Abstract
Background
D-dimer tests have been widely used to rule-out deep venous thrombosis (DVT), but with low specificity. Circulating microRNAs (miRNAs) are novel promising biomarkers in diverse diseases. The purpose of our study was to identify the diagnostic abilities of circulating miRNA-320a/b and to assess their correlation with plasma D-dimer in DVT and post-thrombotic syndrome (PTS) patients.
Material/Methods
Plasma samples were taken from 30 DVT patients, 30 PTS patients, and 30 age- and sex-matched healthy volunteers. Quantitative real-time PCR (qPCR) assay and turbidimetric immunoassay were conducted to assess the concentrations of miRNA-320a/b and D-dimer in plasma.
Results
Circulating miRNA-320a and miRNA-320b were significantly upregulated in DVT patients with fold changes of 1.58 and 1.79, respectively. The receiver operating characteristic (ROC) curve analysis showed area under the curve (AUC) values of 0.70 (95% CI: 0.56–0.83) for miRNA-320a and 0.79 (95% CI: 0.67–0.90) for miRNA-320b. Moreover, plasma levels of miRNA-320b were associated with D-dimer values (r=0.52, 95% CI: 0.19–0.74) in DVT. However, no significant changes in plasma miRNA-320a/b and D-dimer were detected in PTS patients.
Conclusions
Compared with controls, circulating miRNA-320a/b was differentially expressed in DVT. Simultaneous detection of miRNA-320a/b with D-dimer may improve diagnostic accuracy of DVT.
MeSH Keywords: Biological Markers, Diagnosis, Fibrin Fibrinogen Degradation Products, MicroRNAs, Venous Thrombosis
Background
Venous thromboembolism (VTE) is a common cardiovascular disorder. Deep venous thrombosis (DVT) and pulmonary embolism are the 2 most common types of VTE. DVT is a clot formation in a deep vein, with an annual incidence rate of more than 300,000 cases annually in the USA [1]. Pulmonary embolism, a potentially life-threatening condition, occurs when a thrombus detaches from the DVT original site and travels to the lung. Common clinical manifestations of DVT include swelling, erythema, and tenderness in affected extremities, but with less specificity [2]. Some DVT patients have no obvious symptoms, and thus escape timely diagnosis and treatment. Consequently, clinical manifestations must be combined with imaging and biomarkers to assess potential thrombosis [3,4]. What’s worse, 20% to 50% of patients suffering from DVT will develop PTS, a chronic and potentially devastating complication of DVT; 5% to 10% patients will develop severe PTS, and 30% will have a recurrence within the next decade [1,5]. These complications are costly and adversely affect quality of daily life. DVT is a disorder with multiple etiological factors. Even a straightforward case requires complete hypercoagulable workup [6]. Differences in anticoagulant therapy duration and thrombus location may influence the recanalization of DVT [7]. Therefore, it is very important to promptly identify DVT and initiate appropriate interventions.
Numerous studies have attempted to find precise and early diagnostic laboratory biomarkers for DVT. However, to date, plasma D-dimer is the only well-established and clinically applied biomarker to identify DVT. The D-dimer test reflects the level of fibrin degradation. A negative D-dimer result is convincing evidence to rule-out DVT, but a positive D-dimer has low specificity and low positive predictive value (PPV) to diagnose DVT [4,8]. D-dimer concentrations can also be elevated in non-thrombotic disorders, including disseminated intravascular coagulation, infection, and stroke [9]. Therefore, novel candidates or complementary biomarkers are still imperative in DVT diagnosis.
MicroRNAs (miRNAs) are a family of small, noncoding, single-stranded RNAs. Through translation inhibition or inducing target mRNA degradation, miRNAs regulate protein expression post-transcriptionally [10,11]. In peripheral blood, miRNAs are present at a remarkably steady level [12]. Over the past decade, circulating miRNAs have been emerging as attractive potential biomarkers in various diseases [12–14], since Mitchell et al. demonstrated that miRNAs had a potential to detect prostate cancer [15]. However, advances in dysregulated miRNAs in thrombosis are still sparse. Among the limited indications, miRNA-320a/b, 2 members of the miRNA-320 family, have been demonstrated to be differentially expressed in VTE and pulmonary embolism [16–18].
