Summary
Natural killer (NK) cells play a central role in immune responses through direct cytotoxicity and the release of cytokines that prime adaptive immunity. In simian primates, NK cell responses are regulated by interactions between two highly polymorphic sets of molecules—the killer-cell immunoglobulin-like receptors (KIRs) and their MHC class I ligands. KIR-MHC class I interactions in humans have been implicated in the outcome of a number viral diseases and cancers. However, studies to address the role of KIRs in animal models have been limited by the complex immunogenetics and lack of defined ligands for KIRs in non-human primates. Due to the rapid evolution of KIRs, there is little conservation among the KIR genes of different primate species and it is not possible to predict the specificity of KIRs from known KIR-MHC class I interactions in humans. Hence, the MHC class I ligands for KIRs in species other than humans are poorly defined. Here we review the KIR genes of the rhesus macaque, an important animal model for HIV/AIDS and other infectious diseases, and the MHC class I ligands that have been identified for KIRs in this species.
Keywords: Macaque, KIR, MHC, NK cells
Introduction
Natural killer (NK) cells provide a critical early defense against infectious diseases and tumors by virtue of their ability to recognize and kill infected or malignant cells without previous antigenic stimulation (1, 2). In humans and other simian primates, NK cells express killer-cell immunoglobulin-like receptors (KIRs) that regulate these responses through interactions with MHC class I molecules on the surface of potential target cells (1, 2). KIRs are type I integral membrane proteins that consist of two or three extracellular immunoglobulin (Ig)-like domains (2D or 3D), a stem region, a transmembrane domain, and either a long or short cytoplasmic tail (1, 2). Depending on their transmembrane and cytoplasmic domains, KIRs can transduce either inhibitory or activating signals. Whereas inhibitory KIRs have long (L) cytoplasmic domains with a pair of immunoreceptor tyrosine-based inhibitory motifs (ITIMs), activating KIRs have short (S) cytoplasmic tails and a positively charged residue in the membrane-spanning domain that recruits an adaptor protein with an immunoreceptor tyrosine-based activation motif (ITAM) (1, 2). The recognition of MHC class I ligands on the surface of healthy cells by inhibitory KIRs normally suppresses NK cell activation (3-5). However, if ligand recognition is disrupted, for instance as a result of MHC class I downmodulation by a viral pathogen (6-10) or the deletion of MHC class I genes by tumor cells (11), this inhibition is lost, resulting in NK cell degranulation and target cell lysis. The ligands for activating KIRs are not as well defined; however, NK cells expressing activating KIRs respond in an MHC class I-dependent manner to directly lyse target cells (1, 12).
The KIR genes are polygenic with a variable number of loci per haplotype. They are encoded by the leukocyte receptor complex (LRC) on human chromosome 19 and segregate independently from MHC genes (13). In addition to variable gene content, there is considerable allelic variability within each gene. Thus, KIR diversity is a function of variation in gene content and allelic polymorphism. Fifteen expressed KIR genes have been identified in humans with 7 to 12 genes per haplotype (13). In humans, these haplotypes can be grouped into two categories based on the number of genes encoded: a minimal A haplotype that contains 7 genes with only a single activating KIR, or more complex B haplotypes that contain up to 12 genes with various combinations of activating KIR (13). Nevertheless, four genes (KIR3DL3, KIR3DP1, KIR2DL4 and KIR3DL2), referred to as “framework genes”, are present on all human KIR haplotypes (13).
The recognition of MHC class I ligands by KIRs is primarily determined by surface residues in a C-terminal patch of the MHC class I α1 domain and may be influenced by MHC class I-bound peptides. In the case of KIR3DL1, HLA class I ligands are differentiated on the basis of a public epitope corresponding to α1 domain residues 77-83. All HLA-B molecules and some HLA-A molecules can be classified as either Bw4 or Bw6 allotypes based on the sequence in this region (14). Whereas HLA-Bw4 molecules are broadly recognized by KIR3DL1(15), human KIRs capable of recognizing HLA-Bw6 molecules have not been identified. Similarly, HLA-C recognition by KIR2DL1 versus KIR2DL2/3 is governed by a single amino acid polymorphism at position 80. KIR2DL1 recognizes HLA-C group 2 (HLA-C2) molecules with a lysine at position 80, while KIR2DL2 and KIR2DL3 prefer HLA-C group 1 (HLA-C1) molecules with an asparagine at this position (16). Consistent with crystal structures revealing that KIRs contact their HLA class I ligands across C-terminal residues of the bound peptide (17-19), several studies have also shown that HLA class I-bound peptides can modulate these interactions (20-25).
