Abstract
Background
LPS-inhibited osteoblastic differentiation plays an important role in the pathogenesis of osteomyelitis. Thus, searching for drugs that affect LPS-mediated osteoblastic differentiation may be crucial in developing therapies for osteomyelitis. The purpose of this study was to investigate the role and mechanisms of resveratrol, a natural polyphenol present in red wine, on LPS-inhibited osteoblastic differentiation.
Material/Methods
Cell viability was measured by MMT assay. Mitochondrial ATP levels, membrane potential, and superoxide production were measured to evaluate the effects of LPS and resveratrol on mitochondrial functions in osteoblast-like MC3T3-E1 cells. Osteoblast-related genes, including ALP, OCN, OPN, and RUNX2, were measured by ELISA analysis and RT-PCR in differentiated osteoblast cells treated with LPS and resveratrol. Cellular Sirt1 and PCG-1α levels were measured by Western blot to probe the impact of resveratrol treatment in LPS-stimulated MC3T3-E1 osteoblasts.
Results
The results showed that LPS caused significant mitochondrial dysfunctions of MC3T3-E1 cells in a dose-dependent manner, which were attenuated by resveratrol. Furthermore, LPS markedly decreased the expression of ALP, OCN, OPN, and RUNX2 in MC3T3-E1 cells cultivated in osteoblast differentiation medium, suggesting that LPS inhibited the osteoblastic differentiation of MC3T3-E1 cells. However, resveratrol obviously alleviated the suppressive impact of LPS on osteoblast differentiation. In addition, resveratrol increased expression of Sirt1 and PGC-1α in MC3T3-E1 cells treated with LPS.
Conclusions
Taken together, these results show that resveratrol alleviated the suppression of LPS on osteoblast differentiation by improving, at least in part, mitochondrial function.
MeSH Keywords: Cell Differentiation, Lipopolysaccharides, Osteoblasts
Background
Osteomyelitis is a serious chronic inflammatory disease in elderly people that threatens quality of life [1]. It has been reported that chronic osteomyelitis results from dysregulation of bone formation and reabsorption [2]. At present, no effective strategy is available to prevent or suppress this action. Lipopolysaccharides (LPS), a major component of gram-negative bacterial membranes, is recognized to be responsible for the inflammatory osteolytic diseases, including osteomyelitis, implants infection, and septic arthritis [3–5]. Further studies showed that LPS suppresses osteoblastic differentiation and induces bone loss by stimulating the production of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), which affects bone structure through the inhibition of osteoblast function and stimulation of osteoclast formation directly and indirectly at the cellular level [6–8]. Thus, searching for drugs that affect LPS-mediated osteoblastic differentiation may be crucial in developing therapies for osteomyelitis.
Resveratrol (RSV), a natural polyphenol present in red wine, has been well documented to have many beneficial effects to extend the life span and improve health [9,10]. Previous studies suggested that RSV also possesses potent bone-protective properties [11–14]. Mizutani et al. [12] found that RSV promoted osteoblast differentiation in mouse calvaria osteoblast-like cells MC3T3-E1. Durbin et al. [14] showed that RSV consumption prevented bone loss in the hind-limbs of rats. In addition, recent research by Ornstrup et al. suggested that RSV improves LPS-inhibited osteoblast differentiation in vitro [11]. However, the mechanisms by which RSV attenuate LPS-inhibited osteoblast differentiation remain elusive.
RSV was confirmed to mediate cell function through targeting Sirt1 [15], which can positively regulate the peroxisome proliferator-activated receptor γ coactivator, PGC-1α [16,17]. Sirt1 and PGC-1α signaling are involved in the regulation of RSV in mitochondrial function. Thus, it is not clear whether the improvement of mitochondrial function by RSV alleviates LPS-inhibited osteoblast differentiation.
Therefore, in the present study, we used a cellular LPS-induced model of bone degradation in MC3E3-T1 to determine whether RSV has bone-protective effects, and to explore the underlying mechanisms.
