Abstract
Background
The purpose of this study was to investigate the effects of sinomenine (SIN) on CFA-induced inflammatory pain in rats, and to explore the underlying molecular mechanisms.
Material/Methods
To determine the potential influences of SIN in the pathogenesis of inflammatory pain, an inflammatory pain (IP) mouse model was established and rats were treated with SIN (30 mg/kg). Behavioral tests were used to assess the MWT and TWL of the rats. ELISA assay was used to detect the level of inflammation cytokines. Western blotting and qRT-PCR were carried out to measure the related protein and mRNA expression level, respectively.
Results
We found that the MWT and TWL of the CFA-treated rats were markedly lower than that of the control rats, and they were significantly increased by SIN administration. The results suggest that IP rats had higher levels of TNF-α, IL-1β and IL-6 compared with the control rats. SIN administration decreased the levels of TNF-α, IL-1β, and IL-6. In addition, we found that p-p65 and p-p38 expression notably decreased after SIN treatment in IP rats. Moreover, the results showed that SIN inhibited Cox-2 and PGE2 expression in IP rats.
Conclusions
The data indicate that SIN had a protective role in inflammatory pain through repressing inflammatory mediators via preventing the p38MAPK-NF-κB pathway.
MeSH Keywords: Inflammation Mediators, NF-kappa B, p38 Mitogen-Activated Protein Kinases, Pain, Sinomenium
Background
Pain is one of the most common symptoms in clinical practice, and inflammatory pain is the most important type [1]. Chronic inflammation caused by tissue damage and secondary inflammation caused by noxious stimuli can result in inflammatory pain. Inflammatory pain is characterized by hyperalgesia, evoked pain, and ongoing pain, and is recognized as the most common and difficult chronic pain to treat [2]. Inflammatory pain seriously affects quality of life [3,4]. At present, administration of conventional analgesics, such as nonsteroidal anti-inflammatory drugs (NSAIDs), is the main method for inflammatory pain treatment. However, the treatment effect is unsatisfactory and long-term uses of this agent can result in serious adverse effects [5, 6]. Clinical treatment of inflammatory pain remains a huge problem [7]. Thus, it is urgent for researchers to seek novel and effective strategies for the treatment of inflammatory pain.
Sinomenine (7,8-didehydro-4-hydroxy3,7-dimethoxy-17-methyl-9α, 13α, 14α-morphinan-6-one; [SIN]) is extracted from the Chinese medicinal herb Sinomenium acutum and is widely used in the treatment of mesangial proliferative nephritis and rheumatoid arthritis in China [8,9]. SIN has a variety of pharmacological properties, such as anti-cancer, cytoprotection, immunosuppression, and anti-inflammation effects [10–14]. Nevertheless, there is still no published study investigating the effects of SIN on the development of inflammatory pain and exploring the potential underlying molecular mechanisms.
Therefore, in this study, we aimed to study the effects of SIN administration on CFA-induced inflammatory pain in rats, and to explore the underlying molecular mechanisms.
Material and Methods
Materials
SIN was obtained from Sigma-Aldrich (St. Louis, MO). ELISA kits for TNF-α, IL-1β, IL-6, and PGE2 detection were obtained from Cell Signaling Technology, Inc. (Danvers, MA). The primary antibodies (anti-p65, anti-p-p65, anti-p38, anti-p-p38, anti-Cox-2, and anti-β-actin) and the secondary antibodies were supplied by Cell Signaling Technology, Inc. (Danvers, MA). All other chemicals and reagents were supplied by Sangon Biotect (Shanghai) Co., Ltd. (Shanghai, China).
