Abstract
It is well established that protein kinase A (PKA) is involved in hippocampal dependent memory consolidation. Sleep is also known to play an important role in this process. However, whether sleep-dependent memory consolidation involves PKA activation has not been clearly determined. Using behavioral observation, animals were categorized into sleep and awake groups. We show that intrahippocampal injections of the PKA inhibitor Rp-cAMPs in post-contextual fear conditioning sleep produced a suppression of long-term fear memory, while injections of Rp-cAMPs during an awake state, at a similar time point, had no effect. In contrast, injections of the PKA activator Sp-cAMPs in awake state, rescued sleep deprivation-induced memory impairments. These results suggest that following learning, PKA activation specifically in sleep is required for the consolidation of long-term memory.
Memory is initially labile requiring a process of consolidation to become stable. Further, this process is known to involve gene expression and protein synthesis. One of the gene cascades that has been studied extensively in the hippocampus in relation to memory is cyclic adenosine monophosphate (cAMP)/PKA. Activation of PKA leads to the phosphorylation of various downstream kinases and transcription factors (e.g., cAMP response element binding protein; CREB), that are required for memory consolidation (for review, see Abel and Nguyen 2008). Using a hippocampus dependent task (step-down inhibitory avoidance), it has been reported that PKA activity increased significantly immediately and within a 3–6 h time window following training (Vianna et al. 2000; Pereira et al. 2001). Further, pharmacological suppression of cAMP produced memory deficits while its activation, at corresponding time intervals, produced memory enhancements (Vianna et al. 2000; Quevedo et al. 2004; Datta et al. 2009). Suppression of PKA, using either a genetic approach or administration (peripheral or intraventricular/intrahippocampal) of protein synthesis or PKA inhibitors, also disrupts long-term fear memory (Abel et al. 1997; Bourtchouladze et al. 1998; Ahi et al. 2004). Interestingly, suppression of PKA just prior to or within ∼4–6 h following training produced memory deficits while suppression outside this time window was not effective (Bernabeu et al. 1997; Wallenstein et al. 2002; Ahi et al. 2004).
Considerable evidence suggests that memory consolidation could be taking place in sleep and that hippocampus-dependent memory is especially affected (for review, see Kreutzmann et al. 2015). At the behavioral level, it has been reported that following a learning experience there is an increase in sleep (especially rapid eye movement sleep, REM) (Fishbein et al. 1974; Hennevin et al. 1995; Smith and Rose 1997; Hellman and Abel 2007). Conversely, post-learning sleep deprivation produces long-term memory deficits (Fishbein 1971; Smith and Rose 1996; Graves et al. 2003; Ruskin and LaHoste 2008; Hagewoud et al. 2010a,b,c, 2011; Binder et al. 2012; Kumar and Jha 2012). At the cellular level, a reactivation of neuronal activity following awake learning has been reported (Pavlides and Winson 1989; Wilson and McNaughton 1994; Ribeiro et al. 2004; for reviews, see Ribeiro and Nicolelis 2004; Inostroza and Born 2013), which could be part of the consolidation process. A number of studies have also shown that long-term potentiation (LTP), a neural model of learning and memory, is suppressed by sleep deprivation (Romcy-Pereira and Pavlides 2004; Marks and Wayner 2005; Ishikawa et al. 2006; Vecsey et al. 2009; Ravassard et al. 2016). Specific to fear memory, sleep deprivation within a 0–5 but not 5–10 h post-conditioning suppressed hippocampus dependent contextual but not hippocampus independent cued fear memory, when tested 24 h later (Graves et al. 2003).
Recent evidence suggests that sleep-dependent memory consolidation may share similar molecular mechanisms identified in learning/memory and synaptic plasticity (for review, see Graves 2001). It has been reported that sleep deprivation impaired cAMP and PKA signaling as well as enhancing protein levels of phosphodiesterase 4 (PDE4), an enzyme that degrades cAMP in the hippocampus (Vecsey et al. 2009). This in turn produced contextual fear memory deficits and suppressed a PKA-dependent form of hippocampal LTP, which could be reversed with peripheral injections of the PDE4 inhibitor Rolipram. A more recent study by the same group (Havekes et al. 2014) demonstrated that up-regulating cAMP/PKA signaling in the hippocampus via a pharmacogenetic method prevented sleep deprivation-induced memory impairments in an object-place recognition task. Further, it has been reported that cAMP (as well as MAPK) activation shows a circadian pattern being highest during the sleep phase, reported to be most conducive to memory consolidation (Eckel-Mahan et al. 2008). A later study reported that increased levels of phosphorylated PKA were found during REM sleep (although not in slow wave sleep, SWS) (Luo et al. 2013). The above evidence provides strong, albeit indirect, support for the involvement of PKA in sleep-dependent memory consolidation. In the present study, we tested, for the first time, the effects of pharmacologically manipulating hippocampal PKA levels specifically in sleep on long-term fear memory.
