Abstract
Rationale
Despite direct immediate intervention and therapy, ST segment Elevation Myocardial Infarction (STEMI) victims remain at risk for infarct expansion, heart failure, re-infarction, repeat revascularization and death.
Objective
To evaluate the safety and bioactivity of autologous CD34+ cell (CLBS10) intracoronary infusion in patients with left ventricular dysfunction (LVD) post-STEMI.
Methods and Results
Patients who underwent successful stenting for STEMI and had LVD (ejection fraction [EF] ≤48%) ≥4 days post-stent were eligible for enrollment. Subjects (N=161) underwent mini bone marrow harvest and were randomized 1:1 to receive (A) autologous CD34+ cells (minimum 10M±20% cells; N=78) or (B) diluent alone (N=83), via intracoronary infusion. The primary safety endpoint was adverse events (AEs), serious AEs (SAEs) and major adverse cardiac event (MACE). The primary efficacy endpoint was change in resting myocardial perfusion over 6 months. No differences in myocardial perfusion or adverse events were observed between the control and treatment groups, although increased perfusion was observed within each group from baseline to 6 months (p<0.001). In secondary analyses, when adjusted for time of ischemia, a consistently favorable cell dose dependent effect was observed in the change in LVEF and infarct size, and the duration of time subjects were alive and out of hospital (p=0.05). At one year, 3.6% (N=3) and 0% deaths were observed in the control and treatment group, respectively.
Conclusions
This Phase 2, randomized, double-blind, placebo-controlled trial (PreSERVE-AMI) represents the largest study of cell-based therapy for STEMI completed in US and provides evidence supporting safety and potential efficacy in patients with LVD post STEMI who are at risk for death and major morbidity.
Keywords: CD34+ cells, angiogenesis, ST-segment elevation myocardial infarction, cell transplantation, clinical trial, stem cell, endothelial progenitor cells, myocardial ischemia
Subject Terms: Clinical Studies, Cell Therapy, Myocardial Infarction, Vascular Biology
INTRODUCTION
Approximately 1 in 5 patients beyond the age of 45 years who experience acute myocardial infarction (AMI) will be dead within a year. While the incidence of STEMI has declined and survival following STEMI has improved, the prognosis remains poor for those with residual LVD after STEMI.1, 2 AMI causes myocardial necrosis and apoptosis resulting in ventricular remodeling, which is a precursor to subsequent cardiac dysfunction, congestive heart failure and other cardiac adverse events including death.3, 4 The extent of myocardial cell loss is dependent on the duration and location of the coronary artery occlusion, existing collateral coronary circulation and the integrity of the cardiac microvasculature.5–8 Evidence indicates that damage and ongoing attrition of the cardiac microcirculation is a harbinger of worse outcome, and that recovery of microcirculatory function leads to improved LV function and clinical outcomes.9
Mobilization of CD34+ cells from the bone marrow occurs naturally following MI.10 Importantly, high levels of circulating CD34+ cells have been associated with improved outcomes in patients, while poor CD34+ mobilizers have a worse prognosis.11, 12 CD34+ cells are capable of differentiating into endothelial cells and also secrete a variety of paracrine factors that promote neovascularization.13–16 Following an extensive MI, the natural repair mechanism of mobilization and recruitment of CD34+ cells may be insufficient to prevent adverse remodeling. Consequently, new strategies are needed to limit or prevent cardiac dysfunction after AMI and to alter the natural history of the disease in patients with inadequate innate repair mechanisms.17, 18
Over 2600 patients have received intracoronary infusion of autologous bone marrow derived mononuclear cells (BMMNC) post MI.19 While some studies of bone marrow cell therapy post-STEMI have shown significant improvements in cardiac function and reduction in MACE, 20, 21 others have not,22 although the safety of this approach has been established. A meta-analysis of 50 BMMNC studies showed significant improvements in clinical outcomes including increase in ejection fraction (mean 4%) and reductions in infarct size, incidence of death, and recurrent MI.23
The inconsistency of results from BMMNC infusion studies may reflect the fact that not all bone marrow cells contribute to tissue repair 24, 25 Available evidence suggests that specific mononuclear cell subpopulations are integral to driving ischemic tissue repair.26 Most evidence supports CD34+ cells as a critical factor in mediating repair, and pre-clinical data shows that selected CD34+ cells provide superior outcomes when compared to unselected BMMNC, even at equivalent CD34+ cell doses.27
A Phase 1, prospective, multi-center, dose escalating cohort-controlled trial of intracoronary administration of bone marrow derived autologous CD34+ cells in STEMI patients provided preliminary evidence of feasibility and safety and suggested that patients receiving ≥ 10 million CD34+ cells had significant improvement in myocardial perfusion and preservation of LV function at 6 months follow-up.28 In the present Phase 2, randomized, double-blind, placebo-controlled study (PreSERVE-AMI), we aimed to further evaluate the safety and bioactivity of autologous CD34+ cell (CLBS10) intracoronary infusion in patients with residual LVD after STEMI.