Inflammatory pain (IP) model establishment and drug treatment
Healthy young male SD rats (150–200 g, 4–6 week) were supplied by the Vital River Company (Beijing, China). All rats were fed under standard conditions [15]. All the rats ate and drank freely. The present study was approved by the Ethics Committee of the First People’s Hospital of Changzhou and the Third Affiliated Hospital. All experiments were carried out following with National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Rats were randomly divided into 4 groups (n=10 per group): (1) CFA group: 100 μl CFA was used to induced inflammatory pain, and inflammatory pain model was conducted as previously described [15]; (2) saline group: 100 μl saline was injected into the right hind paw of the rats of the control group; (3) saline+SIN group: rats in this group were firstly intraperitoneally injected with 30 mg/kg SIN, and 1 h later, the rats were injected with 100 μl saline. (4) CFA+SIN group: rats in this group were firstly intraperitoneally injected with 30 mg/kg SIN, and 1 h later the rats were injected subcutaneously with 100 μl CFA. Intraperitoneal injection of SIN was performed 2 times every day for 9 days. Intraperitoneal injection of saline was performed in the rats of CFA and saline groups every 12 h for 9 days. SIN was dissolved in saline containing 1% dimethylsulfoxide (DMSO) just before injection. Rats were deeply anesthetized by intraperitoneal injection with pentobarbital (60 mg/kg) 24 h after CFA injection. At the end of the experiment, the L4-L6 dorsal spinal cords were collected for subsequent analysis.
Behavioral tests [15]
The rats underwent behavioral tests at 0 h, 6 h, 1 d, 3 d, 5 d, 7 d, and 9 d after CFA injection. Rats were placed in the wire mesh cages and adapted for 15–20 min, then a series of von Frey filaments (Stoelting, Kiel, WI, USA) were used to test the mechanical hyperalgesia of rats in different groups. Gradually-increasing pressures were applied to the hind paws of the rats. Each filament was applied 10 times for 5–6 s each time. The mechanical withdrawal thresholds (MWTs) were considered as the minimal force that initiated paw withdrawal. To perform the thermal preference testing, a radiant heater (BME-410A, Beijing, China) was used to test the thermal preference of rats in different groups, as previously described [15]. In brief, the radiant heat was placed at the bottom of the plantar surface of the rat hind paw. Every hind paw was tested 3 times, for 3 min each time. When the hind paw of a rat was removed from the heat source every 40 s, the thermal withdrawal latencies (TWLs) were recorded. In addition, the cold allodynia of the rats was tested by use of acetone, as previously described [15].
ELISA assay
The levels of tumor necrosis factor-α (TNF-α), interlukin-1β (IL-1β), interlukin-6 (IL-6), and PGE2 in rats of different groups were measured by ELISA assay according to the manufacturer’s instructions of each kit. Each experiment was repeated at least 3 times.
Quantitative real-time (qRT-PCR)
Total RNA from the tissues was extracted using RNAiso Plus (Takara Bio, Dalian, China) according to the manufacturer’s instructions. cDNAs were generated by performing the reverse transcription experiment with the ThermoScript RT-PCR system (Invitrogen, Grand Island, NY, USA). qPCR was performed to analyze the synthesized cDNAs by using the 2X Maxima SYBR Green/ROX qPCR Master Mix kit. The amplification conditions of qPCR were as follows: 95°C for 5 min, 40 cycles at 95°C for 30 s, 55°C for 30 s, and 72°C for 30s, then 72°C for 10 min. β-actin was used as an internal control. The 2−ΔΔCq method [16] was used to analyze the relative gene expression. Experiments were repeated at least 3 times.
Western blotting
Total proteins from tissues were collected using lysis buffer. The BCA Protein Assay Kit (Thermo Scientific, Pierce Protein Research Products, Rockford, USA) was applied to detect the protein concentration. Equal amounts of protein samples were resolved by 12% SDS-PAGE and then transferred onto polyvinylidene fluoride (PVDF) membranes. Subsequently, the membranes were firstly blocked with 5% non-fat milk for 2 h, and then incubated overnight at 4°C with the primary antibodies, followed by incubating with horseradish peroxidase (HRP)-conjugated secondary antibodies (Cell Signaling) for 2 h. Finally, the chemiluminescent ECL reagent (Millipore, MA, USA) was used to visualize the protein bands. NIH Image J software (NIH Image, Bethesda, MD, USA) was used for data analysis.