Long-Evans rats (n = 34; Institute for Animal Reproduction, Japan), 16 wk old on average, were used in the present study. Animals were provided with water and food ad libitum and maintained on a 12:12 h light:dark cycle (lights on at 12:00 p.m.), in a temperature-controlled environment (23°C). Two weeks before behavioral procedures, animals were implanted with cannulae for drug injections. Animals were anesthetized with sodium pentobarbital (65.0 mg/kg), placed on a stereotaxic frame (Kopf Instruments,), and implanted bilaterally with 22-gauge stainless-steel guide cannulae (C232G/5.0/SPC, PlasticsOne), coupled with dummy cannulae (C232DC/5.0/SPC, PlasticsOne; 0.5 mm protrusion), aimed at the CA1 field of the dorsal hippocampus, at the following coordinates: AP: bregma point −3.5 mm; ML: mid-sagital suture ±2.5 mm; DV: skull surface −3.0 mm. Stereotaxic coordinates were determined according to the atlas of Paxinos and Watson (2007). Following surgery, each rat received a local antibiotic treatment (triple antibiotic ointment, Zeria Pharmaceutical Co, Ltd) around the incision and was injected with 3000 units of Penicillin (Penicillin G potassium, Meiji Co, Ltd), to prevent post-operative infections. Animals were monitored until they fully recovered from anesthesia and were given at least 1 wk to recover. Animals were handled for 5 min on three consecutive days before contextual fear conditioning. Experiments were performed at the beginning of the light cycle. Animals were contextual fear conditioned (Zeitgeber time [ZT] 0–1) by placing them in a conditioning chamber (O'Hara & Co., LTD). The chamber was made of Plexiglas and was 34 × 26 × 28 cm in size. The floor was made of stainless steel rods that were connected to a shock generator (O'Hara & Co., LTD). The chamber was enclosed within a sound-isolated cubicle (52 × 38 × 54 cm) that was evenly illuminated by two fluorescent lights (O'Hara & Co., LTD). A 5-min baseline behavior was recorded, after which electric shocks (3 footshocks, 1 mA, 1 sec, 2 min intershock interval) were given. The animals were left in the chamber for an additional 3 min. Freezing behavior was scored at 5 sec intervals using computer software (Time FZ1; O'Hara & Co., LTD) and was defined as the lack of all movements, except those associated with respiration.
Immediately after fear conditioning, animals were placed in a cylindrical recording chamber (45 cm in diameter × 60 cm high) that was illuminated by white LED lights and contained a video camera for behavioral recordings. There was also a small window on the chamber for direct observation of the animal's behavior. The animals were either kept awake (AW group) or allowed to sleep (SLEEP group) for 4 h. AW animals were kept awake by gently tapping on the chamber (1 tap every 15 min, on average), when they showed drowsiness. This method was chosen based on previous reports that gentle handling is very effective in keeping rodents awake (Meerlo et al. 2001) while producing minimum stress, as determined by plasma corticosterone and adrenocorticotophic (ACTH) levels (Hagewoud et al. 2010b; Gross et al. 2015). Furthermore, sleep deprivation-induced stress has been reported to not be correlated to sleep deprivation-induced memory deficits (Hagewoud et al. 2010b). The animal's behavior was subdivided into seven different states (Pavlides and Winson 1989; Ribeiro et al. 2002). These included four different awake behaviors: (1) active, evidenced by exploration of the chamber with whisking; (2) alert, evidenced by stillness while sitting on the floor; (3) quiet-awake, evidenced by stillness lying down on the floor or grooming with opened eyes; (4) drowsy, evidenced by stillness with eyes semiclosed, just prior to drifting into sleeping state. Two sleeping states were distinguished (van Betteray et al. 1991):(1) slow wave sleep (SWS), evidenced by overall stillness with curled-up or stretched-out body and closed eyes; (2) rapid-eye-movement (REM) sleep, evidenced by ear and whisker movements, heavy and irregular breathing, and head collapsing to the floor of the chamber. Each wake/sleep state was determined independently by two experienced observers. Thus, although sleep–wake states were assessed by behavioral observation alone, we are confident that the accuracy was higher than 90%.
Drugs were injected into the dorsal hippocampus bilaterally at the start of sleep, or at a similar time point for AW, control animals. To ensure a steady sleep state, the injections were initiated following a brief (∼5 min) sleep period. The injections were made with a 25 µL Hamilton syringe driven by an injection pump (KDS510, KD Scientific Inc.) and connected via a two-channel stainless steel swivel (TCS2-23, Eicom) with polyethylene tubes (PE50, Becton, Dickinson & Co.) to the 30-gauge injection cannulae on the animal's head. The injection cannulae were left in place for the remainder of the 4 h period after the injection, not to disturb the animal's sleep. Postmortem verification of cannulae placements was performed after the completion of behavioral procedures. Long-term contextual fear memory was examined 24 h after training for 5 min by measuring the percentage of time spent freezing in the original conditioning context, without the footshocks. To compare the effects of treatment (e.g., SLEEP versus AW), a repeated-measures two-way ANOVA was used, followed by post hoc analysis, using Sidak's multiple comparison test (P-value = 0.05; GraphPad Software, Inc.). All experiments for this study were carried out in accordance with the Regulations for Animal Experiments at the University of Tsukuba and Fundamental Guidelines for Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions.