METHODS
Study population and design
PreSERVE-AMI is a Phase 2, randomized, double-blind, placebo-controlled trial performed at 60 sites in the US [ClinicalTrials.gov identifier: NCT01495364]. From January 2012 to December 2013, patients with LVD (EF≤48% by CMR imaging) ≥4 days after coronary artery stenting were enrolled after providing institutional review board–approved informed written consent. Time from symptom onset to patient receiving coronary artery stent is referred to as total ischemic time. Enrolled subjects underwent mini bone marrow harvest and were randomized 1:1 to receive either autologous CD34+ cells (minimum dose of 10 million (±20%) CD34+ cells in autologous serum) or autologous serum by intracoronary infusion.
The primary safety endpoint was occurrence of AEs, SAEs and MACE (cardiovascular mortality, heart failure, re-infarction, revascularization). The primary efficacy endpoint was change in resting myocardial perfusion over 6 months (gated SPECT). The initial study design included a primary safety endpoint that was restricted to evaluating the safety of bone marrow harvest and infusion, however this was modified during the course of the study to reflect the goal of assessing overall safety. Secondary endpoints including changes in LVEF, end systolic volume (LVESV), end diastolic volume (LVEDV) and infarct size (by CMR) (supplemental materials).
Bone marrow harvest and cell selection
Bone marrow aspiration was performed in all subjects randomized to either CD34+ cells or placebo between 4 and 9 days after stent implantation using conscious sedation and local anesthesia (supplemental materials). CD34+ cells were selected from the harvested cells using the CliniMACS system. CD34+ cell enumeration, purity, and viability were assayed by flow cytometry (Stem-Kit reagents; Beckman Coulter, Brea, CA). Endotoxin levels were determined using Limulus test kits (Lonza, Allendale, NJ). The CD34+ cell product was suspended in 10 mL phosphate buffered saline supplemented with autologous serum and human serum albumin (HSA). All treatment subjects received a minimum dose of ≥ 10 million (±20%) CD34+ cells as defined in release criteria. The cell dose in each subject was the total dose of CD34+ cells produced from their bone marrow aspirate. Control subjects received 10 mL phosphate buffered saline supplemented with autologous serum and HSA without cells.
Cell infusion
Subjects were infused within 72 h of completion of the bone marrow harvest and within 11 days following stent placement. CD34+ cells were infused via an over-the-wire balloon catheter positioned within the stented segment of the infarct related artery using a stop-flow technique described previously28 (supplemental materials).
Imaging
CMR was performed to assess baseline LVEF if a screening echocardiogram LVEF (or equivalent) performed at least 2 days after percutaneous coronary intervention (PCI) revealed an LVEF ≤ 45% (or ≤ 35% if assessed prior to Day 2). The CMR LVEF was performed no sooner than 96 h (4 days) after stenting and had to be ≤ 48% to be eligible for randomization. For subjects who could not undergo CMR (e.g. implanted device), the LVEF was measured with SPECT, and had to be ≤ 45%. Follow-up was also performed with SPECT in these subjects. At 6 months follow-up, LVEF, LVESV, LVEDV), infarct size were assessed by CMR (SPECT substituted in subjects who could not undergo CMR).
A gated rest SPECT MPI scan was used to evaluate perfusion using the resting total severity score (RTSS). Approximately 25% of paired scans (CMR and SPECT) were re-read by the reader to assess reproducibility. In addition both the SPECT and CMR studies were re-read by independent core labs. While there was disparity between the core lab readings on the individual subject level, the overall conclusions from the two core labs were similar. Accordingly the original core lab results are reported here.
Safety monitoring
Safety was assessed by monitoring of AEs, SAEs, MACE, temperature log, physical examinations with vital signs and weight, cardiopulmonary examination, 12-lead electrocardiograms (ECGs), 24h Holter ECG monitoring (after the infusion was completed) and laboratory safety assessments (biochemistry, hematology, coagulations status, cardiac markers). An AE was defined as any untoward medical occurrence in a subject and could include any unfavorable and unintended sign (including an abnormal laboratory finding), symptom, or disease (new or exacerbated). A Clinical Endpoint Committee (CEC), blinded to randomized treatment assignments, was appointed to independently adjudicate investigator-reported MACE and other events of interest (supplemental materials). The study was monitored by an independent Data Safety Monitoring Board.
Statistical design analysis
Using a two-sample t-test at a two-sided 0.05 level of significance, a sample size of 80 subjects in each group was determined to yield 85% power to reject the hypothesis that there is no difference between the two groups in the change in RTSS, after allowance for a 5% drop out. During the course of the study the Contract Research Organization was instructed to perform an unblinded analysis to determine if sample size would be sufficient to distinguish a change in LVEF between treatment and control groups. They reported that the sample size would be insufficient and in fact that the change in LVEF favored the control group at that point. Data is expressed as mean +/− standard deviation and confidence intervals are two-sided and at the 95% confidence level, unless otherwise stated. The primary efficacy analyses for mean change in perfusion (RTSS) from baseline to 6 months between the treatment and control groups, and for secondary endpoints of changes in LVEF were performed using the analysis of covariance and the baseline values as covariates. The primary safety comparison was for differences in the rates of AE, SAE, and MACE between groups for the composite and individual MACE using a chi-square test and two-sample z-test, as appropriate. Pre-specified tertiary analyses to assess the influence of multiple parameters, including cell dose and total ischemic time, on efficacy and safety endpoints were performed using multiple regression models and analysis of covariance. Responses in cell dose subgroups of <14 million cells/kg, > 14 million cells/kg and > 20 million cells/kg were assessed in a post-hoc analysis which evaluated the ratio of MACE. Analyses on the intention to treat (ITT, defined as all randomized subjects) or modified intention to treat (mITT, defined as all randomized subjects who underwent bone marrow harvest and were infused with study product) are reported.