Material and Methods
Cell culture and treatment
A mouse calvaria-derived preosteoblast cell line, MC3T3-E1 (ATCC; Manassas, VA), was used as a model of osteoblasts, as previously reported [18]. Cells were cultured in DME media supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin solution in a humidified culture chamber under conditions of 95% air and 5% CO2 at 37°C. Before treatment, cells were planted in tissue culture-treated plates (Corning, China) and grown to 85% confluence. To examine the effect of LPS on mitochondrial function, a variety of concentrations of LPS were used to incubate the cells (0, 0.5, 1, and 2 μg/ml) for 24 h according to a previously described method [19]. To study the effects of RSV on LPS-influenced mitochondrial function, cells were incubated for 24 h with LPS alone, LPS + RSV, or vehicle solution according to a previously published method with minor modifications [12]. MC3T3-E1 cells with 85% confluence were first cultured in osteogenic differentiation medium. After 48 h, the cells were incubated with LPS alone or in the presence of RSV, and those cells treated with vehicle were used as control.
MTT assay
Cells were seeded in a 96-well plate and subjected to LPS (2 μg/ml) stimulation in the presence or absence of RSV (25 μM). After treatment, 20 μl MTT was added to each well and incubated for 4 h at 37°C, followed by addition of 150 μL DMSO (dimethyl sulfoxide) to dissolve the formed purple formazan dye. Then, a scanning muti-well spectrophotometer (Multiskan MK3, Thermo Scientific, USA) was used to measure the absorbance at 490 NM. The vehicle-treated control group was taken as 100% cell viability and all other groups were normalized to this value.
Measurement of mitochondrial ATP concentration
Determination of ATP concentration in mitochondria of MC3T3-E1 was performed using an ATP assay kit (Beyotime, China) based on a previously published method [20]. Briefly, the culture medium of MC3T3-E1 cells from different groups was discarded, and cells were lysed with lysis buffer on ice. The lysed samples were centrifuged (10,000×g, 5 min, 4°C), and the supernatant was collected for ATP determination. Then, signals from luciferase-catalyzed fluorescein reaction were detected using a microplate reader (Bio-Rad, Hercules, CA). The obtained values were finally calculated to determine the changes in mitochondrial ATP levels of MC3T3-E1.
Measurement of mitochondrial membrane potential (MMP)
MMP was detected using fluorescent probe JC-1 (Beyotime, China) in accordance with the manufacturer’s instructions. Briefly, adherent MC3T3-E1 cells from different groups were rinsed with PBS and incubated with JC-1/DMEM (1: 1) for 20 min at 37°C. After staining, cells were washed twice using JC-1 staining buffer. Then, fluorescent signals were detected using a fluorescence microplate reader (Bio-Rad, Hercules, CA). The wavelengths of excitation/emission (Ex/Em) for red fluorescent J-aggregates were set at 525 nm/590 nm, and at 490 nm/530 nm for green fluorescent monomer. The ratios of J-aggregates and monomer were finally calculated to determine the MMP of MC3T3-E1.
Measurement of mitochondrial superoxide production
Cellular superoxide production in mitochondria was detected using the MitoSOX™ Red mitochondrial superoxide indicator (Molecular Probes, Eugene, OR) according to the manufacturer’s protocol and a previously published method [21]. Briefly, adherent MC3T3-E1 cells from different groups were incubated with MitoSOX™ reagent working solution (5 μM) at 37°C in the dark for 10 min. After incubation, cells were rinsed 3 times using warm Hank’s balanced salt solution containing Ca2+/Mg2+ (HBSS; Gibco, USA). Then, fluorescent signals were detected using a fluorescence microplate reader. The wavelengths of Ex/Em were set at 510 nm/580 nm. Relative fluorescence was finally calculated to indicate the mitochondrial superoxide production of MC3T3-E1.
RNA extraction and quantitative real-time PCR (RT-PCR) analysis
For the detection of osteogenesis-related genes, total RNA was extracted from MC3T3-E1 cells using TRIzol reagent (Takara, Japan), and cDNA was synthesized using PrimeScript™ RT Master Mix (Takara, Tokyo, Japan) by reverse transcription according to the manufacturer’s protocols. Then, the transcription levels were determined by RT-PCR with the SYBR Green PCR kit (Takara, Tokyo, Japan) utilizing the ABI PRISM 7500 sequence detection system (Life Technologies Corporations, Carlsbad, CA). The PCR reaction system consisted of 1 μl cDNA, 10 μl SYBR® Premix Ex Taq (Takara), and 0.25 μM of each paired primer in a total volume of 20 μl. The reaction condition was: initial denaturation at 95°C for 30 s followed by 40 cycles at 95°C for 5 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s [22]. Arithmetic formulae (2-ΔΔCt method) were applied to determine relative changes in gene expression over the internal control, β-actin [23]. The primer sequences of ALP, OCN, OPN, RUNX2, and β-Actin were obtained from Shanghai Sangon Company (Table 1; Sangon, Shanghai, China).
