Abstract
Purpose
This study aimed to assess complications and outcomes of a new approach, that is, combining short course radiotherapy (SRT), concurrent and consolidative chemotherapies, and delayed surgery.
Materials and Methods
In this single arm phase II prospective clinical trial, patients with T3-4 or N+ M0 rectal adenocarcinoma were enrolled. Patients who received induction chemotherapy or previous pelvic radiotherapy were excluded. Study protocol consisted of three-dimensional conformal SRT (25 Gy in 5 fractions in 1 week) with concurrent and consolidation chemotherapies including capecitabine and oxaliplatin. Total mesorectal excision was done at least 8 weeks after the last fraction of radiotherapy. Primary outcome was complete pathologic response and secondary outcomes were treatment related complications.
Results
Thirty-three patients completed the planned preoperative chemoradiation and 26 of them underwent surgery (24 low anterior resection and 2 abdominoperineal resection). Acute proctitis grades 2 and 3 were seen in 11 (33.3%) and 7 (21.2%) patients, respectively. There were no grades 3 and 4 subacute hematologic and non-hematologic (genitourinary and peripheral neuropathy) toxicities and perioperative morbidities such as anastomose leakage. Grade 2 or higher late toxicities were observed among 29.6% of the patients. Complete pathologic response was achieved in 8 (30.8%) patients who underwent surgery. The 3-year overall survival and local control rates were 65% and 94%, respectively.
Conclusion
This study showed that SRT combined with concurrent and consolidation chemotherapies followed by delayed surgery is not only feasible and tolerable without significant toxicity but also, associated with promising complete pathologic response rates.
Keywords: Combined modality therapy, Conformal radiotherapy, Rectal cancer, Iran, Consolidation chemotherapy, Anticancer drug combination, XELOX
Introduction
Patients with early stage rectal cancer can be treated with radical surgery alone or local resection with or without chemoradiation. However, management of locally advanced rectal cancer is somehow more sophisticated. Surgical management of primary rectal cancer per se is associated with high local and distant recurrence that necessitates a multimodality treatment protocol. Following the study of Sauer et al. [1], it was shown that in German Rectal Cancer Trial, neoadjuvant radiochemotherapy significantly increased local control and overall survival at 10 years and was considered as the standard approach towards locally advanced rectal adenocarcinoma. There are two accepted methods for neoadjuvant radiotherapy: first, mainly North American method also called conventional chemoradiation which includes 45 to 50.4 Gy of radiation in 1.8–2 Gy fractions together with 5-fluorouracil (5FU) based concurrent chemotherapy. The second method, commonly practiced in Scandinavia, is short course radiotherapy (SRT) that consists of 25 Gy in 5 consecutive daily fractions followed by immediate surgery within 1 week [2,3]. These two approaches do not differ in the rate of local control, overall survival and even perioperative complications as shown in Trans-Tasman Rectal Trial [4]. In addition, this method has been previously proven to be safe and effective in a high burden radiotherapy facility with long patient waiting list [5]. However, some authors believe that a good neoadjuvant treatment should provide tumor downstaging, improved resectability and sphincter preservation in low rectal tumors as well. However, considering the limited interval between radiotherapy and surgery and also lack of concurrent chemotherapy in short course method, less response occurs in neoadjuvant treatment as compared to long course chemoradiation [6].
Due to the fact that SRT is associated with lower cost and duration of treatment, this method cannot be ignored in countries with limited health expenditure like ours. In addition, other investigators also tried to overcome the major drawback of SRT which is its less pathologic complete response (pCR) and sphincter preservation rate as compared to conventional method. In this regard, some authors proposed increasing the interval between radiotherapy and surgery [7] and reported about 10% increase in pCR in group with delayed surgery. Other investigators tested the addition of preoperative chemotherapy following SRT [8] and delayed surgery with results comparable to conventional chemoradiation. Due to the concerns of increased toxicity, only a few studies are available regarding the addition of concurrent chemotherapy to SRT but with appropriate results and acceptable complications rate [9,10].