There is considerable genetic evidence that KIR and HLA class I polymorphisms can influence the outcome of infection for viral pathogens, including HIV-1 (26, 27), hepatitis C virus (28), human papillomavirus (29) and cytomegalovirus (30). KIR and HLA class I genetics have also been implicated in the success of cancer immunotherapies and reproduction (31-35). However, studies to address the immunological mechanisms underlying these observations have been limited by the lack of a suitable animal model. Mice and other rodent species do not have KIRs, but instead express an expanded repertoire of Ly49 genes, which encode type II transmembrane proteins of the C-type lectin-like receptor family as polymorphic NK cell receptors (36). Moreover, as a consequence of the particularly rapid pace of KIR evolution (37-40), it is generally not possible to predict the specificity of KIRs in non-human primates on the basis of sequence comparisons with human KIRs. Thus, it has not been possible to investigate the regulation of NK cell responses through KIR-MHC class I interactions in species other than humans due to a lack of defined ligands for KIRs in non-human primate models.
Macaques are valuable animal models for HIV-1/AIDS (41) and for other human viral pathogens, including cytomegalovirus, Epstein-Barr virus and Kaposi’s sarcoma-associated herpesvirus (42-45). Characterization of macaque KIR and MHC class I genes has also yielded fascinating insights into the evolution of these loci in primates (46-49). Thus, knowledge of the underlying genetics and molecular interactions for KIR and MHC class I molecules of macaques is both of significant practical value for investigating NK cell responses in non-human primate models of viral disease and fundamental importance for comparative immunogenetics. In this review, we will focus on the immunogenetics and ligand specificity of macaque KIRs, with particular emphasis on the rhesus macaque (Macaca mulatta) as the best characterized of the macaque species.
Genetics of macaque KIRs
Despite considerable divergence in gene content and sequence, the KIR loci of macaques share a number of similarities with their human counterparts at the genomic level. Similar to humans, the KIR genes of macaques are arranged in a head-to-tail manner on chromosome 19 within the LRC (40). The KIR loci of macaques can also be divided into centromeric and telomeric clusters that exhibit extensive variation in the number of genes per haplotype as well as animal-to-animal variation in gene sequence (40, 45, 46, 50). Therefore, in order to understand macaque KIR genetics one must consider the number of genes encoded by a given animal, the identity of those genes and their allelic variation.
Nomenclature
KIR genes are named according to the domain structure of the receptors they encode. The first digit in the gene name indicates the number of Ig-like domains in the molecule, and the ‘D’ denotes ‘domain’. Following the D is either an ‘L’ for long cytoplasmic tail, ‘S’ for short cytoplasmic tail or ‘P’ for pseudogene. The final digits indicate the gene number. For example, the product of KIR3DL02 has three Ig-like domains, a long cytoplasmic tail, and is the second gene corresponding to this domain structure. Rhesus macaque KIRs are prefixed with ’Mamu’ (Macaca mulatta), as in Mamu-KIR3DS02. When comparing KIR genes between species, is important to note that a shared gene name goes not indicate an orthologous relationship. For example, although human KIR3DL1 and Mamu-KIR3DL01 encode molecules with a similar domain structure (three Ig-like domains and a long cytoplasmic tail) the gene number does not indicate sequence similarity or a common evolutionary origin. As might be expected for a developing field, the nomenclature for macaque KIRs has not been consistent across the literature. For this review, we will use Immuno-Polymporhism Database (IPD) conventions (51), and the standardized gene names widely adopted by recent publications in the field (47, 50, 52-54).
Based on sequence similarity, the KIR genes of apes and Old World monkeys can also be classified into broad phylogenetic lineages that are useful for understanding their evolutionary relationships among different species. Four KIR lineages are recognized in humans (I, II, III and V) that correspond to receptors differing in domain structure and ligand specificity; lineage I includes KIR2DL4, KIR2DL5A and KIR2DL5B, lineage II includes KIR3DL1/S1 and KIR3DL2, lineage III includes KIR2DS2, KIR2DL2/3, KIR2DS3/5, KIR2DP1, KIR2DL1, KIR3DP1, KIR2DS1 and KIR2DS4, and lineage V includes KIR3DL3 (55). The expanded KIR3DL/S genes of macaques and other Old World monkeys were originally classified as a fifth lineage (lineage IV) to reflect their sequence divergence and distinct phylogenetic clustering from human lineage II KIRs (56). However, we will follow the precedent of recent publications, which have dropped the lineage IV distinction in favor of grouping these genes with human lineage II KIRs (55, 57).