Table 1.
Primer sequences and used for qRT-PCR.
| Gene | Primer sequence (5′–3′) |
|---|---|
| ALP | F: GAGCGTCATCCCAGTGGAG |
| R: TAGCGGTTACTGTAGACACCC | |
| OCN | F: GAGGGCAATAAGGTAGTGAA |
| R: CATAGATGCGTTTGTAGGC | |
| OPN | F: CTCAGAAGCAGAATCTC |
| R: ATGGTCTCCATCGTCATCAT | |
| RUNX2 | F: TTCAACGATCTGAGATTTGTGGG |
| R: GGATGAGGAATGCGCCCTA | |
| β-actin | F: GGCTGTATTCCCCTCCATCG |
| R: CCAGTTGGTAACAATGCCATGT |
F – Forward; R – Reverse.
ELISA analysis
Cellular ALP, OCN, OPN, and RUNX2 levels were quantitatively determined using commercially available ELISA kits (Excellbio, Shanghai, China) based on the manufacturer’s instructions. Briefly, adherent MC3T3-E1 cells from different groups were lysed and collected. Next, a total of 100 μl samples were added to the microplate and incubated for 2 h. Then, equal volumes of primary antibodies against ALP, OCN, OPN, or RUNX2 were added to each well and incubated for another 1 h, followed by final incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies for 0.5 h. All the above incubations were performed at 37°C, washing 3 times with TBST after each incubation. The optical density (OD) values at 450 nm were detected using a fluorescence microplate reader (Bio-Rad, Hercules, CA). The aforementioned cellular levels were finally calculated according to the OD values obtained [19].
Western blot analysis
After treatments, adherent MC3T3-E1 osteoblasts were rinsed with cold PBS and lysed with cold RIPA lysis buffer (Beyotime, China). The resulting cell lysates were collected and centrifuged (12 000×g, 15 min, 4°C). After centrifugation, total protein of each sample was quantified by BCA method (Thermo Scientific, Rockford, IL) according to the instruction of the manufacturer, and boiled in loading buffer (Beyotime, China). Equal amounts of proteins from each sample were then separated on 10% SDS-PAGE gel and transferred to a nitrocellulose membrane (Coring, China) as previously reported [24]. The membrane was blocked with 5% BSA in Tris-buffered saline containing 0.1% Tween 20 (TBST) for 2 h at 37°C and cultured with primary antibody against Sirt1 (1: 1000; Santa Cruz), PGC-1α (1: 1000; Santa Cruz), or β-actin (1: 1000; Beyotime, China) overnight at 4°C. Then, the membrane was exposed to the appropriate IRDye 800CW-conjugated secondary antibodies (1: 5000; LI-COR Bioscience, Lincoln, NE) for 0.5 h at 37°C. Infrared fluorescence bands were visualized using an Odyssey infrared imaging system (LI-COR Bioscience, Lincoln, NE). The obtained bands were finally quantified with Quantity One software (Bio-Rad, Hercules, CA) and ratios of the protein of interest and β-actin were calculated to determine changes in protein levels [25].
Statistical analysis
Data were collected from 3 or more independent experiments and are shown as means ± standard deviation (SD). Statistical analyses were performed by one-way ANOVA followed by Tukey’s test using GraphPad Prism software (Version 5.01). Statistical significance was set at P<0.05.
Results
LPS-induced mitochondrial dysfunction in MC3T3-E1 cells
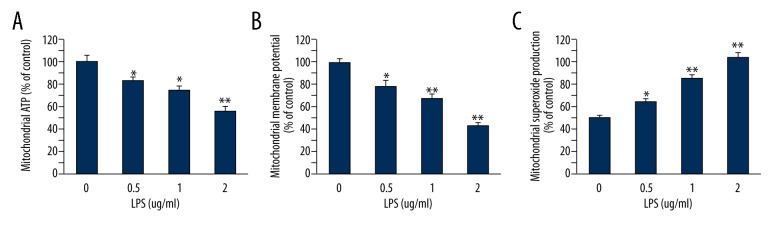
To assess the effect of LPS on mitochondrial function in cultured MC3T3-E1, cells were incubated with 0, 0.5, 1, or 2 μg/ml LPS for 24 h, and biomarkers of mitochondrial dysfunction were then assessed. As shown in Figure 1, cellular mitochondria function in MC3T3-E1 cells were significantly decreased by LPS in a dose-dependent manner, characterized by decreasing mitochondrial ATP production (Figure 1A) and mitochondrial membrane potential (Figure 1B), and increasing mitochondrial superoxide production (Figure 1C).