Considering the promising results achieved in the earlier mentioned approaches regarding the improvement of SRT flaws, in this study, the authors aimed to assess the ability to induce pCR, feasibility and toxicities by a new approach including neoadjuvant SRT with concurrent and preoperative consolidative chemotherapies followed by delayed surgery.
Materials and Methods
1. Study design and participants
This study is a phase 2 single-arm prospective clinical trial. Patients referred to our radiation oncology ward for neoadjuvant treatment with a pathologic report of rectal adenocarcinoma were recruited in this study. The study design was reviewed and approved by the Research Ethics Committee of Tehran University of Medical Sciences (No. 9111188003-100785), to be in line with declaration of Helsinki. The patients participated voluntarily in this study and all possible complications were disclosed to them. Written inform consent was obtained before any treatment. The trial was registered with Iranian Registry of Clinical Trials (http://www.irct.ir), a regional branch of World Health Organization Clinical Trial Registration (No. IRCT2016121424266N2).
2. Pretreatment assessment and inclusion/exclusion criteria
A full clinical and radiologic local staging was performed by physical examination (including digital rectal examination), flexible colonoscopy, pelvic magnetic resonance imaging or rectal endoscopic ultrasonography. Other mandatory diagnostic workup consisted of contrast enhanced thorax and abdominopelvic computed tomography scan, complete blood count, liver and renal function tests and serum carcinoembryonic antigen; positron emission tomography was not in the study protocol because of limited availability. Finally, patients with the following criteria were enrolled for the study: T3, T4 or lymph node positive (N+) rectal adenocarcinoma located up to 15 cm from anal verge, the Eastern Cooperative Oncology Group (ECOG) performance status 0–1. The patients who were candidates of abdominoperineal resection (APR) regardless of response to radiation therapy were also included. Patients with one of the following conditions were excluded from the study: recurrent tumors after previous surgery, synchronous distant metastasis, previous history of pelvic radiotherapy, history of another cancer, impairment of renal function test as much as not to tolerate oxaliplatin or capecitabine, receiving induction chemotherapy and also medical unfitness for surgery.
3. Radiotherapy protocol
All the patients were treated by three-dimensional (3D) conformal radiotherapy with 18 MV photon X-rays. The clinical target volume included the gross tumor and involved nodes together with elective pelvic lymph nodes (presacral, mesorectal and internal iliac lymph nodes in all cases and external and inguinal nodes in appropriate conditions) and entire mesorectum with adequate margins (7–10 mm for elective and involved nodes, and 2 cm for primary tumor respecting anatomic boundaries) [11]. The planning target volume (PTV) was defined as clinical target volume plus 1 cm margin due to patient’s positioning and setup error in the center. The PTV received 25 Gy in 5 daily 5 Gy-fractions for 1 week.
4. Chemotherapy protocol
All the patients received concurrent chemotherapy including intravenous oxaliplatin 85 mg/m2 in d1 and oral capecitabine 825 mg/m2 twice a day in days 1–5 of radiotherapy. In addition, one cycle of consolidative chemotherapy was prescribed 3–4 weeks after completion of radiotherapy, consisting of intravenous oxaliplatin 130 mg/m2 in day 1 and oral capecitabine 1,000 mg/m2/bid in days 1–14. The adjuvant postoperative chemotherapy was at the discretion of attending physician.
5. Surgical procedure
All the participants were referred to surgeon within 6 and 8 weeks after completion of radiotherapy (3–4 weeks after consolidative chemotherapy). The default procedures were low anterior resection (LAR) and APR at the discretion of surgeons. The patients were monitored for perioperative complications by colorectal surgical team for 1 month following operation (in-patient or out-patient) and then referred back with permanent pathology report, to radiation oncology ward.
6. Complication assessment
The secondary endpoints were feasibility of combined SRT with concurrent and consolidative chemotherapies. In other words, the complications related to such approach were the center of attention. Thus, patients were evaluated closely for acute (from the beginning of radiotherapy to 1-month post-surgery) and late (after 3 months post-surgery) gastrointestinal, genitourinary and hematologic toxicities using patient reported complaints, physical examination and laboratory studies (complete blood count and renal function tests). The grading of treatment-related toxicities were according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 and highest grade was recorded for each patient. The subjects with proctitis were treated with loperamide, while in cases with bloody discharge, hydrocortisone suppository or 5-aminosalicilates (5ASA) tablets (e.g., mesalamine) were prescribed. They were also evaluated for perioperative complications including anastomosis leakage, delayed surgical wound healing or dehiscence and formation of enterocutaneous, rectovesical or rectovaginal fistula.
