Abstract
Background
The number of studies on venous thromboembolism (VTE) and thyroid cancer is very scarce and existing data are contradictory. This paper reviews VTE in thyroid cancer.
Methods
The following words were used for a comprehensive literature review using MEDLINE database: Blood coagulation factors; thyroid hormones; blood coagulation tests; venous thromboembolism; receptors thyroid hormone; hemostasis; fibrinolysis; bleeding; blood coagulation disorders; thyroid neoplasms; Thyroid cancer, papillary; Thyroid cancer, follicular; Thyroid carcinoma, anaplastic; Thyroid cancer, Hurthle cell; Familial medullary thyroid carcinoma; venous thrombosis; Pulmonary embolism; Blood coagulation factors. The studies, which include any changes in hemostasis and thyroid cancer were included and reviewed.
Results
Although few studies have shown a possible increase in VTE occurrence in thyroid cancer in patients ≥ 60 years old and in proximity to cancer diagnosis, other studies could not find any difference compared to general population. New thyroid cancer classification excluding common subtype(s) with benign nature, may affect the results of the future studies on association of VTE and thyroid cancer.
Conclusions
Prospective studies on the occurrence of VTE in various types and severities of thyroid cancer and in different age groups are warranted, as the results would affect clinical practice on the necessity of usage of anticoagulants in some thyroid cancer groups.
Keywords: Venous Thromboembolism; Blood Coagulation Factors; Fibrinolysis; Thyroid Neoplasms; Thyroid Hormones; Receptor, Thyroid Hormone
1. Introduction
Risk of venous thromboembolism (VTE) including deep vein thrombosis (DVT) and pulmonary embolism (PE), has been different among various malignancies (1). There is no direct report on risk of VTE in thyroid cancer or thyroid cancer was not included in those studies (1-7).
Thyroid cancer induces thrombosis through compression (8, 9), angioinvasion (10-13), or possibly a prothrombotic state. However, the studies on thyroid cancer and risk of VTE and hypercoagulability are scarce. In this review we focus more on risks of VTE and prothrombotic states in thyroid cancer.
2. Methods
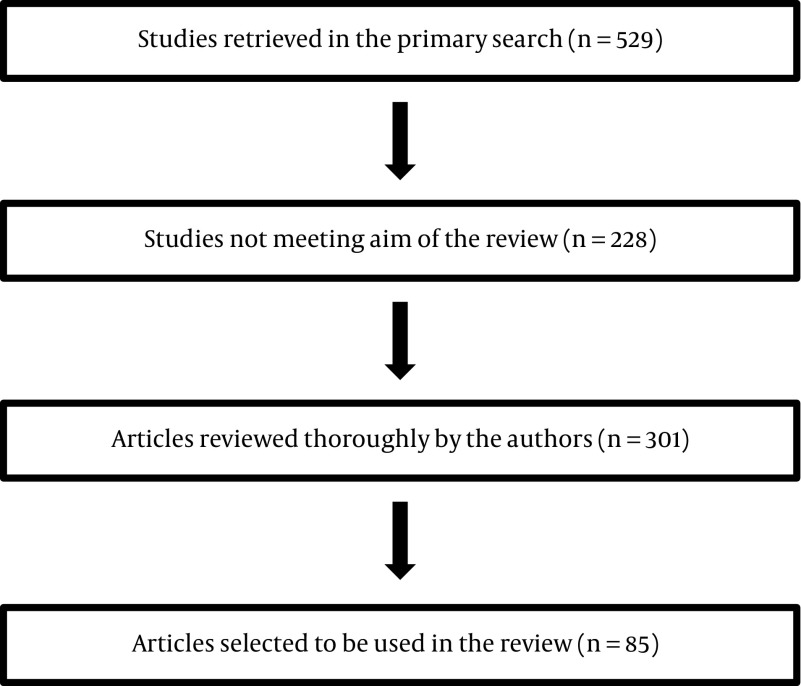
Using MEDLINE database, the following words were used for a throrough literature review until March 2016: A comprehensive review of the literature has been done. The following text words and medical subject heading were used for the MEDLINE search until March 2016: Blood coagulation factors; thyroid hormones; blood coagulation tests; venous thromboembolism; receptors thyroid hormone; hemostasis; fibrinolysis; bleeding; blood coagulation disorders; Thyroid neoplasms; Thyroid cancer, papillary; Thyroid cancer, follicular; Thyroid carcinoma, anaplastic; Thyroid cancer, Hurthle cell; Familial medullary thyroid carcinoma; Venous thrombosis; Pulmonary embolism; Blood coagulation factors. Additionally, the list of references in pertinent chapters in textbooks and in review articles was searched for publications beyong 2000. The studies that assessed occurrence of VTE and/or blood hemostasis alteration among patients with any type thyroid cancer were included. The generated abstracts were reviewed for their title and abstract content by the authors. Those that were not covering hemostatic changes or clinical VTE occurrence or thyroid cancer were excluded. Full article of relevant abstracts were reviewed thoroughly and their eligibility were re-evaluated again. The flowchart of evaluated and selected studies is depicted. Selected and assessed studies are summarized in Figure 1.
Figure 1. Flowchart of Assessment and Selection of the Studies Used in This Review*.