Statistical analysis
Data are displayed as mean ±SD. The t test was used to assess the statistical significance. All statistical analyses were performed using SPSS18.0 (SPSS Inc., Chicago, Illinois, USA). p<0.05 indicated significant differences.
Results
SIN alleviated CFA-induced inflammatory pain in rats
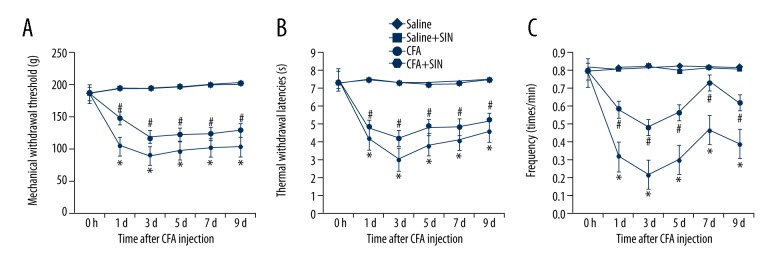
To study the effects of SIN on inflammatory pain, we used behavioral tests (the reaction of the hind paw on the mechanical, thermal, and cold stimuli). The results suggest that CFA significantly decreased the MWTs, TWLs, and frequency responses to cold stimulation, and SIN treatment notably eliminated these decreases at 1 d, 3 d, 5 d, 7 d, and 9 d after CFA injection (Figure 1A–1C). These results indicate that SIN can alleviate CFA-induced inflammatory pain.
Figure 1.
SIN alleviates inflammatory pain induced by CFA. (A) Mechanical withdrawal threshold after injection; (B) Thermal withdrawal latencies after injection; (C) Frequency responses to cold stimulation after injection. Saline: rats were injected with 100 μl saline only; Saline+SIN: rats were injected with 30 mg/kg SIN and 100 μl saline; CFA: rats were injected with 100 μl CFA only; CFA+SIN: rats were injected with 30 mg/kg SIN and 100 μl CFA. * p<0.05 vs. Saline group; # <0.05 vs. CFA group.
SIN inhibits inflammatory cytokines production in IP rats
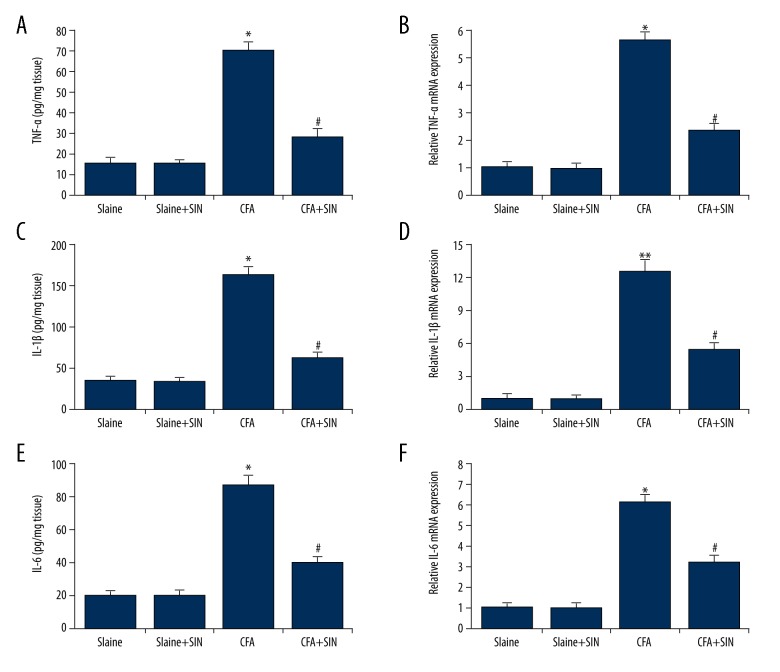
To investigate the exact beneficial role that SIN had in the IP rats, the levels of TNF-α, IL-1β, and IL-6 were determined. As shown in Figure 2, TNF-α, IL-1β, and IL-6 levels were significantly higher in the CAF group than in the control groups. Administration with SIN in IP rats reduced the levels of TNF-α, IL-1β, and IL-6.
