Abstract
Background
The current guideline for oxytocin regimens in the abnormal labor of delivery is continuous infusion. The objective of the present study was to compare effects and safety measures of various available regimens of oxytocin in abnormal labor delivery.
Material/Methods
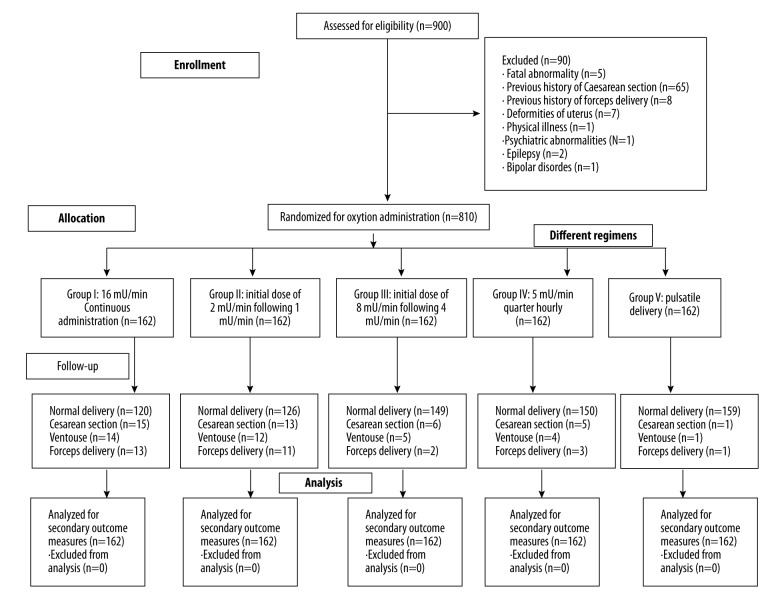
In this clinical experimental study, a total of 900 pregnant women admitted for delivery were randomized into 5 group with 162 each. Pregnant women received oxytocin as continuous administration of 16 mU/min (Group I), 1 mU/min (group II), 4 mU/min (group III), 5 mU/min quarter-hourly (group IV), and through a syringe pump (group V). Measurement of the expense of delivery, the ratio of the instrumental delivery, and the other secondary outcome measures was performed to find the best regimen of oxytocin. The 2-tailed paired t test and Mann-Whitney U test following Dunnett’s multiple comparison tests were used at 95% confidence level.
Results
Pulsatile delivery had least risk of instrumental delivery as compared to continuous infusion (p<0.0001, q=6.663) and normal-frequency low-dose (p<0.0001, q=5.638) of oxytocin. The time required from infusion to delivery was longer for group II (p=0.001, q=2.925), group IV (p<0.0001, q=4.829), and group V (p<0.0001, q=41.456) than for group I. The expense of delivery was: group I < group II < group IV < group III < group V.
Conclusions
High-dose and pulsatile preparation of oxytocin had reduced risks of operative delivery vs. continuous administration.
MeSH Keywords: Delivery, Obstetric; Labor, Induced; Obstetric Labor Complications; Pulsatile Flow; Vasotocin
Background
Failure to have uterine contractions and cervix enhancement are defined as abnormal labor (AL) or dystocic delivery (Figure 1) [1] and is common in childbirth [2]. To overcome such conditions, Caesarean section (CS), forceps delivery (FD), oxytocin (OXT) [3], amniotomy [4], and hydration administration are used before delivery. OXT is a nonapeptide [5] that is naturally secreted by the posterior pituitary gland, and a synthetic form is used in AL for normal vaginal delivery [6]; it stimulates uterine contractility [3] and is also used in the treatment of postpartum hemorrhage [7].
Figure 1.
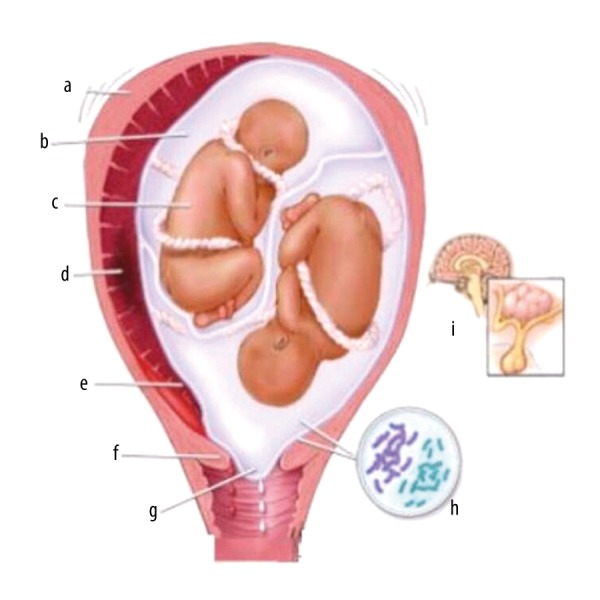
Pictorial presentation of reasons for abnormal labor. a: excess contraction, b: too much fluid, c: multiple fetuses, d: ischemia, e: placental abruption, f: cervical factor, g: rupture of membrane, h: hormonal changes, i: bacterial infection.