7. Treatment response
The secondary endpoint was the pathologic response to neoadjuvant treatment. The pathologic response was assessed based on the report done by experienced pathologist in gastrointestinal malignancies. Tumor depth of invasion (ypT) and number of involved lymph nodes (ypN) for tumor and node down-staging were respectively considered. However, the tumor regression grade (TRG) suggested by Dworak et al. [12] was chosen for response evaluation.
8. Outcomes and analysis
The primary outcomes were complete pathologic response (ypCR) to neoadjuvant short course radiochemotherapy with delayed surgery and secondary outcomes were feasibility and complications of such treatment. The sample size was calculated based on the formula for detecting 15% improvement of ypCR from 12.5% in Stockholm III trial which was reported in short-course radiotherapy with delayed surgery arm [13]. The power was 80% and type I error (α) was 0.05. In order to report ypCR, both intentions to treat and per protocol analysis were performed. The stand-point for evaluation of survival rate with Kaplan-Meyer method was the date of the end of radiotherapy.
Results
1. Pretreatment characteristics
From August 2013 to February 2015, a total 33 patients were enrolled in this study (Fig. 1) and their characteristics are shown in Table 1. Mean age (±standard deviation) of the subjects was 58.5 ± 12.65 years. The average distance from anal verge was 6.5 ± 2.79 cm.
Fig. 1.
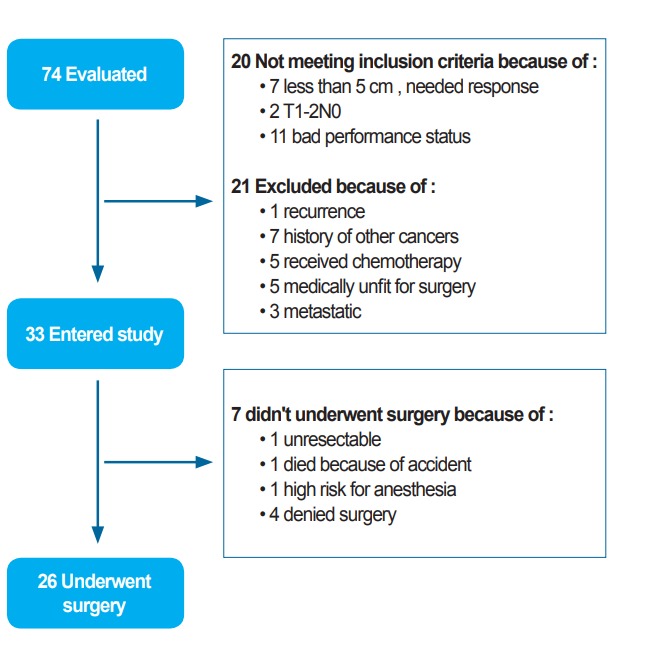
Allocation diagram.
Table 1.
Patients and tumors characteristics
| Characteristic | Value |
|---|---|
| Age (yr) | 61 (32–81) |
| Gender | |
| Male | 24 (72.7) |
| Female | 9 (27.3) |
| ECOG performance status | |
| 0 | 31 (93.9) |
| 1 | 2 (6.1) |
| Distance from AV (cm) | 6 (1–15) |
| <5 | 5 (15.1) |
| 5–10 | 23 (69.7) |
| 11–15 | 5 (15.1) |
| Differentiation | |
| Well (grade 1) | 12 (36.3) |
| Moderate (grade 2) | 13 (39.3) |
| Poor (grade 3) | 8 (24.2) |
| Clinical staging | |
| T3N0 | 8 (24.2) |
| T3N1 | 17 (51.5) |
| T3N2 | 5 (15.1) |
| T4N0 | 1 (3) |
| T4N1 | 1 (3) |
| T4N2 | 1 (3) |
Values are presented as median (range) or number (%).
