Abstract
Background
Overexpression of p16 is associated with improved outcomes among patients with oropharyngeal carcinoma. However, its role in nasopharyngeal cancer patient outcomes remains unclear.
Methods
Eighty-six patients with nasopharyngeal carcinoma treated at MD Anderson from 2000 to 2014 were identified. Epstein bar virus (EBV) and Human papillomavirus (HPV) status were determined by in situ hybridization and p16 by immunohistochemical staining.
Results
EBV positivity was associated with extended overall survival (median 95.0 vs. 44.9 months, P<0.004), progression-free survival (PFS) (median 80.4 vs. 28.1 months, P<0.013) and locoregional control (LRC) (median 104.4 vs. 65.5 months, P<0.043). In patients with EBV-positive tumors, p16 overexpression correlated with improved PFS (median 106.3 vs. 27.1 months, P<0.02) and LRC (median 93.6 vs. 64.5 months, P<0.02).
Conclusion
P16 overexpression is associated with improved PFS and LRC in patients with EBV-positive nasopharyngeal carcinoma. p16 expression may complement EBV status in predicting treatment outcomes for nasopharyngeal carcinoma patients.
Keywords: p16, HPV, EBV, nasopharyngeal carcinoma, nasopharynx cancer
INTRODUCTION
Although relatively uncommon in most parts of the world, nasopharyngeal carcinoma (NPC) is endemic in Southeast Asia, with incidence rates as high as 50 per 100,000 individuals.1,2 In North America, the incidence of NPC has stayed stable in the single-digit range.3 In addition to this epidemiologic diversity, the clinicopathologic characteristics of NPC also distinguish it from other head and neck cancers. Pathologically, NPC can be classified according to the World Health Organization system into keratinizing (WHO type I) and non-keratinizing (WHO types II and III) subtypes.4 Molecularly, association of the Epstein-Barr virus (EBV) genome further stratifies this disease into categories with different clinical outcomes. The recognized presence of nuclear EBV DNA in WHO types II and III, but not WHO type I NPCs, suggests that a unique pathogenesis process exists for each tumor subtype.5
Recently, human papillomavirus (HPV) has been proposed to have a potential etiologic role in the development of non-endemic, EBV negative NPC 3,6–8. In oropharyngeal carcinoma, detection of HPV DNA within cancer cells is strongly associated with improved clinical outcome and serves as a marker of prognosis.9–10 The prospect of a similar relationship between HPV and NPC appears very intriguing, yet conflicting reports on HPV and EBV co-infection rates, small patient numbers and geographic and treatment variations obscure the clinical relevance of HPV in NPC 11–13. Recent studies have suggested oncogenic HPV is associated with EBV negative NPC, primarily among whites and those with WHO type I histology and confers a worse prognosis, whereas others demonstrated that HPV occurrence in NPC is more similar to oral cavity carcinomas with no real prognostic value. 7, 14,15 In addition, methods to detect the presence of HPV DNA in oropharyngeal carcinoma tissues have disadvantages ranging from procedural complexity and high cost to low detection sensitivity (in situ hybridization; ISH) and low specificity (polymerase chase reaction; PCR).16
Immunostaining for p16 protein expression is proposed as a surrogate or complementary procedure for determining HPV status in oropharyngeal cancer, based on a known strong correlation between HPV positivity and p16 overexpression.17–21 Also known as cyclin-dependent kinase inhibitor 2A, p16 is a tumor suppressor protein that is crucial in preventing inappropriate cell proliferation. In HPV-related oropharyngeal cancer, the HPV oncoprotein E7 inactivates retinoblastoma protein (Rb), an important cell cycle checkpoint, to promote proliferation. This releases p16 from negative feedback control and causes a paraodoxical increase in p16 levels to inhibit uncontrolled cellular proliferation. Thus, p16 overexpression in HPV oropharyngeal cancer often represents an unsuccessful attempt to stop cell division. HPV related oropharyngeal carcinomas with p16 overexpression are very sensitive to radiotherapy and have a better prognosis.21 It is unclear whether this relationship also holds true for NPC. Here, we investigated the potential role of p16 and EBV status in patients with NPC, hypothesizing that p16 can complement EBV status as a marker of prognosis.
METHODS
Patient Selection
As a part of an institutional review board–approved study, we identified 312 consecutive patients with NPC treated at MD Anderson Cancer Center between the years 2000 and 2014. After excluding patients with non-primary, recurrent, and non-NPC histology tumors, a total of 86 patients were identified as having tumor tissue specimens available for pathology review and testing for EBV and p16 status. The medical records of all 86 of these patients were then retrospectively reviewed for clinical and pathologic characteristics, as well as details of chemotherapy, surgery, and radiation therapy.
EBV, HPV and p16 Detection
All available tumor specimen blocks were centrally reviewed by two MD Anderson pathologists specializing in head and neck cancer. In situ hybridization for EBV-encoded RNA was used to detect EBV in formalin-fixed, paraffin-embedded tissue sections by using automated BenchMark system (Ventana, Tuscon, AZ, US) according to the manufacturer’s protocol, with appropriate positive and negative controls. Expression of p16 was analyzed by immunohistochemical staining of 4-µm paraffin sections according to standard protocols.3 Briefly, samples were incubated with primary monoclonal antibody targeting the p16 antigen (BD Systems, Franklin Lakes, NJ, US) followed by the addition of a secondary antibody. The stained slides were then washed with incubation buffer and developed with the chromogen diaminobenzidine (DAB) and counterstained with hematoxylin. Similarly, for HPV detection, the Ventana INFORM HPV III Family 16 Probe (Ventana, Tuscon, AZ, US) was used to detect hybridization in the nuclei of high-risk HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 66). Expression of p16 was considered positive when strong nuclear and cytoplasmic staining was present in at least 60% of all tumor cells.
