Abstract
Rituximab (RTX) has become a standard therapy for certain B cell malignancies and autoimmune diseases. We report 2 RTX-treated patients who developed severe tick-borne encephalitis virus (TBEV) infection. The inability to generate new antibody responses renders RTX-treated patients susceptible to TBEV, impedes laboratory diagnosis, and necessitates preventive vaccination in endemic areas.
Keywords: B cell depletion, immunoglobulins, tick-borne encephalitis, vaccination
Tick-borne encephalitis virus (TBEV), a neurotropic member of the genus flavivirus transmitted by ticks of the genus Ixodes, is highly prevalent in Central and Eastern Europe and in Russia [1]. TBEV causes a biphasic disease, ranging from asymptomatic infection to severe meningoencephalitis. Rituximab (RTX) is a chimeric monoclonal anti-CD20 antibody, which in patients causes long-lasting (≤6–9 months) depletion of peripheral B cells. Anti-CD20 antibodies were originally developed for B cell malignancies, but are increasingly used to treat autoimmune diseases [2, 3]. The use of RTX is an established risk factor for hepatitis B virus reactivation [4], progressive multifocal leukoencephalopathy [2], and for severe infections with the tick-borne pathogens Candidatus Neoehrlichia mikurensis and Babesia microti [5, 6]. Here, we report 2 cases of severe tick-borne encephalitis (TBE) after RTX treatment.
CASE PRESENTATIONS
Patient 1
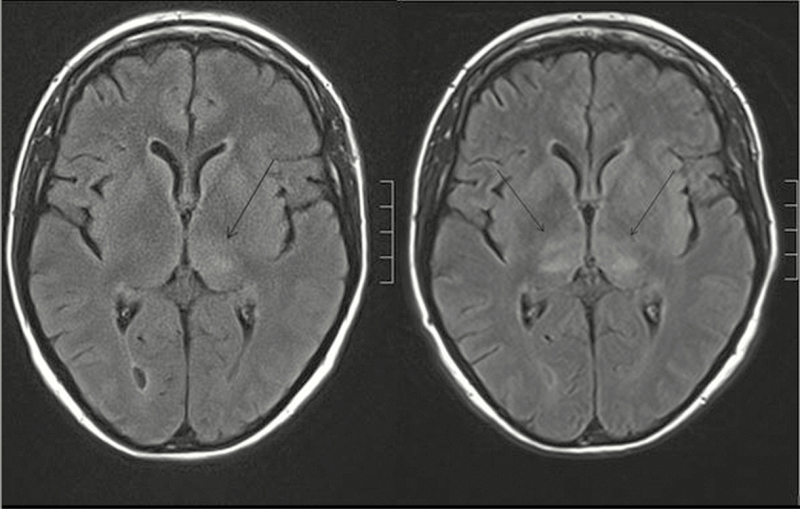
A 54-year-old woman with rheumatoid arthritis was treated with 3 cycles of RTX starting in May 2014 (cumulative dose, 6000 mg) due to therapy failure with methotrexate, leflunomide, etanercept, and tocilizumab. Five months after a further dose of RTX (1000 mg) in April 2016, she was hospitalized with fever, headache, and hemiparesis. Laboratory parameters of systemic inflammation were elevated (11.200 leukocytes/µL, CRP 102 mg/L). Cerebrospinal fluid (CSF) analysis revealed mixed pleocytosis (272 leukocytes/µL) with highly elevated protein (2.3 g/L). Despite antibiotic therapy, the patient deteriorated and developed progressive tetraparesis and decreased vigilance, necessitating mechanical ventilation. Magnetic resonance imaging (MRI) revealed white matter lesions in the thalamus (Figure 1), the midbrain, and the cerebellum. Increasing lesions resulted in progressive obstruction of the aqueduct with subsequent hydrocephalus. Real-time reverse transcription polymerase chain reaction (RT-PCR) analysis of CSF (using an inhouse method targeting the 3’ noncoding region of the TBEV genome [7]) was positive for TBEV RNA on days 4 and 6 after hospitalization, whereas a day 13 CSF sample and a urine specimen (day 7) remained negative. IgM or IgG antibodies specific to TBEV were not detectable in the patient’s serum or CSF on days 4 and 13 (SERION ELISA classic FSME Virus IgG/IgM; Institut Virion\Serion GmbH, Würzburg, Germany). Consistent with RTX treatment, CD19+ B cells were absent in her peripheral blood. After 3 weeks, she was transferred to a rehabilitation unit with a residual high-grade tetraparesis.
Figure 1.
Axial FLAIR magnetic resonance images of the Rituximab-treated patient 1 taken on day 1 (left) and day 3 (right) after hospital admission. Tick-borne encephalitis was characterized by nonenhancing white matter lesions in the thalamus (indicated by arrows). Similar lesions were found in the midbrain and cerebellum (not shown).