Gene content
The first studies to characterize macaque KIRs primarily relied on cDNA sequences (45, 58); however, one complete rhesus macaque haplotype as also been described at the genomic level (40). Later studies sequenced additional full- and partial-length KIR cDNAs, and used segregation analysis to infer the gene content of KIR haplotypes (46, 47, 49, 52). Although the organization of macaque KIR genes is still unclear, the most recent phylogenetic and segregation analyses support the existence of 22 KIR genes (46, 47, 50) (Table 1). As the body of KIR sequences has grown, most have been found to be similar to previously reported sequences, suggesting that the expressed KIR genes of macaques have been identified. Nevertheless, the number of distinct KIRs expressed per animal varies widely, ranging from 6-13 (46). It should also be noted that the vast majority of KIR sequences are derived from cDNA, and although many KIR pseudogenes have been identified, it is likely that others remain to be discovered.
Table 1.
KIR genes and number of alleles defined in rhesus macaques
| Gene | Alleles |
|---|---|
| Mamu-KIR1D | 2 |
| Mamu-KIR2DL04 | 20 |
| Mamu-KIR3DL01 | 28 |
| Mamu-KIR3DL02 | 8 |
| Mamu-KIR3DLW03 | 3 |
| Mamu-KIR3DL04 | 2 |
| Mamu-KIR3DL05 | 10 |
| Mamu-KIR3DL06 | 2 |
| Mamu-KIR3DL07 | 10 |
| Mamu-KIR3DL08 | 10 |
| Mamu-KIR3DL10 | 5 |
| Mamu-KIR3DL11 | 6 |
| Mamu-KIR3DL20 | 7 |
| Mamu-KIR3DS01 | 3 |
| Mamu-KIR3DS02 | 11 |
| Mamu-KIR3DS03 | 3 |
| Mamu-KIR3DS04 | 4 |
| Mamu-KIR3DS05 | 3 |
| Mamu-KIR3DS06 | 6 |
| Mamu-KIR3DS07 | 2 |
| Mamu-KIR3DSW08 | 9 |
| Mamu-KIR3DS09 | 5 |
Centromeric region
The centromeric end of the macaque KIR region is less variable than the telomeric end and comparatively gene poor. There are three KIR loci in this region: Mamu-KIR3DL20, -KIR1D and–KIR2DL04 (Fig. 1). In contrast to most KIR, these three genes have more limited polymorphism. It has therefore been speculated that their receptors may recognize conserved ligands, such as non-classical MHC molecules. However, no ligands have been identified so far the receptors encoded by these genes.
Fig 1. Macaque KIR domain structure and genomic organization.
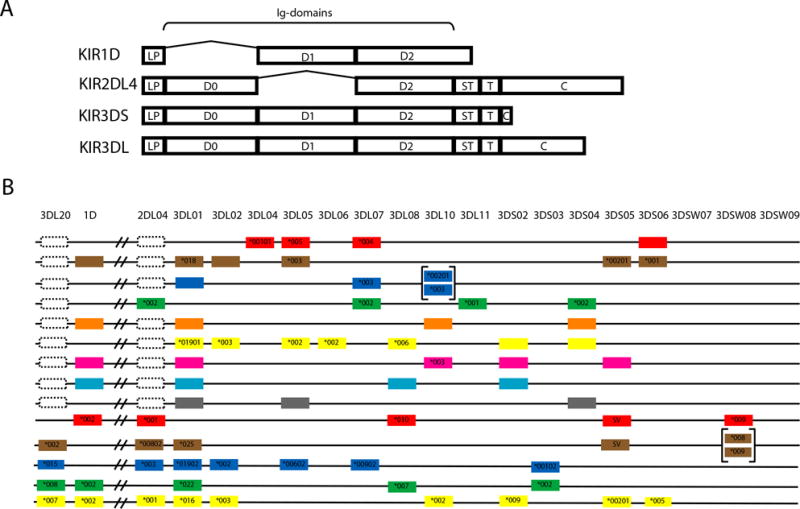
(A) Schematic representation of macaque KIR molecules showing domain structure. Domains are abbreviated as follows: leader peptide (LP), immunoglobulin-like domains (D0-D2), transmembrane region (T), stem (ST) and cytoplasmic region (C). Note: Mamu-KIR1D has a frameshift in the final third of the D2 domain, resulting in a novel domain and truncation at a final length of 244 amino acids. (B) KIR gene content from representative haplotypes observed in rhesus macaques. KIR genes are indicated along the top axis. The identity of the allele is indicated within the schematic boxes if it was determined. The physical map of gene order is arbitrary and brackets indicate gene duplication. Due to limitations in the technique used to generate the first 9 haplotypes, Mamu-KIR2DL04 could not be detected and dotted lines indicate the potential presence of this gene. While Mamu-KIR3DL20 was not detected in any of these haplotypes either, these were generated from cDNA and this KIR can be present as a pseudogene. Dotted lines indicate this possibility.
