Abstract
The study of gravitropism is hindered by the fact that as a root responds, the gravitational stimulus changes. Using a feedback system to connect a rotating stage platform to a video digitizer system, we were able to maintain a constant angle of gravistimulation to Arabidopsis roots for long time periods. The rate of curvature approximated the sine rule for angles of stimulation between 20° and 120°. For a given angle of stimulation, the rate of curvature also remained constant, with no observed diminishment of the response. Although previous reports of Arabidopsis root gravitropism suggest latent periods of approximately 30 min, using a smooth mechanical stage to reorient the root, we observed a mean time lag of approximately 10 min. This more rapid onset of curvature can, in part, be explained by reduced mechanical perturbation during the process of gravistimulation. This suggests that mechanical stimulation associated with rapid root re-orientation may confound investigations of early gravitropic events.
Although the gravitropism of plant roots has been studied for well over 100 years, its mechanisms are still poorly understood. The use of Arabidopsis, with its plethora of mutants showing altered gravity response, promises to aid in the investigation of these mechanisms. Due to the small and fragile nature of the Arabidopsis root, characterizations of mutants are often performed with coarse temporal resolution, with the result that the initiation of the response is unobserved (Hobbie and Estelle, 1995; Kiss et al., 1996; Fukaki et al., 1997; Tian and Reed, 1999). Ishikawa and Evans (1997) and Mullen et al. (1998b) detailed the kinetics of gravitropic curvature in horizontally stimulated Arabidopsis roots. These studies revealed a time lag between 20 and 45 min before curvature was initiated in the distal elongation zone (DEZ). Yet interpretation of data for later time periods is complicated by the process of the graviresponse, which changes the stimulation angle at the root cap, the probable site of gravity perception (for review, see Sack, 1991). The response also creates a large difference in angle of orientation along the elongating region of the root and may involve adaptation to the gravity signal.
Sachs (1882) proposed that the gravitropic response was proportional to the component of the gravity vector perpendicular to the root axis, leading to the “sine rule” approximation of the dependence of response on the stimulation angle. Although the rate of curvature has been found to be related to the sine of the stimulation angle for roots (Larsen, 1969; Perbal, 1974) and coleoptiles (Pickard, 1973; Iino et al., 1996), a simple sine dependence only held for angles of stimulation less than 90°, and the optimal angle of stimulation was in some cases greater than 90°. Further, a recent study of maize roots by Barlow et al. (1993) found no dependence of rate of curvature on stimulation angle, for angles between 20° and 90°, the range in which the sine rule approximation should be most valid.
Since prior studies of the relationship between stimulation angle and response in roots have been equivocal, we have employed a new technique to investigate this in the roots of Arabidopsis. We used a computer feedback system to rotate a seedling growing on agar so that its root tip was constrained to a particular angle from vertical. Thus we were able to maintain a constant stimulus throughout the graviresponse. This allowed us to evaluate the dependence of the response on stimulus angle and to better understand the detailed kinetics of the gravitropic response.