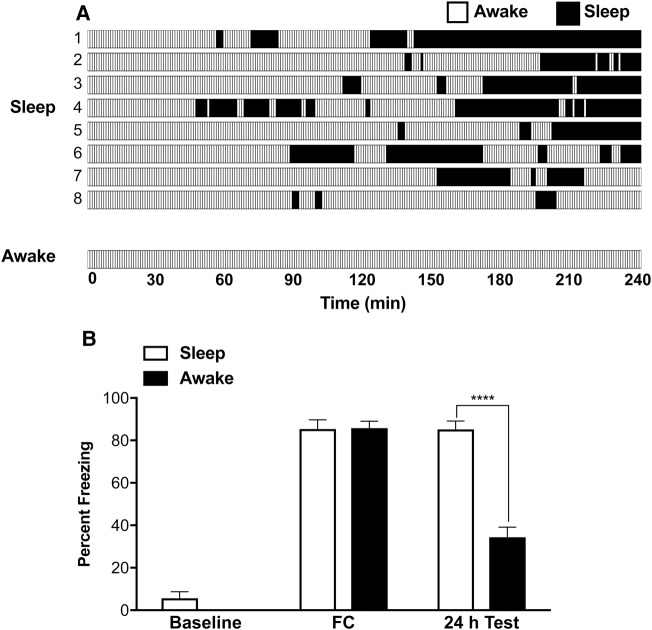
Prior to determining the role of PKA in memory, we examined whether sleep deprivation following fear conditioning indeed affects the consolidation of long-term fear memory. As described above, animals were contextual fear conditioned, then placed in a recording chamber and allowed to sleep or kept awake for 4 h. On the following day, fear memory was tested. The sleep profile is presented in Figure 1A. The mean sleep time in the SLEEP animals was 71.75 ± 14.14 min (over the 4 h period), which corresponds to 29.89% of total observation time. The fear conditioning results are presented in Figure 1B. Animals from all groups showed similar motor activity during the 5-min baseline period, prior to shock. Their freezing behavior increased after the conditioning, to a comparable level for all groups (repeated-measures ANOVA, F(4,60) = 69.37, P < 0.0001). At the 24 h test, the AW animals froze significantly less, compared to the SLEEP animals in the test session (84.99% and 34.38% for SLEEP and AW animals, respectively; F(4,60) = 7.648, P < 0.0001). In line with previous studies, our results confirm that post-learning sleep affects the consolidation of fear memory.
Figure 1.
Effects of sleep on long-term fear memory. (A) Sleep–wake profile following fear conditioning (FC). For the SLEEP group, data is plotted for each individual animal. For AW group, a representative animal is shown. Each vertical bar represents 1 min. The AW animals were in either active, alert or quiet states for 91.22% (219.10 ± 8.71 min) and in a drowsy state for 8.78% (21.10 ± 6.98 min) of total observation time (240 min) and never entered sleep. The SLEEP animals, slept on average 72 min (29.89% of total observation time). (B) Effects of sleep deprivation on long-term contextual fear memory. Both groups of animals showed near-zero freezing behavior during a 5-min baseline recording. Following baseline, all animals were fear conditioned (3 footshocks, 1 mA, 1 sec, 2 min apart). This produced comparable freezing behavior for all groups. Fear memory was measured 24 h later in the same context. The AW animals (n = 8) showed significantly less freezing compared to the SLEEP animals (n = 9). Results are presented as mean ± SEM percent freezing; (****) P < 0.0001.
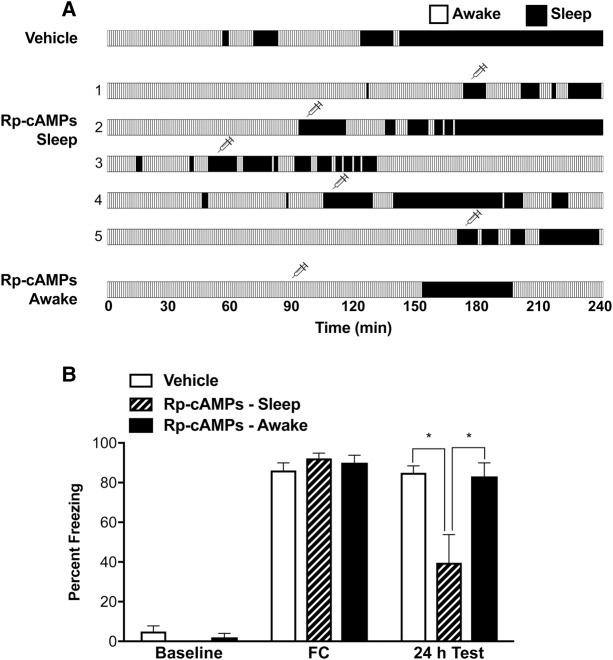
We next tested whether PKA activity is critical for sleep-mediated memory consolidation, by injecting the PKA inhibitor Rp-cAMPs bilaterally into the CA1 field of the dorsal hippocampus. Animals were conditioned as described above, then placed in the recording chamber for 4 h. At the start of sleep, they received 3 µL of Rp-cAMPs (20 mM, BML-CN135, Enzo Life Sciences, Inc.) or vehicle (aCSF, 124 mM NaCl, 3 mM KCl, 26 mM NaHCO3, 2 mM CaCl2/2H2O, 1 mM MgSO4/7H2O, 1.25 mM NaH2PO4, 10 mM d-glucose, pH 7.4). Long-term fear memory was tested 24 h later. As shown in Figure 2, intrahippocampal injection of Rp-cAMPs in SLEEP animals produced a deficit in contextual fear memory, compared to the vehicle-injected controls. Freezing of Rp-cAMPs-injected rats was significantly lower than those of vehicle-injected controls (51.66% and 84.99% for Rp-cAMPs-injected and vehicle-injected animals, respectively; F(4,48) = 4.907, P < 0.05). The effect of Rp-cAMPs injection on fear memory was similar to AW animals in the first experiment.