Mamu-KIR1D
Mamu-KIR1D encodes a single Ig-like domain and no cytoplasmic tail. While the nucleotide sequence of the transcript encodes two Ig-like domains and a cytoplasmic tail similar in length to Mamu-KIR2DL04, the translated amino acid sequence contains only one intact Ig-like domain (D1) and two thirds of the D2 Ig-like domain, which is disrupted by a frameshift mutation (45). Because of this frameshift, the translated protein instead has a novel 55 amino acid domain and truncates without a cytoplasmic tail (Fig. 1). Although the function of Mamu-KIR1D is presently unclear, it is hypothesized to be secreted (58). There is no human ortholog to Mamu-KIR1D; however, variants of human KIR2DS4 have also been described that lack a cytoplasmic tail due to a frameshift mutation and are also predicted to produce a soluble protein (59).
Mamu-KIR2DL04
Mamu-KIR2DL04 is present in the majority of published macaque KIR haplotypes (46). Its gene product shares approximately 84% sequence similarity with human KIR2DL4 at the amino acid level, although one notable difference is that Mamu-KIR2DL04 encodes two ITIM motifs in the cytoplasmic tail in contrast to one in human KIR2DL4 (45). Human KIR2DL4 binds HLA-G (60, 61), and has been shown to play a role in placental development. In macaques, the ortholog to HLA-G is a pseudogene (62). However, macaques express another non-classical molecule in the placenta (Mamu-AG) (63), which shares characteristics of HLA-G and could potentially serve as a ligand for Mamu-KIR2DL04.
Mamu-KIR3DL20
Mamu-KIR3DL20 encodes an inhibitory KIR that is present in most, but not all, macaque KIR haplotypes (46, 52). Several papers have reported macaque KIR2DL sequences similar to human KIR2DL5, which are identical to Mamu-KIR3DL20 transcripts, except that Mamu-KIR3DL20 encodes an additional Ig-like domain (40, 45). These Mamu-KIR2DL5 transcripts are believed to represent alternative splice variants, or an alternative allele, of Mamu-KIR3DL20 in which this exon is not present. The KIR2DL5/KIR3DL20 locus has been speculated to represent an evolutionary intermediate between KIR2DL and KIR3DL genes (40).
Telomeric region
The KIR loci at the telomeric end of the macaque LRC are particularly diverse with variable numbers of polymorphic lineage II KIR3DL/S genes on any given haplotype. As described above, these genes are most similar to human lineage II KIRs, but cluster as a distinct phylogenetic group sometimes referred to as lineage IV (40, 64). The diversity of these genes is one of the features that distinguish macaque and human KIR. Whereas humans have an expanded complement of lineage III KIRs that encode receptors for HLA-C, macaques and other Old World monkeys lack lineage III KIR and MHC-C genes (40, 45, 65). These species instead express an expanded repertoire of KIR3DL/S genes that mirrors the expansion of their MHC-A and –B genes. Accordingly, several MHC-A and –B molecules of macaques have been identified as KIR3DL/S ligands (53, 66, 67).
Mamu-KIR3DL/S genes
Phylogenetic and segregation analyses support the existence of ten Mamu-KIR3DL genes (Mamu-KIR3DL01, -KIR3DL02, -KIR3DLW03, -KIR3DL04, -KIR3DL05, -KIR3DL06, -KIR3DL07, -KIR3DL08, -KIR3DL10 and -KIR3DL11) and nine Mamu-KIR3DS genes (Mamu-KIR3DS01, -KIR3DS02, -KIR3DS03, -KIR3DS04, -KIR3DS05, -KIR3DS06, -KIR3DSW07, -KIR3DSW08 and -KIR3DSW09) in rhesus macaques (45, 47, 50, 52) (Table 1). Individual haplotypes are highly variable and may include additional gene duplications. Moreover, the Mamu-KIR3DL/S genes are characterized by substantial allelic diversity (45, 47, 50, 52). There is also extensive domain sharing among the lineage II KIRs, which is consistent with ancestral recombination and duplication events in the formation of these loci (50).