Figure 2.
SIN inhibits TNF-α, IL-1β, and IL-6 expression in IP rats. (A, B) Protein/mRNA expression of TNF-α; (C, D) Protein/mRNA expression of IL-1β; (E, F) Protein/mRNA expression of IL-6. Saline: rats were injected with 100 μl saline only; Saline+SIN: rats were injected with 30 mg/kg SIN and 100 μl saline; CFA: rats were injected with 100 μl CFA only; CFA+SIN: rats were injected with 30 mg/kg SIN and 100 μl CFA. * p<0.05 vs Saline group; # <0.05 vs. CFA group.
SIN inhibits MAPKp38 and NF-κB activation in IP rats
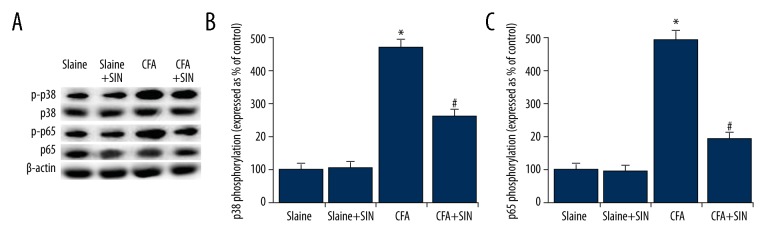
To explore the underlying molecular mechanisms of the effects of SIN on inflammatory pain, p38MAPK pathway and NF-κB pathway were assessed in the present study. As shown in Figure 3, compared with the control groups, p-p65 and p-p38 were significantly increased in the CFA group. SIN significantly decreased the expression levels of p-p65 and p-p38 in the SIN+CFA group in comparison with the CFA group. Thus, SIN can inhibit p38MAPK and NF-κB activation in IP rats.
Figure 3.
SIN inhibits MAPKp38 and NF-κB pathway activation in IP rats. (A) The protein levels of p-p38 and p-p65 were detected by Western blot analysis. (B) The expression levels of p38 and p65 phosphorylation are expressed as percent of the control. Saline: rats were injected with 100 μl saline only; Saline+SIN: rats were injected with 30 mg/kg SIN and 100 μl saline; CFA: rats were injected with 100 μl CFA only; CFA+SIN: rats were injected with 30 mg/kg SIN and 100 μl CFA. * p<0.05 vs. Saline group; # <0.05 vs. CFA group.
SIN inhibits Cox-2 expression in IP rats
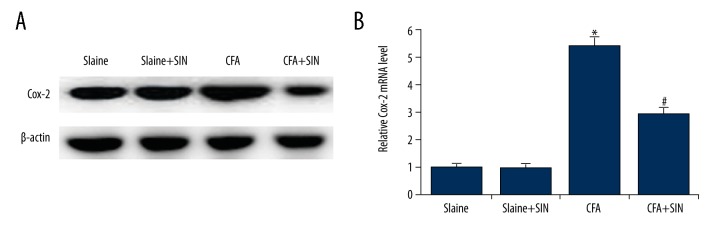
The level of COX-2 was determined, and the results suggest that, compared with the control groups, the protein and mRNA level of COX-2 significantly increased in the CAF group, and the level of COX-2 markedly decreased in the SIN treatment group when compared to the CAF group (Figure 4). The data indicate that SIN inhibits COX-2 expression in IP rats.
Figure 4.