Figure 1.
Effects of LPS on cellular mitochondrial functions in MC3T3-E1 cells. (A) LPS decreased ATP levels in mitochondria of MC3T3-E1 cells in a dose-dependent manner. (B) LPS decreased MMP of MC3T3-E1 cells in a dose-dependent manner. (C) LPS increased superoxide production in mitochondrial of MC3T3-E1 cells in a dose-dependent manner. Data are mean ±SD, n=8. * P<0.05, ** P<0.01 vs. vehicle.
Resveratrol improved cellular dysfunctions in LPS-treated MC3T3-E1 cells
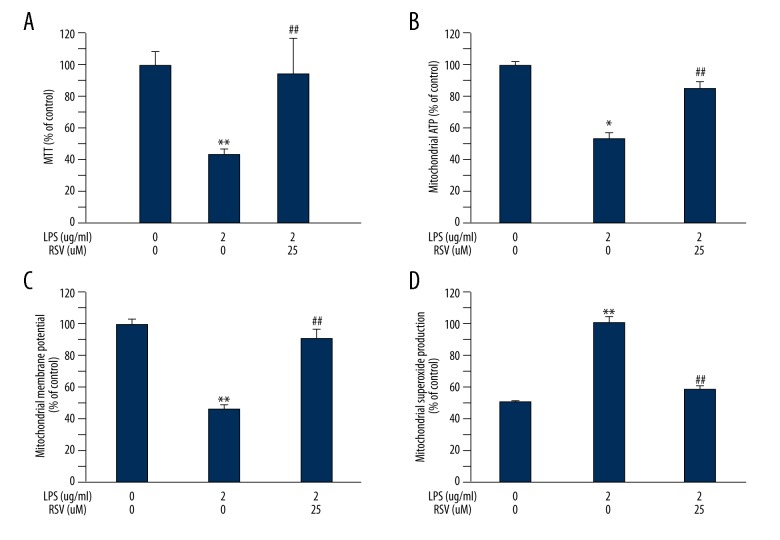
As shown in Figure 2A, MTT assay revealed that the decreased cell viability of MC3T3-E1 induced by LPS was markedly increased by RSV (25 μM) treatment. Next, we observed the effect of resveratrol on LPS-inhibited mitochondrial function of MC3T3-E1 cells through detecting ATP production in mitochondria, mitochondrial membrane potential, and superoxide production. We found that resveratrol significantly attenuated mitochondrial dysfunction of LPS-exposed MC3T3-E1 cells. The ATP production in mitochondria (Figure 2B) and MMP (Figure 2C) were significantly improved in cells treated with resveratrol compared to those in the LPS group, and there was a significant decrease in mitochondrial superoxide production (Figure 2D). These results show that resveratrol significantly attenuated the inhibitory influence of LPS on mitochondrial dysfunction.
Figure 2.
Effect of resveratrol treatment in LPS-stimulated MC3T3-E1 cells on cell viability and mitochondrial dysfunctions. Resveratrol significantly increased cell viability (A), mitochondrial ATP levels, and MMP (B) in MC3T-E1 cells. (C) Resveratrol reduced superoxide production in mitochondria. Data are mean ±SD, n=8. * P<0.05, ** P<0.01 vs. vehicle. ## P<0.01 vs. LPS.