RESULTS
Gravitropism Kinetics
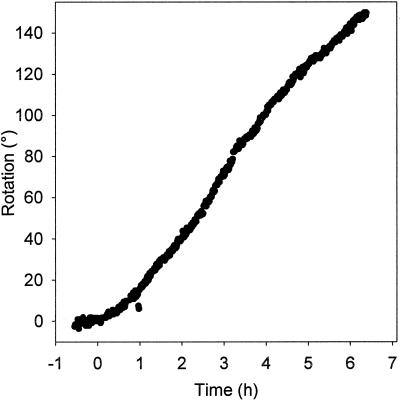
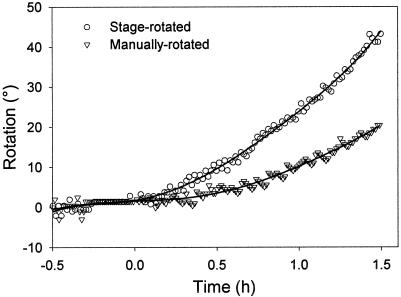
Although experiments measuring the angle of a root tip as a function of time after gravistimulation provide useful measures of graviresponse, the strength of the stimulus is time-dependent in such cases. To better quantitate the kinetics of the graviresponse of Arabidopsis roots, we used the feedback system to smoothly reorient the roots from a vertical to a horizontal position. The tips of the roots were then constrained at an angle of 90° relative to vertical (see Fig. 1). The roots responded quickly to the gravitational stimulus and achieved a constant rate of curvature within an hour of stimulation (Fig. 2). Curvature continued undiminished well beyond 90°, the amount of curvature that would be needed for the root tip to attain vertical orientation if it was not being constrained horizontally. Because the roots responded more quickly than we expected based on previous studies (Ishikawa and Evans, 1997; Mullen et al., 1998b), we estimated the latent period for the response. To obtain an estimate of the latent period, we looked at the time interval of −0.5 to 1.5 h (Fig. 3). A sixth-order polynomial was fit to data in this interval by non-linear regression, and the second derivative with respect to time was taken. The local maximum of the resulting equation, which represents the time of greatest change in the rate of curvature, was used as the time of commencement of the response. Using this method, we calculated a latent period of 10.5 ± 2.4 min (mean ± se, n = 12). To better compare this estimate of the latent period with previous studies, we repeated the experiment, but we gravistimulated the root by removing the Petri dish from the vertical stage, manually rotating the root to a horizontal position, and reattaching the dish to the stage (Fig. 3). The average latent period for manually stimulated roots was 19.6 ± 2.6 min (n = 9), a significant difference (P = 0.02, t test). Thus, it seems that the feedback system provided a reduction in mechanical stresses, which allowed a quicker response.
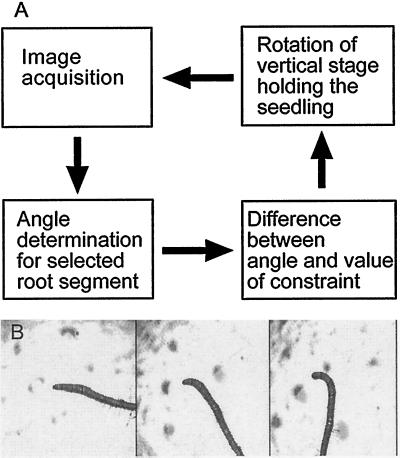
Figure 1.
Feedback system for constraining root orientation. A, After an image of the root is captured, the image is divided into segments, which are a fixed distance from the root tip. The angles of orientation for the segments are then determined and compared with the desired angle of constraint. The vertical stage is then rotated by the amount necessary to achieve the desired angle of orientation. B, Time-lapse images of an Arabidopsis root showing the development of gravitropic curvature while the root tip remains horizontal.
Figure 2.
Kinetics of the gravitropic response of an Arabidopsis root. The root tip was constrained at 90° relative to vertical at 0 h. The measured rotation was the rotation of the vertical stage necessary to keep the root tip horizontal.
Figure 3.
Early time-course for the gravitropic response of typical Arabidopsis roots. The stage-rotated root was reoriented to a horizontal position by means of the rotating vertical stage. The manually rotated root was removed from the stage, reoriented by hand, and reattached to the vertical stage. The fitted lines are sixth-order polynomials from which latent periods were calculated (r2 = 0.995 and 0.976 for the stage-rotated and manually-rotated samples, respectively).
Dependence on Stimulation Angle
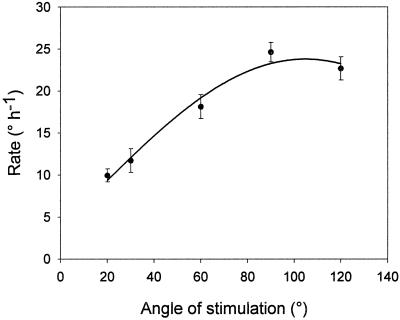
Because the curvature of the root as a function of time is linear over a long time interval (Fig. 2), the average rate of curvature could be determined by linear regression. This value is a useful measure of the strength of the gravitropic response for a given angle of stimulation. To find the relation between the strength of the response and the dose of the gravity signal perpendicular to the root axis, we measured the mean rate of curvature for roots with tips constrained at different angles of stimulation. The rate of curvature increased with angle of stimulation for angles less than 90° (Fig. 4). The relation can be approximated by a sinusoidal function of the form:
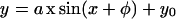 |
where y is the rate of curvature, x is the stimulation angle, and a, φ, and yo are parameters calculated by non-linear regression to be 15.9, −14.7, and 7.9, respectively. A physical interpretation of this equation is that the threshold angle for gravitropic response is 15°, since at this angle the sinusoidal term of the function is nil.