The current guideline for OXT regimens in AL delivery is continuous infusion [8]. However, cardiac effects have been also reported by continuous infusion of OXT [6]. There are various regimens available for OXT, including low-dose [5], high-dose [3], increased frequency [5], and pulsatile preparations [9]. Different regimens have different outcome measures and adverse effects as per the conditions and profile of the patients [3]. To date, available studies are only comparisons of continuous infusion and pulsatile delivery of OXT. However, an individualized treatment does not exist, and arrested labor is handled according to adopted clinical guidelines rather than taking into account the status of the uterus and the pathological and anatomical conditions of pregnant women [1]. Most of the randomized studies had a high rate of operative delivery in abnormal labor [10]. The present study compared all regimens of OXT available in the market for augmentation of labor in child birth, aiming to help obstetrics with the selection of proper regimens of OXT for augmentation of labor. To the best of our knowledge, no single study has compared all dosage forms of OXT for augmentation of labor. The present study was carried out to determine if it is possible to decrease the rate of instrumental deliveries and to increase the health of the mothers without affecting the health of the neonates, and to assess the economic implications of various regimens.
The primary objective of the study was to compare the ratio of instrumental delivery. The secondary endpoint of the study was to compare secondary outcome measures for various available regimens of OXT in AL following delivery for augmentation of labor in Chinese pregnant women.
Material and Methods
Material
Various regimens of OXT injection (Pitocin®) were purchased from Pfizer Inc. (USA). Normal saline was purchased from Baxter (China) Pvt. Ltd.
Ethics statement
The Maternal and Neonatal Ethics Committee of Wuhan Maternal and Child Healthcare Hospital, China approved the protocol of augmentation of delivery, and we followed the ethics guidelines for sexual and reproductive healthcare research on female participants for childbirth in accordance with Chinese law [11].
Inclusion criteria
From 1 January 2013 to 31 December 2015, there were 900 pregnant women admitted for childbirth to Wuhan Maternal and Child Healthcare Hospital, Wuhan, China and to Second People’s Hospital of Huanggang City, China. Wuhan Maternal and Child Healthcare Hospital and Second People’s Hospital of Huanggang City are non-governmental referral bodies of the Ministry of Education and Ministry of Health in PR China. This clinical experimental study enrolled primiparous women with cervical dilation at least 28 mm with no augmentation, the absence of any pregnancy complications, singleton pregnancy, with an arrested labor progress per the partogram, with a need for oxytocin for augmentation of labor. We only included women over 18 years of age, who had gestational age 260–295 days, and who gave informed consent for augmentation of labor and publication of their data in educational magazines, journals, and textbooks in any form or medium (including all forms of electronic publication or distribution) anywhere in the world without time limit (Table 1).
Table 1.
Anatomical and prenatal characteristics of pregnant women.
| Parameters | Group | ||||||
|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | |||
| Treatment of OXT | 16 mU/min Continuous administration | 2 mU/min following 1 mU/min Normal frequency | 8 mU/min following 4 mU/min Normal frequency | 5 mU/min quarter-hourly | Pulsatile preparations | ||
| Sample Size | 162 | 162 | 162 | 162 | 162 | ||
| Age (Mean ±SD) (years) | 25.75±1.84 | 25.46±1.95 | 25.53±2.46 | 26.84±1.62 | 28.44±3.07 | ||
| Gestational age (Mean ±SD) (days) | 282±8 | 281±5 | 278±4 | 281±5 | 283±7 | ||
| Presence of genital Infection | 5 (3) | 4 (2) | 6 (4) | 7 (4) | 8 (5) | ||
| Educated# | 80 (49) | 85 (52) | 86 (53) | 87 (54) | 89 (55) | ||
| Smokers | 12 (7) | 13 (8) | 14 (9) | 15 (9) | 17 (10) | ||
| Alcoholic | 9 (6) | 8 (5) | 13 (8) | 12 (7) | 11 (7) | ||
| First time delivery | 144 (89) | 142 (88) | 138 (85) | 139 (86) | 143 (88) | ||
| Second delivery | 16 (10) | 18 (11) | 21 (13) | 21 (13) | 18 (11) | ||
| More than second delivery | 2 (1) | 2 (1) | 3 (2) | 1 (1) | 1 (1) | ||
| Origin | Chinese | 160 (99) | 161 (99) | 162 (100) | 161 (99) | 162 (100) | |
| Non-Chinese | 2 (1) | 1 (1) | 0 (0) | 1 (1) | 0 (0) | ||