Resveratrol ameliorated LPS-inhibited osteogenic differentiation in MC3T3-E1 cells
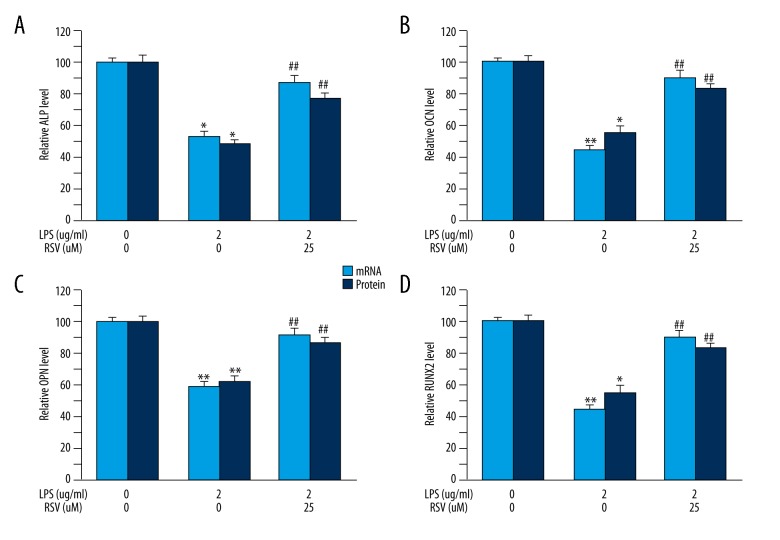
To determine the effect of resveratrol on LPS-inhibited osteogenic differentiation in MC3T3-E1 cells, we detected mRNA and protein level of osteogenic markers, including ALP, OCN, OPN, and RUNX2, through PCR and ELISA methods. We found that resveratrol alleviated the toxic activity of LPS during the differentiation of MC3T3-E1 cells into osteoblasts. In addition, LPS obviously decreased osteogenic differentiation, as indicated by the significantly reduced levels of ALP (Figure 3A), OCN (Figure 3B), OPN (Figure 3C), and RUNX2 (Figure 3D). However, administration of resveratrol attenuated LPS-suppressed MC3T3-E1 osteogenic differentiation, with significantly increased levels of osteogenic markers (Figure 3).
Figure 3.
Effect of resveratrol treatment in LPS-stimulated MC3T3-E1 cells on osteoblast differentiation-associated markers. Resveratrol significantly increased both mRNA and protein levels of ALP (A), OCN (B), OPN (C), and RUNX2 (D) in MC3T3-E1 cells. Data are mean ±SD, (A) n=7–8; (B) n=7 for all experiments. * P<0.05, ** P<0.01 vs. vehicle, ## P<0.01 vs. LPS.
Resveratrol increased Sirt1 and PCG-1α level in LPS-treated MC3T3-E1 cells
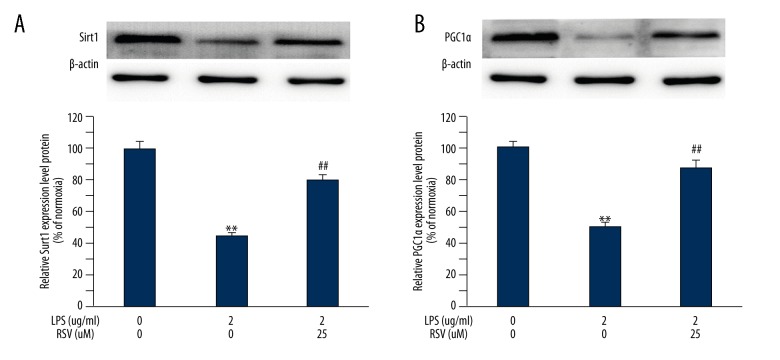
The levels of Sirt1 and PGC-1α were measured in MC3T3-E1 cells treated with LPS at the indicated time points. We found that LPS significantly decreased the expression of Sirt1 and PGC-1α, and this was attenuated by resveratrol. Therefore, we added this agent to the LPS-treated MC3T3-E1 osteoblasts, and cellular Sirt1 and PGC-1α levels were determined at the indicated time points. A significant decrease of the 2 proteins was detected in LPS-treated MC3T3-E1 cells compared to those from the vehicle group (Figure 4). Both Sirt1 (Figure 4A) and PGC-1α were increased in the MC3T3-E1 cells treated with resveratrol (Figure 4B).
Figure 4.
Effect of resveratrol treatment in LPS-stimulated MC3T3-E1 cells on Sirt1 and PCG-1α. Resveratrol increased levels of Sirt1 (A) and PCG-1α (B) in MC3E3-T1 cells. Data are mean ±SD, (A) n=7–8; (B) n=7 for all experiments. ** P<0.01 vs. vehicle, ## P<0.01 vs LPS.
Discussion
In the present study, we demonstrated for the first time that resveratrol can alleviate LPS-inhibited osteoblastic differentiation of MC3T3-E1 cells. In addition, resveratrol attenuated MC3T3-E1 cell impairments caused by LPS, especially for mitochondrial dysfunctions.