3. Results
3.1. Thyroid Cancer and Hypercoagulability
Follicular cell-derived thyroid cancers are comprised of well-differentiated papillary carcinoma, follicular carcinoma, poorly differentiated carcinoma, and anaplastic (undifferentiated) carcinoma (14). Differentiated carcinoma accounts for 95% of all the cases of thyroid cancer (15). Thyroid cancer annual incidence is roughly 1% of all new malignant diseases and has increased over the last decade, mainly due to improved ability to diagnose malignant transformation in small thyroid nodules (16).
An independent association between VTE and thyroid cancer in thyroidectomized individuals could not be found in a retrospective cohort study (17). Overall risk of VTE in thyroidectomy and parathyroidectomy was 0.16 that was six times less frequent than the risk in the entire cohort (0.96), reflecting a very low rate (18). In a large and prospective cohort study, the absolute rate per 1000 person year (95%CI) of the risk of VTE in thyroid cancer patients aged > 60 year was 9.5 (4.3 - 21) compared to 0.6 (0.1 - 4.3) in those aged < 60 years, giving a 15.8 folds higher risk between the two age groups. Regarding time to occurrence of VTE since cancer diagnosis, absolute rate per 1000 person years (95% CI) was 30 (9.6 - 92) in 0 - 3 months compared to 11 (3.5 - 33) in 3 - 12 months and 0.5 (0.1-3.8) in > 12 months. Although authors have initially considered that there was no significant difference in comparison with the background general population; however, age and proximity to the time of diagnosis showed significant effect on this risk ratio (19). Overall, a prothrombotic state might exist by aging, which is reflexed by increases in FV, FVII, FVIII, and FIX. Many laboratory parameters, such as D-dimer, may need age-adjusted normal ranges (20). The large UK study lacks information on the staging of thyroid cancers, thyroid cancer subtypes (19) and therefore, rate adjustments for risk of VTE in cancers with more aggressive compared to indolent behavior, concurrent thyroid function status, and thyroid hormone therapy, as the latter may have a prothrombotic effect (21-23).
Encapsulated follicular variant of papillary thyroid carcinoma (EFVPTC) comprises 10% - 20% of currently diagnosed cancers in Europe and North America are EFVPTC (24, 25). Recently, the new name of “noninvasive follicular thyroid neoplasm with papillary-like nuclear features” (NIFTP) was therefore suggested based on indolent nature and very low risk of adverse outcomes of the disease. This potential reclassification will significantly reduce clinical and psychological burden of a misdiagnosis of cancer (26). Similar concern would be raised with overdiagnosis and overtreatment of thyroid nodules (16). On the other hand, malignancies with high incidence but low VTE rate (such as leukemia, Non-Hodgkin lymphoma, and thyroid cancer with incidence of 13.5, 19.5, and 13.9 per 100,000 men and women per year) can significantly contribute to the overall burden of VTE occurrence (27). Although pancreatic and gastric cancers have the highest rates of VTE, however, more than 30% of VTE have happened in Non-Hodgkin’s lymphoma and leukemia (1). This assumption could be implied to thyroid cancers as well. The true effect of thyroid cancer on the risk of VTE is still unknown (17).
Medullary thyroid cancer has metabolic characteristics and has been associated with paraneoplastic syndromes (28). Elevated catecholamines and serotonin due to pheochromocytoma and/or medullary thyroid carcinoma may contribute to platelet activation and aggregation and hence, a hypercoagulable state (29, 30). Serotonin potentiates overall platelet procoagulant properties through increase in interaction of platelets with tissue-factor-rich microvesicles and platelet activation (31) or through modification of the content of N-glycans on the surface of platelets causing an increase in platelet aggregation, and a prothrombotic state (32). A hypercoagulable state have been considered in the previous reports of a case of widespread medullary carcinoma with recurrent stroke, nonbacterial endocarditis, elevated D-dimer and normal fibrinogen (28), and a case of metastatic medullary thyroid cancer with DVT and PE and superior sagittal sinus thrombosis and mild elevation of FVIII, fibrinogen, and antithrombin III (33).
A hypercoagulable state has also been observed in other types of thyroid cancer, including papillary adenocarcinoma with follicular and clear cell patterns on histological specimen (34), Hurthle cell carcinoma (35), and anaplastic thyroid carcinoma (36).
Cancer, in general, is associated with an imbalance in the hemostatic system and induces a prothrombotic state; however, the pathogenesis of cancer-associated coagulopathy is complex (37, 38). Cancer patients commonly demonstrate abnormal levels of coagulations factors, such as elevated fibrinogen, FV, FVIII, FIX, and FXI, fibrinogen/fibrin degradation products, and platelet counts (39).
Hemostatic profile in thyroid cancer may depend on the severity of the disease (40). Alterations of coagulation-fibrinolytic system in thyroid cancer have not been extensively studied. Mean platelet volume and its elevation have been suggested as a biomarker for the risk of papillary thyroid cancer in patients with thyroid nodule (41).
Hemeoxygenase-1, a carbon monoxide-producing enzyme can be upregulated in thyroid cancer cell lines. Carbon monoxide significantly enhances in vitro and in vivo plasma coagulation and this is achieved through binding to fibrinogen-associated heme group(s) that enhances properties of fibrinogen substrate. In thyroid cancer carboxyhemoglobin concentration is increased (2.4%) and a carbon-monoxide-mediated clot strength is observed resulting in plasmatic a hypercoagulability state. The latter has been determined by a thrombelastographic method. Further investigation on the role of this enzyme in thyroid cancers (specifically those with increased endogenous carbon monoxide production) is warranted (42).