SIN inhibits COX-2 expression in IP rats. (A) The protein level of COX-2 was detected by Western blot analysis. (B) The mRNA level of COX-2 was detected by qRT-PCR. Saline: rats were injected with 100 μl saline only; Saline+SIN: rats were injected with 30 mg/kg SIN and 100 μl saline; CFA: rats were injected with 100 μl CFA only; CFA+SIN: rats were injected with 30 mg/kg SIN and 100 μl CFA. * p<0.05 vs. Saline group; # <0.05 vs. CFA group.
SIN inhibits PGE2 production in IP rats
We also assessed PGE2 production, and found that PGE2 expression was significantly higher in CAF-treatment rats than in the control groups. SIN notably reduced the induction of PGE2 caused by CFA (Figure 5).
Figure 5.
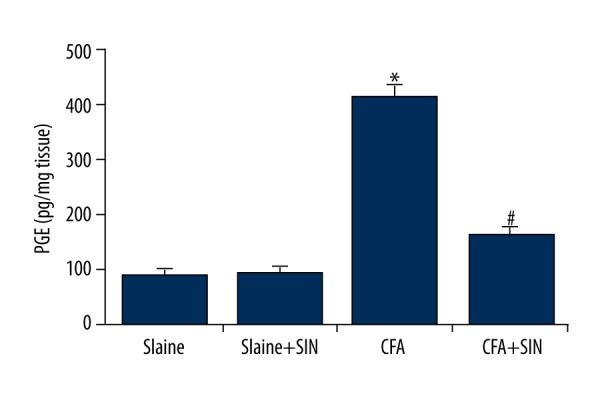
SIN inhibits PGE2 production in IP rats. PGE2 production was determined by ELISA assay. Saline: rats were injected with 100 μl saline only; Saline+SIN: rats were injected with 30 mg/kg SIN and 100 μl saline; CFA: rats were injected with 100 μl CFA only; CFA+SIN: rats were injected with 30 mg/kg SIN and 100 μl CFA. * p<0.05 vs. Saline group; # <0.05 vs. CFA group.
Discussion
This study investigated the effects of SIN on inflammatory pain induced by CFA and explored its mechanisms. The results indicated that administration of SIN via intraperitoneal injection can reduce the pain response and increase the pain threshold. Moreover, we found that SIN relieved inflammatory pain by preventing p38MAPK-NF-κB signaling pathway activation. Our data indicate that SIN may be a new promising drug for inflammatory pain treatment.
Inflammatory pain remains a serious clinical problem worldwide, and the molecular mechanisms underlying inflammatory pain still remain unclear. SIN, a common rattan drug, is widely used in the treatment of various arthritic diseases in traditional Chinese medicine (TCM) clinics. Recent studies indicated that SIN has anti-cancer effects on several cancers, such as breast cancer, hepatocellular carcinoma, and lung cancer [12,13,17]. Evidence also confirmed the neuroprotective effects of SIN [11,18,19]. Furthermore, a growing number of studies have revealed that SIN has multiple pharmacological activities, including antiangiogenic, antirheumatic, immunosuppressive effects, and anti-inflammatory effects [11]. However, there is little research on the influences of SIN in inflammatory pain. Thus, we studied the effects of SIN on inflammatory pain, and explored the underlying mechanisms. To perform our investigation, an inflammatory pain rat model was established using 100 μl CFA.