Bone degradation, characterized by destructive injury to the bone tissues, is clinically characterized by excessive bone resorption. The condition has been thought to be correlated with gram-negative bacterial LPS [26]. It has been well documented that exposure to LPS causes inhibition of osteoblast differentiation [3–5]. In line with the results of previous studies [27], we found that osteogenic differentiation was also significantly decreased in MC3T3-E1 exposed to LPS.
Mitochondria are involved in many biological processes. Recent studies have elucidated that normal mitochondrial function is essential for osteogenic differentiation [28–34]. Dysfunction in mitochondria is associated with the impairment of osteogenesis [31,32]. The toxic effect produced by LPS was demonstrated to be associated with dysfunction in mitochondria [35–37]. In the present study, LPS reduced ATP production in mitochondria and MMP of MC3T3-E1 cells, while the superoxide production in mitochondria was increased.
Resveratrol, which is linked to myriad physiological benefits, was reported to possess bone-protective effects, such as stimulating osteoblast differentiation [12,38]. To the best of our knowledge, the well-established pattern of osteogenic markers generally includes ALP, OCN, OPN, and RUNX2, all of which are related to bone formation and are widely used for indicating osteogenic differentiation [26]. In accordance with previous studies [12,38], we observed that resveratrol significantly increased the osteoblastic marker levels in LPS-treated MC3T3-E1 cells, suggesting that resveratrol alleviated LPS-inhibited osteoblastic differentiation. It was reported that resveratrol can potently improve mitochondrial function [17]. In the present study, after administrating resveratrol, we found increased mitochondrial ATP and MMP production, as well as a decrease in mitochondrial superoxide production. These results indicate that resveratrol caused a significant improvement of cellular mitochondria function. Mitochondrial function is critical for the regulation of osteogenic differentiation, and mitochondria have been proposed as biological targets of resveratrol [29,31,34,39]. Therefore, the improvement of mitochondrial function and subsequent enhancement of osteogenic differentiation in LPS-treated MC3T3-E1 cells may partly contribute to the bone-protective effect of resveratrol.
Among the various molecular targets of resveratrol, Sirt1 and PCG-1α are intensively studied. Several studies suggested that resveratrol exerts its effects by Sirt1 and PCG-1α [10,17], both of which are important regulators in maintaining mitochondrial function [24]. Lagouge et al. demonstrated that resveratrol can increase the activation of Sirt1 and PCG-1α to protect against metabolic disease [17]. However, no convincing data have been presented to date for the mechanisms of its bone-protective action. In the present study, we observed that administration of resveratrol ameliorated LPS-inhibited osteoblast differentiation in MC3T3-E1 cells, which was accompanied by increased cellular Sirt1 and PCG-1α. Given the crucial role of Sirt1 and PCG-1α in maintaining cellular mitochondrial function, the mechanism underlying the bone-protective effect of resveratrol may converge on these 2 molecules. Future studies should examine how these 2 molecules affect the improved LPS-inhibited osteoblast differentiation of resveratrol.
Conclusions
We herein show that resveratrol protected against inhibition of osteoblast differentiation with LPS-treated MC3T3-E1 cells. The evidence of improvement in cellular mitochondrial function suggests that the mitochondria-protective action of resveratrol might partly contribute to ameliorating inhibition of osteoblast differentiation. This study raises the possibility of clinical application of resveratrol in individuals with bone diseases, such as osteomyelitis. Further investigations are indicated to delineate the mechanisms of resveratrol in maintenance of bone homeostasis.
Footnotes
Source of support: This study was sponsored by the National Natural Science Foundation of China (No. 81472129 and No. 81672202), the Science and Technology Commission of Shanghai Municipality (No. 17411971900 and No. 15411950900), the “Shuguang Program” supported by the Shanghai Education Development Foundation and Shanghai Municipal Education Commission (No. 14SG34), the “Outstanding Young Scholar Program” supported by Second Military Medical University, and the “Research Clinician Program” supported by Shanghai Changzheng Hospital
Conflict of interest
None.
