Abstract
Oldenlandia diffusa has been used to treat various cancers. Cytochrome P450, a drug metabolic enzyme, might be influenced by herbal medicine. Currently, the problem that remains is the effective treatment in drug-drug interaction situation. Potential influences of Oldenlandia diffusa were elucidated on the CYP450 activities in rats using a cocktail method. Blood samples were precipitated by acetonitrile. Quantitative determination of target test object was done by ultra-performance liquid chromatography tandem mass spectrometry detection. Influences of oldenlandia diffusa on the activities of five CYP450 subtypes in rats were evaluated by five specific probe drugs (phenacetin for CYP1A2, omeprazole for CYP2C19, tolbutamide for CYP2C9, metoprolol for CYP2D6, and midazolam for CYP3A4) according to the pharmacokinetic parameters changes. No statistically significant difference (P > 0.05) in pharmacokinetic behaviors can be observed in the five probe drugs. There is a potential guidance on clinical drug combination with Oldenlandia diffusa. Oldenlandia diffusa in compound preparation showed well security.
1. Introduction
Oldenlandia diffusa, an herbal medicine, has a very important medicinal role used as a traditional oriental medicine for inflammatory and infectious diseases, such as pneumonia, appendicitis, and urinary tract infections [1, 2]. Moreover, previous studies had been reported that Oldenlandia diffusa and its major compound, ursolic acid have anticancer effects and immunomodulating activities [3, 4]. Ting et al. found that Oldenlandia diffusa had a promising treatment for hepatocellular carcinoma, colorectal cancer, and breast cancer [5–7].
Cytochrome P450 (CYP450), the most important drug metabolic enzyme superfamily, plays major roles in the metabolism of a variety of drugs, other xenobiotics and endogenous molecules [8–10]. Among many CYPs isoforms, human CYP1A2, CYP2C9, CYP2D6, CYP2C19, and CYP3A4 are the major CYP450 isoforms that metabolize over 90% of the clinical drugs [11, 12]. Clinically, induction or inhibition of the CYP activities could influence the pharmacokinetics of drugs, resulting in unexpected and even serious clinical drug-drug interactions (DDIs) [13, 14]. Especially, enzyme inhibition by inhibitor drugs could lead to an increase in plasma concentrations of another drug, thus increasing the risk of adverse drug reactions (ADRs) [15]. It is essential to research the inhibition or induction of CYPs in order to predict the potential DDIs and thereby avoiding the occurrence of adverse events. The cocktail approach has become one of the basic analytical tools to evaluate DDIs in vivo. Kinds of compound specially catalyzed by each CYP isoform, known as probe drugs, have been widely used to assess various individual CYP450 activities in this approach [16, 17].
For determining the safety of clinical combination use, the influences of oldenlandia diffusa on the activities of five CYP450 subtypes in rats is evaluated according to the pharmacokinetic parameters changes using five specific probe drugs (phenacetin for CYP1A2, omeprazole for CYP2C19, tolbutamide for CYP2C9, metoprolol for CYP2D6, and midazolam for CYP3A4); compare oldenlandia diffusa-treated groups with control group. A sensitive and specific ultra-performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) method was used to simultaneously quantify the five probe drugs concentration by a single-run process.
2. Materials and Methods
2.1. Chemicals and Reagents
Phenacetin (purity > 99%), omeprazole (purity > 98%), tolbutamide (purity > 98%), and metoprolol (purity > 99%) were purchased from Toronto Research Chemicals Inc. (Toronto, Ontario, Canada). Midazolam (purity > 99%) was obtained from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Carbamazepine (IS) was purchased from J&K Chemical. (Shanghai, China); Oldenlandia diffusa (purity > 99%) was purchased from Shanghai Canspec Scientific Instruments Co., Ltd. (Shanghai, China). Formic acid (analytical reagent grade) was purchased from Sigma-Aldrich (St. Louis, MO, USA). HPLC-grade methanol and acetonitrile (ACN) were purchased from Merck Company (Darmstadt, Germany). Ultrapure water was produced in a Milli-Q system (Millipore, Bedford, MA, USA). All other chemicals and solvents were at analytical grade level.