RESULTS
Subject disposition and baseline characteristics
Of the 281 subjects consented, 86 failed screening and the remaining 195 were randomized to either the CD34+ cell therapy group (n=100) or the placebo group (n=95) (Figure 1). Of those randomized, 161 subjects (78 in the treated and 83 in the control group) completed the bone marrow harvest procedure and underwent infusion. All surviving subjects have completed the one year follow-up visit (median follow-up time, 18 months at the time of the analysis for this manuscript).
Figure 1. PRESERVE-AMI study flow diagram.
†There were no deaths in the mITT treatment group
*There were 3 deaths in the mITT placebo group
¥Other reasons were: AIDS, low hemoglobin values, hypotension requiring medication, re-occlusion of the infarct-related artery, pulmonary nodules, apical thrombus, CMR not being performed and subject lost prior to treatment.
Baseline characteristics of treated subjects were similar between the cell therapy and placebo groups in terms of gender, race, cardiovascular risk factors and medical history. Index primary PCI parameters were similar with the exception of total ischemic time of the infarct related artery, which was significantly longer in the CD34+ cell group compared with the control group (p=0.04; Table 1). When divided into groups of CD34+ cell dose administered, no differences in baseline characteristics were observed across groups (Supplement Table I).
Table 1.
Baseline characteristics of subjects randomized and treated
| Control (N=83) |
Treated - CLBS10 (N=78) |
P-value* | |
|---|---|---|---|
| Demographics | |||
| Age; mean ± SD | 56.4 ± 10.1 | 57.1 ± 10.1 | 0.65 |
| Female; n (%) | 17 (20%) | 12 (15%) | 0.4 |
| Race; White, n (%) | 62 (75%) | 56 (72%) | 0.87 |
| CV Risk Factors | |||
| Hypertension (%) | 56 (67%) | 53 (68%) | 0.80 |
| Diabetes (%) | 19 (23%) | 27 (35%) | 0.1 |
| Hyperlipidemia (%) | 17 (20%) | 13 (17%) | 0.82 |
| NYHA Class*; mean ± SD | 1.9 ± 0.7 | 1.8 ± 0.6 | 0.59 |
| CV Medical History; n (%) | |||
| Prior CABG | 2 (2%) | 2 (3%) | 0.95 |
| Prior PCI | 15 (18%) | 15 (19%) | 0.85 |
| Prior CHF | 11 (13%) | 11 (14%) | 0.88 |
| Prior MI | 15 (18%) | 13 (17%) | 0.34 |
| Index AMI/PCI; mean ± SD | |||
| Infarct size (grams) | 38.6 ± 19.5 | 33.8 ± 17.4 | 0.16 |
| Pre-discharge LVEF (%) | 34.1 ± 8.4 | 34.3 ± 7.3 | 0.90 |
| LVEDV index | 91.9 ± 20.8 | 98.0 ± 25.6 | 0.12 |
| LVESV index | 58.5 ± 19.9 | 61.2 ± 23.6 | 0.46 |
| Total Ischemic Time (min) | 569 ± 864 | 931 ± 1277 | 0.04 |
| Time from stent to infusion (days) | 9.4 ± 1.43 | 9.3 ± 1.23 | 0.60 |
Abbreviations: AMI: acute myocardial infarction; CABG: coronary artery bypass graft; CV: cardiovascular; CHF: congestive heart failure; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end-systolic volume; NYHA: New York Heart Association; PCI: percutaneous coronary intervention
P-values for quantitative characteristics are based on a t-test. P-values for categorical characteristics are based on a Chi-square test.
Bone marrow harvest and coronary infusion procedure
Bone marrow harvest (mean volume 388 ± 18 mL) yielded total mean viable CD34+ cell count of 41.3 ± 21.5 million and 40.2 ± 25.9 million in the control and treatment groups, respectively (Supplement Table II). The mean CD34+ cell count in the final cell product (CLBS10) was 14.9 ± 8 million cells (range 8 to 43.8 million cells). The mini bone marrow harvest procedure and infusion were well-tolerated, with 5% and 9% of subjects, respectively, experiencing SAEs not considered related to the procedures (Supplement Table III).
SPECT perfusion imaging
The mean RTSS score, the primary endpoint of the study improved between baseline and 6 months in both the control (−149.6 ± 221.16, P=0.01) and cell therapy (−142.7 ± 257.8, P=0.014, Figure 2A) groups, however, this change was not significantly different between the groups. There was no relationship between cell dose and improvement in perfusion in further analyses.
Figure 2. Change in cardiac function and structure over time.