Statistical Analysis
Chi-square or Fisher’s exact tests were used to compare all categorical variables, and t tests were used to compare continuous variables when appropriate. The Kaplan-Meier method was used to analyze overall survival (OS), progression-free survival (PFS), and locoregional control (LRC), and compared between groups with log-rank tests. Time was defined as the date of pathologic diagnosis to the event of interest. PFS was defined as time from diagnosis to disease progression or death from any cause. Locoregional failure was defined as relapse within the primary site or neck lymph node. The influence of covariates on clinical outcomes was determined by multivariate Cox proportional hazard regression analysis. The proportional hazard assumption was tested graphically. All tests were 2-sided, and a P value of <0.05 was considered significant. All statistical analyses were done with IBM SPSS software (V22.0).
RESULTS
Patient and Tumor Characteristics
Patient characteristics are listed in Table 1. The median age for the entire group was 51.4 years. Most patients were male (72%) and white (64%), and majority presented with advanced locoregional disease (T3–4, 69%; N+, 87%) and an Eastern Cooperative Oncology Group performance status score of 0–1 (90%). All patients received intensity-modulated radiation therapy as part of their treatment, with 89.5% receiving a total dose of 70 Gy in 33–35 fractions (range 66–70 Gy in 30–35 fractions). Ninety-four percent of the patients received chemotherapy. Radiation treatment field included the primary tumor and bilateral neck levels II – V. Of the patients receiving chemotherapy, 74% received induction chemotherapy, 83% received concurrent chemotherapy and 8% received adjuvant chemotherapy. A detailed breakdown of chemotherapy use is listed in supplemental Table S1.
Table 1.
Patient Characteristics (n=86)
| Characteristic | Value or Number of Patients (%) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| All | EBV- Positive |
EBV- Negative |
P Value |
p16- Positive |
p16- Negative |
P Value |
|
| Age | |||||||
| Median | 51.4 | 47.3 | 56.2 | 52.3 | 59.1 | ||
| Range | 16.9–84.8 | 16.9–84.8 | 28.2–77.7 | 16.9–77.7 | 17.2–84.8 | ||
| Sex | 0.21 | 0.78 | |||||
| Male | 62 (72) | 34 (77) | 22 (63) | 28 (70) | 19 (73) | ||
| Female | 24 (28) | 10 (23) | 13 (37) | 12 (30) | 7 (27) | ||
| Race | 0.34 | 0.66 | |||||
| White | 55 (64) | 30 (68) | 22 (63) | 26 (65) | 16 (62) | ||
| African American | 9 (10) | 4 (9) | 3 (9) | 3 (8) | 4 (15) | ||
| Asian | 13 (15) | 8 (18) | 4 (11) | 5 (13) | 4 (15) | ||
| Hispanic | 9 (10) | 2 (5) | 6 (17) | 6 (15) | 2 (8) | ||
| Smoking | 49 (57) | 21 (48) | 24 (69) | 0.10 | 16 (40) | 15 (58) | 0.25 |
| WHO Grade | 0.01 | 0.16 | |||||
| I | 17 (20) | 4 (9) | 12 (34) | 13 (33) | 4 (15) | ||
| II & III | 69 (80) | 40 (91) | 23 (66) | 27 (68) | 22 (85) | ||
| T Category | 0.62 | 0.60 | |||||
| T1 | 14 (16) | 8 (18) | 4 (11) | 7 (18) | 5 (19) | ||
| T2 | 13 (15) | 6 (14) | 7 (20) | 6 (15) | 5 (19) | ||
| T3 | 17 (20) | 8 (18) | 9 (26) | 12 (30) | 4 (15) | ||
| T4 | 42 (49) | 22 (50) | 15 (43) | 15 (38) | 12 (46) | ||
| Nodal Disease | 75 (87) | 41 (93) | 28 (80) | 0.10 | 31 (78) | 26 (100) | 0.01 |
| Chemotherapy | 81 (94) | 43 (98) | 31 (89) | 0.16 | 37 (93) | 25 (96) | 0.65 |
| Performance Score | |||||||
| 0–1 | 77 (90) | 41 (93) | 30 (86) | 0.46 | 36 (90) | 25 (96) | 0.64 |
| >1 | 9 (10) | 3 (7) | 5 (14) | 4 (10) | 1 (4) | ||
Abbreviations: EBV, Epstein-Barr virus; WHO, World Health Organization
Of the 86 patients identified, 44 had tumors that were EBV-positive, 40 were p16-positive, and 13 were both EBV-positive and p16-positive. Seven patients (8%) had indeterminate or borderline EBV status and 20 patients (22%) did not have sufficient tissue samples to determine p16 expression status. These patients were excluded from multivariate analysis. In terms of HPV status, 23 tumors were positive, 45 were negative, and 18 had indeterminate status. All 23 patients found to have HPV-positive tumors by in situ hybridization were all also p16-positive, which accounted for 57.5% (23 of 40) of all p16-positive samples. The remaining 17 of the p-16 positive tumors were negative for HPV. HPV status was not associated with OS, PFS or LRC (Supplemental Fig S1).