The Mamu-KIR3DS genes, which are presumed to encode activating KIRs, appear to have evolved from a recombination between ancestral KIR3DL and KIR2DL04 genes after the divergence of apes and Old World monkeys. Whereas exons 1-5 are most similar to extant Mamu-KIR3DL genes, exons 6-9 are most similar to Mamu-KIR2DL04 (45, 48). However, due to a deletion in exon 8 and a single nucleotide substitution in the splice donor of intron 8, Mamu-KIR3DS transcripts express receptors with truncated cytoplasmic tails (48). Thus, the Mamu-KIR3DS genes encode KIRs with 3 Ig-like domains followed by a stem, transmembrane and truncated cytoplasmic domain that lacks the pair of ITIMs present in Mamu-KIR2DL04. These receptors were originally designated KIR3DH, where ‘H’ denotes hybrid, to reflect their hybrid origins (45), but were later re-named KIR3DS in accordance with human nomenclature (47, 50). However, human and macaque KIR3DS genes are clearly not related.
Although Mamu-KIR3DS molecules share features with activating KIRs of humans at the protein level, including a charged residue in the transmembrane domain and short cytoplasmic domain that lacks inhibitory motifs, these features evolved independently. This is perhaps best reflected at the primary amino acid sequence level, where the transmembrane domain of Mamu-KIR3DS contains an arginine rather than the lysine residue found in human activating KIRs. The presence of an arginine in this domain suggests that Mamu-KIR3DS recruits the Fc receptor α chain instead of DAP12 to transduce activating signals (45), as has been demonstrated for human KIR2DL4 (68). Thus, the activating KIRs of humans and macaques evolved independently to have similar features.
In many species, pairs of activating and inhibitory KIRs are observed with highly similar Ig-like domains, with human KIR3DL1/KIR3DS1 being the best-studied example (26, 69-71). Macaques also have pairs of activating and inhibitory KIRs that encode similar Ig-like domains (48, 50). The best-matched pair of activating and inhibitory KIRs is Mamu-KIR3DLw03 and -KIR3DS05. Mamu-KIR3DL07 and -KIR3DSW09 also share two of three Ig-like domains. Although the functional significance of having inhibitory and activating KIRs with similar Ig-like domains is not fully understood, these observations further support the evolution of activating KIRs from inhibitory genes.
KIR genotypes and haplotypes
There is substantial diversity in the number of KIR genes expressed by a given animal and in the gene content of macaque KIR haplotypes. Macaque haplotypes contain 3-7 genes with an average of 11-12 expressed KIR genes per animal (47) (Fig. 1). In contrast to human KIRs, there are no framework genes in macaques, although Mamu-KIR3DL20, -KIR2DL04 and -KIR3DL01 are present on most haplotypes (46, 50, 52). Macaque haplotypes also do not conform to A and B haplotype groups, raising the possibility that they are subject to different selective pressures. The diversity of KIR haplotypes in macaques is therefore much greater than human haplotype diversity (47).
In addition to a lack of framework genes, there is a range of KIR gene frequency. Of the lineage II KIRs, Mamu-KIR3DL01, -KIR3DL02, -KIR3DL05, -KIR3DL07, -KIR3DL08, -KIR3DL10 and -KIR3DS02 are the most common, with at least one copy present in 50-85% of animals (50). Most of the remaining KIR genes were detected in 15-40% of animals. Note, because these frequencies are based on genotype, gene frequencies at the haplotype level will be lower. The KIR genes of rhesus macaques also have different frequencies for populations of distinct geographic origins. For instance, Mamu-KIR3DL04 is observed exclusively in Indian-origin animals, whereas Mamu-KIR3DL06 is primarily observed in animals of Chinese origin (47). Chinese-origin rhesus macaques typically also have one additional inhibitory KIR on average compared to animals of Indian or Burmese origin (47).
Genotyping approaches
Several approaches for KIR genotyping of macaques have been reported. The majority of published genotypes were determined by sequence-specific PCR using primers designed to target specific KIR genes (46, 52, 54). This approach is cost effective and allows relatively high throughput analysis, but typically does not provide allele-specific typing. Panels of microsatellite markers have also been used for KIR genotyping (49). While these markers can be highly informative in certain contexts, this indirect genotyping approach is typically lower in resolution than sequence-specific PCR (49). More recently, a number of groups have turned to methods based on next generation sequencing (50, 72). These approaches generally use primers to conserved regions of KIR genes in order to broadly amplify macaque KIRs and have the advantage of providing allele-specific information.