2. Treatment tolerance
All the subjects completed the course of concurrent chemoradiation successfully. Three patients (12.1%) did not receive full prescribed dose of consolidative chemotherapy (due to intolerance to oral capecitabine). Fifteen patients (45.5%) reported no or only grade 1 acute toxicities. Grades 2 and 3 acute proctitis was seen in 11 (33.3%) and 7 (21.2%) patients, respectively. No grade 4 acute proctitis was recorded. However, no grade 3 cystitis, hematologic toxicities or peripheral sensory neuropathy were observed.
Twenty-six patients underwent total mesorectal excision (24 LAR and 2 APR). The median interval from end of radiotherapy to surgery was 10 months. Amongst others who failed to undergo surgery, 1 patient died in a car crash before surgery, 4 refused the operation despite primary consent, 1 had an unresectable tumor in pre-surgical evaluation (which was unresectable before radiotherapy as well), and the last 1 was medically unfit for surgery due to cardiac disease that progressed during chemoradiation. During 1-month postoperative period, only an event of grade 3 surgical site infection occurred in one diabetic patient. Eleven events of late treatment related to grades 2 or 3 toxicity occurred in 8 patients (24.2%) (Table 2).
Table 2.
Frequency of grade 2–3 late treatment related toxicities at 3 months post-surgery
| Type of late toxicity events | Number |
|---|---|
| Erectile dysfunction | 1 |
| Proctitis | 6 |
| Vaginitis | 1 |
| Bowel obstruction | 1 |
| Urinary urge incontinence | 1 |
| Renal failure | 1 |
3. Pathologic response
Complete pathologic response (TRG0) was reported in 8 patients (24.2%), partial response (TRG1 and TRG2) was reported in 6 patients (18.2%) and poor response (TRG3) was reported in 12 patients (36.4%) out of the 33 study subjects. Among the subjects who underwent surgery, the rates of complete, partial, and poor pathologic response were 30.8%, 23.0%, and 46.2%, respectively. All the patients enjoyed R0 resection.
4. Treatment outcomes
The 3-year overall survival (OS) and disease-free survival (DFS) in all the subjects was 60% and 52%, respectively (Figs. 2 and 3). The local control (LC) and distant control (DC) rates were 84% and 68%, respectively, among all the study subjects. However, among the subjects who underwent surgery per protocol, the rates of OS, DFS, LC, and DC were 65%, 55%, 94%, and 63%, respectively.
Fig. 2.
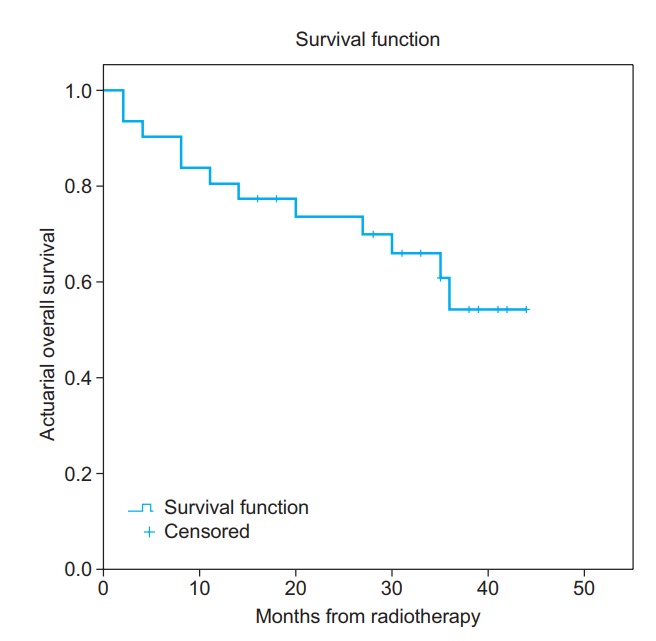
Actuarial overall survival rate.
Fig. 3.
