Abstract
We report on a case of loiasis revealed during an assisted reproductive technology (ART) cycle. Loa loa could limit implantation outcome. We propose to focus on an ART strategy with frozen embryos to treat the patient before any transfer.
Keywords: assisted reproductive technology, filariasis, follicular fluid, in vitro fertilization, Loa loa
Loa loa, the causative agent of loiasis, is transmitted to humans by Chrysops tabanid flies. This filarial parasite is mainly confined to West and Central Africa, where it is known as the “eye worm.” Numerous imported cases have been reported in Western countries [1]. Here, we report on a case of loiasis that was revealed during an assisted reproductive technology (ART) cycle in a 38-year-old HBV-positive woman originally from Cameroon, and we review the literature about the medical care of loiasis during the ART cycle [2, 3].
CASE REPORT
A 38-year-old Cameroonian woman, gravida 2, para 0, 2 abortions, had been treated in our ART center since 2012. The couple had been attempting to conceive since 2010. The woman’s only known infectious disease was viral hepatitis B. In 2012, ophthalmic symptoms prompted the diagnosis of Loa loa infection. An adult worm was then extracted from the right eye during ocular surgery, and the woman was treated with a single dose (200 µg/kg) of the antiparasitic drug ivermectin.
Bilateral obstruction of the fallopian tubes was revealed during diagnostic laparoscopy. Hence, we decided to initiate in vitro fertilization (IVF) and an embryo transfer protocol. The first cycle took place in 2013 and a second in 2014. No microorganism was reported during the medical care, and embryo transfers did not result in pregnancy. A third IVF cycle was initiated in 2016 by the use of a standard antagonist-GnRH protocol for ovarian stimulation, with 300 IU of recombinant FSH. Ten mature follicles were obtained when ovulation was triggered by the administration of choriogonadotropin alfa. Thirty-six hours later, transvaginal ultrasound-guided oocyte retrieval was performed under general anaesthesia. Eleven cumulus-oocyte complexes (COCs) were retrieved from the follicular fluid (FF). No microorganisms were detected during direct screening under the microscope. This cohort was divided into 5 COCs for conventional in vitro fertilization (IVFc) and 6 for intracytoplasmic sperm injection (ICSI). On day 1 after insemination using the IVFc technique, several freely moving microscopic worms were observed during the zygote denudation step. Some of the microscopic worms were trapped by the cumulus cells. No worm was found in the ICSI fertilization media. A parasitologist soon confirmed that these microscopic worms belong to the Loa genus. We performed parasitological assessment on the initial FF sample, which rapidly confirmed Loa loa microfilariosis (Figure 1). A blood sample confirmed the presence of marked hypereosinophilia (0.9 G/L, 13.7%) and microfilariae (800/mL).
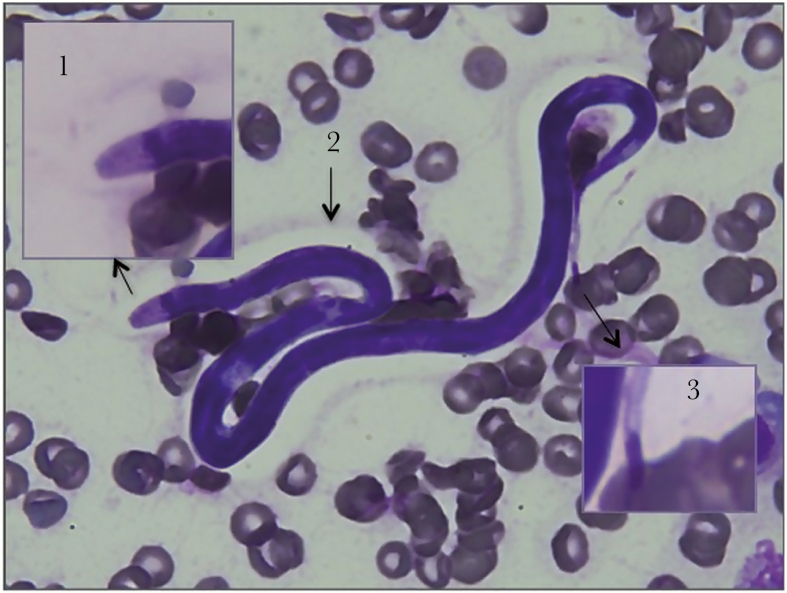
Figure 1.
Loa loa microfilaria in the follicular fluid (magnification: ×100). The resuspended pellet was smeared on a slide and stained with May-Grünwald Giemsa reagent. Arrows indicate details of the cephalic space (1), the unstained sheath (2), and details of the nuclei extending into the tip of the tail (a typical feature of Loa loa) (3).