Other macaque species
In addition to rhesus macaques, KIR sequences have also been reported for pigtailed macaques (M. nemestrina) and cynomolgus macaques (M. fascicularis). Phylogenetic analyses indicate that most of these sequences cluster with rhesus macaque KIRs (49, 66), suggesting that macaques share an orthologous repertoire of KIR genes that was formed prior to the divergence of these species from a common ancestor approximately 3.5 million years ago (73). The KIR genes of Mauritian cynomolgus macaques (MCMs) are the best characterized due to their simplified genetics. A small founder population of cynomolgus macaques, estimated to be as few as four animals, was introduced to the island of Mauritius approximately 500 years ago (74). MCMs have since expanded to form a population of more than 20,000 animals. Due to their limited genetic diversity, it was possible to characterize all of the KIR genes and haplotypes in this unique population. Microsatellite typing was initially used to define eight KIR haplotypes (49), which is comparable to the seven MHC haplotypes previously identified in these animals (75). The KIRs expressed by animals homozygous for each haplotype (except two for which only heterozygotes were available) were sequenced, identifying 3-8 KIR genes per haplotype. Although none of these sequences were identical to previously reported KIR alleles from other species, all are phylogenetically related to putative KIR genes of rhesus macaques (49). These results are therefore consistent with the recent divergence of cynomolgus and rhesus macaques from a common ancestor approximately 1.9 million years ago and the similarity in their MHC class I genes (73, 76). Comparisons with the genetics of KIRs in MCMs have also helped to define and classify the KIR genes of rhesus macaques (47, 50). MCMs may be of special interest for studying the role of KIR-MHC class I interactions in disease pathogenesis, because unlike any other model, the limited number of KIR and MHC haplotypes in this population makes it possible to build cohorts of animals that are KIR and/or MHC identical.
MHC class I ligands of macaque KIRs
Although the ligands for macaque KIRs remain poorly defined, MHC class I ligands for a few of the lineage II KIRs have recently been identified (53, 54, 66, 77). Similar to HLA-A and –B recognition by human lineage II KIRs, these KIRs recognize Mamu-A and –B molecules and differentiate ligands on the basis of α1 domain sequences corresponding to Bw4 and Bw6 epitopes. However, in addition to having KIRs specific for Bw4 ligands, macaques also express KIRs that are capable of recognizing MHC ligands with Bw6 epitopes.
Several Mamu-A molecules were initially identified as ligands for three inhibitory KIRs (Mamu-KIR3DLW03, -KIR3DL05, -KIR3DL11) and one activating KIR (Mamu-KIR3DS05) by staining MHC class I-transfected cells with soluble KIR-immunoglobulin Fc domain (KIR-Fc) fusion proteins (77). Significant increases in staining over background were observed for Mamu-KIR3DLW03*004 bound to cells expressing Mamu-A1*001:01, -A1*008:01 and –A1*011:01, for Mamu-KIR3DL05*007 bound to cells expressing Mamu-A1*001:01, -A1*002:01 and –A3*13:11, for Mamu-KIR3DL11*003 bound to cells expressing Mamu-A1*008:01, and for Mamu-KIR3DS05*003 bound to cells expressing Mamu-A1*001:01 and –A1*011:01 (Table 2). The highest avidity interactions were for Mamu-KIR3DLW03*004/-A1*001:01 and Mamu-KIR3DL05*007/-A3*13:11, which reflect KIR binding to Bw4 versus Bw6 ligands respectively. Reciprocal exchange of the Bw4 and Bw6 residues of Mamu-A1*001:01 and Mamu-A3*13:11 revealed that Mamu-KIR3DLW03*004 binding is strictly dependent on the presence of the Bw4 epitope, whereas Mamu-KIR3DL05*007 can bind to molecules with either epitope, but prefers ligands such as Mamu-A1*002 that contain a Bw6 variant with asparagine at position 77 (77). In the case of Mamu-KIR3DS05*003, the binding of this activating receptor to Mamu-A1*001:01 and –A1*011:01 was surprising given the difficulty in demonstrating MHC class I binding to activating KIRs. However, the avidity of these interactions was especially low, which may be analogous to reports of low avidity HLA class I binding to human activating KIRs (78, 79).
Table 2.