EBV positivity was associated with non-keratinizing WHO type II and III tumors (P<0.01), and p16 positivity was associated with having node-positive disease at presentation (P<0.01). Although p16 positivity showed a higher association with keratinizing WHO grade I tumors compared to non-keratinizing WHO grade II and III tumors (76% vs. 55%), this difference was not statistically significant (P=0.16). No differences were found in EBV or p16 status according to age, race, sex, performance status, smoking status, T classification, and chemotherapy use or sequence (Table 1 and S1).
Clinical Outcomes
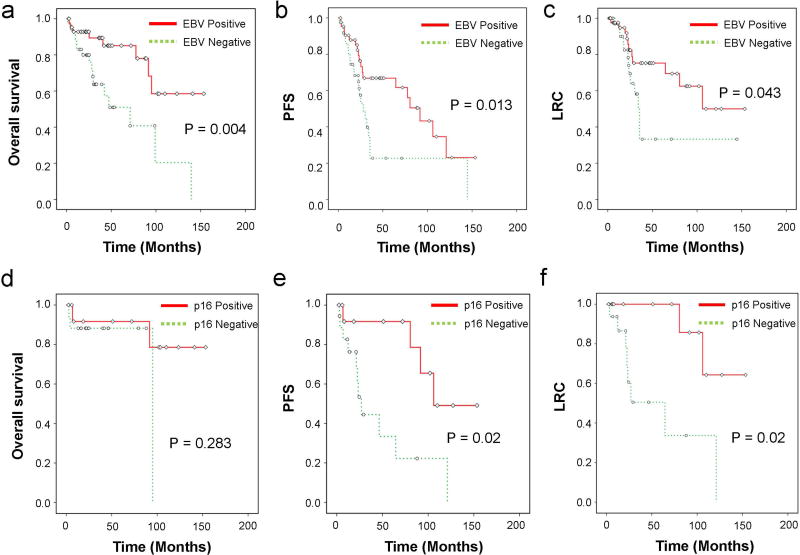
The median follow-up time for all surviving patients was 36 months. The 3-year OS, PFS, and LRC rates for the entire group were 79.4%, 52.5% and 64.5%. Patients with WHO II/III had improved survival and disease control outcomes compared to those with WHO I tumors. Patients with EBV-negative tumors had poorer survival and disease-control outcomes than those with EBV-positive tumors (Fig 1a–c). The median OS times were 95.0 months for patients with EBV-positive tumors versus 44.9 months EBV-negative (P=0.004). Similarly, median PFS times were 80.4 months (EBV+) versus 28.1 months (EBV–) (P=0.007) and median LRC times were 104.4 months (EBV+) versus 34.2 months (EBV–) (P=0.04). Univariate analysis (Table 2) showed that in addition to WHO I and EBV-negative status, older age, and performance status score >1 were associated with worse OS and PFS, whereas receipt of chemotherapy and lower T classification were associated with improved PFS and LRC.
Fig 1.
Survival and disease-control outcomes stratified by Epstein-Barr virus (EBV) and p16 status. Patients with EBV-positive tumors had better overall survival (OS) (a), progression-free survival (PFS) (b), and locoregional control (c) compared with patients with EBV-negative tumors. For patients with EBV-positive nasopharyngeal carcinoma, p16 positivity is not predictive of a) OS, but is associated with significantly improved e) PFS and f) LRC.
Table 2.
Univariate Analysis of Patient-, Disease-, and Treatment-Related Factors Potentially Associated with Outcomes
| Covariate | Overall Survival | Progression-Free Survival | Locoregional Control | |||
|---|---|---|---|---|---|---|
|
| ||||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age* | 1.05 (1.02–1.09) | 0.003 | 1.03 (1.01–1.06) | 0.01 | 1.02 (0.99–1.05) | 0.23 |
| Smoking | 0.24 | 0.045 | 0.20 | |||
| Non-smoker | 1.00 | 1.00 | 1.00 | |||
| Smoker | 3.07 (1.04–9.06) | 2.14 (1.02–4.50) | 1.83 (0.73–4.62) | |||
| WHO Grade | 0.006 | 0.009 | 0.055 | |||
| II&III | 1.00 | 1.00 | 1.00 | |||
| I | 3.18 (1.39–7.27) | 2.51 (1.25–5.02) | 2.39 (0.98–5.87) | |||
| T Category* | 1.03 (0.72–1.49) | 0.86 | 1.44 (1.03–2.02) | 0.04 | 1.68 (1.03–2.74) | 0.037 |
| N Stage | 0.51 | 0.69 | 0.89 | |||
| N− | 1.00 | |||||
| N+ | 0.73 (0.28–1.89) | 1.21 (0.49–2.92) | 0.93 (0.32–2.75) | |||
| Chemotherapy | 0.18 | 0.02 | 0.01 | |||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 0.44 (0.13–1.48) | 0.27 (0.92–0.78) | 0.2 (0.06–0.70) | |||
| Performance Score | 0.01 | 0.007 | 0.46 | |||
| 0–1 | 1.00 | 1.00 | 1.00 | |||
| >1 | 3.71 (1.34–10.25) | 2.98 (1.35–6.59) | 1.59 (0.47–5.39) | |||
Analyzed as continuous covariate.