2.2. Instrument and Conditions
Chromatographic separation was performed on an Agilent UHPLC unit (Agilent Corporation, MA, USA) with a ZORBAX Eclipse Plus C18 column (1.8 μm, 2.1 × 50 mm, I.D. Agilent Corporation, MA, USA) maintained at 30°C. The initial mobile phase consisted of solvent A (0.1% formic acid in water) and solvent B (acetonitrile) with gradient elution as follows: 30% B (0–0.3 min), 30–50% B (0.3–1.3 min), 50–95% B (1.3–1.8 min), and 95–95% B (1.8–2.8 min). The flow rate was 0.40 mL/min. The injection volume was 5 μL. The subsequent reequilibration time was 1.5 min.
The mass spectrometric detection was performed on Agilent 6420 triple-quadrupole mass spectrometer equipped with an electrospray ionization source (Agilent Corporation, MA, USA) in a positive mode. Quantitative analysis was performed in the multiple reaction monitoring (MRM) mode. The Agilent 6420 Quantitative Analysis version B.07.00 analyst data processing software (Agilent Corporation, MA, USA) was used for instrument operation and data acquisition. The precursor-product ion pairs used for the MRM detection and MS parameters are listed in Table 1.
Table 1.
Mass spectrometry information for each probe drugs.
| Compound | Precursor ion (m/z) | Product ion (m/z) | Collision energy (V) | Fragmentor |
|---|---|---|---|---|
| Phenacetin | 180.1 | 109.9 | 24 | 122 |
| Omeprazole | 346.12 | 135.9 | 44 | 108 |
| Tolbutamide | 271.11 | 91 | 36 | 120 |
| Metoprolol | 268.19 | 115.9 | 17 | 130 |
| Midazolam | 326.09 | 290.8 | 44 | 170 |
| Carbamazepine | 237.1 | 194 | 18 | 140 |
2.3. Method Validation
To quantify concentration of five substances, calibration curves were established. Standard of phenacetin, tolbutamide, omeprazole, midazolam, and IS (1 mg/ml) were prepared by methanol. Concentration of each probe drugs was 0.05, 0.1, 0.25, 0.5, 1, 2.5, 5, 10 µg/ml.
2.4. Pharmacokinetic Study
Male Sprague–Dawley (SD) rats (200–220 g) were purchased from Laboratory Animal Center of Wenzhou Medical University (Wenzhou, China). All rats were fasted for 12 h while water was not limited and used to collect 0.5 mL blank blood samples before administration. Oldenlandia diffusa was dissolved by physiological saline. Sixteen rats were randomly divided into two groups: the oldenlandia diffusa group (n=8) and the control group (n=8). All experimental procedures and protocols were reviewed and approved by the Animal Care and Use Committee of Wenzhou Medical University and were in accordance with the Guide for the Care and Use of Laboratory Animals. The control group was given physiological saline by oral administration, while the oldenlandia diffusa group was given oldenlandia diffusa (200 mg/kg) by oral administration every day for 7 days. In 7 days, rats were normally fed, except the last day which needed fasting. After 7 days, all rats were given a cocktail solution by oral administration containing five probe drugs: phenacetin (20 mg/kg), tolbutamide (1 mg/kg), omeprazole (20 mg/kg), metoprolol (20 mg/kg), and midazolam (20 mg/kg). Blood samples (0.3 ml) were collected from the tail vein at 0.167, 0.5, 1, 1.5, 2, 3, 4, 6, 9, 12, and 24 h following the experiment. The samples were immediately centrifuged at 13,000 rpm for 10 min, and the plasma was stored at −20°C.
2.5. Plasma Sample Preparation
Each of 100 μl prepared plasma was added into 1.5 ml tubes that filled with 300 µl ACN, 30 μl IS (1 μg/ml). After shocking through vortex (QL-901, Kylin–Bell Lab instrument) for 2 min, mixture was centrifuged using 13,000 rpm for 10 min. 100 μl supernatant was diluted by 100 μl ultra-water. And 5 μl was injected into UHPLC-MS/MS for analysis. Other working solutions were also progressed like plasma sample.
2.6. Statistics and Analysis
Plasma probe drug concentration versus time data for each rat was analyzed by DAS software (version 3.0). The main pharmacokinetic parameters of the oldenlandia diffusa group and control group were analyzed using SPSS l8.0 statistical software. P < 0.05 was considered statistically significant.