MHC class I ligands of macaque KIRs
| KIR | Ligands | Specificity | KIR-Specific Reagents | References |
|---|---|---|---|---|
| Mamu-KIR3DL01 | Mamu-B*007:01, -B*041:01, -B*058:02, -B*065:01 | Bw4 | NKVFS1 (D233 allotypes) | (53) |
| Mamu-KIR3DLW03 | Mamu-A1*001:01, -A1*008:01, -A1*011:01 | Bw4 | 2H9 | (77, 84) |
| Mamu-KIR3DL05 | Mamu-A1*001:01, -A1*002:01, -A3*13:11 | Bw4 & Bw6 | 2H5, Mamu-A1*002-Gag GY9 | (54, 77, 84) |
| Mamu-KIR3DL11 | Mamu-A1*008:01 | undefined | none | (77) |
| Mamu-KIR3DS05 | Mamu-A1*001:01, -A1*011:01 | undefined | 2H9 | (77, 84) |
| KIR049-4 | Mane-A1*082, -A1*084 | Bw4 & Bw6 | Mane-A1*082-Gag DI9 | (66) |
A concurrent study identified Mamu-A1*002 as ligand for Mamu-KIR3DL05 and demonstrated that this interaction is strongly influenced by Mamu-A1*002-bound peptides (54). Mamu-A1*002 tetramers folded with certain SIV peptides, but not others, bound to primary NK cells and to transfected cells expressing multiple allotypes of Mamu-KIR3DL05 (Table 2). A comparison of the binding of seven Mamu-KIR3DL05 allotypes to four different tetramers, each representing a different Mamu-A1*002-restricted CD8+ T cell epitope (Gag GY9, Env RY8, Nef YY9 or Vif IW9), revealed that most allotypes bound with highest avidity to Mamu-A1*002 in complex with the Gag GY9 peptide, followed by the Env RY8 and Nef YY9 peptides (54). In the case of Gag GY9, the interaction was strong enough to directly observe tetramer staining of unstimulated primary NK cells isolated from peripheral blood (54). However, none of the Mamu-KIR3DL05 allotypes bound to Mamu-A1*002 folded with the Vif IW9 peptide, even under conditions of protein over-expression in transfected cells. This study therefore identified SIV peptides that both stabilize and disrupt Mamu-A1*002 binding to Mamu-KIR3DL05.
The identification of SIV peptides that stabilize MHC class I binding to an inhibitory KIR suggests an intriguing mechanism of immune evasion. Mamu-A1*002 and –KIR3DL05 are both commonly expressed in Indian origin rhesus macaques at frequencies of approximately 20% and 40% (54, 80), respectively, and might therefore be expected to exert selective pressure on virus replication in a significant percentage of animals. Moreover, the SIV peptides that enhance Mamu-A1*002 binding to Mamu-KIR3DL05 (Gag GY9, Env RY8 and Nef YY9) correspond to immunodominant CD8+ T cell epitopes that are bound with particularly high affinity by Mamu-A1*002 (81), suggesting that these peptides are abundantly presented on the surface of virus-infected cells. Thus, SIV may have acquired changes in epitopes that stabilize Mamu-A1*002 interactions with Mamu-KIR3DL05 to prevent the elimination of virus-infected cells by NK cells expressing this KIR. This possibility is consistent with the finding that HIV-1 is under selective pressure to acquire changes in KIR2DL2+ individuals, which increase KIR2DL2 binding to virus-infected cells and impair the ability of NK cells to suppress virus replication in vitro (82). By acquiring changes in viral epitopes that stabilize the binding of common MHC class I molecules to inhibitory KIRs, immunodeficiency viruses, and possibly other viral pathogens, may reduce the susceptibility of infected cells to certain NK cell subsets as a mechanism of immune evasion.
Polymorphisms in the D0 and D1 domains of Mamu-KIR3DL05 accounted for differences in the avidity and peptide preference of binding to Mamu-A1*002 (54). For one allotype, a shift in peptide preference mapped to a cluster of amino acids in a surface-exposed loop of the D1 domain. Whereas most Mamu-KIR3DL05 allotypes preferentially bound to Mamu-A1*002 folded with Gag GY9, mmKIR3D05x (did not receive an IPD designation) only bound to Mamu-A1*002 folded with Nef YY9. Reciprocal exchange of six amino acids corresponding to D1 residues 164-170 of mmKIR3D05x and Mamu-KIR3DL05*008 switched the specificity of the resulting recombinants for Mamu-A1*002-bound peptides (54); an observation consistent with the three-dimensional structure of KIR3DL1 in complex with HLA-B*57 showing that these residues coincide with a loop that contacts the MHC class I α1-domain adjacent to C-terminal residues of the bound peptide (Fig. 2) (19). Interestingly, sequence comparisons of mmKIR3DL05 with other rhesus macaque KIRs revealed that, whereas exons 5-9 are most similar to Mamu-KIR3DL05, exons 1-4 are identical to Mamu-KIR3DS02 (54). Thus, the unique D1 domain of mmKIR3D05x appears to be the result of a recombination event between Mamu-KIR3DS02 and -KIR3DL05. These observations provide a glimpse into the role of domain shuffling and D1 polymorphisms in shaping the selectivity of KIRs for MHC class I-bound peptides.