Figure 4.
The dependence of the rate of root curvature on the stimulation angle. Error bars indicate se (n = 10–12). The curve is a sinusoidal function fitted to the data by non-linear regression.
Threshold Angle for Gravitropic Response
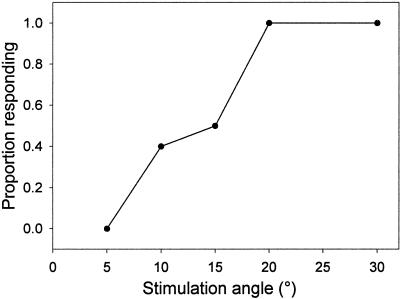
Since the previous data suggested that roots must be reoriented at least 15° from vertical before a response is elicited, we made measurements of gravitropic responses at low stimulation angles to test this prediction. Because the gravitropic response is characteristic in its rapid onset and constant rate of curvature (Fig. 2), we were able to measure the proportion of roots, at a given angle of constraint, which responded to the gravitational stimulus. Figure 5 shows the proportion of roots responding as a function of stimulation angle. The angle at which 50% of the population responds gravitropically is approximately 15°, in good agreement with the prediction from the rate of curvature data.
Figure 5.
Threshold of gravitropic response in Arabidopsis roots. Roots were scored as responders if the root began curving within an hour of stimulation and continued curving throughout the course of the experiment (n = 10).
Localized Contributions
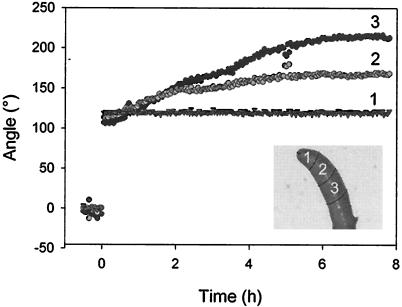
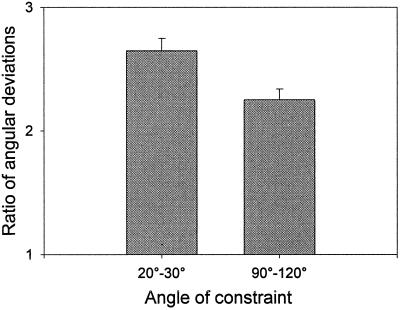
Following gravistimulation, a root enters a phase of curvature development; but, after several hours, curvature in the elongating region, both within the apical region of the elongation zone (240–480 μm from the tip) and the middle of the central elongation zone (CEZ, 480–720 μm from the tip) reaches a steady state (Fig. 6, segments 2 and 3, respectively). This steady state arises from a balance between continuing curvature formation and the migration of curvature out of the elongation zone as the root grows. A comparison of the deviation of steady-state angles for different regions of the elongation zone from the angle of the root tip can be used as an index of relative contributions to the gravitropic response. Comparing the deviation of the apical region of the elongation zone to that of the middle of the CEZ (for elongation zone analysis, see Mullen et al., 1998a), we found that the apical region contributed the majority of the curvature when the root was reoriented to large angles (Fig. 7). However, at smaller angles of stimulation, the more basal region of the elongation zone had significantly greater importance in the response than it had at larger stimulation angles (P = 0.01).
Figure 6.
Localized changes in Arabidopsis root orientation upon gravistimulation. The data show the angles of orientation of different regions of a representative root when the tip-most segment is constrained at 120°. Inset is an image of the root, illustrating the division of the apical region into segments, numbered 1 to 3, corresponding to the angle data.
Figure 7.