References
- 1.Suda T, Takahashi N, Udagawa N, et al. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20(3):345–57. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 2.Roodman D. Advances in bone biology: The osteoclast. Endocr Rev. 1996;17:308–32. doi: 10.1210/edrv-17-4-308. [DOI] [PubMed] [Google Scholar]
- 3.Wang M, Shen J, Jin H, et al. Recent progress in understanding molecular mechanisms of cartilage degeneration during osteoarthritis. Ann N Y Acad Sci. 2011;1240:61–69. doi: 10.1111/j.1749-6632.2011.06258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo C, Yuan L, Wang J, et al. Lipopolysaccharide (LPS) induces the apoptosis and inhibits osteoblast differentiation through JNK pathway in MC3T3-E1 cells. Inflammation. 2014;37(2):621–31. doi: 10.1007/s10753-013-9778-9. [DOI] [PubMed] [Google Scholar]
- 5.Kadono H, Kido J, Kataoka M, et al. Inhibition of osteoblastic cell differentiation by lipopolysaccharide extract from Porphyromonas gingivalis. Infect Immun. 1999;67(6):2841–46. doi: 10.1128/iai.67.6.2841-2846.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 7.Lin SK, Kok SH, Kuo MY, et al. Nitric oxide promotes infectious bone resorption by enhancing cytokine-stimulatedinterstitial collagenase synthesis in osteoblasts. J Bone Miner Res. 2003;18:39–46. doi: 10.1359/jbmr.2003.18.1.39. [DOI] [PubMed] [Google Scholar]
- 8.Zhang YH, Heulsmann A, Tondravi MM, et al. Tumor necrosis factor-alpha (TNF) stimulates RANKLinduced osteoclastogenesis via coupling of TNF type 1 receptor and RANK signaling pathways. J Biol Chem. 2001;276:563–68. doi: 10.1074/jbc.M008198200. [DOI] [PubMed] [Google Scholar]
- 9.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: The in vivo evidence. Nat Rev Drug Discov. 2006;5(6):493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 10.Tennen RI, Michishita-Kioi E, Chua KF. Finding a target for resveratrol. Cell. 2012;148(3):387–89. doi: 10.1016/j.cell.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 11.Ornstrup MJ, Harslof T, Sorensen L, et al. Resveratrol increases osteoblast differentiation in vitro independently of inflammation. Calcif Tissue Int. 2016;99(2):155–63. doi: 10.1007/s00223-016-0130-x. [DOI] [PubMed] [Google Scholar]
- 12.Mizutani K, Ikeda K, Kawai Y, Yamori Y. Resveratrol stimulates the proliferation and differentiation of osteoblastic MC3T3-E1 cells. Biochem Biophys Res Commun. 1998;253(3):859–63. doi: 10.1006/bbrc.1998.9870. [DOI] [PubMed] [Google Scholar]
- 13.Mobasheri A, Shakibaei M. Osteogenic effects of resveratrol in vitro: Potential for the prevention and treatment of osteoporosis. Ann N Y Acad Sci. 2013;1290:59–66. doi: 10.1111/nyas.12145. [DOI] [PubMed] [Google Scholar]
- 14.Durbin SM, Jackson JR, Ryan MJ, et al. Resveratrol supplementation preserves long bone mass, microstructure, and strength in hindlimb-suspended old male rats. J Bone Miner Metab. 2014;32(1):38–47. doi: 10.1007/s00774-013-0469-2. [DOI] [PubMed] [Google Scholar]
- 15.Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191–96. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 16.Canto C, Auwerx J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell Mol Life Sci. 2010;67(20):3407–23. doi: 10.1007/s00018-010-0454-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127(6):1109–22. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Nagao M, Tanabe N, Manaka S, et al. LIPUS suppressed LPS-induced IL-1α through the inhibition of NF-κB nuclear translocation via AT1-PLCβ pathway in MC3T3-E1 cells. J Cell Physiol. 2017 doi: 10.1002/jcp.25777. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Hao W, Wang X, Su H. miR-23b targets Smad 3 and ameliorates the LPS-inhibited osteogenic differentiation in preosteoblast MC3T3-E1 cells. J Toxicol Sci. 2016;41(2):185–93. doi: 10.2131/jts.41.185. [DOI] [PubMed] [Google Scholar]