| Pre-pregnancy BMI (kg/m2) | Underweight (≤18.5) | 11 (7) | 10 (6) | 12 (7) | 13 (8) | 9 (5) | |
| Normal (>18.5 but ≤24.9) | 136 (83) | 135 (83) | 135 (83) | 134 (83) | 139 (86) | ||
| Overweight (≥25 but ≤29.9) | 12 (7) | 13 (8) | 12 (7) | 9 (5) | 9 (5) | ||
| Obese (class I) (≥30 but ≤34.9) | 1 (1) | 2 (1) | 1 (1) | 4 (2) | 3 (2) | ||
| Obese (class II) (≥35 but ≤39.9) | 1 (1) | 1 (1) | 1 (1) | 1 (1) | 1 (1) | ||
| Extreme obesity (class III) (≥40) | 1 (1) | 1 (1) | 1 (1) | 1 (1) | 1 (1) | ||
| Blood pressure (mmHg) | Systolic | ≥120 but ≤149 | 157 (97) | 156 (96) | 154 (95) | 155 (96) | 159 (98) |
| ≥150 | 5* (3) | 6 (4) | 8 (5) | 7* (4) | 3 (2) | ||
| Diastolic | ≥90 but ≤99 | 160 (99) | 157 (97) | 159 (98) | 158 (98) | 161 (99) | |
| ≥100 | 2 (1) | 5 (3) | 3* (2) | 4 (2) | 1 (1) | ||
BMI – body mass index; OXT – oxytocin. Data were represented as Number (Percentage).
Cardiac patient,
Graduate or more.
Insignificant differences in anatomical and prenatal characteristics (p≥0.01). Patients with history of serious diseases were excluded from the study.
Exclusion criteria
Pregnant women who had a fetal abnormality or previous history of CS and FD were excluded for augmentation of labor. Women who had deformities of the uterus, physical illness, psychiatric abnormalities, epilepsy, or bipolar disorders were excluded from the study, as were women under 18 years of age, who had gestational age less than 259 days, and who had a history of serious diseases.
Prior sample size calculations
Open Epi source 3.01 (Epidemiologic Statistics for Public Health) was used for prior sample size calculations. The prior sample size was found to be 162 in each of 5 group (simple randomization). The parameters were considered as population size (N): 900, hypothesized% frequency (p): 85±5%, confidence limit: 95%, and design effect: 1 [12]. The 5-arm study tree is presented in Figure 2.
Figure 2.
The 5-arm clinical experimental study tree for different oxytocin regimens administration in abnormal labor condition.
Intervention
Continuous administration of 16 mU/min OXT was performed according to obstetrics guidelines (group I) [13]. There was an initial dose of 2 mU/min after 1 mU/min (group II), and the initial dose of 8 mU/min after 4 mU/min (group III) OXT [3]. There was 5 mU/min OXT quarter-hourly (group IV) administered [14]. The syringe pump (Wuhan Union Medical Technology Co., Ltd., Wuhan, China) was used for pulsatile OXT delivery (group V). The pump delivered a one-eighth portion of the continuous administration[9].
Primary outcome measures
The rate of operative delivery or the instrumental delivery, including CS, Ventouse, and FD, was evaluated for each group [15].
Secondary outcome measures
We evaluated secondary outcomes such as failed vaginal birth within 24 h after oxytocin administration, time required from infusion to delivery, and time required for pain relief. Maternal death, child death, uterine hyperstimulation, post-delivery hemorrhage, Apgar score, and the time required for the first stage of labor to delivery were counted for each group [9].
Sample collection
At delivery, samples were collected before and after labor room administration. Data on CS, Ventouse, and FD were collected from midwifery. The urine samples and vaginal biopsies were taken for uterine hyperstimulation, post-delivery hemorrhage, Apgar score, and the time required for the first stage of labor to delivery [16]. A vaginal biopsy was performed by an obstetrician using urethra cystoscope flexible biopsy forceps urologic instruments (Henan Forever Medical Co., Ltd., Guangzhou, China). The sample collected was immediately sent to the Pathology Department for further analysis. The pathologist was blinded to the regimens used.
Expense of delivery
The cost for pathology, hospitalization, interventions, and follow-up of each woman in the study were evaluated. The pathology cost included the cost of pathology before delivery. The intervention cost included intervention, cold-chain logistics, wastage, and distribution costs. The hospitalization cost included case charges, indoor room charges, ICU charges, and ventilator charges. The follow-up cost was included after discharge treatment and pathology [17].
Postnatal questionnaires
A structured questionnaire was used, including questions on physical complaints, only post-delivery-related pain, sleep, energy, self-evaluated life stress, and perceived life satisfaction at 3-month intervals during the postnatal period [18].