Abbreviations: OS, overall survival; PFS, progression-free survival; LRC, locoregional control; HR, hazard ratio; CI, confidence interval; WHO, World Health Organization
P16 Positivity Predicts Improved PFS and LRC in EBV-positive tumors
When all patients were stratified based on p16 status of their tumors, the median OS times were 139.5 months for those with p16+ tumors and 95.1 months for those with p16– tumors (P=0.46). Although this was not statistically significant, we did observed a trend for improved PFS and LRC for patients with p16-positive (compared with p16-negative), with median PFS times of 91.6 months (p16+) versus 27.1 months (p16–) (P=0.07), and median LRC times 106.1 months (p16+) versus 30.2 months (p16–) (P=0.09).
Next, we assessed the predictive value of p16 status in the subgroup of patients with EBV-positive NPC. Among EBV-positive patients, p16 status did not correlate with OS but was significantly associated improved PFS and LRC (Fig 1d–f). The median OS times were 139.5 months (EBV+/p16+ patients) versus 95.1 months (EBV+/p16 patients). The median PFS times were 106.3 months for EBV+/p16+ patients versus 27.1 months for EBV+/p16– patients (P=0.02). Similarly, patients with EBV+/p16+ tumors had a median LRC time of 93.6 months compared with 64.5 months for patients with EBV+/p16– tumors (P=0.02).
Similarly, among patients with WHO II/III status, which is associated with EBV driven pathology,22,23 p16 positivity was associated with significantly improved PFS and LRC. The median PFS times were 105.3 months for p16+ tumors vs 46.4 months for patients with p16 – tumors (P=0.014). The median LRC times were 106.5 months for patients with p16+ tumors compared with 56.8 months for those with p16- tumors (P=0.041).
To account for competing covariates, we used multivariate analyses after adjustment for age, smoking status, WHO grade, T classification, and p16 status in EBV-positive patients (Table 3). We excluded the use of chemotherapy and baseline performance status as covariates because most of the patients with EBV-positive tumors had received chemotherapy (98.5%) and had performance status scores of ≤1 (93.2%). In our multivariate model, p16 positivity remained a strong predictor of improved PFS (P=0.02) and LRC (P=0.04) among patients with EBV-positive tumors. Similarly, WHO status and T classification remained significantly associated with PFS.
Table 3.
Multivariate Analysis of Patient- and Disease-Related Factors Affecting Outcomes Among Patients with EBV-Positive Tumors
| Covariate | Overall Survival | Progression-Free Survival | Locoregional Control | |||
|---|---|---|---|---|---|---|
|
| ||||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| p16 | 0.49 | 0.02 | 0.04 | |||
| Negative | 1.00 | 1.00 | 1.00 | |||
| Positive | 0.44 (0.04–4.77) | 0.11 (0.02–0.64) | 0.13 (0.02–0.90) | |||
| Age* | 1.05 (0.96–1.14) | 0.28 | 1.01 (0.95–1.05) | 0.97 | 0.99 (0.93–1.05) | 0.67 |
| Smoking | 0.16 | 0.78 | 0.77 | |||
| Non-smoker | 1.00 | 1.00 | 1.00 | |||
| Smoker | 0.51 (0.10–3.28) | 0.82 (0.22–3.14) | 1.55 (0.26–9.17) | |||
| WHO Grade | 0.18 | 0.02 | 0.334 | |||
| II&III | 1.00 | 1.00 | 1.00 | |||
| I | 2.70 (0.64–11.11) | 4.95 (1.25–19.6) | 3.45 (0.41–19.52) | |||
| T Category* | 0.49 (0.13–1.82) | 0.29 | 1.92 (1.01–3.64) | 0.05 | 2.05 (0.15–28.83) | 0.59 |
analyzed as continuous covariate
Abbreviations: EBV, Epstein-Barr virus; HR, hazard ratio; CI, confidence interval; WHO, World Health Organization
DISCUSSION
Our current study showed that p16 positivity correlated with improved PFS and LRC for patients with EBV-positive NPC, raising the possibility it may be an independent predictor of outcomes in this sub-group of patients. p16 is an important tumor suppressor protein that is essential to the regulation of the Rb1 cell cycle pathway. p16 induces cell cycle arrest via the inhibition of cyclin-dependent kinase 2 and 4 and prevents unchecked cellular growth and proliferation.24 Inactivation of p16 has been found at high frequencies in several types of cancer in humans, including carcinomas of the head and neck.25 Paradoxically, despite its role as an inhibitor of cell proliferation, overexpression of p16 has been linked with tumorigenesis, particularly in the setting of HPV-related neoplasms.26 The association between p16 overexpression and HPV infection may reflect the presence of the HPV oncoprotein E7, which disables the Rb protein leading to cell cycle progression. In response to this “HPV-associated” disruption of the Rb cell cycle checkpoint, p16 is then overexpressed to compensate for uncontrolled cellular proliferation.27
However, several mechanisms other than HPV can disable Rb function and cause p16 overexpression, as have been demonstrated in breast, lung, and bladder cancers.28–30 In addition to being overexpressed by inactivation of the Rb pathway, p16 can also be overexpressed via Rb-independent pathways, as is the case during the p38-mediated stress response.31 Therefore, p16 overexpression may be an intrinsic cellular response to increased proliferation rather than a direct consequence of HPV infection. This is especially relevant to NPC, in which the incidence of Rb inactivation is low.32 Thus although p16 negativity most likely rules out HPV infection, p16 overexpression in tumors can be attributed to multiple causes. This supposition was confirmed in our study, in which the 23 patients found to have HPV-positive tumors by in situ hybridization were all also found to be p16-positive. However, HPV was positive in only 57.5% of p16-postive tumors. Further, the lack of correlation found between p16 status and WHO classification suggests that overexpression of this tumor suppressor protein is multifactorial.