Similar thromboplastin activity of monocytes between malignant versus benign neoplasm groups is deducted from a study with serious methodological limitations and therefore is not generalizable (43). Similarly, due to very small sample size of thyroid carcinoma, the conclusion over higher procoagulant activity in metastatic vs non-metastatic malignant tumors lacks generalizability (44).
The role of various components of Plasminogen activating system (PAS), especially urokinase-plasminogen activator (uPA) and plasminogen activator inhibitor type 1 (PAI-1), in thyroid cancer progression, metastasis, and prognosis and progression-free survival is established. Although significantly higher expression of uPA and PAI-1 (45-48), and uPA receptor (49) has been observed in malignant compared to benign, and in more aggressive compared to less aggressive thyroid cancers, its effect on hemostasis alteration has yet to be elucidated.
In summary, it is still unproven whether thyroid cancer is associated with an increased risk of VTE. Our information is even less when it comes to mechanism(s) predisposing to a prothrombotic state. Increased platelet activation and/or aggregation secondary to abnormal catecholamine and/or serotonin metabolism might be a possible mechanism as mean platelet volume has been suggested as biomarker of thyroid cancer. Any derangement of endothelial function and in primary- and secondary hemostatic cascades and in fibrinolytic pathways (especially in components of PAS) would contribute to this tendency and warrants further evaluation.
3.2. Thyroid Cancer and Angioinvasion and Compression
Tumor thrombus in thyroid malignancies and propensity to spread by contiguous vascular extension is not the goal of this review, but it may potentiate prothrombotic cascades when occurs in thyroid cancer. Occasionally, both high-risk papillary and follicular thyroid cancer invade great vessels of neck and upper mediastinum (10-13), but in rare occasions tumor embolism can affect remote anatomical sites such as middle cerebral artery (50). Clinical presentation in great cervical veins can vary from asymptomatic presentation to florid superior vena cava syndrome (51-54). Nine out of 5,507 thyroidectomized thyroid malignancies had tumor thrombi (55).
Although tumor compression and/or tumor invasion/thrombus are not primarily considered as prothrombotic risk factors for VTE (and should not be included in epidemiological studies regarding evaluation of VTE risk in thyroid cancer), both conditions have the potential to increase the risk of thrombus/embolus formation. These cancerous patients may be at a higher risk of VTE compared to those thyroid cancer patients without these pathologies. Further studies are warranted in this regard.
3.3. Thyroid Cancer Therapy
The application of medical and surgical management strategies of thyroid cancers continue to evolve (56). Considering possible prothrombotic effect of levothyroxine therapy (21-23), it is unknown to what extent TSH-suppressive thyroid hormone therapy and/or cancer (57) may contribute to VTE events in thyroid cancer patients.
In general, in cancer patients, cytotoxic chemotherapy has been associated with the development of VTE (58-60). There are several ongoing clinical trials on Selumetinib, Everolimus, Everolimus and Vatalanib, and Lenvatinib and treatment of various types of thyroid cancer (61). Despite of all these ongoing trials, FDA-approved biologically targeted chemotherapies for differentiated thyroid cancer at the present, are Doxorubicin, Sorafenib, and Lenvatinib (62) and for medullary thyroid cancer are Vandetanib (63) and Cabozantinib (64). These medications may also have some promising effects on advanced PDTC and anaplastic thyroid cancers as well (61).
Multikinase inhibitors (MKI) or Tyrosine Kinase Inhibitors (TKI) are used in various types of thyroid cancer such as medullary thyroid carcinoma and differentiated thyroid cancer refractory to radioiodine (65, 66). MKIs such as FDA-approved Sorafenib used for differentiated thyroid cancer are associated with both arterial thrombosis and VTE (65, 67, 68). TKIs are also associated with hypothyroidism with an incidence as high as 32% in Vandetanib (66, 69). As moderate and/or subclinical hypothyroidism may induce a prothrombotic state, this is unclear whether medication-induced hypothyroidism contributes to these VTE adverse events. Cabozantinib has been used in the treatment of locally advanced or metastatic medullary thyroid carcinoma and has a VTE incidence of ~4% among the cancer patients (70). Hypothyroidism and VTE have been reported in usage of Motesanib in the treatment of aggressive differentiated thyroid cancer with various histologies (71). Treatment-related PE was not significantly different among cancer vs. placebo groups using Lenvatinib (72). Although VTE is listed among pharmacological adverse effects of TKIs, whether using Vandetanib (Caprelsa) or Cabozantinib (Cometriq) is associated with increased VTE in thyroid cancer treatment has yet to be elucidated (63, 64, 73, 74).
Pulmonary embolism was detected in 11% of Lenalidomide users (75). VTE events occur in 9% - 22% of Pomalidomide, Lenalidomide, and Thalidomide users (65). Everolimus has been used in anaplastic thyroid cancer treatment (76). Daily everolimus plus low-dose weekly cisplatin was accompanied with 11% venous thrombosis in cancer patients (77). Vemurafenib, used in anaplastic carcinoma, has caused disseminated intravascular coagulation in a patient with advanced malignant melanoma (78). Vorinostat, a histone deacetylase inhibitor, has been used in differentiated and meduallry thyroid carcinomas and DVT was reported among the Grade 3 adverse events attributed to the drug usage (79).