Figure 2.
Effects of PKA inhibition on long-term fear memory. (A) Sleep–wake profile following fear conditioning (FC), along with time point of bilateral intrahippocampal injections, as marked by syringe symbol. Note that the AW animals were injected at comparable times to the SLEEP animals. For vehicle-injected control animals, only representative data is plotted. For Rp-cAMPs-injected animals, the data were individually plotted. Each vertical bar represents 1 min. Average total time spent in sleep was not significantly different between vehicle-injected (71.75 ± 14.15 min), Rp-cAMPs-injected in sleep (75.2 ± 14.45 min), and Rp-cAMPs-injected in awake state (43.6 ± 13.18 min) (P > 0.1 for all comparisons). (B) Effects of bilateral intrahippocampal injection of the PKA inhibitor Rp-cAMPs on long-term contextual fear memory. All animals showed near-zero freezing behavior during baseline recording and robust freezing following conditioning. Fear memory was measured 24 h later in the same context. Injection of Rp-cAMPs (20 mM, 3 µL) in SLEEP animals (n = 5) produced significantly impaired consolidation of contextual fear memory, compared to the vehicle injected controls (n = 9). In contrast, for animals injected with Rp-cAMPs in the awake state (n = 5), no long-term memory deficits were observed. Results are presented as mean ± SEM percent freezing; (*) P < 0.05.
We, further, examined whether Rp-cAMPs also had a detrimental effect on long-term memory when injected during the awake state. After conditioning, Rp-cAMPs was injected into the CA1 of the dorsal hippocampus during wakefulness, at a comparable time as the SLEEP group (Fig. 2). The animals were then kept awake for ∼1 h, following which they were allowed to sleep spontaneously for the remainder of the recording time. They were tested 24 h later for fear memory. As shown in Figure 2, Rp-cAMPs animals injected in the awake state showed freezing levels comparable to vehicle-injected controls (83.14% and 84.99% for Rp-cAMPs-injected in AW and vehicle-injected controls, respectively; F(2,24) = 0.4359, P = 0.6517), but significantly higher freezing levels, compared to Rp-cAMPs injected in sleep (83.14% and 51.66% for Rp-cAMPs-injected in AW and Rp-cAMPs-injected in SLEEP, respectively; F(4,32) = 2.816, P < 0.05). Taken together, these results indicate that PKA signaling specifically during sleep following a learning experience has a significant impact on memory consolidation.
Finally, we tested whether PKA activation could prevent the memory impairment induced by post-training sleep deprivation. Animals were fear conditioned (same as above), injected intrahippocampally bilaterally during awake state with either the PKA activator Sp-cAMPs (20 mM; BML-CN136, Enzo Life Sciences, Inc.) or vehicle (aCSF, 124 mM NaCl, 3 mM KCl, 26 mM NaHCO3, 2 mM CaCl2/2H2O, 1 mM MgSO4/7H2O, 1.25 mM NaH2PO4, 10 mM D-glucose, pH 7.4), 2.5 h after contextual fear conditioning, at a time interval yoked to SLEEP animals of the first two experiments. The animals were kept awake for the 4 h recording period. Their long-term fear memory was assessed 24 h later. As shown in Figure 3, Sp-cAMPs injected into CA1 significantly rescued the memory deficit observed following post-training sleep deprivation. Sp-cAMPs-injected animals exhibited significantly higher freezing responses compared to the vehicle-injected animals (72.00% and 34.38% for Sp-cAMPs-injected and vehicle-injected controls, respectively; F(4,44) = 7.922, P < 0.001). This result suggests that activating PKA can, in and of itself, attenuate the detrimental effects of sleep deprivation on memory consolidation.
Figure 3.