Relative local contributions to gravitropic curvature. The deviations in the orientation of the root tip from the central portion of the elongation zone and from the apical region of the elongation zone are compared to provide an index of the relative contributions of these regions to the gravitropic curvature. The ratio of these deviations was determined both for low angles of stimulation (n = 10) and high angles (n = 15). Error bars indicate se. A large ratio indicates a relatively greater contribution to curvature by the central portion of the elongation zone, whereas a small ratio indicates a relatively greater contribution by the apical region of the elongation zone.
DISCUSSION
By measuring the kinetics of Arabidopsis root gravitropism with a feedback system, we observed a rapid onset of curvature, averaging approximately 10 min. Therefore, the perceived stimulus must be transduced to a response in the DEZ and CEZ more rapidly than previously expected. The sedimentation time of amyloplasts has been measured to be approximately 5 min (MacCleery and Kiss, 1999). The presentation time for Arabidopsis roots has been estimated to be between 20 s and 5 min (Kiss et al., 1989, 1996). It has previously been suggested that harsh gravistimulations may affect the sensing process (Barlow et al., 1993). Although we did not attempt to measure presentation time using the rotating stage device, we expect that any changes in the presentation time will be reflected in the shorter latent period. Although potential electrical (Ishikawa and Evans, 1990; Weisenseel et al., 1992) and chemical (Perera et al., 1999) signals have been detected within this time interval, caution must be exercised when assessing components of the early phases of the gravitropic response. The manner in which roots were gravistimulated affected the observed latent period. Roots gravistimulated smoothly by the feedback system exhibited a reduced gravitropic latent period. This suggests that mechanical stress associated with rapid reorientation may alter the response and could confound the identification of true gravitropic signal transduction components.
Once the roots commenced bending, the rate of curvature remained constant over long time periods. This rate of curvature varied with the angle of stimulation and followed a sinusoidal dependence with a phase shift of 15°. This contrasts with observations in maize roots (Barlow et al., 1993), which found no relation between the rate of curvature and stimulation angle. These results also differ from older reports (Audus, 1964; Perbal, 1974) suggesting a “modified sine rule” with maximal curvature at angles of 120° to 135°. However, we were able to make observations at a constant root tip orientation for long periods of time. Since the root cap is the major site of perception of the gravity signal (Sack, 1991; Blancaflor et al., 1998), allowing changes in the orientation of the root tip during gravitropic response can confound results. It seems likely that the large curvature observed at high angles of stimulation in other studies (Audus, 1964) was caused by the root tip spending more time oriented at large angles rather than a larger magnitude response at these high angles.
The changes in the magnitude of the gravitropic response with stimulation angle were accompanied by changes in the location of the response (Fig. 6). At small reorientation angles, much of the curvature occurred in the CEZ. However, as the stimulation angle increased, differential growth in the DEZ and the apical portion of the CEZ increased more than in the central CEZ, shifting the region of greatest curvature acropetally. This type of response is consistent with a model of gravitropism in which the signal originates in the root cap and dampens with distance from the cap only if one assumes that the central CEZ is more sensitive to the signal than the more apical region of the elongation zone. To accommodate the observation that the region of maximal curvature shifts acropetally when the root is stimulated at higher angles (Fig. 7), one may speculate that the apical region, because of its proximity to the signal source and the higher strength of the signal at higher stimulation angles, now receives a signal sufficiently above its response threshold to account for a large contribution to overall curvature. Alternatively, the apical region of the elongation zone might be capable of directly perceiving a gravistimulus for sufficiently large angles of stimulation, though this is a model which is difficult to reconcile with the observation that the gravitropic response is lost when the columella cells of the root cap are destroyed (Blancaflor et al., 1998).
This pattern of response is also consistent with the observed similarity between circumnutation and low-angle gravistimulation (Barlow et al., 1993) since circumnutation primarily occurs in the CEZ (Okada and Shimura, 1990; Mullen et al., 1998b). Study of the growth patterns of roots constrained to angles below the gravitropic threshold may shed light on the relationship between circumnutation and gravitropism. The ability of the feedback system to smoothly rotate the seedling in order to maintain a constant angle of orientation for a specific region of the root, such as the root cap, provides a means for measuring curvature development while keeping the gravitropic stimulus constant. This ability should prove useful in understanding the influence of gravity on other growth responses.