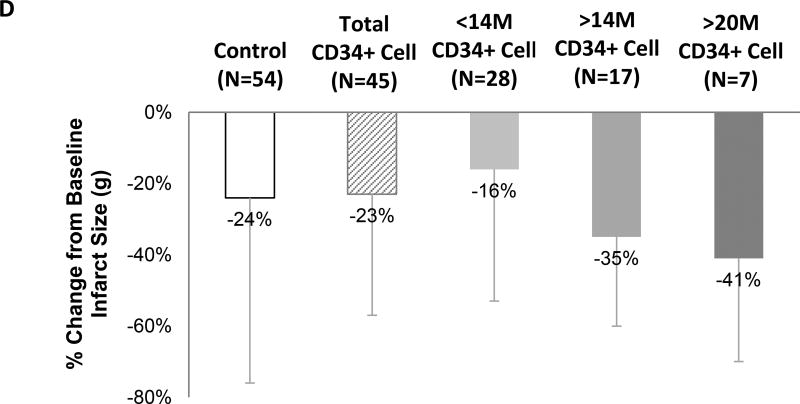
A) Change in RTSS score from baseline to 6 months for the control (P=0.010) and CLBS10 treated (P=0.014) group. B) Change in LVEF from baseline to 6 months for the control and CLBS10 treated group (both P<0.001). No significant difference in mean RTSS or LVEF between the control vs CLBS10 treated group observed. Thick lines represent mean change in RTSS or LVEF score. Thin lines represent individual subjects. C) The mean LVEF change from baseline (±SE) in pooled treatment and cell dose subgroups was compared to control using a t-test of the means. D) Mean percent change from baseline in infarct size measured by CMR. P=NS for all comparisons.
Post-infusion clinical events
Mortality and MACE
In the mITT population (all randomized subjects who underwent bone marrow harvest and were infused with study product), there were no deaths in the CD34+ cell group compared to 3 deaths in the control group (p=0.04, z-test) with trends in the probability of survival favoring the CD34+ cell group (p=0.055; Figure 3A). Deaths occurred at post-infusion day 3, 22, and 695 due to ventricular fibrillation, cardiogenic shock, and heart failure, respectively. In the ITT population (all randomized subjects), 3 deaths occurred in subjects randomized to the control group and 1 death occurred in a subject randomized to CD34+ cell treatment who did not undergo infusion (Supplement Figure I).
Figure 3. Mortality and MACE in the mITT population (patients that received and infusion of CD34 cells or control).
Kaplan-Meier plots of the probability of A) survival for the CLBS10 and control treated subjects. P-values reflect a log-rank test of treatment vs control. B) Percentage of subjects experiencing MACE during the post-infusion follow-up period (median follow-up: 12 months).
While the incidence of MACE was similar in the CD34+ cell and control groups (Supplement Table III), a regression analysis adjusting for total ischemic time showed a trend toward decreased MACE incidence as the CD34+ cell dose increased (p=0.06; Table 2). As a result, post-hoc analyses were performed to determine cell dose thresholds above and below which an impact on MACE was observed. A trend toward a reduced MACE incidence in the >14 and >20 million cell dose subgroups was observed, but was not statistically significant (Figure 3B).
Table 2.
Influence of cell dose on safety and efficacy endpoints
| Parameter of change from baseline to 6 months | CD34+ Cell Dose (106) Parameter Estimate (SE) |
p-value |
|---|---|---|
| MACE incidence | −0.111 (0.058) | 0.06 |
| Days Alive and Out of Hospital | 0.16 (0.08) | 0.05 |
| LVEF | 2.21 (1.08) | 0.05 |
| LVESV | −0.70 (0.53) | 0.19 |
| LVEDV | −0.53 (0.63) | 0.40 |
| % Change from Baseline in Infarct Size | −1.4 (0.59) | 0.02 |
Abbreviations: CHF: congestive heart failure; Hx = medical history; MI: myocardial infarction; LVEF: left ventricular ejection fraction
Data represents CMR with SPECT data imputed. Analyses performed using analysis of covariance after adjustment for total ischemic time.
Each 1 million increase in CD34+ cell dose led to a decrease in log (odds) of 0.111 in MACE. For all other parameters, a linear regression model was fitted. Each 1 million increase in CD34+ cell dose led to a change of 0.16, 2.21, −0.70, −0.53, and −1.4 for days alive and out of hospital, LVEF, LVESV, LVEDV, and % change from baseline in infarct size, respectively.
Multiple regression model
Safety
The incidence of AEs and SAEs at 12 month follow-up (18 month median follow-up) were similar in the cell therapy and control groups (Supplement Table III). When adjusted for total ischemic time, increasing CD34+ cell dose was associated with increased number of days alive and out of the hospital (p=0.05; Table 2).
Left ventricular function
A statistically significant increase in LVEF from baseline to 6 months was observed within the CD34+ cell group (P<0.001) and within the control group (P<0.001) (Figure 2B). While there was not a difference in LVEF change from baseline between the control group and CD34+ cell group as a whole, tertiary analyses demonstrated a significant association between the change in LVEF and cell dose after adjusting for total ischemic time (p=0.045; Table 2). Post-hoc analyses of cell dose subgroups showed that the LVEF change in those receiving CD34+ cell doses >20 million cells (10.2 ± 9.8%) was significantly greater compared to the control group (4.9% ± 8.8%) (P<0.05, Figure 2C). There did not appear to be an association between changes in LVESV and LVEDV and CD34+ cell dose (Table 2).