Five embryos were obtained in IVFc technique, 4 by the use of ICSI. We decided to cancel the ongoing IVF attempt after providing the couple with as much information as possible and obtaining their consent. We froze 6 embryos on day 3 postfertilization: 5 and 1 from IVFc and ICSI, respectively.
Three weeks after the administration of a single dose (12 mg) of ivermectin, the level of microfilaraemia and of hypereosinophilia had fallen to 50/mL and 0.7 G/L (9%), respectively. Treatment with diethylcarbamazine (DEC) and corticosteroids was then initiated. One month later, a blood smear analysis confirmed that the patient was cured. We then resumed the embryo transfer process. No pregnancy was obtained.
DISCUSSION
Worldwide, around 10 million people are infected by Loa loa [1, 4]. Fertility centers should always adapt their practice as a function of their patients’ backgrounds and medical histories. Loiasis is rarely observed in travelers, although some researchers have reported the development of loiasis after 12 days of exposure [5]. When considering cases imported into France, the 4 most exposed countries are Cameroon (n = 22), Gabon (n = 14), the Central African Republic (n = 4), and the Democratic Republic of the Congo (n = 3) [6]. Other endemic countries are the Republic of the Congo, Angola, Chad, Equatorial Guinea, Nigeria, and Sudan [7]. Here, we describe a case of imported Loiasis from Cameroon. Since the patient went back several times to Cameroon between 2013 and 2015, we first thought that she was reinfected. However, ivermectin is not active against Loa loa adults but only on microfilaraemia. Ivermectin is recommended for reducing microfilaraemia because it binds to invertebrate-specific glutamate-gated chloride channels present in the membrane of neuron and myocyte. This results in cellular hyperpolarization, paralysis, and death of the parasite [8]. However, in contrast to DEC, ivermectin is not active against adult Loa loa worms [1, 4]. In 2012, our patient only received a single dose of ivermectin, and its efficacy was not assessed. Hence, loiasis was likely to recur. We failed to diagnose loiasis during the previous 2 ICSI cycles, despite the retrospective observation of hypereosinophilia in a blood sample collected in 2014. The diurnal periodicity of microfilaraemia might have prevented us from detecting the infection earlier.
This brief report is the third observation of Loa loa in ovarian FF during oocyte retrieval [2, 3]. The mechanism by which the parasite ended up in the FF is unclear. One hypothesis is the transition from the blood to the FF during folliculogenesis. But the most plausible explanation is blood contamination, which should always be considered when assessing FF. Microfilariae present in the bloodstream of small blood vessels on the surface of the ovary might have passed into the FF during ovarian puncture. This hypothesis is supported by the hemorrhagic aspect of the FF and the observation of red blood cells under the microscope (Figure 1). Given the parasitic load, any blood contamination of any samples can be contaminated with microfilariae.
As mentioned by Wisanto et al., we know little about the possible effects of close contact with microfilariae on human oogenesis, fertilization, and early embryo development [2]. Furthermore, the present study is the first to assess the likelihood of Loa loa contamination during IVFc vs ICSI. The disease was diagnosed during the IVFc process on day 1 during spermatozoa survival evaluation. Thanks to removal of the microfilariae during the oocyte washing and denudation steps, the ICSI protocol ruled out parasitic contamination before fertilization. In contrast, the COCs were exposed to the parasite during fertilization in the IVFc process, and some worms adhered to granulosa cells. Importantly, longer exposure to Loa loa in the IVFc process did not seem to impact early embryo development. The latter was of better quality in IVFc than in the ICSI protocol, which yielded 5 and 1 frozen embryos, respectively. The 2 previous reports and our present one indicate that exposure to Loa loa before and during fertilization has little or no impact on early in vitro embryo development.
In Wisanto and colleagues’ case report on Loa loa microfilariae in IVFc, 2 fresh embryos were transferred as usual because there was no report of adverse effects of Loa loa on pregnancy at that time. However, the transfer resulted in an early miscarriage [2]. Embryo implantation is known to be related to the endometrium’s inflammatory and immune status and the Th1/Th2 balance. The endometrium’s immune status changes during the implantation window, with the production of pro-inflammatory, pro-Th1 cytokines (eg, TNF-α and Il-6) [9]. Strongyloïdes stercoralis and Loa loa, 2 common worms, induce marked hypereosinophilia and immune activation in the human host [10, 11]. Given that the endometrium is highly vascularized, one cannot rule out a possible effect of parasitic infections on embryo implantation and embryogenesis. The cumulative failure of embryos’ transfers supports this hypothesis.