Consequently, in the present study, we detected the expressions of miRNA-320a/b in DVT and PTS patients to investigate their diagnostic values. Furthermore, the correlations between miRNA-320a/b and D-dimer concentrations in plasma were explored, hoping to provide more information for the diagnosis and development in DVT and PTS.
Material and Methods
Patient recruitment
From October 2015 to December 2016, we recruited a total of 60 consecutive patients, 20–80 years old, including 30 DVT patients and 30 PTS patients, admitted in the First Affiliated Hospital of Zhengzhou University. During the same period, a total of 30 consecutive healthy volunteers were enrolled as the control group, with an average age of 52 years old. All participants had provided their written informed consent and this work was approved by the Medical Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
All DVT patients were confirmed by color Doppler ultrasonography. Due to PTS, the Villalta scale was the main diagnostic tool. The exclusion criteria were as follows: ongoing anticoagulation treatment for more than 3 months, pregnancy, duration of symptoms for more than 1 year, and previous thrombosis within the last year.
Plasma preparation and preservation
Three milliliters of fasting peripheral venous blood were extracted from each study subject in duplicate. All samples were collected in vacuum tubes containing trisodium citrate and were mixed. Following centrifugation at 120 000×g for 10 min at 4°C, the plasma supernatants were transferred into RNase/DNase-free tubes and immediately stored at −80°C for further analysis.
RNA isolation and quantitative real-time PCR (qPCR) assay
The levels of miRNA-320a/b were evaluated by qPCR assay. Briefly, total RNA was extracted from thawed plasma using TRI REAGENT® BD (MRC, TB-126, Cincinnati, OH, USA) according to the manufacturer’s protocol, and then RNA was reversely transcribed using a PrimeScript™ RT Reagent Kit (TaKaRa, DRR047A, Tokyo, Japan). Finally, RT-PCR was performed on the LightCycler® 480 Real-Time PCR System (Roche Diagnostics, Mannheim, Germany) in triplicate with SYBROeR Premix Ex Taq™ II (TaKaRa, DRR820S, Tokyo, Japan). To normalize the results, miRNA-16 was used as an internal reference gene. The sequences for miRNA-16 primers were as follows: forward primer: 5′-CGGGTCGTAGCAGCACGTAAATA-3′; RT primer: 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACT GGATACGACCGCCAA-3′. The forward primers for miRNA-320a and miRNA-320b were 5′-AAAAGCTGGGTTGAGAGGGCGA-3′ and 5′-AAAAGCTGGGTTGAGAGGGCAA-3′, separately. The universal reverse primer sequence was 5′-ACTCAGTGCAGGGTCCGAGG-3′. The crossing point (Cp) was used to calculate the expression levels of miRNAs, and a Cp greater than 36 was considered very low expression and required no further analysis. The individual relative expression was calculated by the equation 2−ΔCp (ΔCp=CpmiRNA-320a/b–CpmiRNA-16), and 2−ΔΔCp was expressed as fold change [19].
Plasma D-dimer detection
To assess plasma D-dimer levels, samples were taken to the clinical laboratory of the First Affiliated Hospital of Zhengzhou University and measured by HemosIL D-Dimer HS (Instrumentation Laboratory, Bedford, MA, USA) on a ACL TOP® 700 LAS (Instrumentation Laboratory) device. Immunoturbidimetric assay was performed to guarantee the quality of D-dimer values.
Targets identification of miRNAs
MiRTarbase (http://mirtarbase.mbc.nctu.edu.tw/), miRWalk 2.0 (http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/) together with TargetScan (http://www.targetscan.org/) were applied to identify the predicted target genes. After that, the gene ontology (GO) and KEGG pathway analysis of putative target genes were performed by DAVID Bioinformatics Resources 6.8 (https://david.ncifcrf.gov/).
Statistical analysis
For continuous variables, values are presented as mean ± standard deviation (SD), and as counts and percentages for categorical variables. Differences in miRNAs relative abundance between patients and controls were compared using the t test for continuous variables. Differences among the 3 cohorts were assessed by one-way analysis of variance (one-way ANOVA) for normally distributed data, chi-squared test for categorical variables, and Mann-Whitney U test for skewed data. Receiver operating characteristic (ROC) curves and the area under the curve (AUC) were used to estimate the diagnostic power and accuracy, respectively, of biomarkers. Correlations between the levels of D-dimer and miRNAs were evaluated using Pearson correlation. Two-tailed P values less than 0.05 were deemed statistically significant. All statistical analyses were performed using IBM SPSS Statistics software version 21.0 (IBM Inc., Armonk, NY, USA) and Prism (GraphPad, La Jolla, CA, USA).