Infarct size
The mean LV infarct size decreased from baseline to 6 months in both the control (−24 ± 52%, P< 0.001) and the cell therapy (−23 ± 34%, P<0.001) groups, however, this change was not significantly different between the groups (Figure 2D). Tertiary analyses showed that CD34+ cell dose was associated with reduction in infarct size (p=0.02) after adjustment for total ischemic time (Table 2). Post-hoc analyses showed non-significant trends toward greater percent reductions in infarct size at the >14 million and >20 million cell doses (Figure 2D).
Relationship between bone marrow CD34+ cell content and clinical outcomes
Bone marrow CD34+ cell content was not associated with differences in baseline characteristics, except for the prevalence of diabetes being increased among patients with higher bone marrow CD34+ count (Supplement Table IV). Bone marrow CD34+ cell content did not correlate with the rates of MACE (p=0.2) or SAEs (p=0.4) (Tables 3 and 4).
Table 3.
Bone marrow CD34 count and MACE incidence at 6 months in the control group
| Bone Marrow CD34 Cell Counts (107) | ||||||
|---|---|---|---|---|---|---|
| MACE Category | Total (N=83) |
≤2.02 (N=22) |
>2.02 to ≤3.66 (N=20) |
>3.66 to ≤4.95 (N=21) |
>4.95 (N=20) |
P-value |
| Any MACE Event | 12 (14%) | 2 (9%) | 3 (15%) | 3 (14%) | 4 (20%) | 0.77 |
| Cardiac Death | 2 (2%) | 1 (5%) | 1 (5%) | 0 | 0 | 0.86 |
| Revascularization | 8 (10%) | 1 (5%) | 2 (10%) | 1 (5%) | 4 (20%) | 0.38 |
| HF Hospitalization | 4 (5%) | 1 (5%) | 0 | 2 (10%) | 1 (5%) | 0.79 |
| Re-infarction | 1 (1%) | 0 | 1 (5%) | 0 | 0 | 0.48 |
Abbreviations: HF: heart failure; MACE: major adverse cardiac events; MI: myocardial infarction; SAE: severe adverse events; SE: standard error
P-values are comparing the incidence of MACE events across all four cell groups are based on Chi-square test.
Table 4.
Influence of bone marrow CD34 cell counts on MACE and SAE in the control group
| Parameter of change | CD34+ Cell Count (108) Parameter Estimate (SE) |
p-value |
|---|---|---|
| Overall MACE* | 1.1 (0.9) | 0.2 |
| Cardiovascular death | −2.3 (3.4) | 0.5 |
| Recurrent MI | −2.7 (6.0) | 0.7 |
| Coronary revascularization | 1.6 (1.2) | 0.2 |
| HF hospitalization | −0.1 (1.7) | 0.9 |
| CV hospitalization | 0.8 (1.0) | 0.4 |
| SAE | 0.8 (0.9) | 0.4 |
Abbreviations: CV: cardiovascular; HF: heart failure; MACE: major adverse cardiac events; MI: myocardial infarction; SAE: severe adverse events; SE: standard error
Regression analysis on MACE (composite and individual components) as a function of bone marrow CD34 cell count
DISCUSSION
This Phase 2 study demonstrates that intracoronary infusion of CD34+ cells after STEMI is associated with favorable safety and clinical outcomes. Though the primary efficacy endpoint of improvement in resting myocardial perfusion over 6 months was not met, this study provides new insight into the influence of administered CD34+ cell dose on clinical safety and efficacy outcomes. When adjusted for ischemic time, there was a significant relationship between the CD34+ cell dose received and the change in infarct size, LVEF and days alive and out of hospital. The lack of mortality among active treatment subjects is also noteworthy. The low mortality rates observed in both the control (3.6%) and the CLBS10 treatment (0%) group may reflect both the clinical success of guideline driven STEMI treatment as well as the CD34 therapy protocol.
Infarct size reduction tended to be greater with higher cell doses, and patients treated with >20 million cells showed greater LVEF change. The infarct region perfusion improvement that was observed in the Phase 1 study for subjects treated with ≥ 10 million cells, was not observed in this Phase 2 study. Though perfusion was significantly improved from baseline to 6 months within both the control and CLBS10 treated group, no difference between the two groups was found. These results indicate that an imaging based endpoint such as SPECT myocardial perfusion may not be a suitable surrogate due to limitations in reproducibility of the technique (e.g. image acquisition and processing involving patient motion, filtering, collimation, alignment, and scaling).40 Although our findings confirm the earlier Phase 1 safety profiles, longer follow up is needed particularly with respect to cell dose threshold related outcomes.
Failure to promptly revascularize after STEMI increases myocardial necrosis and may result in ventricular dilatation and progressive congestive heart failure.29, 30 Despite the success of early thrombolysis and primary PCI in the management of STEMI, patients continue to be at risk for mortality and morbidity hazards due to persistent heart failure.31, 32 There is evidence that microvascular abnormalities in this population may contribute to late complications despite epicardial revascularization, but few therapeutic options have been available to date. In response to myocardial infarction, bone marrow derived CD34+ cells are believed to be recruited into the circulation, to home to ischemic tissues, and to participate in the repair and regeneration process. However, this mechanism is not sufficiently beneficial in patients who develop congestive heart failure (CHF) post MI.