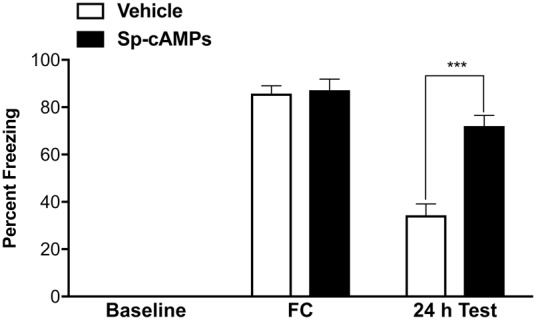
Effects of bilateral intrahippocampal injections of the PKA activator Sp-cAMPs on contextual fear memory in sleep-deprived animals. Similar to previous experiment, both groups of animals showed near-zero freezing behavior during baseline recording and robust freezing following fear conditioning (FC). Fear memory was measured 24 h later in the same context. Bilateral intrahippocampal injection of the PKA activator Sp-cAMPs (20 mM, 3µL) in AW animals (n = 5) reversed the detrimental effects of sleep deprivation on the consolidation of long-term fear memory, compared to the vehicle injected controls (n = 8). Results are presented as mean ± SEM percent freezing; (***) P < 0.001.
Previous studies have suggested that PKA plays a critical role in memory consolidation (for review, see Hernandez and Abel 2011). PKA levels increase following training in a number of hippocampal-dependent tasks (Bernabeu et al. 1997; Izquierdo and Medina 1997; Pereira et al. 2001). On the other hand, interfering with PKA expression just prior to or shortly after learning produces memory deficits (Schafe et al. 1999; Wallenstein et al. 2002). Interestingly, two time points for PKA effects have been reported, one during the initial acquisition just after training and the other between 3–6 h after training (Bourtchouladze et al. 1998). A shortcoming of the previous studies is that they did not take into account the animal's behavioral state (i.e., sleep/awake). Besides the effects of learning and memory on PKA expression (or deficits resulting from its suppression), sleep deprivation has also been reported to suppress PKA expression (Vecsey et al. 2009), as well as downstream targets of PKA such as CREB (Hagewoud et al. 2011). The combined evidence suggests that sleep-dependent memory consolidation may involve similar molecular mechanisms as have been demonstrated previously for hippocampal-dependent memory (for review, see Havekes et al. 2015).
In the present study, we first replicated previous findings (Graves et al. 2003) that a brief period (4 h) of sleep deprivation following learning produces long-term fear memory deficits. Second, we show that suppressing PKA levels in the hippocampus specifically in sleep following contextual fear conditioning disrupts long-term fear memory. This provides direct evidence that PKA expression during sleep is necessary for long-term fear memory consolidation. PKA suppression during the awake state but at a similar time point following conditioning, did not produce memory deficits. This probably reflects the fact that these animals were allowed to sleep at a later time point (although within the 4 h critical period), as the drug effects had subsided and PKA levels increased. On the other hand, intrahippocampal injections of the PKA activator Sp-cAMPs at a comparable time point in awake animals was sufficient to overcome the sleep deprivation-induced memory deficits. It is also pertinent to state that Rp-cAMPs has been reported to produce peak PKA suppression within 15 min, which then rapidly diminishes and is ineffective by 90 min after intrahippocampal injection (Vianna et al. 2000). Thus, it is safe to assume that our drug injections produced their effects mainly in the intended behavioral state—that is, either sleep or wakefulness.
As stated above, two peaks of PKA expression, at 0 and 3–6 h, following step-down inhibitory avoidance training are observed (Bernabeu et al. 1997). Interestingly, PKA suppression at 0 or 3 h disrupted long-term memory while PKA suppression at intervals within a 90 min range disrupted short-term memory (Vianna et al. 2000). Further, while inhibition of PKA (and MAPK) 15 min before or immediately after training in an inhibitory avoidance task suppressed both short- and long-term memory, inhibition of protein synthesis produced only a long-term memory deficit (Quevedo et al. 2004). A different study (Grecksch and Matthies 1980) also reported two sensitive periods for amnesic effects by the protein synthesis inhibitor anisomycin. Intrahippocampal administration of anisomycin 10 min before and at 80 min and 4 and 6 h after retention of a brightness discrimination task disrupted memory, while no amnesia was observed with injections at 45 and 165 min (∼3 h) post-training. Two critical periods at 0 and 4 h for PKA and protein synthesis for memory consolidation were also reported for contextual fear memory in mice (Bourtchouladze et al. 1998). Further, genetic inhibition of PKA disrupted late- but not early-phase LTP, spatial memory and long-term but not short-term contextual fear memory (Abel et al. 1997). However, the precise time-course of PKA activation and possible correlation with behavioral state following learning remains to be determined.