- 20.Wang CN, Duan GL, Liu YJ, et al. Overproduction of nitric oxide by endothelial cells and macrophages contributes to mitochondrial oxidative stress in adrenocortical cells and adrenal insufficiency during endotoxemia. Free Radic Biol Med. 2015;83:31–40. doi: 10.1016/j.freeradbiomed.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 21.Vincent M, Kato K, McLean LL, et al. Sensory neurons and schwann cells respond to oxidative stress by increasing antioxidant defense mechanisms. Antioxid Redox Signal. 2009;11(3):425–38. doi: 10.1089/ars.2008.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li T, Cheng H, Yuan H, et al. Antitumor activity of cGAMP via stimulation of cGAS-cGAMP-STING-IRF3 mediated innate immune response. Sci Rep. 2016;6:19049. doi: 10.1038/srep19049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Du JK, Cong BH, Yu Q, et al. Upregulation of microRNA-22 contributes to myocardial ischemia-reperfusion injury by interfering with the mitochondrial function. Free Radic Biol Med. 2016;96:406–17. doi: 10.1016/j.freeradbiomed.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Han X, Deng Y, Yu J, et al. Acarbose accelerates wound healing via Akt/eNOS signaling in db/db Mice. Oxid Med Cell Longev. 2017;2017:7809581. doi: 10.1155/2017/7809581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bandow K, Maeda A, Kakimoto K, et al. Molecular mechanisms of the inhibitory effect of lipopolysaccharide (LPS) on osteoblast differentiation. Biochem Biophys Res Commun. 2010;402(4):755–61. doi: 10.1016/j.bbrc.2010.10.103. [DOI] [PubMed] [Google Scholar]
- 27.Guo C, Yuan L, Wang JG, et al. Lipopolysaccharide (LPS) induces the apoptosis and inhibits osteoblast differentiation through JNK pathway in MC3T3-E1 cells. Inflammation. 2014;37(2):621–31. doi: 10.1007/s10753-013-9778-9. [DOI] [PubMed] [Google Scholar]
- 28.Chen CT, Shih YR, Kuo TK, et al. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26(4):960–68. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- 29.Pietila M, Lehtonen S, Narhi M, et al. Mitochondrial function determines the viability and osteogenic potency of human mesenchymal stem cells. Tissue Eng Part C Methods. 2010;16(3):435–45. doi: 10.1089/ten.tec.2009.0247. [DOI] [PubMed] [Google Scholar]
- 30.Arakaki N, Yamashita A, Niimi S, Yamazaki T. Involvement of reactive oxygen species in osteoblastic differentiation of MC3T3-E1 cells accompanied by mitochondrial morphological dynamics. Biomed Res. 2013;34(3):161–66. doi: 10.2220/biomedres.34.161. [DOI] [PubMed] [Google Scholar]
- 31.Kwon IK, Lee SC, Hwang YS, Heo JS. Mitochondrial function contributes to oxysterol-induced osteogenic differentiation in mouse embryonic stem cells. Biochim Biophys Acta. 2015;1853(3):561–72. doi: 10.1016/j.bbamcr.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Tsai PH, Chien Y, Chuang JH, et al. Dysregulation of mitochondrial functions and osteogenic differentiation in Cisd2-deficient murine induced pluripotent stem cells. Stem Cells Dev. 2015;24(21):2561–76. doi: 10.1089/scd.2015.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shum LC, White NS, Mills BN, et al. Energy metabolism in mesenchymal stem cells during osteogenic differentiation. Stem Cells Dev. 2016;25(2):114–22. doi: 10.1089/scd.2015.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q, Gao Z, Chen Y, Guan MX. The role of mitochondria in osteogenic, adipogenic and chondrogenic differentiation of mesenchymal stem cells. Protein Cell. 2017;8(6):439–45. doi: 10.1007/s13238-017-0385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun M, Wang R, Han Q. Inhibition of leukotriene B4 receptor 1 attenuates lipopolysaccharide-induced cardiac dysfunction: Role of AMPK-regulated mitochondrial function. Sci Rep. 2017;7:44352. doi: 10.1038/srep44352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu C, Dai X, Yang Y, et al. Dexmedetomidine attenuates lipopolysaccharide-induced acute lung injury by inhibiting oxidative stress, mitochondrial dysfunction and apoptosis in rats. Mol Med Rep. 2017;15(1):131–38. doi: 10.3892/mmr.2016.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunter R, Ojha U, Bhurtel S, et al. Lipopolysaccharide-induced functional and structural injury of the mitochondria in the nigrostriatal pathway. Neurosci Res. 2017;114:62–69. doi: 10.1016/j.neures.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Boissy P, Andersen TL, Abdallah BM, et al. Resveratrol inhibits myeloma cell growth, prevents osteoclast formation, and promotes osteoblast differentiation. Cancer Res. 2005;65(21):9943–52. doi: 10.1158/0008-5472.CAN-05-0651. [DOI] [PubMed] [Google Scholar]
- 39.Ikeda K. Mitochondrial function in bone resorption and formation. Nihon Rinsho. 2011;69(7):1203–8. [PubMed] [Google Scholar]