3.4. Thromboprophylaxis
Our knowledge on risk of VTE in various types, severity, and stages of thyroid cancers and side effects of their treatment is inadequate and these require population-based prospective cohort studies and clinical trials. Until then, our recommendations for thromboprophylaxis in thyroid cancers in various clinical situations are similar to those provided by cancer societies for prevention and treatment of cancer-associated VTE (See Table 1) (80-84). Patients’ education about the signs and symptoms of VTE should be done by oncology professionals (85). Using a validated risk assessment tool such as Khorana score (58) for discrimination between low-, intermediate-, and high-risk patients is recommended (83).
Table 1. Various Dosing Regimens for Prevention and Treatment of VTE in Cancerous Patients (Including Thyroid Cancer) 83-85.
| Druga | Regimenb | |
|---|---|---|
| Hospitalized medical patients c,d | UFH | 5000 U every 8 h |
| Enoxaparin | 40 mg once daily | |
| Fondaparinux | 2.5 mg once daily | |
| Treatment of established venous thromboembolism | ||
| Initial | ||
| UFHe | 80 U/kg IV bolus, then 18 U/kg per h IV; adjust dose based on aPTTf | |
| Enoxaparine,g | 1 mg/kg once every 12 h; 1.5 mg/kg once daily | |
| Fondaparinuxe | < 50 kg, 5.0 mg once daily; 50 - 100 kg, 7.5 mg once daily; > 100 kg, 10 mg once daily | |
| Long-termh | Enoxaparing | 1.5 mg/kg once daily; 1 mg/kg once every 12 h |
| Warfarin | Adjust dose to maintain INR 2 - 3 |
Abbreviations: aPTT, activated partial thromboplastin time; UFH, Unfractionated heparin.
aSome of the commonly used anticoagulation medications are listed here. Full list of the medications are listed elsewhere (83, 84).
bAll doses are administered as subcutaneous injections except as indicated. In renal dysfunction, doses may need adjustments.
cDuration for medical patients is length of hospital stay or until fully ambulatory; for surgical patients, prophylaxis should be continued for at least 7 - 10 days. Extended prophylaxis for up to 4 weeks should be considered for high-risk patients.
dChemoprophylaxis for surgical patients are not listed here. Information regarding are listed elsewhere (83, 84).
eParenteral anticoagulants should overlap with warfarin for 5 - 7 days minimum and continued until INR is in the therapeutic range for 2 consecutive days.
fUnfractionated heparin infusion rate should be adjusted to maintain the aPTT within the therapeutic range in accordance with local protocol to correspond with a heparin level of 0.3 - 0.7 U/mL using a chromogenic Xa essay.
gTwice-daily dosing may be more efficacious than once-daily dosing for enoxaparin based on post hoc data.
hTotal duration of therapy depends on clinical circumstances. For detailed information please see refs. 83 and 84.
4. Discussion
Although cancer, in general, increases a prothrombotic tendency, thyroid cancer has scarcely been evaluated in this regard. Older age (> 60 years of age) and proximity to the time of diagnosis have been reported as possible risk factors of VTE (19). However, these assumptions require further confirmation due to lack of adequate information. Studies on VTE incidence in various subtypes and/or severities of thyroid cancer are warranted. Thyroid cancer may not only predispose a prothrombotic state per se, but also its treatment [e.g., biologically targeted chemotherapies (65, 67, 68, 70) and suppressive levothyroxine treatment (21-23)] and/or side effects of its treatment [e.g., biological chemotherapy-induced thyroid dysfunctions (66, 69)] may add to that risk.
There is a paucity of information on mechanisms involved in hemostatic changes in thyroid cancer and its subtypes and severities (29, 39, 43, 45-49). Studies on changes in platelet adhesion and aggregation (29), various coagulation factors (39), components of PAS (45-49), endothelial functioning, and procoagulant molecules [e.g., tissue factor-positive micropeptides, cancer-derived tissue factor, cancer procoagulant, and heparanase (37, 38)] are required.
4.1. Overall Conclusions
Well-designed, large-scale, and prospective studies and clinical trials are required to elucidate whether VTE is associated with various types and stages of thyroid cancer.
Acknowledgments
We are thankful to Mr. Fred King, Medical Librarian at Medstar Washington hospital Center for his technical assistance.
Footnotes
Conflict of Interest:None to declare.
Contributor Information
Arash Ordookhani, Email: arash.ordookhani@yahoo.com.
Abbas Motazedi, Email: fymotazedi@aol.com.