Recent findings from the Cell-based Cardiac Studies (ACCRUE) show that unselected BMMNCs may be ineffective for treatment of MI without measurable clinical benefit or changes in LV function.22 The present study supports the concept that focused therapy with a specific “active agent” cell type, administered at a specific concentration, may be a more effective therapeutic strategy. Preclinical models have already established that compared to the unselected BMMNC population, CD34+ cells more efficiently incorporate into the ischemic myocardium and increase capillary density.27, 33 Clinical studies evaluating the therapeutic potential of cells selected for CD34 expression have also demonstrated a consistently favorable impact on outcomes.34–37 In each of the prior 5 randomized, placebo-controlled clinical trials of CD34 cell therapy, observations were made of superior safety and clinical benefit in the treated vs. control subjects.28, 34, 36, 38, 39 In this context, this current study is consistent with the evidence for CD34 cell therapy safety and efficacy and supports further exploration of the CD34 therapeutic strategy.
The results of this PreSERVE-AMI Phase 2 study provide support for the concept that autologous administration of bone marrow CD34+ cells is safe. Additionally, exploratory analysis indicates potential efficacy benefit at higher cell doses in patients with STEMI. Such hypothesis should be examined in future randomized controlled trials. Our findings mirror prior studies in refractory angina, critical limb ischemia (CLI), and heart failure. Additionally, our study offers insights into the CD34+ cell dose threshold that is required for meaningful clinical efficacy.
Supplementary Material
Novelty and Significance.
What Is Known?
Benefits of intracoronary administration of bone marrow mononuclear cells in patients after acute myocardial infarction (MI) remain uncertain.
We investigated whether high dose autologous bone marrow-derived CD34+ cells will improve outcomes post-MI.
What New Information Does This Article Contribute?
This Phase 2, randomized, double-blind, placebo-controlled trial (PreSERVE-AMI) showed that intracoronary autologous CD34+ cell therapy is safe after MI and may reduce adverse outcomes and improve function, especially in those receiving higher cell doses.
Autologous bone marrow mononuclear cell therapy has been used in patients with left ventricular dysfunction post acute MI. However, its beneficial effects remain uncertain. We conducted a Phase II randomized, blinded, placebo-controlled trial to investigate whether intra-coronary administration of high doses of autologous bone marrow CD34+ cells, known to be enriched for progenitor cells, will be safe and efficacious in patients with left ventricular dysfunction after an acute MI. We found that CD34+ cell therapy was safe. In secondary measures, mortality was lower and there was a dose-dependent increase in left ventricular function and reduction in infarct size in the treated group compared to placebo.
Acknowledgments
SOURCES OF FUNDING
Caladrius Biosciences, Inc.
DISCLOSURES
Dr. Arshed Quyyumi is a member of the Caladrius Biosciences Board. Caladrius Biosciences funded and conducted the study as the sponsor of this clinical trial.
Nonstandard Abbreviations and Acronyms
- AE
adverse events
- AMI
acute myocardial infarction
- BMMNC
bone marrow derived mononuclear cells
- CEC
Clinical Endpoint Committee
- CHF
congestive heart failure
- CMR
cardiac magnetic resonance
- CV
Cardiovascular
- ECG
Electrocardiogram
- EF
ejection fraction
- HSA
human serum albumin
- ITT
intent-to-treat
- LVD
left ventricular dysfunction
- LVEDV
left ventricular end diastolic volume
- LVEF
left ventricular ejection fraction
- LVESV
left ventricular end systolic volume
- MACE
major adverse cardiovascular endpoints
- MI
myocardial infarction
- mITT
modified intent-to-treat
- PCI
percutaneous coronary interventions
- RTSS
resting total severity score
- SAE
severe adverse events
- SDF-1
stromal cell-derived factor-1
- SPECT
single photon emission computed tomography
- STEMI
ST segment elevation myocardial infarction
Footnotes
ClinicalTrials.gov identifier: NCT01495364
References
- 1.Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738–3744. doi: 10.1161/CIRCULATIONAHA.105.561423. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB American Heart Association Statistics, C Stroke Statistics S. Heart disease and stroke statistics--2015 update: A report from the american heart association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 4.Pfeffer MA. Left ventricular remodeling after acute myocardial infarction. Annu Rev Med. 1995;46:455–466. doi: 10.1146/annurev.med.46.1.455. [DOI] [PubMed] [Google Scholar]
- 5.Hirai T, Fujita M, Nakajima H, Asanoi H, Yamanishi K, Ohno A, Sasayama S. Importance of collateral circulation for prevention of left ventricular aneurysm formation in acute myocardial infarction. Circulation. 1989;79:791–796. doi: 10.1161/01.cir.79.4.791. [DOI] [PubMed] [Google Scholar]