MATERIALS AND METHODS
Plant Material
Seeds of Arabidopsis (ecotype Columbia) were surface-sterilized by agitation in 5.25% (v/v) NaOCl solution for 5 min, followed by several rinses in sterile distilled water. Seeds were sown in a row (three seeds per row) on a sterile agar (1% w/v) medium in Petri dishes (60-mm diameter, 15 mm high) sealed with Parafilm (American Can Co., Greenwich, CT). The agar medium contained 1% (w/v) Suc, one-half-strength Murashige-Skoog medium (Murashige and Skoog, 1962), and 1 mm 2-(N-morpholino) ethanesulfonic acid (pH 5.8). The Petri dishes were either immediately placed vertically in a culture room under continuous white light from fluorescent lamps (Sylvania, Danvers, MA; F30T8-CW, fluence rate approximately 60 μm m−2 s−1) at 24°C or refrigerated for 1 to 5 d before being transferred to the culture room. The seedlings were used for experimentation at age 4 to 5 d, when the roots had a length of 10 to 20 mm.
Feedback System
The seedling to be observed was first repositioned so that its root tip was at the center of the Petri dish (center of rotation). This minimized the translational movement of the root during the stimulation process. Repositioning was accomplished by placing forceps under the hypocotyl, lifting slightly, and sliding the plant along the agar. The dish containing the seedling was then attached to a vertical stage, and the seedling was allowed to recover for at least 1.5 h, during which time the growth rate of the root returned to normal (J.L. Mullen and C. Wolverton, unpublished data). The feedback system was then started, so that the root tip was constrained at the desired angle relative to vertical. The feedback system consisted of a CCD camera (Marshall Electronics, Culver City, CA) focused on a seedling root, illuminated by an infrared light-emitting diode (Radio Shack, Fort Worth, TX). The camera was connected to a computer via a frame grabber circuit board (Imagenation, Beaverton, OR). A rotatable vertical stage (Optec Ltd., Tokyo) was also connected to the computer and controlled by custom software. This software utilized the algorithm described by Mullen et al. (1998b) to calculate the angle of the root tip, as well as that of other root segments. The software constrained the user-defined segment of the root to the desired angle, making corrections as frequently as every 20 s. Changes in the angle of the constrained segment caused a stepper motor to rotate the vertical stage in the necessary direction, correcting the angle of that segment (Fig. 1). The custom software recorded the angle of the root segments and the rotation of the vertical stage. The root could be reoriented to a horizontal position (90°) within approximately 60 s by means of individual steps of the motor corresponding to 0.08°.
Footnotes
This work was supported by the National Aeronautics and Space Administration (NASA; grant nos. NAG5–6385 and NAG2–1190), by the NASA/National Science Foundation Joint Program in Plant Biology, Network for Research on Plant Sensory Systems (grant no. IBN–9421856), and by the Institute of Space and Astronautical Science (Japan).
This paper is dedicated to the memory of Paul B. Green whose exceptional creativity in the design of novel equipment for plant growth studies and whose exemplary approaches to quantitative plant biology have been an inspiration to the authors.