Recent findings have suggested a relationship between HPV infection and NPC, but its clinical significance has been hard to establish because of inconsistencies in reported findings, including the incidence of viral coinfection with EBV.2,3,7,14,15 An analysis of NPC patients treated in the United Kingdom in which a multi-tier approach was used to assess HPV positivity, first by screening for p16 by immunohistochemical staining followed by confirmation with high-risk HPV in situ hybridization, showed that HPV-associated NPC was more likely to occur in whites and was not associated with differences in survival.14 However, a study from Johns Hopkins suggested that HPV-associated NPCs may in fact be subepithelial extensions of oropharyngeal tumors rather than true nasopharyngeal primary tumors, because of the lack of anatomic barriers that separate the two compartments.15 In that study, 3 of 4 patients with HPV-positive NPC were found to have oropharyngeal extension; further, p16 was shown to be highly correlated with HPV status. In contrast, others have suggested that HPV-associated NPC represents a distinctive clinicopathologic entity similar to their HPV-associated oropharynx counterpart, with unique epidemiologic and pathologic features.7 This is partially based on the hypothesis that EBV and HPV infections may be mutually exclusive. Findings from MD Anderson Cancer Center showed a high correlation between HPV positivity and lack of EBV coinfection in patients with WHO grade I NPC, implicating a role for EBV-independent, HPV-driven carcinogenesis in such patients.3 Similarly, no cases of coinfection were found in an analysis of 61 patients treated at the University of Michigan in which 18 tumors were HPV-positive and none of those 18 tumors were EBV-positive. That study further indicated that HPV-positive NPC was associated with worse prognosis compared with NPC that was negative for both EBV and HPV.7 However, whether EBV and HPV infections in NPC are truly mutually exclusive remains elusive.11–13 Several studies, including the current one, have demonstrated that EBV status alone is a significant predictor of prognosis in NPC.33,34 Thus, the conclusion from the Michigan study in which patients with HPV-positive NPC have worse outcomes than those with HPV-negative tumors could be attributed to the difference in EBV status rather than the HPV status, as no co-infection of HPV and EBV were found in their tumor samples. Close examination of their data showed patients with HPV+/EBV– tumors actually had better clinical outcomes than did those with HPV–/EBV– tumors, with significantly improved 5-year rates of OS (47.9% vs. 17.6%, P=0.003), PFS (34.2% vs. 11.8%, P=0.001), and LRC (48.1% vs. 26.4%, P=0.002).
Although p16 overexpression is often used as a surrogate for HPV infection in oropharyngeal carcinoma, our results suggest it may be an independent biomarker for predicting response to treatment in NPC. These findings are consistent with the underlying biology of p16 protein, whose levels are increased as a direct compensatory response to uninhibited cellular proliferation.35 We did observe a trend towards improved PFS and LRC among patients with p16-positive tumors independent of EBV status. These differences may become more evident with longer follow-up time and larger number of patients. When patients were stratified based on EBV-positivity, the prognostic and predictive power of p16 became statistically significant. This is mainly due to improved locoregional control as there were only 3 distant failures in our group.
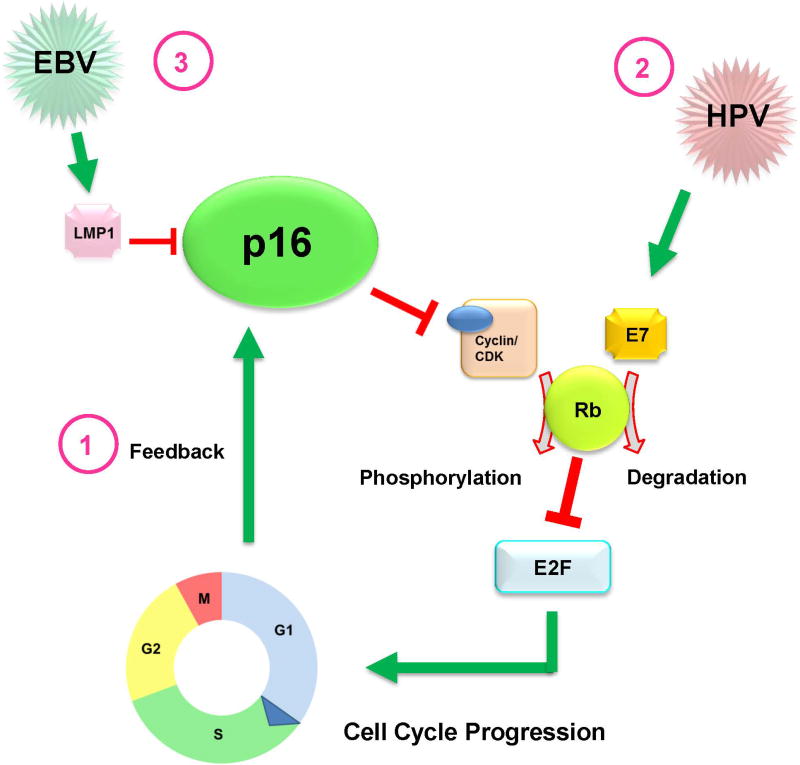
Viral infection by EBV can disrupt several intracellular signaling processes involved in cell cycle regulation. For example, the EBV-associated oncoprotein latent membrane protein 1 (LMP1) can block p16 expression and inhibit downstream effectors, including E2F4 and E2F5 transcription factors, to promote cellular proliferation.36,37 LMP1 is expressed in 65% of EBV-positive nasopharyngeal tumors and has been linked with more aggressive features.38,39 Therefore, it is possible that within EBV-positive NPCs, p16 expression status can identify tumors with an aggressive phenotype with suppressed compensatory up-regulation of this tumor suppressor (Fig 2). Interestingly, all 3 distant failures in the current series occurred in patients with p16-negative tumors. These findings, together with the pooled results from single-institution IMRT studies and the Radiation Therapy Oncology Group (RTOG 0225) trial, which have consistently shown excellent locoregional control up to 90% at 2 years, with distant metastases rate as high as 33% suggest that distant failure may be in part be influenced by p16-status, and merit further analysis of p16-status in cohorts from these trials as well as those from the U.S. Intergroup 0099 and endemic randomized trials.40–43
Fig 2.