Results
Characteristics of study population
Baseline characteristics of patients and controls are shown in Table 1. No significant differences were identified in the distribution of basic features, such as age, sex, BMI, or smoking status between thrombosis patients and healthy controls.
Table 1.
Basic characteristics of study subjects.
| Variables | DVT patients (n=30) | Controls (n=30) | PTS patients (n=30) | P value |
|---|---|---|---|---|
| Ages (years) | 52.56±15.42 | 51.57±12.68 | 53.27±10.69 | 0.880 |
| Male/Female | 16/14 | 20/10 | 17/13 | 0.551 |
| BMI (kg/m2) | 26.59±4.46 | 25.39±5.54 | 26.06±3.71 | 0.603 |
| Smoking (yes/no) | 10/20 | 5/25 | 7/23 | 0.319 |
| Family history (yes/no) | 3/27 | 0/30 | 2/28 | 0.227 |
| Diabetes (yes/no) | 2/28 | 6/24 | 1/29 | 0.075 |
Expressions of circulating miRNA-320a/b in DVT, PTS, and controls
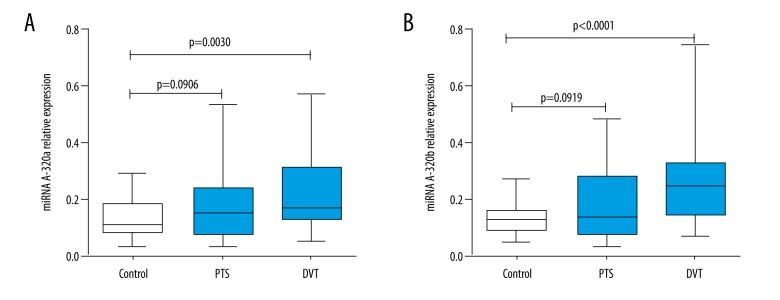
We chose the comparative Cp method (2−ΔCp) to represent the relative abundance of miRNAs. As shown in Figure 1, both miRNA-320a (P=0.0030) and miRNA-320b (P<0.0001) were notably upregulated in DVT patients compared with controls. The fold changes were 1.58 and 1.79, respectively. Previous profiling research demonstrated that a fold change in miRNA expression more than 1.5 potentially influenced the cellular biology [20]. However, neither miRNA-320a (P=0.0906) nor miRNA-320b (P=0.0919) showed significant differences between PTS patients and normal controls.
Figure 1.
Differential expressions of circulating miRNA-320a/b among DVT patients, PTS patients, and healthy controls. The relative abundances normalized by miRNA-16 of miRNA-320a (A) and miRNA-320b (B) were significantly higher than those of healthy controls.
Diagnostic values of plasma miRNA-320a/b for DVT
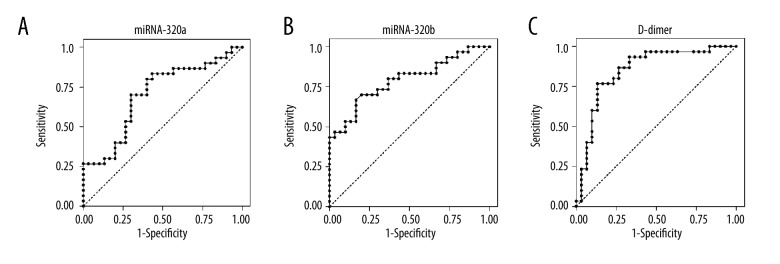
ROC curve analyses were performed to determine the diagnostic sensitivity and specificity of miRNA-320a/b together with D-dimer in DVT. The AUCs for miRNA-320a/b were 0.70 (95% CI: 0.56–0.83) and 0.79 (95% CI: 0.67–0.90), respectively, suggesting that miRNA-320a/b, and especially miRNA-320b, could differentiate DVT patients from healthy individuals, although both of them were lower than the AUC for D-dimer (0.85, 95% CI: 0.75–0.95) (Figure 2).
Figure 2.