3. Results and Discussion
3.1. UHPLC-MS/MS
Figure 1 showed parent and daughter ions for each probe drugs. The post time was 0.891 min (phenacetin), 2.566 min (tolbutamide), 0.478 min (omeprazole), 0.504 min (midazolam), 0.400 min (metoprolol), and 1.588 min (IS). They had a better separation effect.
Figure 1.
Parent and daughter ions for six substances: (a) metoprolol; (b) omeprazole; (c) phenacetin; (d) tolbutamide; (e) midazolam; (f) IS.
3.2. Pharmacokinetics
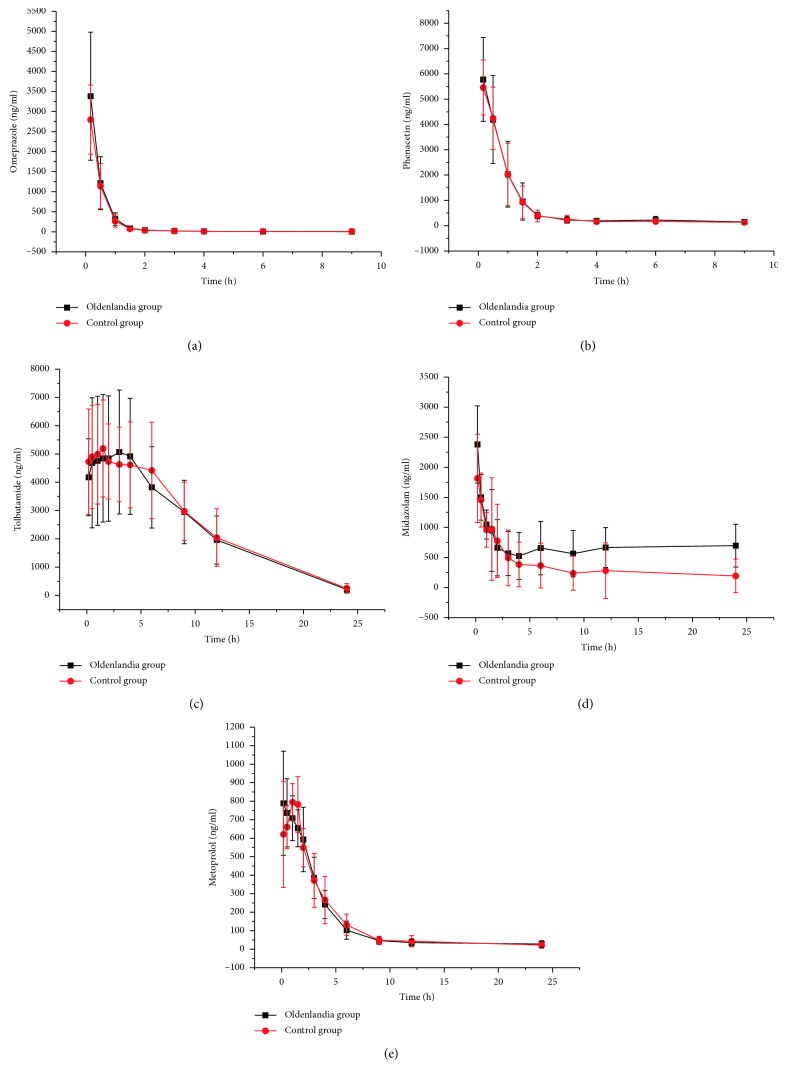
The method was applied to the pharmacokinetic study of five probe drugs in rats. The mean plasma concentration-time curves are shown in Figure 2. The main pharmacokinetic parameters after administration of phenacetin, tolbutamide, omeprazole, metoprolol, and midazolam from noncompartment model analysis are summarized in Table 2.
Figure 2.
The mean plasma concentration-time curves of (a) omeprazole, (b) phenacetin, (c) tolbutamide, (d) midazolam, and (e) metoprolol.
Table 2.