Using the IP rat model, we found that the mechanical hyperalgesia threshold, hyperalgesia latencies, and cold hyperalgesia frequency were markedly reduced in IP rats, and they were all markedly increased by SIN administration, indicating that SIN treatment relieves pain. Inflammation cytokines (TNF-α, IL-1β, and IL-6) were assessed in our study. We found that IP rats had a higher level of TNF-α, IL-1β, and IL-6, and SIN treatment reduced the level of TNF-α, IL-1β, and IL-6. To explore the mechanisms of the effects of SIN on inflammatory pain, we assessed the p38MAPK pathway and NF-κB pathway. It is well known that p38MAPK activation plays an important role in inflammatory pain [20,21]. Evidence has revealed the increased level of p-p38 in inflammation and neuropathic pain [22–24]. Inhibition of the p38MAPK signaling pathway is an effective method for inflammatory pain treatment [25,26]. NF-κB, a protein complex that controls transcription of DNA, cytokine production, and cell survival, plays a key role in regulating the immune response to infection [27]. Incorrect regulation of NF-κB has been linked to cancer and inflammation. The present study found that SIN repressed the p-p38 and p-p65 expression induced by CFA. Moreover, Cox-2 and PGE2, which play important roles in inflammation regulation, were also assessed in our study. The results show that compared with the control groups, Cox-2 and PGE2 levels were significantly higher in CFA-treated rats, and that SIN treatment can inhibit the expression of COX-2 and PGE2 in IP rats.
Taken together, our results indicate that SIN presents a protective role in inflammatory pain induced by CFA in rats through inhibiting inflammatory mediators via suppressing the p38MAPK-NF-κB pathway, providing a more theoretical basis for inflammatory pain treatment.
Conclusions
Our data suggest that SIN plays a protective role in inflammatory pain through inhibiting inflammatory mediators production via repressing the p38MAPK-NF-κB pathway. SIN may be a promising drug for inflammatory pain treatment.
Footnotes
Source of support: This study was supported by the 36th Science and Technology Plan Project (No. CS20109003)
Conflict of interest
None.
References
- 1.Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: Targeting pain at the source. Nat Rev Drug Discov. 2009;8:55–68. doi: 10.1038/nrd2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu KW, Hsu CK, Hsieh CL, et al. Probing the effects and mechanisms of electroacupuncture at ipsilateral or contralateral ST36-ST37 acupoints on CFA-induced inflammatory pain. Sci Rep. 2016;6:22123. doi: 10.1038/srep22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinman RS, Bennell KL, Crossley KM, McConnell J. Immediate effects of adhesive tape on pain and disability in individuals with knee osteoarthritis. Rheumatology. 2003;42:865–69. doi: 10.1093/rheumatology/keg233. [DOI] [PubMed] [Google Scholar]
- 4.Janssens HJ, Janssen M, van de Lisdonk EH, et al. Use of oral prednisolone or naproxen for the treatment of gout arthritis: A double-blind, randomised equivalence trial. Lancet. 2008;371:1854–60. doi: 10.1016/S0140-6736(08)60799-0. [DOI] [PubMed] [Google Scholar]
- 5.Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000;343:1520–28. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 6.Zhang B, He XL, Ding Y, Du GH. Gaultherin, a natural salicylate derivative from Gaultheria yunnanensis: Towards a better non-steroidal anti-inflammatory drug. Eur J Pharmacol. 2006;530:166–71. doi: 10.1016/j.ejphar.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 7.Cheppudira BP, Garza TH, Petz LN, et al. Anti-hyperalgesic effects of AG490, a Janus kinase inhibitor, in a rat model of inflammatory pain. Biomed Rep. 2015;3:703–6. doi: 10.3892/br.2015.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng Y, Li F, Wang D, et al. Sinomenine inhibits the expression of PDL1 in the peripheral blood mononuclear cells of mesangial proliferative nephritis patients. Mol Med Rep. 2013;7:1223–28. doi: 10.3892/mmr.2013.1302. [DOI] [PubMed] [Google Scholar]
- 9.Xu M, Liu L, Qi C, et al. Sinomenine versus NSAIDs for the treatment of rheumatoid arthritis: A systematic review and meta-analysis. Planta Med. 2008;74:1423–29. doi: 10.1055/s-2008-1081346. [DOI] [PubMed] [Google Scholar]