The majority of studies, thus far, have investigated the role of cAMP/PKA/CREB in the CA1 field of the dorsal hippocampus. However, besides the hippocampus, there is an increase in PKA activity in the entorhinal cortex following training in a step-down inhibitory task (Pereira et al. 2001). Further, interfering with this signaling pathway in other brain areas including the CA3, anterior cingulate, and posterior parietal and entorhinal cortex, also produce memory deficits (Ardenghi et al. 1997; Barros et al. 2000; Datta et al. 2009; for review, see Izquierdo et al. 2006). Specific to fear memory, suppression of PKA and protein synthesis with intraventricular (Schafe et al. 1999) or intralateral amygdaloid nucleus (Schafe and LeDoux 2000) injections prior to or just after conditioning produced contextual and cued long- but not short-term fear memory deficits, while intralateral amygdala injections 6 h later had no effect. A similar suppression of long-, but not short-term, contextual and cued fear memory following basolateral amygdala injections of the PKA/PKC inhibitor H7 was reported (Goosens et al. 2000). Interestingly PKA appears to also be involved in the reconsolidation of long-term fear memory. Injection of Rp-cAMPs into the basolateral amygdala following reexposure impaired reconsolidation, while injections of the PKA activator 6-BNZ-cAMPS enhanced it (Tronson et al. 2006). In another study, intra-amygdala injection of a cholera toxin, which suppresses PKA, inhibited acquisition of a Pavlovian approach behavior while injection of Sp-cAMPs enhanced acquisition of this task (Jentsch et al. 2002). However, using a passive avoidance task, it has been reported that PKA inhibition in the hippocampus but not amygdala caused memory deficits (Bevilaqua et al. 1997). As mentioned earlier, inhibition of PKA in the hippocampus at 0, 3, and 6 h caused amnesia (although 1.5 and 9 h were ineffective), while PKA inhibition in the amygdala was ineffective either at 0, 3, or 6 h (Ardenghi et al. 1997; Bevilaqua et al. 1997; Barros et al. 2000).
The role that PKA and protein synthesis may play in sleep-dependent memory consolidation in other brain areas as well as other time points following learning has not been investigated. Further, although the majority of studies have stressed the importance of PKA, a recent report (Ma et al. 2009) suggests that Epac, another downstream target of cAMP, is also critical for memory consolidation in a PKA independent manner. Besides cAMP/PKA/CREB, a number of other signaling pathways and proteins have been shown to play a significant role in memory consolidation (for reviews, see Schafe et al. 2001; Izquierdo et al. 2006; Hernandez and Abel 2011; Abel et al. 2013), which may work differently in different parts of the brain as well as during awake and sleep states. These possibilities will have to be further investigated.
Acknowledgments
This work was supported by Kiban B grant 26285161H to C.P. J.C. was a recipient of the Otsuka Toshimi Scholarship Foundation. The authors like to thank members of Dr. Ogawa's laboratory for their support. We also want to express our deepest gratitude to the Institute for Animal Reproduction, Japan, for providing the animals used in the research.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.046458.117.
References
- Abel T, Nguyen PV. 2008. Regulation of hippocampus-dependent memory by cyclic AMP-dependent protein kinase. Prog Brain Res 169: 97–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. 1997. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 88: 615–626. [DOI] [PubMed] [Google Scholar]
- Abel T, Havekes R, Saletin JM, Walker MP. 2013. Sleep, plasticity and memory from molecules to whole-brain networks. Curr Biol 23: R774–R788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahi J, Radulovic J, Spiess J. 2004. The role of hippocampal signaling cascades in consolidation of fear memory. Behav Brain Res 149: 17–31. [DOI] [PubMed] [Google Scholar]
- Ardenghi P, Barros D, Izquierdo LA, Bevilaqua L, Schröder N, Quevedo J, Rodrigues C, Madruga M, Medina JH, Izquierdo I. 1997. Late and prolonged post-training memory modulation in entorhinal and parietal cortex by drugs acting on the cAMP/protein kinase A signalling pathway. Behav Pharmacol 8: 745–751. [DOI] [PubMed] [Google Scholar]
- Barros DM, Izquierdo LA, Mello e Souza T, Ardenghi PG, Pereira P, Medina JH, Izquierdo I. 2000. Molecular signalling pathways in the cerebral cortex are required for retrieval of one-trial avoidance learning in rats. Behav Brain Res 114: 183–192. [DOI] [PubMed] [Google Scholar]
- Bernabeu R, Bevilaqua L, Ardenghi P, Bromberg E, Schmitz P, Bianchin M, Izquierdo I, Medina JH. 1997. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc Natl Acad Sci 94: 7041–7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilaqua L, Ardenghi P, Schröder N, Bromberg E, Schmitz PK, Schaeffer E, Quevedo J, Bianchin M, Walz R, Medina JH, et al. 1997. Drugs acting upon the cyclic adenosine monophosphate/protein kinase A signalling pathway modulate memory consolidation when given late after training into rat hippocampus but not amygdala. Behav Pharmacol 8: 331–338. [DOI] [PubMed] [Google Scholar]