- 6.Braunwald E, Bristow MR. Congestive heart failure: Fifty years of progress. Circulation. 2000;102:14IV–23. doi: 10.1161/01.cir.102.suppl_4.iv-14. [DOI] [PubMed] [Google Scholar]
- 7.Sheiban I, Fragasso G, Rosano GM, Dharmadhikari A, Tzifos V, Pagnotta P, Chierchia SL, Trevi G. Time course and determinants of left ventricular function recovery after primary angioplasty in patients with acute myocardial infarction. J Am Coll Cardiol. 2001;38:464–471. doi: 10.1016/s0735-1097(01)01407-3. [DOI] [PubMed] [Google Scholar]
- 8.Ren G, Michael LH, Entman ML, Frangogiannis NG. Morphological characteristics of the microvasculature in healing myocardial infarcts. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2002;50:71–79. doi: 10.1177/002215540205000108. [DOI] [PubMed] [Google Scholar]
- 9.Niccoli G, Scalone G, Lerman A, Crea F. Coronary microvascular obstruction in acute myocardial infarction. Eur Heart J. 2015 doi: 10.1093/eurheartj/ehv484. [DOI] [PubMed] [Google Scholar]
- 10.Massa M, Rosti V, Ferrario M, Campanelli R, Ramajoli I, Rosso R, De Ferrari GM, Ferlini M, Goffredo L, Bertoletti A, Klersy C, Pecci A, Moratti R, Tavazzi L. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood. 2005;105:199–206. doi: 10.1182/blood-2004-05-1831. [DOI] [PubMed] [Google Scholar]
- 11.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 12.Patel RS, Li Q, Ghasemzadeh N, Eapen DJ, Moss LD, Janjua AU, Manocha P, Al Kassem H, Veledar E, Samady H, Taylor WR, Zafari AM, Sperling L, Vaccarino V, Waller EK, Quyyumi AA. Circulating cd34+ progenitor cells and risk of mortality in a population with coronary artery disease. Circ Res. 2015;116:289–297. doi: 10.1161/CIRCRESAHA.116.304187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science (New York, N.Y. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 14.Mackie AR, Losordo DW. Cd34-positive stem cells in the treatment of heart and vascular disease in human beings. Texas Heart Institute Journal. 2011;38:474–485. [PMC free article] [PubMed] [Google Scholar]
- 15.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of vegfr-2 and ac133 by circulating human cd34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 16.Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, Perlman H, Qin G, Kishore R, Losordo DW. Exosomes from human cd34+ stem cells mediate their proangiogenic paracrine activity. Circ Res. 2011;109:724–728. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van 't Hof A, Widimsky P, Zahger D. Esc guidelines for the management of acute myocardial infarction in patients presenting with st-segment elevation. Eur Heart J. 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 18.Gerber Y, Weston SA, Berardi C, McNallan SM, Jiang R, Redfield MM, Roger VL. Contemporary trends in heart failure with reduced and preserved ejection fraction after myocardial infarction: A community study. American journal of epidemiology. 2013;178:1272–1280. doi: 10.1093/aje/kwt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM. Autologous bone marrow stem cells to treat acute myocardial infarction: A systematic review. Eur Heart J. 2008;29:1807–1818. doi: 10.1093/eurheartj/ehn220. [DOI] [PubMed] [Google Scholar]
- 20.Huikuri HV, Kervinen K, Niemela M, Ylitalo K, Saily M, Koistinen P, Savolainen ER, Ukkonen H, Pietila M, Airaksinen JK, Knuuti J, Makikallio TH. Effects of intracoronary injection of mononuclear bone marrow cells on left ventricular function, arrhythmia risk profile, and restenosis after thrombolytic therapy of acute myocardial infarction. Eur Heart J. 2008;29:2723–2732. doi: 10.1093/eurheartj/ehn436. [DOI] [PubMed] [Google Scholar]
- 21.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 22.Gyongyosi M, Wojakowski W, Lemarchand P, Lunde K, Tendera M, Bartunek J, Marban E, Assmus B, Henry TD, Traverse JH, Moye L, Suerder D, Corti R, Huikuri HV, Miettinen JA, Woehrle J, Obradovic S, Roncalli J, Malliaras K, Pokushalov E, Romanov A, Kastrup J, Bergmann MW, Atsma D, Diederichsen AC, Edes I, Benedek I, Benedek T, Pejkov H, Nyolczas N, Pavo N, Bergler-Klein J, Pavo IJ, Sylven C, Berti S, Navarese EP, Maurer GM. Meta-analysis of cell-based cardiac studies (accrue) in patients with acute myocardial infarction based on individual patient data. Circ Res. 2015 doi: 10.1161/CIRCRESAHA.116.304346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: A systematic review and meta-analysis. Circulation. 2012;126:551–568. doi: 10.1161/CIRCULATIONAHA.111.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global burden of disease study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 25.Rosenzweig A. Cardiac cell therapy — mixed results from mixed cells. New England Journal of Medicine. 2006;355:1274–1277. doi: 10.1056/NEJMe068172. [DOI] [PubMed] [Google Scholar]