Statistical analysis
All data are represented as mean ± SD (standard deviation) of 5 independent results. The 2-tailed paired t test (InStat Statistica, GraphPad Software, Inc., CA, USA) considering β=0.1 and α=0.05 [12] following Dunnett’s multiple comparative test (InStat Statistica, GraphPad Software, Inc., CA, USA) considering q>2.44 as significant value [19] were used for anatomical and prenatal characteristics of pregnant women, primary outcome measures after treatment, and expense of delivery. The Mann-Whitney U test (InStat Statistica, GraphPad Software, Inc., CA, USA) considering Mann-Whitney U-statistic (U’) <14,823 as the significant value [15] following Dunnett’s multiple comparative tests considering q>2.211 as the significant value [20] was used for secondary outcome measures and answers to questionnaires during postnatal follow-up. The secondary outcome measures and answers to questionnaires during follow-up of the postnatal period had lacking normal distributions; therefore, a Mann-Whitney U-statistic was performed. The data were considered as significant at 95% of confidence level and Intention-To-Treat (ITT) analysis [21]. Missing data were balanced across groups by re-inclusion [22].
Results
The enrolled pregnant women in all groups had the same kind of anatomical and prenatal characteristics at the time of augmentation of labor (p≥0.01 for all).
Normal-frequency high-dose (p<0.0001, q=4.954), high-frequency low-dose (p<0.0001, q=5.125), and pulsatile delivery (p<0.0001, q=6.663) of OXT had negligible risk of instrumental delivery as compared to traditional continuous infusion administration of OXT. However, risks were not reduced in normal-frequency low-dose OXT (p=0.0138, q=1.025) vs. continuous infusion of OXT. Moreover, pulsatile delivery of OXT had the least risk of instrumental delivery as compared to normal-frequency low-dose (p<0.0001, q=5.638) therapies of OXT (Table 2).
Table 2.
Comparisons of primary outcome measures.
| Type of delivery | Measures | Group | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | SA | III | SA | IV | SA | V | SA | ||||||||
| P (group II vs. I) | p (group III vs. I) | Q (group III vs. I) | p (group III vs. II) | q (group III vs. II) | p (group IV vs. I) | q (group IV vs. I) | p (group IV vs. III) | p (group V vs. I) | q (group V vs. I) | |||||||
| Instrumental delivery | CS | 15 (9) | 13 (8) | 0.1579 | 6 (4) | 0.0025 | 2.326 | 0.0078 | 1.569 | 5 (3) | 0.0014 | 2.585 | 0.3188 | 1 (1) | 0.0001 | 3.619 |
| Ventouse | 14 (9) | 12 (7) | 0.16 | 5 (3) | 0.0026 | 2.446 | 0.0079 | 1.926 | 4 (2) | 0.0013 | 2.718 | 0.319 | 1 (1) | 0.0002 | 3.533 | |
| FD | 13 (8) | 11 (7) | 0.3188 | 2 (1) | 0.0008 | 3.27 | 0.0025 | 2.636 | 3 (2) | 0.0014 | 2.972 | 0.312 | 1 (1) | 0.0004 | 3.567 | |
| Normal delivery | 120 (74) | 126 (78) | 0.0138 | 149 (92) | <0.0001 | 4.954 | <0.0001 | 3.93 | 150 (93) | <0.0001 | 5.125 | 0.318 | 159 (97) | <0.0001 | 6.663 | |
N/A – not applicable. Sample size: n=162 for all groups. CS – Cesarean section; FD – forceps delivery; SA – statistical analysis value. Data were represented as Number (Percentage). For statistical analysis, instrumental delivery was considered as 1 and normal delivery was considered as 0.
The time required for pain relief for women was the same in all groups (p overall≥0.05, Figure 3).
Figure 3.
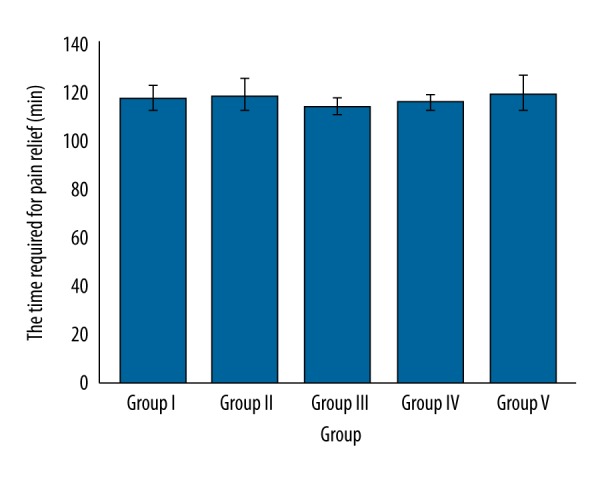
Comparisons of the time required for pain relief. No significant difference among treatments (p overall ≥0.05). Data are represented as mean ±SD, n=162.