References
- 1.Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(29):4839–47. doi: 10.1200/JCO.2009.22.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levitan N, Dowlati A, Remick SC, Tahsildar HI, Sivinski LD, Beyth R, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine (Baltimore). 1999;78(5):285–91. doi: 10.1097/00005792-199909000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Thodiyil PA, Kakkar AK. Variation in relative risk of venous thromboembolism in different cancers. Thromb Haemost. 2002;87(6):1076–7. [PubMed] [Google Scholar]
- 4.Heit JA. Cancer and venous thromboembolism: scope of the problem. Cancer Control. 2005;12 Suppl 1:5–10. doi: 10.1177/1073274805012003S02. [DOI] [PubMed] [Google Scholar]
- 5.Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9(7):e1001275. doi: 10.1371/journal.pmed.1001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cronin-Fenton DP, Sondergaard F, Pedersen LA, Fryzek JP, Cetin K, Acquavella J, et al. Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997-2006. Br J Cancer. 2010;103(7):947–53. doi: 10.1038/sj.bjc.6605883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293(6):715–22. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 8.Gomes MN, Hufnagel CA. Superior vena cava obstruction: a review of the literature and report of 2 cases due to benign intrathoracic tumors. Ann Thorac Surg. 1975;20(3):344–59. doi: 10.1016/s0003-4975(10)64229-4. [DOI] [PubMed] [Google Scholar]
- 9.Hyer SL, Dandekar P, Newbold K, Haq M, Wechalakar K, Thway K, et al. Thyroid cancer causing obstruction of the great veins in the neck. World J Surg Oncol. 2008;6:36. doi: 10.1186/1477-7819-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhargav PR. Tumor thromboembolization into the internal jugular vein through its draining vein: a preoperative radiologic feature of high-risk thyroid cancer. Surgery. 2014;155(1):196–7. doi: 10.1016/j.surg.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Mishra A, Agarwal A, Agarwal G, Mishra SK. Internal jugular vein invasion by thyroid carcinoma. Eur J Surg. 2001;167(1):64–7. doi: 10.1080/110241501750069855. [DOI] [PubMed] [Google Scholar]
- 12.Motohashi S, Sekine Y, Iizasa T, Nakano K, Numata T, Fujisawa T. Thyroid cancer with massive invasion into the neck and mediastinal great veins. Jpn J Thorac Cardiovasc Surg. 2005;53(1):55–7. doi: 10.1007/s11748-005-1012-8. [DOI] [PubMed] [Google Scholar]
- 13.Franco IF, Gurrado A, Lissidini G, Di Meo G, Pasculli A, Testini M. Floating left innominate vein neoplastic thrombus: a rare case of mediastinal extension of follicular thyroid carcinoma. Phlebology. 2015;30(2):140–4. doi: 10.1177/0268355513515209. [DOI] [PubMed] [Google Scholar]
- 14.Boelaert K. Thyroid gland: Revised guidelines for the management of thyroid cancer. Nat Rev Endocrinol. 2010;6(4):185–6. doi: 10.1038/nrendo.2010.17. [DOI] [PubMed] [Google Scholar]
- 15.American Thyroid Association Guidelines Taskforce on Thyroid N, Differentiated Thyroid C, Cooper DS, Doherty GM, Haugen BR, Kloos RT, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 16.Burman KD, Wartofsky L. CLINICAL PRACTICE. Thyroid Nodules. N Engl J Med. 2015;373(24):2347–56. doi: 10.1056/NEJMcp1415786. [DOI] [PubMed] [Google Scholar]
- 17.Reinke CE, Hadler RA, Karakousis GC, Fraker DL, Kelz RR. Does the presence of thyroid cancer increase the risk of venous thromboembolism in patients undergoing thyroidectomy? Surgery. 2011;150(6):1275–85. doi: 10.1016/j.surg.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 18.Roy M, Rajamanickam V, Chen H, Sippel R. Is DVT prophylaxis necessary for thyroidectomy and parathyroidectomy? Surgery. 2010;148(6):1163–8. doi: 10.1016/j.surg.2010.09.013. discussion 1168-9. [DOI] [PubMed] [Google Scholar]
- 19.Walker AJ, Card TR, West J, Crooks C, Grainge MJ. Incidence of venous thromboembolism in patients with cancer - a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49(6):1404–13. doi: 10.1016/j.ejca.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 20.Favaloro EJ, Franchini M, Lippi G. Aging hemostasis: changes to laboratory markers of hemostasis as we age - a narrative review. Semin Thromb Hemost. 2014;40(6):621–33. doi: 10.1055/s-0034-1384631. [DOI] [PubMed] [Google Scholar]
- 21.Van Zaane B, Squizzato A, Debeij J, Dekkers OM, Meijers JC, Van Zanten AP, et al. Alterations in coagulation and fibrinolysis after levothyroxine exposure in healthy volunteers: a controlled randomized crossover study. J Thromb Haemost. 2011;9(9):1816–24. doi: 10.1111/j.1538-7836.2011.04430.x. [DOI] [PubMed] [Google Scholar]
- 22.Horne M3, Singh KK, Rosenfeld KG, Wesley R, Skarulis MC, Merryman PK, et al. Is thyroid hormone suppression therapy prothrombotic? J Clin Endocrinol Metab. 2004;89(9):4469–73. doi: 10.1210/jc.2004-0536. [DOI] [PubMed] [Google Scholar]