- 26.Li S, Zhou B, Han ZC. Therapeutic neovascularization by transplantation of mobilized peripheral blood mononuclear cells for limb ischemia. A comparison between cd34+ and cd34− mononuclear cells. Thrombosis and haemostasis. 2006;95:301–311. doi: 10.1160/TH05-06-0442. [DOI] [PubMed] [Google Scholar]
- 27.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, Kearney M, Zak V, Asahara T, Losordo DW. Cd34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 28.Quyyumi AA, Waller EK, Murrow J, Esteves F, Galt J, Oshinski J, Lerakis S, Sher S, Vaughan D, Perin E, Willerson J, Kereiakes D, Gersh BJ, Gregory D, Werner A, Moss T, Chan WS, Preti R, Pecora AL. Cd34+ cell infusion after st elevation myocardial infarction is associated with improved perfusion and is dose dependent. American heart journal. 2011;161:98–105. doi: 10.1016/j.ahj.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 29.Abbate A, Bussani R, Biondi-Zoccai GG, Santini D, Petrolini A, De Giorgio F, Vasaturo F, Scarpa S, Severino A, Liuzzo G, Leone AM, Baldi F, Sinagra G, Silvestri F, Vetrovec GW, Crea F, Biasucci LM, Baldi A. Infarct-related artery occlusion, tissue markers of ischaemia, and increased apoptosis in the peri-infarct viable myocardium. Eur Heart J. 2005;26:2039–2045. doi: 10.1093/eurheartj/ehi419. [DOI] [PubMed] [Google Scholar]
- 30.Angeja BG, Gunda M, Murphy SA, Sobel BE, Rundle AC, Syed M, Asfour A, Borzak S, Gourlay SG, Barron HV, Gibbons RJ, Gibson CM. Timi myocardial perfusion grade and st segment resolution: Association with infarct size as assessed by single photon emission computed tomography imaging. Circulation. 2002;105:282–285. doi: 10.1161/hc0302.103588. [DOI] [PubMed] [Google Scholar]
- 31.Brodie BR, Stuckey TD, Hansen CJ, Muncy DB, Weintraub RA, Kelly TA, Berry JJ. Timing and mechanism of death determined clinically after primary angioplasty for acute myocardial infarction. The American journal of cardiology. 1997;79:1586–1591. doi: 10.1016/s0002-9149(97)00203-8. [DOI] [PubMed] [Google Scholar]
- 32.Busk M, Maeng M, Kristensen SD, Thuesen L, Krusell LR, Mortensen LS, Steinmetz ER, Nielsen TT, Andersen HR. Timing, causes, and predictors of death after three years' follow-up in the danish multicenter randomized study of fibrinolysis versus primary angioplasty in acute myocardial infarction (danami-2) trial. The American journal of cardiology. 2009;104:210–215. doi: 10.1016/j.amjcard.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 33.Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, Ganser A, Knapp WH, Drexler H. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 34.Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, Socan A, Schrepfer S, Torre-Amione G, Haddad F, Wu JC. Effects of intracoronary cd34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circ Res. 2013;112:165–173. doi: 10.1161/CIRCRESAHA.112.276519. [DOI] [PubMed] [Google Scholar]
- 35.Lezaic L, Socan A, Poglajen G, Peitl PK, Sever M, Cukjati M, Cernelc P, Wu JC, Haddad F, Vrtovec B. Intracoronary transplantation of cd34(+) cells is associated with improved myocardial perfusion in patients with nonischemic dilated cardiomyopathy. Journal of cardiac failure. 2015;21:145–152. doi: 10.1016/j.cardfail.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Losordo DW, Henry TD, Davidson C, Lee JS, Costa MA, Bass T, Mendelsohn F, Fortuin FD, Pepine CJ, Traverse JH, Amrani D, Ewenstein BM, Riedel N, Story K, Barker K, Povsic TJ, Harrington RA, Schatz RA. Intramyocardial, autologous cd34+ cell therapy for refractory angina. Circ Res. 2011 doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poglajen G, Sever M, Cukjati M, Cernelc P, Knezevic I, Zemljic G, Haddad F, Wu JC, Vrtovec B. Effects of transendocardial cd34+ cell transplantation in patients with ischemic cardiomyopathy. Circulation. Cardiovascular interventions. 2014;7:552–559. doi: 10.1161/CIRCINTERVENTIONS.114.001436. [DOI] [PubMed] [Google Scholar]
- 38.Losordo DW, Kibbe MR, Mendelsohn F, Marston W, Driver VR, Sharafuddin M, Teodorescu V, Wiechmann BN, Thompson C, Kraiss L, Carman T, Dohad S, Huang P, Junge CE, Story K, Weistroffer T, Thorne TM, Millay M, Runyon JP, Schainfeld R. A randomized, controlled pilot study of autologous cd34+ cell therapy for critical limb ischemia. Circulation. Cardiovascular interventions. 2012;5:821–830. doi: 10.1161/CIRCINTERVENTIONS.112.968321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vrtovec B, Poglajen G, Sever M, Lezaic L, Domanovic D, Cernelc P, Haddad F, Torre-Amione G. Effects of intracoronary stem cell transplantation in patients with dilated cardiomyopathy. Journal of cardiac failure. 2011;17:272–281. doi: 10.1016/j.cardfail.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Sherwood M, Hage FG, Heo J, Shaw LJ, Cerqueira MD, Iskandrian AE. Spect myocardial perfusion imaging as an endpoint. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2012;19:891–894. doi: 10.1007/s12350-012-9583-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.