One can expect the parasitic risk to be very low or null if the embryo culture medium is negative, as described in the prevention of viral risk in ART [12]. The use of washing steps and denudation means that the oocytes should not be discarded—even when the FF has a bloody aspect. Furthermore, oocytes are protected by the zona pellucida. The vertical transmission of Loa loa during pregnancy has never been reported. Microscopy assessments and nested PCR assays of cord blood and peripheral blood of a newborn from an infected mother were negative for microfilariae [1]. In contrast, a few microfilariae were seen in the intervillar lacunae of the mother’s placenta [1]. Two other case reports of asymptomatic loiasis discovered during pregnancy indicated that the mothers were treated with DEC after delivery and that there was no evidence of microfilariae having crossed the placenta [13].
We considered that freezing all the embryos was the safest option to limit Loa loa’s possible impact on implantation and pregnancy. Moreover, the treatment of loiasis is not recommended during pregnancy because of the risk of serious side effects, such as encephalopathy, kidney failure, and hepatitis [1]. This prompted us to treat the patient before any embryo transfer. This study demonstrates the importance of adapting ART to the patient’s medical history. We hypothesize that the worms could impact the embryo implantation rate. The interaction between endometrial immune system and Loa loa infection should be investigated.
Acknowledgements
The authors thank Professor F. Bani-Sadr for the patient’s health care. They also thank Doctor Thomas Martin and David Fraser (Biotech Communication SARL, Ploudalmézeau, France) for copyediting assistance.
Financial support. This study was funded by University Hospital Amiens, France.
Potential conflicts of interest. All authors: no reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Antinori S, Schifanella L, Million M et al. Imported Loa loa filariasis: three cases and a review of cases reported in non-endemic countries in the past 25 years. Int J Infect Dis 2012; 16:e649–62. [DOI] [PubMed] [Google Scholar]
- 2. Wisanto A, Laureys M, Camus M et al. Loa loa microfilariae aspirated during oocyte retrieval. Hum Reprod 1993; 8:2096–7. [DOI] [PubMed] [Google Scholar]
- 3. Chang LW, Reller ME, Bishop JA et al. A 41-year-old woman from Cameroon with infertility. Clin Infect Dis 2008; 47:141–3, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Metzger WG, Mordmüller B. Loa loa—does it deserve to be neglected? Lancet Infect Dis 2014; 14:353–7. [DOI] [PubMed] [Google Scholar]
- 5. Moffett S, Wills CP. Images in emergency medicine. Young man with foreign-body sensation in the right eye. Loaiasis (African eye worm). Ann Emerg Med 2010; 55:578, 583. [DOI] [PubMed] [Google Scholar]
- 6. Gantois N, Rapp C, Gautret P et al. Imported loiasis in France: a retrospective analysis of 47 cases. Travel Med Infect Dis 2013; 11:366–73. [DOI] [PubMed] [Google Scholar]
- 7. Zouré HG, Wanji S, Noma M et al. The geographic distribution of Loa loa in Africa: results of large-scale implementation of the Rapid Assessment Procedure for Loiasis (RAPLOA). PLoS Negl Trop Dis 2011; 5:e1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yates DM, Wolstenholme AJ. An ivermectin-sensitive glutamate-gated chloride channel subunit from Dirofilaria immitis. Int J Parasitol 2004; 34:1075–81. [DOI] [PubMed] [Google Scholar]
- 9. Lédée N, Petitbarat M, Chevrier L et al. The uterine immune profile may help women with repeated unexplained embryo implantation failure after in vitro fertilization. Am J Reprod Immunol 2016; 75:388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Breloer M, Abraham D. Strongyloides infection in rodents: immune response and immune regulation. Parasitology 2017; 144:295–315. [DOI] [PubMed] [Google Scholar]
- 11. Nutman TB, Reese W, Poindexter RW, Ottesen EA. Immunologic correlates of the hyperresponsive syndrome of loiasis. J Infect Dis 1988; 157:544–50. [DOI] [PubMed] [Google Scholar]
- 12. Devaux A, Soula V, Sifer C et al. Hepatitis C virus detection in follicular fluid and culture media from HCV+ women, and viral risk during IVF procedures. Hum Reprod 2003; 18:2342–9. [DOI] [PubMed] [Google Scholar]
- 13. Shaw S, Pegrum GD. Filariasis as an incidental finding in pregnancy. Postgrad Med J 1965; 41:37–9. [DOI] [PMC free article] [PubMed] [Google Scholar]