- Binder S, Baier PC, Mölle M, Inostroza M, Born J, Marshall L. 2012. Sleep enhances memory consolidation in the hippocampus-dependent object-place recognition task in rats. Neurobiol Learn Mem 97: 213–219. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. 1998. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem 5: 365–374. [PMC free article] [PubMed] [Google Scholar]
- Datta S, Siwek DF, Huang MP. 2009. Improvement of two-way active avoidance memory requires protein kinase A activation and brain-derived neurotrophic factor expression in the dorsal hippocampus. J Mol Neurosci 38: 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel-Mahan KL, Phan T, Han S, Wang H, Chan GC-K, Scheiner ZS, Storm DR. 2008. Circadian oscillation of hippocampal MAPK activity and cAMP: implications for memory persistence. Nat Neurosci 11: 1074–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein W. 1971. Disruptive effects of rapid eye movement sleep deprivation on long-term memory. Physiol Behav 6: 279–282. [DOI] [PubMed] [Google Scholar]
- Fishbein W, Kastaniotis C, Chattman D. 1974. Paradoxical sleep: prolonged augmentation following learning. Brain Res 79: 61–75. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Holt W, Maren S. 2000. A role for amygdaloid PKA and PKC in the acquisition of long-term conditional fear memories in rats. Behav Brain Res 114: 145–152. [DOI] [PubMed] [Google Scholar]
- Graves L. 2001. Sleep and memory: a molecular perspective. Trends Neurosci 24: 237–243. [DOI] [PubMed] [Google Scholar]
- Graves LA, Heller EA, Pack AI, Abel T. 2003. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem 10: 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecksch G, Matthies H. 1980. Two sensitive periods for the amnesic effect of anisomycin. Pharmacol Biochem Behav 12: 663–665. [DOI] [PubMed] [Google Scholar]
- Gross BA, Vanderheyden WM, Urpa LM, Davis DE, Fitzpatrick CJ, Prabhu K, Poe GR. 2015. Stress-free automatic sleep deprivation using air puffs. J Neurosci Meth 251: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagewoud R, Havekes R, Novati A, Keijser JN, Van der zee EA, Meerlo P. 2010a. Sleep deprivation impairs spatial working memory and reduces hippocampal AMPA receptor phosphorylation. J Sleep Res 19: 280–288. [DOI] [PubMed] [Google Scholar]
- Hagewoud R, Havekes R, Tiba PA, Novati A, Hogenelst K, Weinreder P, Van der zee EA, Meerlo P. 2010b. Coping with sleep deprivation: shifts in regional brain activity and learning strategy. Sleep 33: 1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagewoud R, Whitcomb SN, Heeringa AN, Havekes R, Koolhaas JM, Meerlo P. 2010c. A time for learning and a time for sleep: the effect of sleep deprivation on contextual fear conditioning at different times of the day. Sleep 33: 1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagewoud R, Bultsma LJ, Barf RP, Koolhaas JM, Meerlo P. 2011. Sleep deprivation impairs contextual fear conditioning and attenuates subsequent behavioural, endocrine and neuronal responses. J Sleep Res 20: 259–266. [DOI] [PubMed] [Google Scholar]
- Havekes R, Bruinenberg VM, Tudor JC, Ferri SL, Baumann A, Meerlo P, Abel T. 2014. Transiently increasing cAMP levels selectively in hippocampal excitatory neurons during sleep deprivation prevents memory deficits caused by sleep loss. J Neurosci 34: 15715–15721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havekes R, Meerlo P, Abel T. 2015. Animal studies on the role of sleep in memory: from behavioral performance to molecular mechanisms. Curr Top Behav Neurosci 25: 183–206. [DOI] [PubMed] [Google Scholar]
- Hellman K, Abel T. 2007. Fear conditioning increases NREM sleep. Behav Neurosci 121: 310–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennevin E, Hars B, Maho C, Bloch V. 1995. Processing of learned information in paradoxical sleep: relevance for memory. Behav Brain Res 69: 125–135. [DOI] [PubMed] [Google Scholar]
- Hernandez PJ, Abel T. 2011. A molecular basis for interactions between sleep and memory. Sleep Med Clin 6: 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inostroza M, Born J. 2013. Sleep for preserving and transforming episodic memory. Annu Rev Neurosci 36: 79–102. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Kanayama Y, Matsumura H, Tsuchimochi H, Ishida Y, Nakamura S. 2006. Selective rapid eye movement sleep deprivation impairs the maintenance of long-term potentiation in the rat hippocampus. Eur J Neurosci 24: 243–248. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Medina JH. 1997. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem 68: 285–316. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Bevilaqua LRM, Rossato JI, Bonini JS, Medina JH, Cammarota M. 2006. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci 29: 496–505. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, Nestler EJ, Taylor JR. 2002. Stimulation of protein kinase A activity in the rat amygdala enhances reward-related learning. Biol Psychiatry 52: 111–118. [DOI] [PubMed] [Google Scholar]
- Kreutzmann JC, Havekes R, Abel T, Meerlo P. 2015. Sleep deprivation and hippocampal vulnerability: changes in neuronal plasticity, neurogenesis and cognitive function. Neuroscience 309: 173–190. [DOI] [PubMed] [Google Scholar]