Schematic demonstrating possible molecular mechanisms involving p16 expression, Epstein-Barr virus (EBV) status, and human papillomavirus (HPV) status. 1) p16 is a tumor suppressor protein that inhibits cell cycle progression by preventing the formation of cyclin D/CDK complex, which is needed to phosphorylate retinoblastoma protein (Rb). Rb binds and prevents E2F from entering the nucleus to initiate cell cycle progression. (2) When cells are infected by HPV, the oncoprotein E7 binds to Rb, and signals it for degradation. As a result, E2F is released into the nucleus to initiate cell cycle progression. The uncontrolled cell proliferation initiates a negative feedback loop to signal p16 upregulation to counter this effect. (3) During EBV infection, this protective feedback mechanism can be disrupted by the oncoprotein latent membrane protein 1 (LMP1) by blocking the expression of p16.
Despite these findings, the use of p16 expression as a marker to further stratify various risk groups of NPC faces many challenges.16,44 For example, substantial differences exist in the definition of p16 overexpression, with definitions of p16 positivity ranging from 30% to 90% positive tumor cell staining.3,6,7 This heterogeneity in staining patterns likely reflects differences in the immunohistochemical staining protocols and probes used. Suggestions have been made that a positive p16 score should require at least 70% tumor cell staining.44 Currently, no universally adopted staining protocol has been identified, and monoclonal antibodies can vary among suppliers. Therefore, accurate determination of p16 expression status on an individual basis requires that the staining protocol be optimized for a particular set of probes and use of proper standardized controls to minimize the risk of false results.
There are several limitations to this study. Notably, this is a non-randomized, retrospective review from a single-institution and patient numbers are limited. While treatments for our patients were relatively homogeneous (100% received definitive dose IMRT and over 83% received concurrent chemotherapy), many patients (75%) received induction chemotherapy, which consisted of varied regimens. In addition, around 20% of the patients had insufficient tissue sample for definitive p16 analysis, which may have lowered the statistical power of our study to detect additional differences in patient outcomes. Finally, all 23 HPV-positive tumors were also p16 positive, accounting for 57.5% of all p16-positive samples. Although not explicitly tested in our series, this raises the question as to whether patients with tumors harboring HPV-driven p16 up-regulation have a different prognosis compared to HPV independent p16-upregulation.
CONCLUSION
In this study, we showed that in patients with EBV-positive tumors, the lack of p16 expression conferred a worse PFS and LRC. These patients may benefit from further local or systemic treatment intensification such as radiotherapy dose-escalation or addition of a radiosensitizing agent. Alternatively, the need for chemotherapy can be assessed in those with potentially “low risk” stage II disease who have non-keratinizing (WHO II/III), EBV-positive and p16-positive tumors. Although these findings raises a possibility of p16 status, independent of HPV, as a potential complementary marker of prognosis in addition to EBV status for patients with NPC, given the limited number of the patients in our study, and its retrospective nature, a prospective multi-institutional effort would provide the sample size and statistical power to definitively determine the prognostic relationship, if any, among p16 expression level, EBV status, and clinical outcomes for patients with NPC. Accurate identification and validation of p16 as a complementary biomarker could further stratify these patients with the goal of optimizing treatment outcomes and guide future study design.
Supplementary Material
Acknowledgments
Funding Support:
Funding support include the SWOG-Hope Foundation Dr. Charles A. Coltman, Jr. Fellowship in Clinical Trials, the National Institutes of Health Paul Calabresi Clinical Trial Program (K12 CA088084-14), Clinician Scientist Loan Repayment Program (L30 CA136381-02), MD Anderson/Elekta AB MR-LinAc Seed Grant, the MD Anderson Center for Advanced Biomedical Imaging/General Electric Healthcare In-Kind Award, the Center for Radiation Oncology Research at MD Anderson Cancer Center Seed Grant, and the MD Anderson Institutional Research Grant Program (C.D.F); The Gerstner Family Foundation Career Development Award and Center for Regenerative Medicine Career Development Award (B.Y.S.K). The funding organizations played no role in the study design, the collection, analysis and interpretation of data, writing of the manuscript, nor in the decision to submit the manuscript.