- 23.Stuijver DJ, van Zaane B, Squizzato A, Meijers JC, Otten HM. The effects of an extremely high dose of levothyroxine on coagulation and fibrinolysis. J Thromb Haemost. 2010;8(6):1427–8. doi: 10.1111/j.1538-7836.2010.03854.x. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Singh B, Tallini G, Carlson DL, Katabi N, Shaha A, et al. Follicular variant of papillary thyroid carcinoma: a clinicopathologic study of a problematic entity. Cancer. 2006;107(6):1255–64. doi: 10.1002/cncr.22138. [DOI] [PubMed] [Google Scholar]
- 25.Jung CK, Little MP, Lubin JH, Brenner AV, Wells SJ, Sigurdson AJ, et al. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab. 2014;99(2):E276–85. doi: 10.1210/jc.2013-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA Oncol. 2016;2(8):1023–9. doi: 10.1001/jamaoncol.2016.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Cancer Institute . Cancer Stat Fact Sheets, National Cancer Institute, Surveillance, epidemiology, and end results program. Maryland : National Institute of Health; 2015. Available from: http://seer.cancer.gov/statfacts/ [Google Scholar]
- 28.Lal G, Brennan TV, Hambleton J, Clark OH. Coagulopathy, marantic endocarditis, and cerebrovascular accidents as paraneoplastic features in medullary thyroid cancer--case report and review of the literature. Thyroid. 2003;13(6):601–5. doi: 10.1089/105072503322238872. [DOI] [PubMed] [Google Scholar]
- 29.Pishdad GR. Ventricular thrombosis in Sipple's syndrome. South Med J. 2000;93(11):1093–5. [PubMed] [Google Scholar]
- 30.de Groot JW, Kema IP, Breukelman H, van der Veer E, Wiggers T, Plukker JT, et al. Biochemical markers in the follow-up of medullary thyroid cancer. Thyroid. 2006;16(11):1163–70. doi: 10.1089/thy.2006.16.1163. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Vilchez I, Diaz-Ricart M, White JG, Escolar G, Galan AM. Serotonin enhances platelet procoagulant properties and their activation induced during platelet tissue factor uptake. Cardiovasc Res. 2009;84(2):309–16. doi: 10.1093/cvr/cvp205. [DOI] [PubMed] [Google Scholar]
- 32.Mercado CP, Quintero MV, Li Y, Singh P, Byrd AK, Talabnin K, et al. A serotonin-induced N-glycan switch regulates platelet aggregation. Sci Rep. 2013;3:2795. doi: 10.1038/srep02795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tiede DJ, Tefferi A, Kochhar R, Thompson GB, Hay ID. Paraneoplastic cholestasis and hypercoagulability associated with medullary thyroid carcinoma. Resolution with tumor debulking. Cancer. 1994;73(3):702–5. doi: 10.1002/1097-0142(19940201)73:3<702::aid-cncr2820730333>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 34.Raveh E, Cohen M, Shpitzer T, Feinmesser R. Carcinoma of the thyroid: a cause of hypercoagulability? Ear Nose Throat J. 1995;74(2):110–2. [PubMed] [Google Scholar]
- 35.Luo H, Tulpule S, Alam M, Patel R, Sen S, Yousif A. A Rare Silent Killer: Right Atrial Metastasis of Thyroid Hurthle Cell Carcinoma. Case Rep Oncol. 2015;8(2):233–7. doi: 10.1159/000430948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stickel JS, Mayer F, Vollmer JP, Zurn CS, Gawaz M, Kanz L, et al. Ventricular thrombus and thrombocytopenia as first presentation of anaplastic thyroid carcinoma. J Clin Oncol. 2013;31(12):e194–6. doi: 10.1200/JCO.2012.43.4894. [DOI] [PubMed] [Google Scholar]
- 37.Falanga A, Schieppati F, Russo D. Cancer Tissue Procoagulant Mechanisms and the Hypercoagulable State of Patients with Cancer. Semin Thromb Hemost. 2015;41(7):756–64. doi: 10.1055/s-0035-1564040. [DOI] [PubMed] [Google Scholar]
- 38.Mitrugno A, Tormoen GW, Kuhn P, McCarty OJ. The prothrombotic activity of cancer cells in the circulation. Blood Rev. 2016;30(1):11–9. doi: 10.1016/j.blre.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rickles FR, Edwards RL. Activation of blood coagulation in cancer: Trousseau's syndrome revisited. Blood. 1983;62(1):14–31. [PubMed] [Google Scholar]
- 40.Mina A, Favaloro EJ, Koutts J. Hemostatic dysfunction associated with endocrine disorders as a major risk factor and cause of human morbidity and mortality: a comprehensive meta-review. Semin Thromb Hemost. 2007;33(8):798–809. doi: 10.1055/s-2007-1000372. [DOI] [PubMed] [Google Scholar]
- 41.Baldane S, Ipekci SH, Sozen M, Kebapcilar L. Mean platelet volume could be a possible biomarker for papillary thyroid carcinomas. Asian Pac J Cancer Prev. 2015;16(7):2671–4. doi: 10.7314/apjcp.2015.16.7.2671. [DOI] [PubMed] [Google Scholar]
- 42.Nielsen VG, Garol BD, Zelman EA, Guerrero MA. Hemeoxygenase-1 mediated hypercoagulability in a patient with thyroid cancer. Blood Coagul Fibrinolysis. 2013;24(6):663–5. doi: 10.1097/MBC.0b013e328363ab86. [DOI] [PubMed] [Google Scholar]