LITERATURE CITED
- Audus LJ. Geotropism and the modified sine rule: an interpretation based on the amyloplast statolith theory. Physiol Plant. 1964;17:737–745. [Google Scholar]
- Barlow PW, Parker JS, Butler R, Brain P. Gravitropism of primary roots of Zea mays L. at different displacement angles. Ann Bot. 1993;71:383–388. [Google Scholar]
- Blancaflor EB, Fasano JM, Gilroy S. Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol. 1998;116:213–222. doi: 10.1104/pp.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaki H, Fujisawa H, Tasaka M. The RHG gene is involved in root and hypocotyl gravitropism in Arabidopsis thaliana. Plant Cell Physiol. 1997;38:804–810. doi: 10.1093/oxfordjournals.pcp.a029238. [DOI] [PubMed] [Google Scholar]
- Hobbie L, Estelle M. The axr4 auxin-resistant mutants of Arabidopsis thaliana define a gene important for root gravitropism and lateral root initiation. Plant J. 1995;7:211–220. doi: 10.1046/j.1365-313x.1995.7020211.x. [DOI] [PubMed] [Google Scholar]
- Iino M, Tarui Y, Uematsu C. Gravitropism of maize and rice coleoptiles: dependence on the stimulation angle. Plant Cell Environ. 1996;19:1160–1168. doi: 10.1111/j.1365-3040.1996.tb00431.x. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Evans ML. Gravity-induced changes in intracellular potentials in elongating cortical cells of mung bean roots. Plant Cell Physiol. 1990;31:457–462. [PubMed] [Google Scholar]
- Ishikawa H, Evans ML. Novel software for analysis of root gravitropism: comparative response patterns of Arabidopsis wild-type and axr1 seedlings. Plant Cell Environ. 1997;20:919–928. doi: 10.1046/j.1365-3040.1997.d01-129.x. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Hertel R, Sack FD. Amyloplasts are necessary for full gravitropic sensitivity in roots of Arabidopsis thaliana. Planta. 1989;177:198–206. [PubMed] [Google Scholar]
- Kiss JZ, Wright JB, Caspar T. Gravitropism in roots of intermediate-starch mutants of Arabidopsis. Physiol Plant. 1996;97:237–244. doi: 10.1034/j.1399-3054.1996.970205.x. [DOI] [PubMed] [Google Scholar]
- Larsen P. The optimum angle of geotropic stimulation and its relation to the starch statolith hypothesis. Physiol Plant. 1969;22:469–488. [Google Scholar]
- MacCleery SA, Kiss JZ. Plastid sedimentation kinetics in roots of wild-type and starch-deficient mutants of Arabidopsis. Plant Physiol. 1999;120:183–192. doi: 10.1104/pp.120.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen JL, Ishikawa H, Evans ML. Analysis of changes in relative elemental growth rate patterns in the elongation zone of Arabidopsis roots upon gravistimulation. Planta. 1998a;206:598–603. doi: 10.1007/s004250050437. [DOI] [PubMed] [Google Scholar]
- Mullen JL, Turk E, Johnson K, Wolverton C, Ishikawa H, Simmons C, Söll D, Evans ML. Root-growth behavior of the Arabidopsis mutant rgr1: roles of gravitropism and circumnutation in the waving/coiling phenomenon. Plant Physiol. 1998b;118:1139–1145. doi: 10.1104/pp.118.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Phy-siol Plant. 1962;15:473–497. [Google Scholar]
- Okada K, Shimura Y. Reversible root tip rotation in Arabidopsis seedlings induced by obstacle-touching stimulus. Science. 1990;250:274–276. doi: 10.1126/science.250.4978.274. [DOI] [PubMed] [Google Scholar]
- Perbal G. L'action des statolithes dans la réponse géo- tropique des racines de Lens culinaris. Planta. 1974;116:153–174. doi: 10.1007/BF00380650. [DOI] [PubMed] [Google Scholar]
- Perera IY, Heilmann I, Boss WF. Transient and sustained increases in inositol 1,4,5-trisphosphate precede differential growth response in gravistimulated maize pulvini. Proc Nat Acad Sci USA. 1999;96:5838–5843. doi: 10.1073/pnas.96.10.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard BG. Geotropic response patterns of the Avena coleoptile: I. Dependence on angle and duration of stimulation. Can J Bot. 1973;51:1003–1021. [Google Scholar]
- Sachs J. Über orthotrope und plagiotrope Pflanzenteile. Arb Bot Inst Wûrzburg. 1882;2:226–284. [Google Scholar]
- Sack FD. Plant gravity sensing. Int Rev Cytol. 1991;127:193–252. doi: 10.1016/s0074-7696(08)60695-6. [DOI] [PubMed] [Google Scholar]
- Tian Q, Reed JW. Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development. 1999;126:711–721. doi: 10.1242/dev.126.4.711. [DOI] [PubMed] [Google Scholar]
- Weisenseel MH, Becker HF, Ehlgoetz JG. Growth, gravitropism, and endogenous ion currents of cress roots (Lepidium sativum L): measurements using a novel three-dimensional recording probe. Plant Physiol. 1992;100:16–25. doi: 10.1104/pp.100.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]