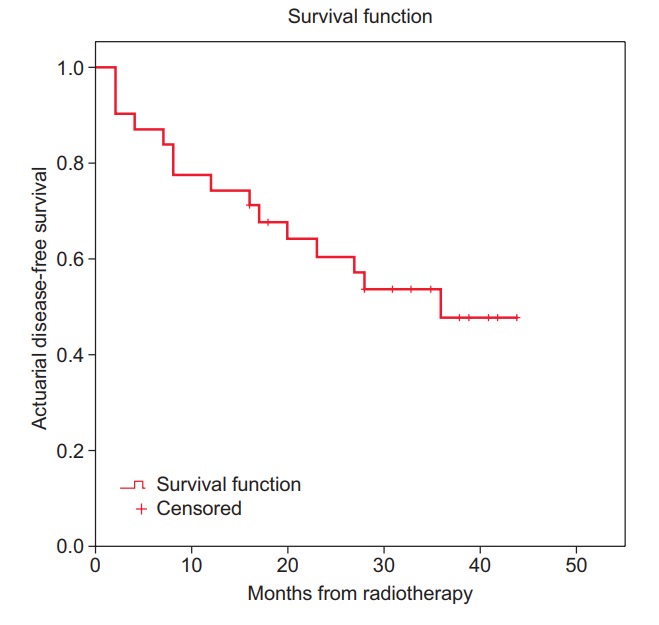
Actuarial disease-free survival rate.
Discussion and Conclusion
Neoadjuvant treatment is the standard of care for patients with locally advanced rectal cancer. Chemoradiation or SRT are both widely accepted as neoadjuvant treatments and can be practiced as standard protocols for improving LC in this group of patients. To address the differences in these approaches, two phase III randomized trials were conducted and the results are available. Polish trial and Trans-Tasman Radiation Oncology (TROG) group did not show a significant difference in survival and local recurrence rate between chemoradiation and SRT [4,14].
TROG trial showed that SRT had significantly less acute adverse events as compared to long course chemoradiotherapy, while the post-operative complications were comparable [15]. Nevertheless, the major concern that keep colorectal oncologist from concurrent short course chemoradiotherapy is the fear of escalated treatment-related acute and late complications [6]. There is agreement among various studies that by lengthening the interval between radiotherapy and surgery, these increased risk of complications is negligible as compared to long course chemoradiotherapy [16]. The concept of delayed surgery after SRT was also tested in Stockholm III trial and it was shown that the risk of post-operative complications are significantly lower in SRT with delayed surgery group as compared to immediate surgery (38% vs. 50%) but the oncological outcomes were statistically equal [17].
Some studies aimed to test chemotherapy in a neoor adjuvant sequence to radiotherapy before surgery. For instance, in Dutch rectal cancer trial, rectal cancer patients with synchronous metastasis to liver showed acceptable complication rate with preoperative chemotherapy following SRT [18]. Accordingly, Japanese investigators showed the safety of induction chemotherapy plus cetuximab and SRT [19]. However, there are limited experiences with concurrent chemotherapy and SRT. In one of these rare instances, KROG 10-01 phase II trial, the rate of grade 3 or more toxicities were considerably high (about 38%) [20]. In contrast, another Korean study by Chung et al. [9] showed similar toxicities between short and long course chemoradiotherapies. This difference regarding toxicities could be due to the selection of concurrent chemotherapy regimen. The KROG 10-01 trial utilized bolus 5FU, while the latter utilized infusional 5FU which is expectedly less toxic. Interestingly, in their subsequent trial on the so-called KROG 11-02, same authors changed the regimen to oral capecitabine and this time, reported more acceptable safety profiles [21]. In our study, concurrent oral capecitabine in adjunct to intravenous oxaliplatin regimen was used to improve compliance and safety together with high pathologic response rate, and both goals were met fortunately. Another explanation for the acceptable rates of acute and late complications could be the differences among ethnicities regarding response to chemotherapy [22,23]. Perhaps, the Caucasian unlike Asian populations are more resistant to concurrent 5FU based chemotherapy regimens.