Patient 2
A 74-year-old man with non-Hodgkin lymphoma was treated with 8 cycles of RTX every 4 weeks in combination with bendamustine starting in July 2010, followed by continuation therapy every 2 months with RTX from May 2011 for another 2 years. The RTX dose was 375 mg/m2 for each cycle. More than 3 years after termination of the RTX treatment, the patient was admitted to the hospital with fever and flaccid pareses of the upper limbs. Neurological symptoms rapidly progressed and included hypophonia, diplopia, increasing tetraparesis, and impaired consciousness, necessitating mechanical ventilation. The patient had recently experienced several tick bites and had no history of vaccination against TBEV. A CSF specimen showed 5 leukocytes/µL and moderately elevated protein levels (0.7 g/L). MRI of the brain did not reveal any pathologies, while MRI scan of the spinal cord demonstrated a ventral hyperintense signal alteration in the cervical spinal cord. IgM and IgG antibodies to TBEV in serum and CSF were negative, while real-time RT-PCR analyses of CSF (day 1 and day 6 after hospitalization) and urine (day 1) were positive for TBEV RNA. Intravenous treatment with human immunoglobulin (IVIG; Privigen) for 3 days (cumulative dose, 2 g/kg bodyweight) did not lead to an improvement of the neurologic status. Three months after disease onset, flow cytometry analysis showed 105 CD19+ B cells/µL (normal range, 60–300/µL), and the serology for TBEV was still negative (SERION ELISA classic FSME Virus IgG/IgM).
DISCUSSION
Several aspects of the 2 cases of devastating TBEV infection after RTX treatment reported here merit discussion. First, together with 2 cases of fatal TBE after RTX treatment recently reported from Sweden [8], they indicate that TBE is a previously unrecognized severe infectious complication of RTX therapy. Second, these cases illustrate the need for PCR-based detection of TBEV in RTX-treated patients because of their inability to produce antibodies. Third, the severe course of TBEV infections in RTX-treated patients documents the importance of antibodies in blocking virus spreading, raising the question whether the use of intravenous immunoglobulins could be therapeutically effective. Last, these cases underscore the crucial value of active TBEV vaccination prior to humoral immunosuppression in endemic areas.
In both cases, the absence of a specific B cell response and the prolonged replication of TBEV in the central nervous system (CNS) compartment strongly implicate an impact of the prior RTX therapy on the course of the TBEV infection, leading to severe encephalitis. The discrepancy between normal peripheral CD19+ B cell count and the lack of a specific humoral immune response in patient 2 even 3 years after RTX treatment might be explained by a reduced diversity of the clonal B cell repertoire, which could also be a synergistic effect of the combined RTX and cytotoxic therapy of the B-NHL. Initial analyses indicate a reduced receptor revision after RTX therapy [9]. Thus, although the pool of peripheral B cells has been quantitatively restored, the B cell compartment might consist of a reduced number of clones and therefore lack TBEV-specific B cells.
In immunocompetent patients, the laboratory diagnosis of TBE is based on serology because TBEV IgM and usually also IgG are present in serum when CNS symptoms appear, while TBEV RNA is only rarely detected in the CSF at this time [1]. This rule does not apply to RTX-treated patients, in whom the capacity for a primary humoral immune response against new pathogens is abolished. In our patients, diagnosis was established by the detection of TBEV RNA in several CSF samples by real-time RT-PCR. Notably, the detection of TBEV RNA in urine samples obtained during the encephalitic phase was recently reported [10]. We found TBEV RNA in the urine from patient 2, but not from patient 1. It is essential to initiate diagnostic PCR as soon as possible because viral RNA in the CSF may be present for only a short period of time (in patient 1, the day 13 CSF sample was negative). Therafter, a TBEV infection following RTX therapy can be diagnosed neither by serology nor by PCR. Thus, it is possible that TBEV infections remain underdiagnosed in RTX-treated patients with meningoencephalitis.
Pathophysiologically, RTX-induced depletion of B cells might aggravate the disease by increasing neuroinvasiveness and neurovirulence of the virus. The absence of TBEV-specific antibodies may result in a higher viremia, which correlates with the capacity of the virus to enter the CNS. In addition, the lack of intrathecal neutralizing antibodies likely allows prolonged spreading of the virus between neurons, which is supported by the detection of TBEV RNA in the CSF of our patients. A recently observed cluster of lethal TBEV infections in patients after solid organ transplantation suggests that different forms of (iatrogenic) immunosuppression may represent a risk factor for more severe disease [11]. There is currently no specific antiviral treatment for TBE. However, the early administration of high-dose IVIG has been considered [12]. Particularly, in cases of significant viral replication in the CNS and concomitant absence of endogenous TBEV antibodies, passively transferred neutralizing antibodies may improve the outcome. However, therapeutic administration of IVIG requires a risk-benefit analysis as antibody-dependent enhancement of infection has been suspected in children after postexposure prophylaxis with TBEV-specific hyperimmune serum [12]. High-dose IVIG has been used in RTX-treated patients with West Nile Virus infection, another neurotropic flavivirus, but did not show a clear clinical benefit [13]. It is worth noting that the TBEV-neutralizing antibody content in IVIG preparations depends on the geographic origin of the plasma donors [14]. Therefore, we measured the level of anti-TBEV-IgG in 4 IVIG preparations (Privigen, Gamunex 10%, KIOVIG, and Intratect), which were 202, 436, 4755, and 12 070 U/mL, respectively (cutoff for positive results ≥200 U/mL). These data underline the need to select an IVIG lot with high levels of TBEV-specific IgG to possibly achieve neutralizing intrathecal antibody levels.