The pharmacokinetics parameters of phenacetin, omeprazole, tolbutamide, midazolam, and metoprolol in rat plasma after administration of oldenlandia diffusa.
| Probe drug | Pharmacokinetics parameters | Control group | Oldenlandia diffusa-treated group |
|---|---|---|---|
| Phenaceton | AUC (0–t) (mg/L∗h) | 6.00 ± 1.60 | 6.28 ± 1.81 |
| AUC (0–∞) (mg/L∗h) | 7.37 ± 2.12 | 13.1 ± 9.31 | |
| t1/2 (h) | 5.98 ± 3.58 | 24.67 ± 39.93 | |
| Tmax (h) | 0.22 ± 0.14 | 0.17 ± 0.00 | |
| Vz/F (L/kg) | 23.3 ± 10.5 | 34.6 ± 34.8 | |
| CLz/F (L/h/kg) | 2.90 ± 0.80 | 1.98 ± 0.82 | |
| Cmax (mg/L) | 5.78 ± 1.66 | 5.72 ± 0.76 | |
| Omeprazole | AUC (0–t) (mg/L∗h) | 1.45 ± 0.522 | 1.63 ± 0.754 |
| AUC (0–∞) (mg/L∗h) | 1.48 ± 0.528 | 1.64 ± 0.759 | |
| t1/2 (h) | 3.28 ± 1.47 | 1.86 ± 1.08 | |
| Tmax (h) | 0.17 ± 0.00 | 0.17 ± 0.00 | |
| Vz/F (L/kg) | 77.8 ± 56.0 | 33.2 ± 12.0 | |
| CLz/F (L/h/kg) | 16.1 ± 9.20 | 15.5 ± 9.03 | |
| Cmax (mg/L) | 2.80 ± 0.862 | 3.38 ± 1.60 | |
| Tolbutamide | AUC (0–t) (mg/L∗h) | 60.2 ± 22.7 | 58.2 ± 22.3 |
| AUC (0–∞) (mg/L∗h) | 61.9 ± 23.7 | 62.5 ± 26.3 | |
| t1/2 (h) | 4.21 ± 0.69 | 5.32 ± 1.84 | |
| Tmax (h) | 2.00 ± 1.67 | 2.27 ± 1.21 | |
| Vz/F (L/kg) | 0.11 ± 0.03 | 0.13 ± 0.05 | |
| CLz/F (L/h/kg) | 0.02 ± 0.01 | 0.02 ± 0.01 | |
| Cmax (mg/L) | 5.29 ± 1.78 | 5.45 ± 2.29 | |
| Midazolam | AUC (0–t) (mg/L∗h) | 8.24 ± 8.25 | 13.8 ± 9.57 |
| AUC (0–∞) (mg/L∗h) | 36.1 ± 83.0 | 77.9 ± 81.7 | |
| t1/2 (h) | 29.3 ± 63.4 | 60.0 ± 68.3 | |
| Tmax (h) | 0.88 ± 0.82 | 0.17 ± 0.00 | |
| Vz/F (L/kg) | 28.0 ± 17.2 | 44.1 ± 80.2 | |
| CLz/F (L/h/kg) | 4.93 ± 4.69 | 3.42 ± 4.44 | |
| Cmax (mg/L) | 2.00 ± 0.608 | 2.38 ± 0.641 | |
| Metoprolol | AUC (0–t) (mg/L∗h) | 3.33 ± 0.798 | 3.16 ± 0.576 |
| AUC (0–∞) (mg/L∗h) | 3.54 ± 1.05 | 3.94 ± 1.53 | |
| t1/2 (h) | 6.80 ± 5.09 | 15.7 ± 18.5 | |
| Tmax (h) | 0.920 ± 0.510 | 0.690 ± 0.630 | |
| Vz/F (L/kg) | 53.9 ± 30.8 | 89.7 ± 79.3 | |
| CLz/F (L/h/kg) | 6.06 ± 1.58 | 5.72 ± 1.95 | |
| Cmax (mg/L) | 0.865 ± 0.142 | 0.933 ± 0.172 |
From Table 2, in the experiment for the oldenlandia diffusa and control groups, there was insignificant difference (P > 0.05) in pharmacokinetic behaviors could be observed for phenacetin, tolbutamide, omeprazole, metoprolol, and midazolam. Results of the present study showed that oldenlandia diffusa might not affect the activity of CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 in rats. CYP450 was responsible for ∼90% clinical drugs, including anti-inflammatory, cardiovascular, and cancer drugs [18]. Oldenlandia diffuse had a possibility to participate in drug combination. And according to Wu et al.'s research, classical five probe drugs were selected [19]. These Oldenlandia diffusa was applied as a traditional Chinese medicine in Asia which was mainly distributed in south of the Yangtze River [20]. There were many chemical compositions in Oldenlandia diffusa, but flavonoids, anthraquinone, and iridoid glucosides were mainly active pharmacological effect [21, 22]. The further research needs to know if these monomers have interaction on CYP that pharmacokinetics of five probe drugs had negligible change. And whether oldenlandia diffusa affects the drugs through other metabolic pathways or not is still an issue.