Apart from the issue of feasibility and tolerance, a relatively high rate of about 31% of complete pathologic response (cPR) was observed among the patients who underwent extirpative surgery. Three simultaneous deviations from routine SRT were attempted which could explain the promising results in terms of cPR including delayed surgery, concurrent and consolidative chemotherapies. In orthodox SRT method that is practiced first in Sweden, patients would undergo total mesorectal excision within 1 week after completion of radiotherapy sessions. With early surgery, evaluating the ultimate pathologic response is not too possible, the optimal response may be difficult to achieve and the rate of positive margin will be expectedly high. However, if one can postpone the surgery for some 4–6 weeks, the pathologic response would be theoretically more mature even in primarily unresectable appearing cases [24]. Response to radiotherapy is a continuous process and the optimum interval between completion of radiotherapy and surgery is not clear [25]. Beppu et al. [16] showed that SRT with delayed surgery is non-inferior to long course chemoradiation [26] in terms of down-staging effects and complications. A metaanalytical study also proved that by delaying surgery, the rate of cPR would be 10% greater than that of earlier surgery.
Besides the role of delayed surgery, the addition of concurrent chemotherapy to conventional fractionated radiotherapy and consolidative chemotherapy in the resting period between radiation completion and surgery, both have significantly increased the pCR rate and LC in locally advanced rectal cancer and have acceptable and tolerable toxicity [27,28]. Following these successful observations, some authors implemented chemotherapy as adjunct to neoadjuvant SRT. Myerson et al. [8] tested the efficacy of consolidation chemotherapy in the interval between SRT and surgery. The rate of pCR and 2-year LC were 25% and 95%, respectively. Some investigators assume that, like gastric cancer, neoadjuvant chemotherapy might be associated with improved survival. Currently, this hypothesis is being tested in adjunct to SRT in RAPIDO trial [29]. However, the above studies used chemotherapy in a sequential fashion with SRT but in trials implementing concurrent chemotherapy protocols, the rate of cPR ranged from 1.4% to 21.1% [9,10,20,21]. It is noteworthy that regimens that contain oral capecitabine are associated with absolutely better and higher responses than bolus and infusional 5FU. However, our superior results could be explained by the addition of oxaliplatin to oral capecitabine, that has been shown to significantly improve response rate (TRG0 and TRG1) both in ACCORD trial [30] and in our center previous experience [31]. In order to introduce a new concurrent regimen with SRT, the results of several metaanalysis were used, suggesting the benefit of adding oxaliplatin to 5FU based concurrent long course chemoradiotherapy [32-34].
The main limitation of this study is the small size and nonrandomized design. Another limitation that is worth mentioning is the need for longer follow-up for late effects of radiation and the rate of LC. The observed promising results need to be tested in a randomized manner in comparison with conventional long course chemoradiotherapy.
In conclusion, this study showed that SRT with concurrent chemotherapy and consolidation chemotherapy with delayed surgery are not only feasible and tolerable without significant toxicity but are also associated with promising pathologic response rates. However, this is a small and nonrandomized one-arm study with relatively short follow-up which needs longer assessments for monitoring of late complications. Further investigation is needed to compare this protocol with conventional fractionated protocol in a phase III randomized trial.