- 43.Osterud B, Due JJ. Blood coagulation in patients with benign and malignant tumours before and after surgery. Special reference to thromboplastin generation in monocytes. Scand J Haematol. 1984;32(3):258–64. [PubMed] [Google Scholar]
- 44.Sarode R, Marwaha N, Gupta NM. Procoagulant activity of human tumours: existence of Xa and thrombin-like activities. Br J Cancer. 1990;61(1):29–31. doi: 10.1038/bjc.1990.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horvatic Herceg G, Herceg D, Kralik M, Kulic A, Bence-Zigman Z, Tomic-Brzac H, et al. Urokinase plasminogen activator and its inhibitor type-1 as prognostic factors in differentiated thyroid carcinoma patients. Otolaryngol Head Neck Surg. 2013;149(4):533–40. doi: 10.1177/0194599813496374. [DOI] [PubMed] [Google Scholar]
- 46.Horvatic Herceg G, Herceg D, Kralik M, Bence-Zigman Z, Tomic-Brzac H, Kulic A. Urokinase-type plasminogen activator and its inhibitor in thyroid neoplasms: a cytosol study. Wien Klin Wochenschr. 2006;118(19-20):601–9. doi: 10.1007/s00508-006-0703-1. [DOI] [PubMed] [Google Scholar]
- 47.Baldini E, Sorrenti S, D'Armiento E, Di Matteo FM, Catania A, Ulisse S. The urokinase plasminogen activating system in thyroid cancer: clinical implications. G Chir. 2012;33(10):305–10. [PubMed] [Google Scholar]
- 48.Sid B, Langlois B, Sartelet H, Bellon G, Dedieu S, Martiny L. Thrombospondin-1 enhances human thyroid carcinoma cell invasion through urokinase activity. Int J Biochem Cell Biol. 2008;40(9):1890–900. doi: 10.1016/j.biocel.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 49.Ulisse S, Baldini E, Toller M, Marchioni E, Giacomelli L, De Antoni E, et al. Differential expression of the components of the plasminogen activating system in human thyroid tumour derived cell lines and papillary carcinomas. Eur J Cancer. 2006;42(15):2631–8. doi: 10.1016/j.ejca.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 50.Banerjee AK, Chopra JS. Cerebral embolism from a thyroid carcinoma. Report of a case. Arch Neurol. 1972;27(2):186–7. doi: 10.1001/archneur.1972.00490140090014. [DOI] [PubMed] [Google Scholar]
- 51.Onoda N, Nakamura M, Hosono M, Sasaki Y, Kawajiri H, Takashima T, et al. Successful surgical treatment of advanced follicular thyroid carcinoma with tumor thrombus infiltrating the superior vena cava: report of a case. Surg Today. 2012;42(2):185–90. doi: 10.1007/s00595-011-0033-4. [DOI] [PubMed] [Google Scholar]
- 52.Mugunthan N, Kumar AR, Thomas P. Tumor thrombus in the great veins from papillary carcinoma of the thyroid: 131I scan findings. J Clin Endocrinol Metab. 2010;95(6):2527–8. doi: 10.1210/jc.2009-2031. [DOI] [PubMed] [Google Scholar]
- 53.Wiseman O, Preston PG, Clarke JM. Presentation of thyroid carcinoma as a thrombosed external jugular vein, with intraluminal tumour thrombus in the great veins. Eur J Surg Oncol. 2000;26(8):816–7. doi: 10.1053/ejso.2000.1011. [DOI] [PubMed] [Google Scholar]
- 54.Ingle SA, Karnik ND, Pradhan NN, Ranadive NU, Dhurde A, Kotalwar M. Papillary carcinoma of thyroid presenting as posterior mediastinal mass with superior vena cava syndrome. J Assoc Physicians India. 2004;52:1004–5. [PubMed] [Google Scholar]
- 55.Kobayashi K., Hirokawa M., Yabuta T., Fukushima M., Kihara M., Higashiyama T., et al. Tumor thrombus of thyroid malignancies in veins: importance of detection by ultrasonography. Thyroid . 2011;21(5):527–31. doi: 10.1089/thy.2010.0099. [DOI] [PubMed] [Google Scholar]
- 56.Filetti S, Tuttle RM, Sherman S. Medical Management of differentiated epithelial cell thyroid cancer. In: Braverman LE, Cooper DS, editors. Werner and Ingbar's The Thyroid. A fundamental and clinical text. Philadelphia: Wolters Kluver Lippincott Williams & Wilkins; 2013. pp. 725–74. [Google Scholar]
- 57.Sherman SI. Thyroid carcinoma. Lancet. 2003;361(9356):501–11. doi: 10.1016/s0140-6736(03)12488-9. [DOI] [PubMed] [Google Scholar]
- 58.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902–7. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khorana AA, Francis CW, Culakova E, Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005;104(12):2822–9. doi: 10.1002/cncr.21496. [DOI] [PubMed] [Google Scholar]
- 60.Haddad TC, Greeno EW. Chemotherapy-induced thrombosis. Thromb Res. 2006;118(5):555–68. doi: 10.1016/j.thromres.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 61.Viola D, Valerio L, Molinaro E, Agate L, Bottici V, Biagini A, et al. Treatment of advanced thyroid cancer with targeted therapies: ten years of experience. Endocr Relat Cancer. 2016;23(4):R185–205. doi: 10.1530/ERC-15-0555. [DOI] [PubMed] [Google Scholar]
- 62.National cancer institute Drugs Approved for Thyroid Cancer. 2016 Available from: http://www.cancer.gov/about-cancer/treatment/drugs/thyroid.