4. Conclusion
This research showed that oldenlandia diffusa had no effect on CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. And there was a potential guidance on clinical drug combination that oldenlandia diffusa might be considered as a safety combination drug with five CYPs metabolism drugs in clinical. But the interaction between oldenlandia diffusa and drugs which not involved in CYP450 metabolism still need attention.
Acknowledgments
The work was supported by Applied Research of Public Welfare Technology Foundation of Zhejiang Province (Grant no. 2017C37115). And authors thanked to members of the laboratory of Wenzhou Medical University for technical help.
Conflicts of Interest
The authors had no conflicts of interest.
Authors' Contributions
Yiping Lin designed the protocol. Yiping Lin, Xiaoxia Hu, and Mingxin Ding conducted the protocol. Xiaoxia Hu and Yanli Wei performed the experiment. Xiaoxia Hu analyzed protocol and wrote article.
References
- 1.Kim S. J., Kim Y. G., Kim D. S., et al. Oldenlandia diffusa ameliorates dextran sulphate sodium-induced colitis through inhibition of NF-kappab activation. American Journal of Chinese Medicine. 2011;39(5):957–969. doi: 10.1142/s0192415x11009330. [DOI] [PubMed] [Google Scholar]
- 2.Wajima T., Anzai Y., Yamada T., Ikoshi H., Noguchi N. Oldenlandia diffusa extract inhibits biofilm formation by haemophilus influenzae clinical isolates. PLoS One. 2016;11(11) doi: 10.1371/journal.pone.0167335.e0167335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song Y. H., Jeong S. J., Kwon H. Y., Kim B., Kim S. H., Yoo D. Y. Ursolic acid from Oldenlandia diffusa induces apoptosis via activation of caspases and phosphorylation of glycogen synthase kinase 3 beta in SK-OV-3 ovarian cancer cells. Biological and Pharmaceutical Bulletin. 2012;35(7):1022–1028. doi: 10.1248/bpb.b110660. [DOI] [PubMed] [Google Scholar]
- 4.Chung H. S., Jeong H. J., Hong S. H., et al. Induction of nitric oxide synthase by Oldenlandia diffusa in mouse peritoneal macrophages. Biological and Pharmaceutical Bulletin. 2002;25(9):1142–1146. doi: 10.1248/bpb.25.1142. [DOI] [PubMed] [Google Scholar]
- 5.Ting C. T., Kuo C. J., Hu H. Y., Lee Y. L., Tsai T. H. Prescription frequency and patterns of Chinese herbal medicine for liver cancer patients in Taiwan: a cross-sectional analysis of the National Health Insurance Research Database. BMC Complementary and Alternative Medicine. 2017;17(1):p. 118. doi: 10.1186/s12906-017-1628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S., Shim J. H., Gim H., Park H. S., Kim B. J. Ethanol extract of Oldenlandia diffusa-an effective chemotherapeutic for the treatment of colorectal cancer in humans: -anti-cancer effects of Oldenlandia diffusa. Journal of Pharmacopuncture. 2016;19(1):51–58. doi: 10.3831/kpi.2016.19.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonofiglio D., Giordano C., De Amicis F., Lanzino M., Ando S. Natural products as promising antitumoral agents in breast cancer: mechanisms of action and molecular targets. Mini-Reviews in Medicinal Chemistry. 2016;16(8):596–604. doi: 10.2174/1389557515666150709110959. [DOI] [PubMed] [Google Scholar]
- 8.Lewis D. F. Human cytochromes P450 associated with the phase 1 metabolism of drugs and other xenobiotics: a compilation of substrates and inhibitors of the CYP1, CYP2 and CYP3 families. Current Medicinal Chemistry. 2003;10(19):1955–1972. doi: 10.2174/0929867033456855. [DOI] [PubMed] [Google Scholar]