Footnotes
The authors report no conflict of interests.
Approval and Consent:
This study had institutional review board approval and informed consent was waived.
References
- 1.Sun Y, Tang LL, Chen L, et al. Promising treatment outcomes of intensity-modulated radiation therapy for nasopharyngeal carcinoma patients with N0 disease according to the seventh edition of the AJCC staging system. BMC Cancer. 2012;12:68. doi: 10.1186/1471-2407-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Punwaney R, Brandwein MS, Zhang DY, et al. Human papillomavirus may be common within nasopharyngeal carcinoma of Caucasian Americans: investigation of Epstein-Barr virus and human papillomavirus in eastern and western nanopharyngeal carcinoma using ligation-dependent polymerase chain reaction. Head Neck. 1999;21:21–29. doi: 10.1002/(sici)1097-0347(199901)21:1<21::aid-hed3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Lo EJ, Bell D, Woo JS, et al. Human papillomavirus and WHO type I nasopharyngeal carcinoma. Laryngoscope. 2010;120:1990–1997. doi: 10.1002/lary.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei WI, Sham JST. Nasopharyngeal carcinoma. Lancet. 2005;365:2041–2054. doi: 10.1016/S0140-6736(05)66698-6. [DOI] [PubMed] [Google Scholar]
- 5.Raab-Traub N. Epstein–Barr virus in the pathogenesis of NPC. Semin Cancer Biol. 2002;12:431–441. doi: 10.1016/s1044579x0200086x. [DOI] [PubMed] [Google Scholar]
- 6.Lin Z, Khong B, Kwok S, et al. Human papillomavirus 16 detected in nasopharyngeal carcinomas in white Americans but not in endemic southern Chinese patients. Head Neck. 2014;36:709–714. doi: 10.1002/hed.23362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stenmark MH, McHugh JB, Schipper M, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys. 2013;88:580–588. doi: 10.1016/j.ijrobp.2013.11.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/p16-positive EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32:562–567. doi: 10.1002/hed.21216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munger K, Baldwin A, Edwards KM. Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78:1451–1460. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nature. 2010;10:550–560. doi: 10.1038/nrc2886. [DOI] [PubMed] [Google Scholar]
- 11.Mirzamani N, Salehian P, Farhadi M, et al. Detection of EBV and HPV in nasopharyngeal carcinoma by in situ hybridization. Exp Mol Pathol. 2006;81:231–234. doi: 10.1016/j.yexmp.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Rassekh CH, Rady PL, Arany I, et al. Combined Epstein-Barr virus and human papillomavirus infection in nasopharyngeal carcinoma. Laryngoscope. 1998;108:362–367. doi: 10.1097/00005537-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Tyan YS, Liu ST, Ong WR, et al. Detection of Epstein-Barr virus and human papillomavirus in head and neck tumors. J Clin Microbiol. 1993;31:53–56. doi: 10.1128/jcm.31.1.53-56.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson M, Suh YE, Paleri V, et al. Oncogenic human papillomavirus-associated nasopharyngeal carcinoma: an observational study of correlation with ethnicity, histological subtype and outcome in a UK population. Inf Agent Cancer. 2013;8:30. doi: 10.1186/1750-9378-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singhi AD, Califano J, Westra WH. High-risk human papillomavirus in nasopharyngeal carcinoma. Head Neck. 2012;34:213–218. doi: 10.1002/hed.21714. [DOI] [PubMed] [Google Scholar]
- 16.El-Naggar AK, Westra WH. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck. 2012;34:459–461. doi: 10.1002/hed.21974. [DOI] [PubMed] [Google Scholar]
- 17.Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2001;116:2166–2173. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- 18.Kalof AN, Cooper K. p16INK4a immunoexpression: surrogate marker of high-risk HPV and high-grade cervical intraepithelial neoplasia. Adv Anat Pathol. 2006;13:190–194. doi: 10.1097/00125480-200607000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T. Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol. 1998;153:1741–1748. doi: 10.1016/S0002-9440(10)65689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klussmann JP, Gultekin E, Weissenborn SJ, et al. Expression of p16 protein identified a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol. 2003;162:747–753. doi: 10.1016/S0002-9440(10)63871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niedobitek G, Hansmann ML, Herbst H, et al. Epstein-Barr virus and carcinomas: undifferentiated carcinomas but not squamous cell carcinomas of the nasopharynx are regularly associated with the virus. J Pathol. 1991;165:17–24. doi: 10.1002/path.1711650105. [DOI] [PubMed] [Google Scholar]