Another important lesson from the 2 cases reported here concerns vaccination against TBEV, which is safe and efficient and recommended by national vaccination committees for endemic areas, eg, in Germany and Austria. It is especially important to vaccinate patients at risk for TBEV exposure before initiation of RTX therapy, which leaves protective antibody production by vaccine-induced long-lived CD20-negative plasma cells intact. Rapid vaccination schedules with 3 injections of the inactivated TBEV vaccine given on days 0, 7, and 21 have been developed [1], helping clinicians to protect patients from a potentially devastating infection when they are subsequently treated with RTX for autoimmune diseases. However, as the immunogenicity of TBEV vaccination will be impaired in patients also prior to RTX therapy, if they are immunosuppressed by other drugs [15], exposure prophylaxis to avoid tick bites in endemic areas is particularly relevant in this cohort.
Acknowledgements
Financial support. The authors have no competing financial interests. The authors acknowledge support by Deutsche Forschungsgemeinschaft and Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU) within the funding programme Open Access Publishing.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lindquist L, Vapalahti O. Tick-borne encephalitis. Lancet 2008; 371:1861–71. [DOI] [PubMed] [Google Scholar]
- 2. Faurschou M, Jayne DR. Anti-B cell antibody therapies for inflammatory rheumatic diseases. Annu Rev Med 2014; 65:263–78. [DOI] [PubMed] [Google Scholar]
- 3. Hauser SL, Bar-Or A, Comi G et al. ; OPERA I and OPERA II Clinical Investigators Ocrelizumab versus interferon Beta-1a in relapsing multiple sclerosis. N Engl J Med 2017; 376:221–34. [DOI] [PubMed] [Google Scholar]
- 4. Chen MH, Chen MH, Liu CY et al. Hepatitis B virus reactivation in rheumatoid arthritis patients undergoing biologics treatment. J Infect Dis 2017; 215:566–73. [DOI] [PubMed] [Google Scholar]
- 5. Grankvist A, Andersson PO, Mattsson M et al. Infections with the tick-borne bacterium “Candidatus Neoehrlichia mikurensis” mimic noninfectious conditions in patients with B cell malignancies or autoimmune diseases. Clin Infect Dis 2014; 58:1716–22. [DOI] [PubMed] [Google Scholar]
- 6. Krause PJ, Gewurz BE, Hill D et al. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis 2008; 46:370–6. [DOI] [PubMed] [Google Scholar]
- 7. Schwaiger M, Cassinotti P. Development of a quantitative real-time RT-PCR assay with internal control for the laboratory detection of tick borne encephalitis virus (TBEV) RNA. J Clin Virol 2003; 27:136–45. [DOI] [PubMed] [Google Scholar]
- 8. Knight A, Pauksens K, Nordmark G, Kumlien E. Fatal outcome of tick-borne encephalitis in two patients with rheumatic disease treated with rituximab. Rheumatology (Oxford) 2017; 56:855–6. [DOI] [PubMed] [Google Scholar]
- 9. Palanichamy A, Muhammad K, Roll P et al. Rituximab therapy leads to reduced imprints of receptor revision in immunoglobulin κ and λ light chains. J Rheumatol 2012; 39:1130–8. [DOI] [PubMed] [Google Scholar]
- 10. Caracciolo I, Bassetti M, Paladini G et al. Persistent viremia and urine shedding of tick-borne encephalitis virus in an infected immunosuppressed patient from a new epidemic cluster in North-Eastern Italy. J Clin Virol 2015; 69:48–51. [DOI] [PubMed] [Google Scholar]
- 11. Lipowski D, Popiel M, Perlejewski K et al. A cluster of fatal tick-borne encephalitis virus infection in organ transplant setting. J Infect Dis 2017; 215:896–901. [DOI] [PubMed] [Google Scholar]
- 12. Růžek D, Dobler G, Niller HH. May early intervention with high dose intravenous immunoglobulin pose a potentially successful treatment for severe cases of tick-borne encephalitis? BMC Infect Dis 2013; 13:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morjaria S, Arguello E, Taur Y et al. West Nile virus central nervous system infection in patients treated with rituximab: implications for diagnosis and prognosis, with a review of literature. Open Forum Infect Dis 2015; 2: doi: 10.1093/ofid/ofv136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rabel PO, Planitzer CB, Farcet MR, Kreil TR. Tick-borne encephalitis virus-neutralizing antibodies in different immunoglobulin preparations. Clin Vaccine Immunol 2012; 19:623–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hertzell KB, Pauksens K, Rombo L et al. Tick-borne encephalitis (TBE) vaccine to medically immunosuppressed patients with rheumatoid arthritis: a prospective, open-label, multi-centre study. Vaccine 2016; 34:650–5. [DOI] [PubMed] [Google Scholar]