- 63.Wells SJ, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30(2):134–41. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elisei R, Schlumberger MJ, Muller SP, Schoffski P, Brose MS, Shah MH, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31(29):3639–46. doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dy GK, Adjei AA. Understanding, recognizing, and managing toxicities of targeted anticancer therapies. CA Cancer J Clin. 2013;63(4):249–79. doi: 10.3322/caac.21184. [DOI] [PubMed] [Google Scholar]
- 66.Shah DR, Shah RR, Morganroth J. Tyrosine kinase inhibitors: their on-target toxicities as potential indicators of efficacy. Drug Saf. 2013;36(6):413–26. doi: 10.1007/s40264-013-0050-x. [DOI] [PubMed] [Google Scholar]
- 67.Schutz FA, Je Y, Richards CJ, Choueiri TK. Meta-analysis of randomized controlled trials for the incidence and risk of treatment-related mortality in patients with cancer treated with vascular endothelial growth factor tyrosine kinase inhibitors. J Clin Oncol. 2012;30(8):871–7. doi: 10.1200/JCO.2011.37.1195. [DOI] [PubMed] [Google Scholar]
- 68.Pitoia F, Jerkovich F. Selective use of sorafenib in the treatment of thyroid cancer. Drug Des Devel Ther. 2016;10:1119–31. doi: 10.2147/DDDT.S82972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brassard M, Neraud B, Trabado S, Salenave S, Brailly-Tabard S, Borget I, et al. Endocrine effects of the tyrosine kinase inhibitor vandetanib in patients treated for thyroid cancer. J Clin Endocrinol Metab. 2011;96(9):2741–9. doi: 10.1210/jc.2010-2771. [DOI] [PubMed] [Google Scholar]
- 70.Hoy SM. Cabozantinib: a review of its use in patients with medullary thyroid cancer. Drugs. 2014;74(12):1435–44. doi: 10.1007/s40265-014-0265-x. [DOI] [PubMed] [Google Scholar]
- 71.Sherman SI, Wirth LJ, Droz JP, Hofmann M, Bastholt L, Martins RG, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. 2008;359(1):31–42. doi: 10.1056/NEJMoa075853. [DOI] [PubMed] [Google Scholar]
- 72.Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372(7):621–30. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- 73.Clinical Trials.Gov A Service of the U.S. National Institute of Health. Efficacy of XL184 (Cabozantinib) in Advanced Medullary Thyroid Cancer (EXAM. 2016 Available from: https://clinicaltrials.gov/show/NCT00704730.
- 74.NIH National Library of Medicine. Medline Plus. Cabozantinib. 2016 Available from: https://www.nlm.nih.gov/medlineplus/druginfo/meds/a613015.html#side-effects.
- 75.Segler A, Tsimberidou AM. Lenalidomide in solid tumors. Cancer Chemother Pharmacol. 2012;69(6):1393–406. doi: 10.1007/s00280-012-1874-2. [DOI] [PubMed] [Google Scholar]
- 76.Wagle N, Grabiner BC, Van Allen EM, Amin-Mansour A, Taylor-Weiner A, Rosenberg M, et al. Response and acquired resistance to everolimus in anaplastic thyroid cancer. N Engl J Med. 2014;371(15):1426–33. doi: 10.1056/NEJMoa1403352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fury MG, Sherman E, Haque S, Korte S, Lisa D, Shen R, et al. A phase I study of daily everolimus plus low-dose weekly cisplatin for patients with advanced solid tumors. Cancer Chemother Pharmacol. 2012;69(3):591–8. doi: 10.1007/s00280-011-1734-5. [DOI] [PubMed] [Google Scholar]
- 78.van den Brom RR, Makelburg AB, Schroder CP, de Vries EG, Hospers GA. Vemurafenib-Induced Disseminated Intravascular Coagulation in Metastatic Melanoma. J Clin Oncol. 2015;33(36):e133–4. doi: 10.1200/JCO.2013.51.4471. [DOI] [PubMed] [Google Scholar]
- 79.Woyach JA, Kloos RT, Ringel MD, Arbogast D, Collamore M, Zwiebel JA, et al. Lack of therapeutic effect of the histone deacetylase inhibitor vorinostat in patients with metastatic radioiodine-refractory thyroid carcinoma. J Clin Endocrinol Metab. 2009;94(1):164–70. doi: 10.1210/jc.2008-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kahn SR, Lim W, Dunn AS, Cushman M, Dentali F, Akl EA, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e195S–226S. doi: 10.1378/chest.11-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Streiff MB, Bockenstedt PL, Cataland SR, Chesney C, Eby C, Fanikos J, et al. Venous thromboembolic disease. J Natl Compr Canc Netw. 2011;9(7):714–77. doi: 10.6004/jnccn.2011.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.NICE Venous thromboembolism: reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital. 2015 Available from: https://www.nice.org.uk/guidance/cg92/evidence/full-guideline-243920125. [PubMed]
- 83.Khorana AA, Carrier M, Garcia DA, Lee AY. Guidance for the prevention and treatment of cancer-associated venous thromboembolism. J Thromb Thrombolysis. 2016;41(1):81–91. doi: 10.1007/s11239-015-1313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lyman GH, Khorana AA, Kuderer NM, Lee AY, Arcelus JI, Balaban EP, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(17):2189–204. doi: 10.1200/JCO.2013.49.1118. [DOI] [PubMed] [Google Scholar]
- 85.Lyman GH, Bohlke K, Khorana AA, Kuderer NM, Lee AY, Arcelus JI, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: american society of clinical oncology clinical practice guideline update 2014. J Clin Oncol. 2015;33(6):654–6. doi: 10.1200/JCO.2014.59.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]