ROC curves for miRNA-320a, miRNA-320b, and D-dimer for DVT. The AUCs for miRNA-320a, miRNA-320b, and D-dimer were 0.70 (A), 0.79 (B), and 0.85 (C), respectively, suggesting that miRNA-320a/b can distinguish DVT from healthy controls.
Correlation of D-dimer with miRNA-320a/b in plasma
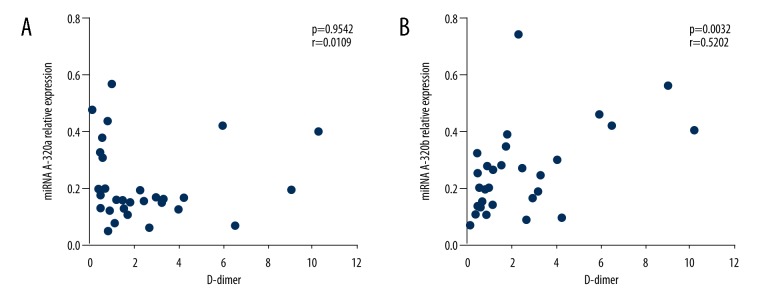
Significantly increased levels of D-dimer were detected in DVT patients (P=0.005), which was not seen in PTS patients (P=0.666). Based on the comprehensive analysis of miRNA-320a/b and D-dimer expressions in different groups, we further explored the association between miRNAs and D-dimer levels in DVT patients. As illustrated in Figure 3, upregulated miRNA-320b (P=0.0032, r=0.5202) had an absolute correlation with D-dimer in DVT patients, while expression of miRNA-320a (P=0.9542, r=0.0109) did not show any correlation.
Figure 3.
Correlations of circulating miRNA-320a and miRNA-320b with D-dimer in DVT patients. Plasma miRNA-320a/b values were plotted against D-dimer levels. No significant correlation existed between miRNA-320a and D-dimer (A). MiRNA-320b showed correlation with D-dimer (B) in DVT patients.
Bioinformatics analysis of miRNA-320a/b
To fully understand the roles of miRNA-320a/b in DVT, putative targets and pathway analysis were investigated. In silico research revealed that miRNA-320a/b regulated important cellular functions, such as cell migration, endothelial cell proliferation, and apoptotic process. Moreover, cytokine activity was involved in the enriched GO terms, indicating that miRNA-320 might regulate the inflammatory response. KEGG enrichment terms were converged on TGF-beta signaling pathway, PI3K-Akt signaling pathway, proteoglycans in cancer, and focal adhesion (Table 2).
Table 2.
KEGG pathway analysis of miRNA-320.
| KEGG terms | Number of genes | P value |
|---|---|---|
| Ribosome | 17 | 3.6E-13 |
| TGF-beta signaling pathway | 8 | 2.6E-4 |
| PI3K-Akt signaling pathway | 12 | 2.2E-3 |
| Rap1 signaling pathway | 9 | 6.1E-3 |
| Proteoglycans in cancer | 8 | 1.9E-2 |
| HIF-1 signaling pathway | 6 | 1.8E-2 |
| Focal adhesion | 8 | 1.7E-2 |
| TNF signaling pathway | 6 | 2.1E-2 |
| Glioma | 5 | 2.2E-2 |
| Melanoma | 5 | 2.8E-2 |
Discussion
In the present work, qPCR was used to detect the expression of miRNA-320a/b in patients with DVT or PTS. The results indicated that, for the DVT group, concentrations of miRNA-320a/b were significantly upregulated. Further ROC analysis revealed that circulating miRNA-320a/b has diagnostic potential in DVT. Moreover, the elevated plasma miRNA-320b level was correlated with D-dimer levels, suggesting that the combination of plasma miRNA-320b and D-dimer detection could improve the diagnostic accuracy of DVT. For PTS patients, neither miRNA-320a/b nor D-dimer showed any statistical difference. PTS is a chronic complication of DVT, and the expression of plasma biomarkers can greatly vary over time. Plasma miRNAs may fluctuate during the development process. It is of great significance to explore novel diagnostic biomarkers so as to minimize the damage from DVT. One-third of DVT patients develop PTS [5], resulting in significant impact on patient quality of life. Moreover, for populations susceptible to DVT, such as iliac vein compression syndrome (IVCS) in hip fracture patients, more aggressive measures are needed when DVT occurs [21]. Thus, timely and accurate diagnosis is important to implement active therapy and prevent the deterioration of DVT.