- 23.Nicholls JM, Agathanggelou A, Fung K, et al. The association of squamous cell carcinomas of the nasopharynx with Epstein-Barr virus shows geographical variation reminiscent of Burkitt’s lymphoma. J Pathol. 1997;183:164–168. doi: 10.1002/(SICI)1096-9896(199710)183:2<164::AID-PATH919>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 24.Lukas J, Parry D, Aagaard L, et al. Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature. 1995;375:503–506. doi: 10.1038/375503a0. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez S, Serrano M. A new mechanism of inactivation of the INK4/ARF locus. Cell Cycle. 2006;5:1382–1384. doi: 10.4161/cc.5.13.2901. [DOI] [PubMed] [Google Scholar]
- 26.Mulvany NJ, Allen DG, Wilson SM. Diagnostic utility of p16INK4a: a reappraisal of its use in cervical biopsies. Pathology. 2008;40:335–344. doi: 10.1080/00313020802035907. [DOI] [PubMed] [Google Scholar]
- 27.Reuschenbach M, Waterboer T, Wallin KL, et al. Characterization of humoral immune responses against p16, p53, HPV16 E6 and HPV16 E7 in patients with HPV-associated cancers. Int J Cancer. 2008;23:2626–2631. doi: 10.1002/ijc.23837. [DOI] [PubMed] [Google Scholar]
- 28.Dublin EA, Patel NK, Gillett CE, et al. Retinoblastoma and p16 proteins in mammary carcinoma: their relationship to cyclin D1 and histopathological parameters. Int J Cancer. 1998;79:71–75. doi: 10.1002/(sici)1097-0215(19980220)79:1<71::aid-ijc14>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 29.Gorgoulis VG, Zacharatos P, Kotsinas A, et al. Alterations of the p16-pRb pathway and the chromosome locus 9p21-22 in non-small-cell lung carcinomas: relationship with p53 and MDM2 protein expression. Am J Pathol. 1998;153:1749–1765. doi: 10.1016/S0002-9440(10)65690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benedict WF, Lerner SP, Zhou J, et al. Level of retinoblastoma protein expression correlates with p16 (MTS-1/INK4A/CDKN2) status in bladder cancer. Oncogene. 1999;18:1197–1203. doi: 10.1038/sj.onc.1202452. [DOI] [PubMed] [Google Scholar]
- 31.Spallarossa P, Altieri P, Barisione C, et al. P38 MAPK and JNK antagonistically control senescence and cytoplasmic p16 INK4A expression in doxorubicin-treated endothelial progenitor cells. PLoS One. 2010;5:e15583. doi: 10.1371/journal.pone.0015583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun Y, Hegamyer G, Colburn NH. Nasopharyngeal carcinoma shows no detectable retinoblastoma susceptibility gene alterations. Oncogene. 1993;8:791–795. [PubMed] [Google Scholar]
- 33.Shi W, Pataki I, MacMillan C, et al. Molecular pathology parameters in human nasopharyngeal carcinoma. Cancer. 2002;94:1997–2006. doi: 10.1002/cncr.0679. [DOI] [PubMed] [Google Scholar]
- 34.Yip KW, Shi W, Pintilie M, et al. Prognostic significance of the Epstein-Barr virus, p53, Bcl-2, and survivin in nasopharyngeal cancer. Clin Cancer Res. 2006;12:5726. doi: 10.1158/1078-0432.CCR-06-0571. [DOI] [PubMed] [Google Scholar]
- 35.Mäkitie AA, MacMillan C, Ho J, et al. Loss of p16 expression has prognostic significance in human nasopharyngeal carcinoma. Clin Cancer Res. 2003;9:2177–2184. [PubMed] [Google Scholar]
- 36.Yang X, He Z, Xin B, Cao L. LMP1 of Epstein-Barr virus suppresses cellular senescence associated with the inhibition of p16INK4a expression. Oncogene. 2000;19:2002–2013. doi: 10.1038/sj.onc.1203515. [DOI] [PubMed] [Google Scholar]
- 37.Ohtani N, Brennan P, Gaubatz S, et al. Epstein-Barr virus LMP1 blocks p16INK4a-RB pathway by promoting nuclear export of E2F4/5. J Cell Biol. 2003;162:173–183. doi: 10.1083/jcb.200302085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu LF, Chen F, Zhen QF, et al. Differences in the growth pattern and clinical course of EBV-LMP1 expressing and non-expressing nasopharyngeal carcinomas. Eur J Cancer. 1995;31:658–660. doi: 10.1016/0959-8049(94)00468-k. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Y, Wang Y, Zeng S, Hu X. LMP1 expression is positively associated with metastasis of nasopharyngeal carcinoma: evidence from a meta-analysis. J Clin Pathol. 2012;65:41–45. doi: 10.1136/jclinpath-2011-200198. [DOI] [PubMed] [Google Scholar]
- 40.Lee N, Harris J, Garden AS, et al. Intensity-Modulated Radiation Therapy With or Without Chemotherapy for Nasopharyngeal Carcinoma: Radiation Therapy Oncology Group Phase II Trial 0225. J Clin Oncol. 2009;27:3684–3690. doi: 10.1200/JCO.2008.19.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310–7. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 42.Chen L, Hu CS, Chen XZ, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2012;13:163–171. doi: 10.1016/S1470-2045(11)70320-5. [DOI] [PubMed] [Google Scholar]
- 43.Wee J, Tan EH, Tai BC, et al. Randomized Trial of Radiotherapy Versus Concurrent Chemoradiotherapy Followed by Adjuvant Chemotherapy in Patients With American Joint Committee on Cancer/International Union Against Cancer Stage III and IV Nasopharyngeal Cancer of the Endemic Variety. J Clin Oncol. 2005;23:6730–6738. doi: 10.1200/JCO.2005.16.790. [DOI] [PubMed] [Google Scholar]
- 44.Larsen CG, Gyldenlove M, Jensen DH, et al. Correlation between human papillomavirus and p16 overexpression in oropharyngeal tumours: a systematic review. Br J Cancer. 2014;110:1587–1594. doi: 10.1038/bjc.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.