The time required from infusion to delivery was longer for group II (p=0.001, q=2.925), group IV (p<0.0001, q=4.829), and group V (p<0.0001, q=41.456) as compared to group I. Group IV (p<0.0001, q=4.247) also had a longer time from infusion to delivery than in group III. However, the time required from infusion to delivery was the same between group I and III (p=0.6912) and between group II and III (p=0.0103, q=0.407) (Figure 4).
Figure 4.
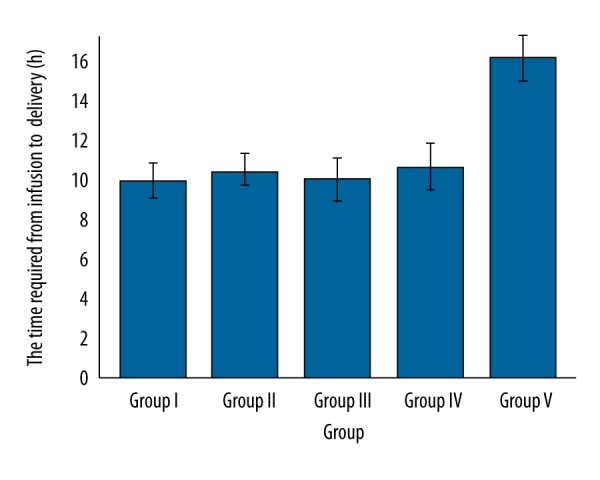
Comparisons of the time required from infusion to delivery (h) between groups. Data are represented as mean ±SD, n=162.
More time was required for the first stage of labor to delivery for group V (p<0.0001, q=5.796) vs. group I. However, the time required for the first stage of labor to delivery was the same between the other groups (p≥0.05, U’>14,823, Figure 5).
Figure 5.
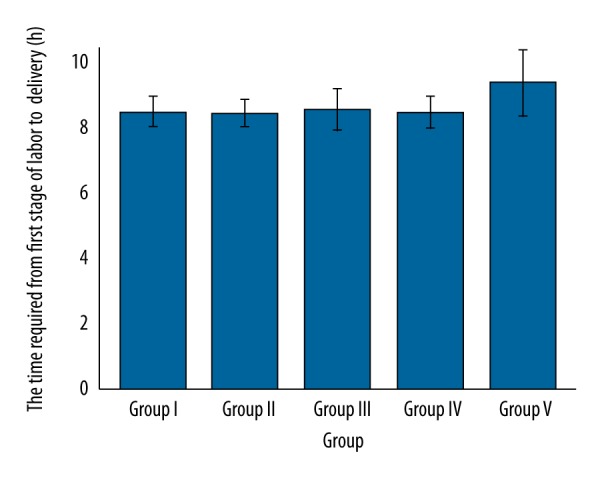
Comparisons of the time required for the first stage of labor to delivery. Higher for group V (p<0.0001, q=5.796) vs. group I. Insignificant differences between the other treatments (U’>14,823). Data are represented as mean ±SD, n=162.
Failed vaginal birth within 24 h, maternal death, death of a child, post-delivery hemorrhage, and Apgar score <7 at 5 min were more common (U’>14,823) in group V compared to group I (U’<14,823, q>2.211) (Table 3).
Table 3.
Comparisons of secondary outcome measures.
| Measures | Group | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | SA | III | SA | IV | SA | V | SA | ||||||||
| p (group II vs. I) | Q (group II vs. I) | p (group III vs. I) | p (group III vs. II) | q (group III vs. II) | p (group IV vs. I) | q (group IV vs. I) | p (group IV vs. III) | q (group IV vs. III) | p (group V vs. I) | q (group V vs. I) | ||||||
| FVB | 40 (25) | 42 (26) | 0.1579 | N/A | 38 (23) | 0.1579 | 0.0452 | 0.5613 | 35 (22) | 0.0249 | 0.6987 | 0.0833 | N/A | 12 (7) | <0.0001 | 3.913 |
| DW | 3 (2) | 4 (2) | 0.919 | N/A | 5 (3) | 0.8385 | 0.9191 | N/A | 2 (1) | 0.9189 | N/A | 0.7595 | N/A | 2 (1) | 0.9189 | N/A |
| DC | 5 (3) | 4 (2) | 0.9191 | N/A | 4 (2) | 0.9191 | N/A | N/A | 2 (1) | 0.7592 | N/A | 0.8382 | N/A | 3 (2) | 0.8384 | N/A |
| PDH | 23 (14) | 15 (9) | 0.4209 | N/A | 25 (15) | 0.4819 | 0.3146 | N/A | 18 (11) | 0.6158 | N/A | 0.4824 | N/A | 2 (1) | 0.033 | 4.563 |
| AS | 9 (6) | 8 (5) | 0.9195 | N/A | 7 (4) | 0.8391 | 0.9194 | N/A | 6 (4) | 0.7603 | N/A | 0.9193 | N/A | 1 (1) | 0.4135 | N/A |
N/A – not applicable. Sample size: n=162 for all groups. FVB – failed vaginal birth within 24 h; DW – death of women; DC – death of child; PDH – post-delivery hemorrhage; AS – Apgar score <7 at 5 min; SA: – statistical analysis value. Data were represented as Number (Percentage). For statistical analysis, secondary outcome event was considered as 1 and absence of that event was considered as 0.