Footnotes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 2.Cedermark B, Johansson H, Rutqvist LE, Wilking N. The Stockholm I trial of preoperative short term radiotherapy in operable rectal carcinoma: a prospective randomized trial. Stockholm Colorectal Cancer Study Group. Cancer. 1995;75:2269–75. doi: 10.1002/1097-0142(19950501)75:9<2269::aid-cncr2820750913>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Martling A, Holm T, Johansson H, Rutqvist LE, Cedermark B, Stockholm Colorectal Cancer Study Group The Stockholm II trial on preoperative radiotherapy in rectal carcinoma: long-term follow-up of a population-based study. Cancer. 2001;92:896–902. doi: 10.1002/1097-0142(20010815)92:4<896::aid-cncr1398>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 4.Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol. 2012;30:3827–33. doi: 10.1200/JCO.2012.42.9597. [DOI] [PubMed] [Google Scholar]
- 5.Farhan F, Fazeli MS, Samiei F, et al. Morbidity and mortality following short course preoperative radiotherapy in rectal carcinoma. Acta Med Iran. 2015;53:627–32. [PubMed] [Google Scholar]
- 6.Minsky BD. Short-course radiation versus long-course chemoradiation for rectal cancer: making progress. J Clin Oncol. 2012;30:3777–8. doi: 10.1200/JCO.2012.45.0551. [DOI] [PubMed] [Google Scholar]
- 7.Bujko K, Partycki M, Pietrzak L. Neoadjuvant radiotherapy (5 × 5 Gy): immediate versus delayed surgery. Recent Results Cancer Res. 2014;203:171–87. doi: 10.1007/978-3-319-08060-4_12. [DOI] [PubMed] [Google Scholar]
- 8.Myerson RJ, Parikh PJ, Tan B, et al. A single-institution phase II trial of five fractions of radiotherapy followed by four courses of FOLFOX chemotherapy as preoperative therapy for rectal adenocarcinoma. J Clin Oncol. 2012;30(4_Suppl):553. [Google Scholar]
- 9.Chung MJ, Kim DW, Chung WK, et al. Preoperative short- vs. long-course chemoradiotherapy with delayed surgery for locally advanced rectal cancer. Oncotarget. 2016;8:60479–86. doi: 10.18632/oncotarget.10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasulov AO, Gordeyev SS, Barsukov YA, et al. Short-course preoperative radiotherapy combined with chemotherapy, delayed surgery and local hyperthermia for rectal cancer: a phase II study. Int J Hyperthermia. 2017:1–9. doi: 10.1080/02656736.2016.1272138. [DOI] [PubMed] [Google Scholar]
- 11.Myerson RJ, Garofalo MC, El Naqa I, et al. Elective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys. 2009;74:824–30. doi: 10.1016/j.ijrobp.2008.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19–23. doi: 10.1007/s003840050072. [DOI] [PubMed] [Google Scholar]
- 13.Pettersson D, Cedermark B, Holm T, et al. Interim analysis of the Stockholm III trial of preoperative radiotherapy regimens for rectal cancer. Br J Surg. 2010;97:580–7. doi: 10.1002/bjs.6914. [DOI] [PubMed] [Google Scholar]
- 14.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215–23. doi: 10.1002/bjs.5506. [DOI] [PubMed] [Google Scholar]
- 15.Ansari N, Solomon MJ, Fisher RJ, et al. Acute adverse events and postoperative complications in a randomized trial of preoperative short-course radiotherapy versus long-course chemoradiotherapy for T3 adenocarcinoma of the rectum: Trans-Tasman Radiation Oncology Group Trial (TROG 01.04) Ann Surg. 2017;265:882–8. doi: 10.1097/SLA.0000000000001987. [DOI] [PubMed] [Google Scholar]
- 16.Beppu N, Matsubara N, Kakuno A, et al. Feasibility of modified short-course radiotherapy combined with a chemoradiosensitizer for T3 rectal cancer. Dis Colon Rectum. 2015;58:479–87. doi: 10.1097/DCR.0000000000000323. [DOI] [PubMed] [Google Scholar]
- 17.Erlandsson J, Holm T, Pettersson D, et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017;18:336–46. doi: 10.1016/S1470-2045(17)30086-4. [DOI] [PubMed] [Google Scholar]
- 18.van Dijk TH, Tamas K, Beukema JC, et al. Evaluation of shortcourse radiotherapy followed by neoadjuvant bevacizumab, capecitabine, and oxaliplatin and subsequent radical surgical treatment in primary stage IV rectal cancer. Ann Oncol. 2013;24:1762–9. doi: 10.1093/annonc/mdt124. [DOI] [PubMed] [Google Scholar]