- 10.Cheng Y, Zhang J, Hou W, et al. Immunoregulatory effects of sinomenine on the T-bet/GATA-3 ratio and Th1/Th2 cytokine balance in the treatment of mesangial proliferative nephritis. Int Immunopharmacol. 2009;9:894–99. doi: 10.1016/j.intimp.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Qian L, Xu Z, Zhang W, et al. Sinomenine, a natural dextrorotatory morphinan analog, is antiinflammatory and neuroprotective through inhibition of microglial NADPH oxidase. J Neuroinflammation. 2007;4:23. doi: 10.1186/1742-2094-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu XL, Zeng J, Chen YL, et al. Sinomenine hydrochloride inhibits human hepatocellular carcinoma cell growth in vitro and in vivo: Involvement of cell cycle arrest and apoptosis induction. Int J Oncol. 2013;42:229–38. doi: 10.3892/ijo.2012.1704. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Li PP, Liu C, et al. Sinomenine hydrochloride inhibits breast cancer metastasis by attenuating inflammation-related epithelial – mesenchymal transition and cancer stemness. Oncotarget. 2017;8:13560–74. doi: 10.18632/oncotarget.14593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q, Li XK. Immunosuppressive and anti-inflammatory activities of sinomenine. Int Immunopharmacol. 2011;11:373–76. doi: 10.1016/j.intimp.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Chen WJ, Guo SD, Wang SG. MicroRNA-16 alleviates inflammatory pain by targeting Ras-related protein 23 (RAB23) and inhibiting p38 MAPK activation. Med Sci Monit. 2016;22:3894–901. doi: 10.12659/MSM.897580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao L, Lu X, Cao Y. MicroRNA and signal transduction pathways in tumor radiation response. Cell Signal. 2013;25:1625–34. doi: 10.1016/j.cellsig.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang SL, Gao YB, Hou W, et al. Sinomenine inhibits A549 human lung cancer cell invasion by mediating the STAT3 signaling pathway. Oncol Lett. 2016;12:1380–86. doi: 10.3892/ol.2016.4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Wang H, Li L, et al. Sinomenine provides neuroprotection in model of traumatic brain injury via the Nrf2-ARE [athway. Front Neurosci. 2016;10:580. doi: 10.3389/fnins.2016.00580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu J, Wang M, Zhang J, et al. The neuroprotection of Sinomenine against ischemic stroke in rats by suppressing NLRP3 inflammasome via AMPK signaling. Int Immunopharmacol. 2016;40:492–500. doi: 10.1016/j.intimp.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 20.Ji RR. Peripheral and central mechanisms of inflammatory pain, with emphasis on MAP kinases. Curr Drug Targets Inflamm Allergy. 2004;3:299–303. doi: 10.2174/1568010043343804. [DOI] [PubMed] [Google Scholar]
- 21.Dong Y, Li P, Ni Y, et al. Decreased microRNA-125a-3p contributes to up-regulation of p38 MAPK in rat trigeminal ganglions with orofacial inflammatory pain. PLoS One. 2014;9:e111594. doi: 10.1371/journal.pone.0111594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji RR, Samad TA, Jin SX, et al. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heathyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 23.Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–22. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crown ED, Gwak YS, Ye Z, et al. Activation of p38 MAP kinase is involved in central neuropathic pain following spinal cord injury. Exp Neurol. 2008;213:257–67. doi: 10.1016/j.expneurol.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang JQ, Du JY, Liang Y, Fang JF. Intervention of electroacupuncture on spinal p38 MAPK/ATF-2/VR-1 pathway in treating inflammatory pain induced by CFA in rats. Mol Pain. 2013;9:13. doi: 10.1186/1744-8069-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin X, Wang M, Zhang J, Xu R. p38 MAPK: A potential target of chronic pain. Curr Med Chem. 2014;21:4405–18. doi: 10.2174/0929867321666140915143040. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto S, Ohta N, Matsumoto A, et al. Haloperidol suppresses NF-kappaB to inhibit lipopolysaccharide-induced pro-inflammatory response in RAW 264 cells. Med Sci Monit. 2016;22:367–72. doi: 10.12659/MSM.895739. [DOI] [PMC free article] [PubMed] [Google Scholar]