There was not a significant reduction in uterine hyperstimulation in group II (p=0.4811), group III (p=0.6154), or group IV (p=0.2694) compared to group I, as reported in Figure 6. However, there was a significant reduction in uterine hyperstimulation in pulsatile OXT regimen (p=0.0328, q=4.422) vs. continuous administration.
Figure 6.
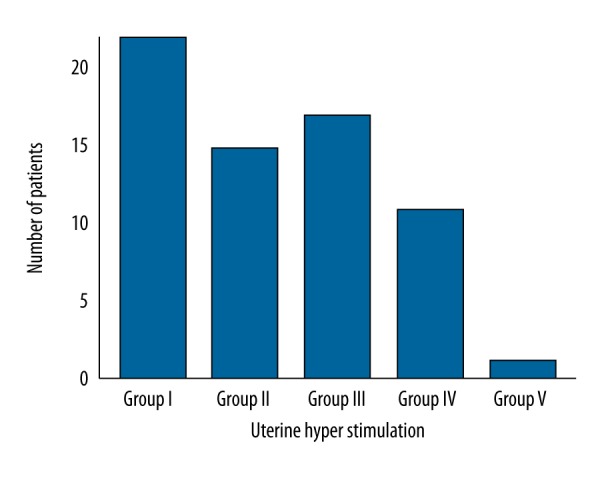
Uterine hyperstimulation effect due to different oxytocin regimens. The significant reduction in group V (p=0.0328, q=4.422) vs. group I. For statistical analysis, uterine hyperstimulation event was considered as 1 and absence of that event was considered as 0.
The cost of OXT administration is shown in Table 4: group I < group II < group IV < group III < group V.
Table 4.
Total expense of the delivery (Euro).
| Cost parameters | Group | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | SA | SA | SA | SA | ||||||||||||
| P (group II vs. I) | q-(group II vs. I) | III | P (group III vs. I) | q (group III vs. I) | P (group III vs. II) | q (group III vs. II) | IV | p (group IV vs. I) | q (group IV vs. I) | p (group IV vs. III) | q (group IV vs. III) | V | p (group V vs. I) | q (group V vs. I) | |||
| P | 14.2 ±1.85 | 135 ±1.79 | 0.0008 | 2.801 | 14.73 ±2.57 | 0.397 | N/A | <0.0001 | 4.615 | 16.14 ±2.72 | <0.0001 | 7.716 | <0.0001 | 5.31 | 17.57 ±2.22 | <0.0001 | 13.993 |
| H | 147.32 ±1.79 | 162.34 ±4.59 | <0.0001 | 33.273 | 165.02 ±2.48 | <0.0001 | 39.206 | <0.0001 | 4.99 | 156.96 ±6.54 | <0.0001 | 21.535 | <0.0001 | 15.015 | 174.3 ±3.05 | <0.0001 | 59.752 |
| I | 77.84 ±1.95 | 95.03 ±3.56 | <0.0001 | 38.504 | 103.63 ±1.72 | <0.0001 | 57.762 | <0.0001 | 15.522 | 107.41 ±7.67 | <0.0001 | 66.223 | <0.0001 | 6.82 | 127.26 ±1.53 | <0.0001 | 110.69 |
| F | 177.34 ±1.71 | 182.67 ±1.38 | <0.0001 | 16.898 | 187.38 ±2.24 | <0.0001 | 31.8 | <0.0001 | 15.641 | 188.07 ±3.88 | <0.0001 | 33.991 | 0.055 | 2.3 | 188.58 ±3.93 | <0.0001 | 35.614 |
| T | 416.7 ±6.63 | 453.55 ±6.6 | <0.0001 | 51.42 | 470.76 ±4.78 | <0.0001 | 75.437 | <0.0001 | 20.771 | 468.58 ±10.02 | <0.0001 | 72.396 | 0.0122 | 2.63 | 507.71 ±5.29 | <0.0001 | 127 |
N/A – not applicable. Sample size: n=162 for all groups. P – pathology; H – hospitalization; I – intervention; F – follow-up; T – total; SA – statistical analysis value. Data were represented as Mean ±SD.
The statistical analysis showed that questionnaire answers during follow-up of the postnatal period were the same for all participants irrespective of the therapy used for augmentation of labor (data are not presented).
Discussion
The pulsatile OXT regimen is much more expensive than the other treatments. The previous studies on high- vs. low-dose [3,23], continuous vs. pulsatile [9], and no other studies on different regimens of OXT administration have evaluated the expense of delivery, which was assessed for first time in the present study.