Although D-dimer is the most widely used biomarker for DVT assessment, its specificity is low. Novel or surrogate biomarkers are still warranted, which can provide optimal therapy choice for DVT patients. Circulating miRNAs, emerging as promising biomarkers in various diseases, have also been shown to play key roles in thrombotic disorders [16,22,23]. Several advantages of circulating miRNAs may account for their popularity. First, by encapsulating in microparticles and exosomes or bonding to Argonaute2 (Ago2) protein and HDL, miRNAs remain remarkably intact and reproducible in plasma [24,25]. Second, the harvest of plasma miRNAs is simple and less invasive. Finally, they are easily detected by qPCR.
Recent studies have identified the diagnostic values of miRNAs in DVT. Xiao et al. demonstrated that plasma levels of miRNA-136-5p and miR-424-5p were associated with DVT and markers of hypercoagulability [26]. Qin et al. showed that serum miR-582, miR-195 and miR-532 might be potential biomarkers to detect DVT [27]. In addition, Xie et al. found that comprehensive analysis of platelets miR-96 and plasma D-dimer could accurately predict DVT after orthopedic surgery [28]. Our data revealed that plasma miRNA-320a/b were upregulated and miRNA-320b was correlated with D-dimer, which is consistent with previous results. Of these, 1 study found that plasma miRNA-320a/b concentrations of unprovoked VTE were significantly higher compared with normal controls [16]. The other 2 found that plasma miRNA-320 was also elevated in PE patients at the screening phase [17,18]. Moreover, miRNA-320 was reported to function in heart disease. For instance, in ischemic cardiomyopathy and aortic stenosis, miRNA-320 from left ventricular samples was notably altered, and overexpressed miRNA-320 enhanced cell death and apoptosis in rat cardiomyocytes [29]. In a study of Chinese patients with acute myocardial infarction (AMI), Huang et al. reveal that plasma miRNA-320b was significantly lower and can participate in the pathogenesis, probability through influencing the TGF-β signaling pathway and cytokine–cytokine receptor interaction [30].
Functional analysis showed that miRNA-320a/b regulated important functions, which may help explain the underlying pathogenesis of thrombosis. The critical mechanisms might be through the TGF-β signaling pathway and PI3K signaling pathway. TGF-β is a pivotal regulator both in pathophysiological angiogenesis and tissue remodeling [31–33]. In microvascular endothelial cell lines, TGF-β1 can promote angiogenesis by regulating thrombospondin-4 (TSP-4) [34], and IL-37 exerts its pro-angiogenic responses through the TGF-β-ALK1 signaling pathway [35]. A previous study showed that the inhibition of PI3K signaling might be a promising therapeutic target for thrombotic diseases [36]. Activation of the PI3K signaling pathway could facilitate the migration of endothelial cells, which regulates the vascular homeostasis and angiogenesis. In DVT rat models, overexpressed miR-126 enhanced vasculogenesis through prompting PI3K-Akt activation, leading to neovascularization and recanalization in DVT [37]. Notably, miRNA-320 was most highly expressed in human platelets, while circulating miRNA-320b could be transferred to vascular endothelial cells (VECs) and regulated intercellular adhesion molecule-1 expression [38], suggesting a potential role of acting as extracellular communicators. Although the exact mechanism regulated by miRNA-320a/b in the pathophysiological pathways of DVT is still ambiguous, the preliminary functional analysis also provides mechanistic insight regarding circulating miRNAs in the pathogenesis of DVT.
Several general limitations in our research should be acknowledged when considering our conclusions. First, our results were based on a relative small sample size, which might weaken the statistical power. More samples would enhance the reliability of significant differences. Second, a follow-up was not performed. Thrombosis is a dynamic process, affected by many factors, including sampling time, primary diseases, and treatment. Comparing the change between before and after treatment in the same DVT patient would be very meaningful. Moreover, self-control or monitoring the dynamic change of microRNA-320 may provide more information. Third, only miRNA-320a/b were investigated in the present study. Given that a single gene can be potentially targeted by many different miRNAs [39], a panel of miRNAs be more reliable biomarkers.