- Kumar T, Jha SK. 2012. Sleep deprivation impairs consolidation of cued fear memory in rats. PLoS One 7: e47042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Phan TX, Yang Y, Garelick MG, Storm DR. 2013. Increases in cAMP, MAPK activity, and CREB phosphorylation during REM sleep: implications for REM sleep and memory consolidation. J Neurosci 33: 6460–6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Abel T, Hernandez PJ. 2009. Exchange protein activated by cAMP enhances long-term memory formation independent of protein kinase A. Learn Mem 16: 367–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks CA, Wayner MJ. 2005. Effects of sleep disruption on rat dentate granule cell LTP in vivo. Brain Res Bull 66: 114–119. [DOI] [PubMed] [Google Scholar]
- Meerlo P, de Bruin EA, Strijkstra AM, Daan S. 2001. A social conflict increases EEG slow-wave activity during subsequent sleep. Physiol Behav 73: 331–335. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Winson J. 1989. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J Neurosci 9: 2907–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. 2007. The rat brain in stereotaxic coordinates, 6th ed Academic, New York. [DOI] [PubMed] [Google Scholar]
- Pereira P, Ardenghi P, Souza T, Medina JH, Izquierdo I. 2001. Training in the step-down inhibitory avoidance task time-dependently increases cAMP-dependent protein kinase activity in the entorhinal cortex. Behav Pharmacol 12: 217–220. [DOI] [PubMed] [Google Scholar]
- Quevedo J, Vianna MRM, Martins MR, Barichello T, Medina JH, Roesler R, Izquierdo I. 2004. Protein synthesis, PKA, and MAP kinase are differentially involved in short- and long-term memory in rats. Behav Brain Res 154: 339–343. [DOI] [PubMed] [Google Scholar]
- Ravassard P, Hamieh AM, Joseph MA, Fraize N, Libourel P-A, Lebarillier L, Arthaud S, Meissirel C, Touret M, Malleret G, et al. 2016. REM sleep-dependent bidirectional regulation of hippocampal-based emotional memory and LTP. Cereb Cortex 26: 1488–1500. [DOI] [PubMed] [Google Scholar]
- Ribeiro S, Nicolelis MAL. 2004. Reverberation, storage, and postsynaptic propagation of memories during sleep. Learn Mem 11: 686–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro S, Mello CV, Velho T, Gardner TJ, Jarvis ED, Pavlides C. 2002. Induction of hippocampal long-term potentiation during waking leads to increased extrahippocampal zif-268 expression during ensuing rapid-eye-movement sleep. J Neurosci 22: 10914–10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro S, Gervasoni D, Soares ES, Zhou Y, Lin S-C, Pantoja J, Lavine M, Nicolelis MAL. 2004. Long-lasting novelty-induced neuronal reverberation during slow-wave sleep in multiple forebrain areas. PLoS Biol 2: E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romcy-Pereira R, Pavlides C. 2004. Distinct modulatory effects of sleep on the maintenance of hippocampal and medial prefrontal cortex LTP. Eur J Neurosci 20: 3453–3462. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, LaHoste GJ. 2008. Aspects of learned fear related to the hippocampus are sleep-dependent. Behav Brain Res 191: 67–71. [DOI] [PubMed] [Google Scholar]
- Schafe GE, LeDoux JE. 2000. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci 20: RC96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Nadel NV, Sullivan GM, Harris A, LeDoux JE. 1999. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learn Mem 6: 97–110. [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Nader K, Blair HT, LeDoux JE. 2001. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci 24: 540–546. [DOI] [PubMed] [Google Scholar]
- Smith C, Rose GM. 1996. Evidence for a paradoxical sleep window for place learning in the Morris water maze. Physiol Behav 59: 93–97. [DOI] [PubMed] [Google Scholar]
- Smith C, Rose GM. 1997. Posttraining paradoxical sleep in rats is increased after spatial learning in the Morris water maze. Behav Neurosci 111: 1197–1204. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Wiseman SL, Olausson P, Taylor JR. 2006. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci 9: 167–169. [DOI] [PubMed] [Google Scholar]
- van Betteray JN, Vossen JM, Coenen AM. 1991. Behavioural characteristics of sleep in rats under different light/dark conditions. Physiol Behav 50: 79–82. [DOI] [PubMed] [Google Scholar]
- Vecsey CG, Baillie GS, Jaganath D, Havekes R, Daniels A, Wimmer M, Huang T, Brown KM, Li X-Y, Descalzi G, et al. 2009. Sleep deprivation impairs cAMP signalling in the hippocampus. Nature 461: 1122–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna MR, Izquierdo LA, Barros DM, Ardenghi P, Pereira P, Rodrigues C, Moletta B, Medina JH, Izquierdo I. 2000. Differential role of hippocampal cAMP-dependent protein kinase in short- and long-term memory. Neurochem Res 25: 621–626. [DOI] [PubMed] [Google Scholar]
- Wallenstein GV, Vago DR, Walberer AM. 2002. Time-dependent involvement of PKA/PKC in contextual memory consolidation. Behav Brain Res 133: 159–164. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. 1994. Reactivation of hippocampal ensemble memories during sleep. Science 265: 676–679. [DOI] [PubMed] [Google Scholar]