We found no significant changes in child death among all treatment regimens. Previous studies reported that pulsatile or delayed OXT regimens were associated with longer labor and more chances of child death [9,24]. Previous studies reported such adverse effects of pulsatile OXT regimen because of large sample size [25] or lack of proper knowledge or exposure regarding administration of dosage forms [26]. In association with pharmaceutical technology adopted in the study, the study was authentic regarding secondary outcome measures.
The study was carried out without administration of amniotic fluid. To date, available studies do not show the superiority of different dosage regimens of OXT in labor [27] because of administration of amniotomy [28] and hydration along with OXT. When amniotic fluid is given along with OXT, it may augment the action of OXT [4]. In the combined action of amniotic fluid and OXT in augmentation of labor, it is difficult to evaluate the action of different regimens of OXT [29]. In respect to the choice of the single OXT regimen in AL, the finding was clear about confounding effects on outcome measures.
Group III had a significant reduction in instrumental delivery patients as compared to group I and group II. High-dose OXT decreased the risk of CS, Ventouse, and FD, and shortens the labor [27]. In respect to dose of OXT in the regimen, the high-dose OXT decreased maternal anxiety.
Regarding limitations of the study, clinicians and the obstetrics team had adequate knowledge of each dosage regimen of OXT from pharmaceutical companies before the study. The program was costly and time-consuming. The study was somewhat tedious because the questionnaire was used every 3 months during the postnatal period. Our literature review was limited to literature published in English. The study did not evaluate the secondary effect of oxytocin regimens with the other labor-augmenting agents like bicarbonates or magnesium sulfate. Finally, the study only evaluated singleton pregnancies.
Conclusions
This clinical experimental study provided comparisons of the effects and the cost of therapy of different regimens of oxytocin, which can be useful for abnormal labor following delivery. The high-dose and pulsatile preparation of oxytocin had reduced risks of operative delivery and had an excessive cost of therapy as compared to continuous administration of oxytocin. A larger study is required to evaluate the secondary effect of different oxytocin regimens along with amniotomy and hydration.
Acknowledgement
Authors are thankful to all technicians and to medical and non-medical staff of Wuhan Maternal and Child Healthcare Hospital, Tongji Medical College, Huazhong University of Science & Technology, and Second People’s Hospital of Huanggang City, who enabled the study success.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Wiberg-Itzel E, Wray S, Åkerud H. A randomized controlled trial of a new treatment for labor dystocia. J Matern Fetal Neonatal Med. 2017 doi: 10.1080/14767058.2017.1339268. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Danilack VA, Gee RE, Berthelot DP, et al. Public health data in action: an analysis of using louisiana vital statistics for quality improvement and payment reform. Matern Child Health J. 2017;21(5):988–94. doi: 10.1007/s10995-016-2254-z. [DOI] [PubMed] [Google Scholar]
- 3.Shu-Qin W, Zhong-Cheng L, Hui-Ping Q, et al. High-dose vs. low-dose oxytocin for labor augmentation: A systematic review. Am J Obstet Gynecol. 2010;203:296–304. doi: 10.1016/j.ajog.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Wiberg-Itzel E, Akerud H, Andolf E, et al. Association between adverse neonatal outcome and lactate concentration in amniotic fluid. Obstet Gynecol. 2011;118:135–42. doi: 10.1097/AOG.0b013e318220c0d4. [DOI] [PubMed] [Google Scholar]
- 5.Kither H, Samangaya R. Abnormal labour. Obstet Gynecol Reprod Med. 2013;23:121–25. [Google Scholar]
- 6.Tanaka H, Tanaka K, Tsuji M, et al. Non-invasive monitoring of the cardiac effects of continuous intravenous oxytocin infusion during cesarean delivery. Int J Gynaecol Obstet. :2017. doi: 10.1002/ijgo.12305. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Jobst A, Krause D, Maiwald C, et al. Oxytocin course over pregnancy and postpartum period and the association with postpartum depressive symptoms. Arch Womens Ment Health. 2016;19:571–79. doi: 10.1007/s00737-016-0644-2. [DOI] [PubMed] [Google Scholar]