- 9.Nishimuta H., Nakagawa T., Nomura N., Yabuki M. Species differences in hepatic and intestinal metabolic activities for 43 human cytochrome P450 substrates between humans and rats or dogs. Xenobiotica. 2013;43(11):948–955. doi: 10.3109/00498254.2013.787155. [DOI] [PubMed] [Google Scholar]
- 10.Pelkonen O. Human CYPs: in vivo and clinical aspects. Drug Metabolism Reviews. 2002;34(1-2):37–46. doi: 10.1081/dmr-120001388. [DOI] [PubMed] [Google Scholar]
- 11.Rendic S., Di Carlo F. J. Human cytochrome P450 enzymes: a status report summarizing their reactions, substrates, inducers, and inhibitors. Drug Metabolism Reviews. 1997;29(1-2):413–580. doi: 10.3109/03602539709037591. [DOI] [PubMed] [Google Scholar]
- 12.Lewis D. F. P450 structures and oxidative metabolism of xenobiotics. Pharmacogenomics. 2003;4(4):387–395. doi: 10.1517/phgs.4.4.387.22752. [DOI] [PubMed] [Google Scholar]
- 13.Geng T., Si H., Kang D., et al. Influences of Re Du Ning Injection, a traditional Chinese medicine injection, on the CYP450 activities in rats using a cocktail method. Journal of Ethnopharmacology. 2015;174:426–436. doi: 10.1016/j.jep.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 14.Li G., Huang K., Nikolic D., van Breemen R. B. High-throughput cytochrome p450 cocktail inhibition assay for assessing drug-drug and drug-botanical interactions. Drug Metabolism and Disposition. 2015;43(11):1670–1678. doi: 10.1124/dmd.115.065987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ingelman-Sundberg M. Pharmacogenetics of cytochrome P450 and its applications in drug therapy: the past, present and future. Trends in Pharmacological Sciences. 2004;25(4):193–200. doi: 10.1016/j.tips.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Breimer D. D., Schellens J. H. A ‘cocktail’ strategy to assess in vivo oxidative drug metabolism in humans. Trends in Pharmacological Sciences. 1990;11(6):223–225. doi: 10.1016/0165-6147(90)90245-4. [DOI] [PubMed] [Google Scholar]
- 17.Lin W., Zhang J., Ling X., et al. Evaluation of the effect of TM208 on the activity of five cytochrome P450 enzymes using on-line solid-phase extraction HPLC-DAD: a cocktail approach. Journal of Chromatography B. 2013;923-924:29–36. doi: 10.1016/j.jchromb.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 18.Rendic S. Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metabolism Reviews. 2002;34(1-2):83–448. doi: 10.1081/dmr-120001392. [DOI] [PubMed] [Google Scholar]
- 19.Wu Q., Zhang Q., Wen C., Hu L., Wang X., Lin G. The effect of MS-275 on CYP450 isoforms activity in rats by cocktail method. International Journal of Clinical and Experimental Pathology. 2015;8:9360–9367. [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S. J., Chung W. S., Kim S. S., Ko S. G., Um J. Y. Antiinflammatory effect of Oldenlandia diffusa and its constituent, hentriacontane, through suppression of caspase-1 activation in mouse peritoneal macrophages. Phytotherapy Research. 2011;25(10):1537–1546. doi: 10.1002/ptr.3443. [DOI] [PubMed] [Google Scholar]
- 21.Kim Y., Park E. J., Kim J., Kim Y., Kim S. R., Kim Y. Y. Neuroprotective constituents from Hedyotis diffusa. Journal of Natural Products. 2001;64(1):75–78. doi: 10.1021/np000327d. [DOI] [PubMed] [Google Scholar]
- 22.Lu C. M., Yang J. J., Wang P. Y., Lin C. C. A new acylated flavonol glycoside and antioxidant effects of Hedyotis diffusa. Planta Medica. 2000;66(4):374–377. doi: 10.1055/s-2000-8544. [DOI] [PubMed] [Google Scholar]