitted to the hospital with severe benzodiazepine dependence. J Clin Psychopharmacol. 1996;16(1):51–7. doi: 10.1097/00004714-199602000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Malcolm R, Brady KT, Johnston AL, Cunningham M. Types of benzodiazepines abused by chemically dependent inpatients. J Psychoactive Drugs. 1993;25(4):315–9. doi: 10.1080/02791072.1993.10472289. [DOI] [PubMed] [Google Scholar]
- 37.Ross HE. Benzodiazepine use and anxiolytic abuse and dependence in treated alcoholics. Addiction. 1993;88:209–18. doi: 10.1111/j.1360-0443.1993.tb00804.x. [DOI] [PubMed] [Google Scholar]
- 38.Koski A, Ojanpera I, Vuori E. Alcohol and benzodiazepines in fatal poisonings. Alcohol Clin Exp Res. 2002;26(7):956–9. doi: 10.1097/01.ALC.0000021337.78063.67. [DOI] [PubMed] [Google Scholar]
- 39.Ogbu UC, Lotfipour S, Chakravarthy B. Polysubstance abuse: alcohol, opioids and benzodiazepines require coordinated engagement by society, patients, and physicians. The western journal of emergency medicine. 2015;16(1):76–9. doi: 10.5811/westjem.2014.11.24720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mueller TI, Goldenberg IM, Gordon AL, Keller MB, Warshaw MG. Benzodiazepine use in anxiety disordered patients with and without a history of alcoholism. J Clin Psychiatry. 1996;57(2):83–9. [PubMed] [Google Scholar]
- 41.Mueller TI, Pagano ME, Rodriguez BF, Bruce SE, Stout RL, Keller MB. Long-term use of benzodiazepines in participants with comorbid anxiety and alcohol use disorders. Alcohol Clin Exp Res. 2005;29(8):1411–8. doi: 10.1097/01.alc.0000175016.01790.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev. 2003;7(6):523–39. doi: 10.1016/s1087-0792(03)90005-0. [DOI] [PubMed] [Google Scholar]
- 43.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, Version 2.0) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 44.Stuppaeck CH, Pycha R, Miller C, Whitworth AB, Oberbauer H, Fleischhacker WW. Carbamazepine versus oxazepam in the treatment of alcohol withdrawal: a double-blind study. Alcohol Alcohol. 1992;27(2):153–8. [PubMed] [Google Scholar]
- 45.Malcolm R, Ballenger JC, Sturgis ET, Anton R. Double-blind controlled trial comparing carbamazepine to oxazepam treatment of alcohol withdrawal. Am J Psychiatry. 1989;146(5):617–21. doi: 10.1176/ajp.146.5.617. [DOI] [PubMed] [Google Scholar]
- 46.Pettinati HM, Weiss RD, Miller WR, Donovan DM, Ernst DB, Rounsaville BJ. Medical Management (MM) treatment manual: A clinical research guide for medically trained clinicians providing pharmacotherapy as part of the treatment for alcohol dependence. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2004. [Google Scholar]
- 47.Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. British journal of addiction. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 48.Miller WR, Tonigan JS, Longabaugh R. Test Manual. Vol. 4. Rockville, MD: US Government Printing Office; 1995. The Drinker Inventory of Consequences (DrInC): An instrument for assessing adverse consequences of alcohol abuse. [Google Scholar]
- 49.Flannery BA, Volpicelli JR, Pettinati HM. Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res. 1999;23(8):1289–95. [PubMed] [Google Scholar]
- 50.Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: The revised Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-Ar) British journal of addiction. 1989;84:1353–7. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 51.Hamilton M. The assessment of anxiety status by rating. British Journal of Medical Psychology. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 52.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheehan DV, Lecrubier Y, Janavs J, Knapp E, Weiller E, Bonora LI, et al. Mini International Neuropsychiatric Interview (MINI) Tampa, Florida and Paris, France: University of South Florida Institute for Research in Psychiatry, and INSERM-Hôpital de la Salpêtrière; 1994. [Google Scholar]
- 54.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 4–57. [PubMed] [Google Scholar]
- 55.Johnson BA, Ait-Daoud N, Roache JD. The COMBINE SAFTEE: a structured instrument for collecting adverse events adapted for clinical studies in the alcoholism field. J Stud Alcohol Suppl. 2005;15:157–67. doi: 10.15288/jsas.2005.s15.157. discussion 40. [DOI] [PubMed] [Google Scholar]
- 56.Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. San Diego: Academic Press; 1985. [Google Scholar]