Conclusions
Our results indicate that circulating miRNA-320a/b are differentially expressed and miRNA-320b concentrations correlate to D-dimer levels in DVT. The simultaneous detection of abnormal plasma miRNA-320 and D-dimer could contribute to the diagnosis of DVT. Further large cohort studies are needed to estimate the clinical values of circulating miRNAs.
Acknowledgements
The authors thank Yongfeng Wang for technical assistance and the Department of Clinical Laboratory Medicine of the First Affiliated Hospital of Zhengzhou University for providing facilities.
Footnotes
Source of support: This study was funded by the Key Project of the Scientific and Technological Department of Henan (No. 152102410067 and No. 162102310142)
Conflicts of interest
None.
References
- 1.Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016;41:3–14. doi: 10.1007/s11239-015-1311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hollenhorst MA, Battinelli EM. Thrombosis, hypercoagulable states, and anticoagulants. Prim Care. 2016;43:619–35. doi: 10.1016/j.pop.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Min SK, Kim YH, Joh JH, et al. Diagnosis and treatment of lower extremity deep vein thrombosis: Korean practice guidelines. Vasc Specialist Int. 2016;32:77–104. doi: 10.5758/vsi.2016.32.3.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaefer JK, Jacobs B, Wakefield TW, Sood SL. New biomarkers and imaging approaches for the diagnosis of deep venous thrombosis. Curr Opin Hematol. 2017;24:274–81. doi: 10.1097/MOH.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 5.Kahn SR. The post-thrombotic syndrome. Hematology Am Soc Hematol Educ Program. 2016;2016:413–18. doi: 10.1182/asheducation-2016.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miao J, Naik G, Muddana S, et al. An uncommon case of lower limb deep vein thrombosis with multiple etiological causes. Am J Case Rep. 2017;18:313–16. doi: 10.12659/AJCR.902391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang C, Fu Q, Zhao Y, et al. Short-term anticoagulant therapy and thrombus location are independent risk factors for delayed recanalization of deep vein thrombosis. Med Sci Monit. 2016;22:219–25. doi: 10.12659/MSM.895228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rectenwald JE, Myers DD, Jr, Hawley AE, et al. D-dimer, P-selectin, and microparticles: Novel markers to predict deep venous thrombosis. A pilot study. Thromb Haemost. 2005;94:1312–17. doi: 10.1160/TH05-06-0426. [DOI] [PubMed] [Google Scholar]
- 9.Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e351S–418S. doi: 10.1378/chest.11-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 11.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 12.Lindner K, Haier J, Wang Z, et al. Circulating microRNAs: Emerging biomarkers for diagnosis and prognosis in patients with gastrointestinal cancers. Clin Sci (Lond) 2015;128:1–15. doi: 10.1042/CS20140089. [DOI] [PubMed] [Google Scholar]
- 13.Liu CJ, Kao SY, Tu HF, et al. Increase of microRNA miR-31 level in plasma could be a potential marker of oral cancer. Oral Dis. 2010;16:360–64. doi: 10.1111/j.1601-0825.2009.01646.x. [DOI] [PubMed] [Google Scholar]
- 14.Ali HEA, Abdel Hameed R, Effat H, et al. Circulating microRNAs panel as a diagnostic tool for discrimination of HCV-associated hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2017;41(4):e51–e62. doi: 10.1016/j.clinre.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105:10513–18. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starikova I, Jamaly S, Sorrentino A, et al. Differential expression of plasma miRNAs in patients with unprovoked venous thromboembolism and healthy control individuals. Thromb Res. 2015;136:566–72. doi: 10.1016/j.thromres.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Xiao J, Jing ZC, Ellinor PT, et al. MicroRNA-134 as a potential plasma biomarker for the diagnosis of acute pulmonary embolism. J Transl Med. 2011;9:159. doi: 10.1186/1479-5876-9-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou X, Wen W, Shan X, et al. MiR-28-3p as a potential plasma marker in diagnosis of pulmonary embolism. Thromb Res. 2016;138:91–95. doi: 10.1016/j.thromres.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–18. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 20.Mestdagh P, Van Vlierberghe P, De Weer A, et al. A novel and universal method for microRNA RT-qPCR data normalization. Genome Biol. 2009;10:R64. doi: 10.1186/gb-2009-10-6-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Liu P, Xia K, et al. Iliac Vein Compression Syndrome (IVCS): An under-recognized risk factor for left-sided Deep Venous Thrombosis (DVT) in old hip fracture patients. Med Sci Monit. 2018;24:2078–82. doi: 10.12659/MSM.901639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujii S, Sugiura T, Dohi Y, Ohte N. MicroRNA in atherothromobosis: Is it useful as a disease marker? Thromb J. 2016;14(Suppl 1):21. doi: 10.1186/s12959-016-0112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolz S, Gorriz D, Tembl JI, et al. Circulating microRNAs as Novel biomarkers of stenosis progression in asymptomatic carotid stenosis. Stroke. 2017;48:10–16. doi: 10.1161/STROKEAHA.116.013650. [DOI] [PubMed] [Google Scholar]
- 24.Arroyo JD, Chevillet JR, Kroh EM, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011;108:5003–8. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vickers KC, Palmisano BT, Shoucri BM, et al. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–33. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Sundquist K, Elf JL, et al. Diagnostic potential of plasma microRNA signatures in patients with deep-vein thrombosis. Thromb Haemost. 2016;116:328–36. doi: 10.1160/TH16-01-0071. [DOI] [PubMed] [Google Scholar]
- 27.Qin J, Liang H, Shi D, et al. A panel of microRNAs as a new biomarkers for the detection of deep vein thrombosis. J Thromb Thrombolysis. 2015;39:215–21. doi: 10.1007/s11239-014-1131-0. [DOI] [PubMed] [Google Scholar]
- 28.Xie X, Liu C, Lin W, et al. Deep vein thrombosis is accurately predicted by comprehensive analysis of the levels of microRNA-96 and plasma D-dimer. Exp Ther Med. 2016;12(3):1896–900. doi: 10.3892/etm.2016.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song CL, Liu B, Diao HY, et al. The protective effect of microRNA-320 on left ventricular remodeling after myocardial ischemia-reperfusion injury in the rat model. Int J Mol Sci. 2014;15:17442–56. doi: 10.3390/ijms151017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang S, Chen M, Li L, et al. Circulating MicroRNAs and the occurrence of acute myocardial infarction in Chinese populations. Circ Cardiovasc Genet. 2014;7:189–98. doi: 10.1161/CIRCGENETICS.113.000294. [DOI] [PubMed] [Google Scholar]
- 31.Pardali E, Goumans MJ, ten Dijke P. Signaling by members of the TGF-beta family in vascular morphogenesis and disease. Trends Cell Biol. 2010;20:556–67. doi: 10.1016/j.tcb.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Samarakoon R, Overstreet JM, Higgins PJ. TGF-beta signaling in tissue fibrosis: Redox controls, target genes and therapeutic opportunities. Cell Signal. 2013;25:264–68. doi: 10.1016/j.cellsig.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toma I, McCaffrey TA. Transforming growth factor-beta and atherosclerosis: Interwoven atherogenic and atheroprotective aspects. Cell Tissue Res. 2012;347:155–75. doi: 10.1007/s00441-011-1189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muppala S, Xiao R, Krukovets I, et al. Thrombospondin-4 mediates TGF-beta-induced angiogenesis. Oncogene. 2017;36(36):5189–98. doi: 10.1038/onc.2017.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao M, Hu Y, Jin J, et al. Interleukin 37 promotes angiogenesis through TGF-beta signaling. Sci Rep. 2017;7:6113. doi: 10.1038/s41598-017-06124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su XL, Su W, Wang Y, et al. The pyrrolidinoindoline alkaloid Psm2 inhibits platelet aggregation and thrombus formation by affecting PI3K/Akt signaling. Acta Pharmacol Sin. 2016;37:1208–17. doi: 10.1038/aps.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng Q, Wang W, Yu X, et al. Upregulation of microRNA-126 contributes to endothelial progenitor cell function in deep vein thrombosis via its target PIK3R2. J Cell Biochem. 2015;116:1613–23. doi: 10.1002/jcb.25115. [DOI] [PubMed] [Google Scholar]
- 38.Gidlof O, van der Brug M, Ohman J, et al. Platelets activated during myocardial infarction release functional miRNA, which can be taken up by endothelial cells and regulate ICAM1 expression. Blood. 2013;121:3908–17. S1–26. doi: 10.1182/blood-2012-10-461798. [DOI] [PubMed] [Google Scholar]
- 39.Grimson A, Farh KK, Johnston WK, et al. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]