- 8.Clark SL, Simpson KR, Knox GE, Garite TJ. Oxytocin: New perspectives on an old drug. Am J Obstet Gynecol. 2009;200:35.e1–6. doi: 10.1016/j.ajog.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Tribe RM, Crawshaw SE, Seed P, et al. Pulsatile versus continuous administration of oxytocin for induction and augmentation of labor: Two randomized controlled trials. Am J Obstet Gynecol. 2012;206:230.e1–8. doi: 10.1016/j.ajog.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Viola N, Leyla R, Marie B, et al. Routine interventions in childbirth before and after initiation of an action research project. Sex Reprod Healthc. 2017;11:86–90. doi: 10.1016/j.srhc.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Karlberg JP, Speers MA. Reviewing clinical trials: A Guide for the Ethics Committee, the University of Hong Kong. Clinical Trials Centre; Hong Kong: 2010. [Google Scholar]
- 12.Gao Z, Cui F, Cao X, et al. Local infiltration of the surgical wounds with levobupivacaine, dexibuprofen, and norepinephrine to reduce postoperative pain: A randomized, vehicle – controlled, and preclinical study. Biomed Pharmacother. 2017;92:459–67. doi: 10.1016/j.biopha.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 13.National Institute for Health & Care Excellence (NICE) Induction of labour – Clinical Guideline (CG70) 2nd Edn. RCOG Press; London: 2008. [Google Scholar]
- 14.Budden A, Chen LJ, Henry A. High-dose versus low-dose oxytocin infusion regimens for induction of labour at term (review) Cochrane Database Syst Rev. 2014;(10):CD009701. doi: 10.1002/14651858.CD009701.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bugg GJ, Stanley E, Baker PN, et al. Outcomes of labours augmented with oxytocin. Eur J Obstet Gynecol Reprod Biol. 2006;124(1):37–41. doi: 10.1016/j.ejogrb.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Blanks AM, Vatish M, Allen MJ, et al. Paracrine oxytocin and estradiol demonstrate a spatial increase in human intrauterine tissues with labor. J Clin Endocr Metab. 2003;88(7):3392–400. doi: 10.1210/jc.2002-021212. [DOI] [PubMed] [Google Scholar]
- 17.Vlassoff M, Diallo A, Philbin J, et al. Cost-effectiveness of two interventions for the prevention of postpartum hemorrhage in Senegal. Int J Gynecol Obstet. 2016;133:307–11. doi: 10.1016/j.ijgo.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang K, Tao F, Liu L, Wu X. Does delivery mode affect women’s postpartum quality of life in rural China? J Clin Nurs. 2012;21(11–12):1534–43. doi: 10.1111/j.1365-2702.2011.03941.x. [DOI] [PubMed] [Google Scholar]
- 19.Leung V, Zhang E, Pang DS. Real-time application of the rat grimace scale as a welfare refinement in laboratory rats. Sci Rep. 2016;6:31667. doi: 10.1038/srep31667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng KH, Peh WCG. Presenting the statistical results. Singapore Med J. 2009;50:11–14. [PubMed] [Google Scholar]
- 21.Modest DP, Ricard I, Stintzing S, et al. for FIRE-3/AIOKRK0306-investigators. Evaluation of survival across several treatment lines in metastatic colorectal cancer: Analysis of the FIRE-3 trial (AIO KRK0306) Eur J Cancer. 2017;84:262–69. doi: 10.1016/j.ejca.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 22.Bugg GJ, Siddiqui F, Thornton JG. Oxytocin versus no treatment or delayed treatment for slow progress in the first stage of spontaneous labour (review) Cochrane Database Syst Rev. 2013;(6):CD007123. doi: 10.1002/14651858.CD007123.pub3. [DOI] [PubMed] [Google Scholar]
- 23.Patka JH, Lodolce AE, Johnston AK. High-versus low-dose oxytocin for augmentation or induction of labor. Ann Pharmacother. 2005;39:95–101. doi: 10.1345/aph.1E037. [DOI] [PubMed] [Google Scholar]
- 24.Tan PC, Soe MZ, Sulaiman S, Omar SZ. Immediate compared with delayed oxytocin after amniotomy labor induction in parous women: A Randomized Controlled Trial. Obstet Gynecol. 2013;121:253–59. doi: 10.1097/AOG.0b013e31827e7fd9. [DOI] [PubMed] [Google Scholar]
- 25.Nayak BK. Understanding the relevance of sample size calculation. Indian J Ophthalmol. 2010;58:469–70. doi: 10.4103/0301-4738.71673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veldhuis JD, Keenan DM, Pincus SM. Motivations and methods for analyzing pulsatile hormone secretion. Endocr Rev. 2009;29:823–64. doi: 10.1210/er.2008-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jamal A, Kalantari R. High and low dose oxytocin in augmentation of labor. Int J Gynecol Obstet. 2004;87:6–8. doi: 10.1016/j.ijgo.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Wiberg-Itzel E, Pembe AB, JaErnbert-Pettersson H, et al. Lactate in amniotic fluid: Predictor of labor outcome in oxytocin-augmented primiparas’ deliveries. PLoS One. 2016;11(10):e0161546. doi: 10.1371/journal.pone.0161546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hadji P, Boekhoff J, Hahn M, et al. Pregnancy-associated transient osteoporosis of the hip: Results of a case-control study. Arch Osteoporos. 2017;12(1):11. doi: 10.1007/s11657-017-0310-y. [DOI] [PubMed] [Google Scholar]