- 19.Beppu N, Yoshie H, Kimura F, et al. The short-term outcomes of induction SOX (S-1 + oxaliplatin) ± cetuximab chemotherapy followed by short-course chemoradiotherapy in patients with poor-risk locally advanced rectal cancer. Surg Today. 2016;46:1123–31. doi: 10.1007/s00595-015-1284-2. [DOI] [PubMed] [Google Scholar]
- 20.Yeo SG, Oh JH, Kim DY, et al. Preoperative short-course concurrent chemoradiation therapy followed by delayed surgery for locally advanced rectal cancer: a phase 2 multicenter study (KROG 10-01) Int J Radiat Oncol Biol Phys. 2013;86:34–9. doi: 10.1016/j.ijrobp.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Lee JH, Kim JG, Oh ST, et al. Two-week course of preoperative chemoradiotherapy followed by delayed surgery for rectal cancer: a phase II multi-institutional clinical trial (KROG 11-02) Radiother Oncol. 2014;110:150–4. doi: 10.1016/j.radonc.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 22.O'Donnell PH, Dolan ME. Cancer pharmacoethnicity: ethnic differences in susceptibility to the effects of chemotherapy. Clin Cancer Res. 2009;15:4806–14. doi: 10.1158/1078-0432.CCR-09-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phan VH, Moore MM, McLachlan AJ, Piquette-Miller M, Xu H, Clarke SJ. Ethnic differences in drug metabolism and toxicity from chemotherapy. Expert Opin Drug Metab Toxicol. 2009;5:243–57. doi: 10.1517/17425250902800153. [DOI] [PubMed] [Google Scholar]
- 24.Hatfield P, Hingorani M, Radhakrishna G, et al. Short-course radiotherapy, with elective delay prior to surgery, in patients with unresectable rectal cancer who have poor performance status or significant co-morbidity. Radiother Oncol. 2009;92:210–4. doi: 10.1016/j.radonc.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Kwak YK, Kim K, Lee JH, et al. Timely tumor response analysis after preoperative chemoradiotherapy and curative surgery in locally advanced rectal cancer: a multi-institutional study for optimal surgical timing in rectal cancer. Radiother Oncol. 2016;119:512–8. doi: 10.1016/j.radonc.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Beppu N, Matsubara N, Noda M, et al. Short-course radiotherapy with delayed surgery versus conventional chemoradiotherapy: a comparison of the short- and longterm outcomes in patients with T3 rectal cancer. Surgery. 2015;158:225–35. doi: 10.1016/j.surg.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Habr-Gama A, Perez RO, Sabbaga J, Nadalin W, Sao Juliao GP, Gama-Rodrigues J. Increasing the rates of complete response to neoadjuvant chemoradiotherapy for distal rectal cancer: results of a prospective study using additional chemotherapy during the resting period. Dis Colon Rectum. 2009;52:1927–34. doi: 10.1007/DCR.0b013e3181ba14ed. [DOI] [PubMed] [Google Scholar]
- 28.Gerard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620–5. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson PJ, van Etten B, Hospers GA, et al. Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer: the RAPIDO trial. BMC Cancer. 2013;13:279. doi: 10.1186/1471-2407-13-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28:1638–44. doi: 10.1200/JCO.2009.25.8376. [DOI] [PubMed] [Google Scholar]
- 31.Haddad P, Miraie M, Farhan F, et al. Addition of oxaliplatin to neoadjuvant radiochemotherapy in MRI-defined T3, T4 or N+ rectal cancer: a randomized clinical trial. Asia Pac J Clin Oncol. 2017;13:416–22. [Google Scholar]
- 32.van der Pols JC, Russell A, Bauer U, Neale RE, Kimlin MG, Green AC. Vitamin D status and skin cancer risk independent of time outdoors: 11-year prospective study in an Australian community. J Invest Dermatol. 2013;133:637–41. doi: 10.1038/jid.2012.346. [DOI] [PubMed] [Google Scholar]
- 33.Yang YJ, Cao L, Li ZW, et al. Fluorouracil-based neoadjuvant chemoradiotherapy with or without oxaliplatin for treatment of locally advanced rectal cancer: an updated systematic review and meta-analysis. Oncotarget. 2016;7:45513–24. doi: 10.18632/oncotarget.9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng J, Feng X, Hu W, Wang J, Li Y. Systematic review and meta-analysis of preoperative chemoradiotherapy with or without oxaliplatin in locally advanced rectal cancer. Medicine (Baltimore) 2017;96:e6487. doi: 10.1097/MD.0000000000006487. [DOI] [PMC free article] [PubMed] [Google Scholar]