Fig. 2. Polymorphisms in the D1 domain of Mamu-KIR3DL05 account for preferential binding to Mamu-A1*002-bound peptides.
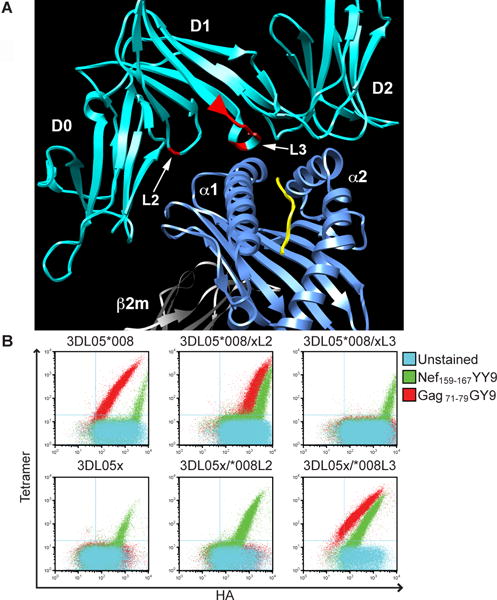
(A) Residues corresponding to surface exposed loops of the D1 domain (L2 and L3) that differ between Mamu-KIR3DL05*008 and mmKIR3DL05x (red) were mapped onto the three-dimensional crystal structure of KIR3DL1 (cyan) bound to HLA-B*57 (blue) (19). The HLA-B*57-bound peptide is indicated in yellow and the positions of the D0, D1 and D2 domains of KIR3DL1 relative to the α1 and α2 domains of HLA-B*57 are labeled. (B) Jurkat cells expressing L2 and L3 recombinants of Mamu-KIR3DL05*008 and mmKIR3DL05x were stained with Mamu-A1*002 tetramers folded with the SIV Gag GY9 and Nef YY9 peptides and an antibody to the HA tag engineered into the D0 domain to confirm cell surface expression (54).
Another inhibitory KIR of rhesus macaques, Mamu-KIR3DL01, was recently shown to recognize MHC class ligands with a Bw4 motif (53). The identification of ligands for this KIR was facilitated by the discovery of a monoclonal antibody to human KIR2D molecules that cross-reacts with allotypes of Mamu-KIR3DL01 that contain an aspartic acid residue at position 233 (D233) (Table 2) (53). This antibody was used to isolate Mamu-KIR3DL01+ NK cells and show that their cytolytic activity is suppressed by target cells expressing Mamu-B*007:01, -B*041:01, -B*058:02 or –B*065:01 (Table 2) (53). Although the Bw4 epitope of these molecules was necessary for Mamu-KIR3DL01 recognition, it was not sufficient, since Mamu-B*017:01, which also contains a Bw4 motif, failed to suppress Mamu-KIR3DL01+ NK cell responses. However, inhibition of these NK cells could be restored by replacing three amino acids of Mamu-B*017:01 (residues 76, 142 and 149) with the corresponding residues of other Mamu-Bw4 ligands at sites predicted to contact Mamu-KIR3DL01 based on the KIR3DL1-HLA-B*57 structure (53). Thus, Mamu-KIR3DL01 and human KIR3DL1 have similar specificities for Bw4 ligands. Yet, despite recognition of similar ligands and a coincidental similarity in nomenclature, Mamu-KIR3DL01 and KIR3DL1 are not othologous and share fewer than half of the residues predicted to contact MHC class I molecules based on the KIR3DL1-HLA-B*57 crystal structure (Fig. 3). These observations reveal remarkable conservation of Bw4 recognition despite the divergence of KIRs since humans and macaques last shared a common ancestor approximately 25 million years ago (83).
Fig. 3. Divergence of the surfaces of Mamu-KIR3DL01 and human KIR3DL1 predicted to contact Bw4 ligands.
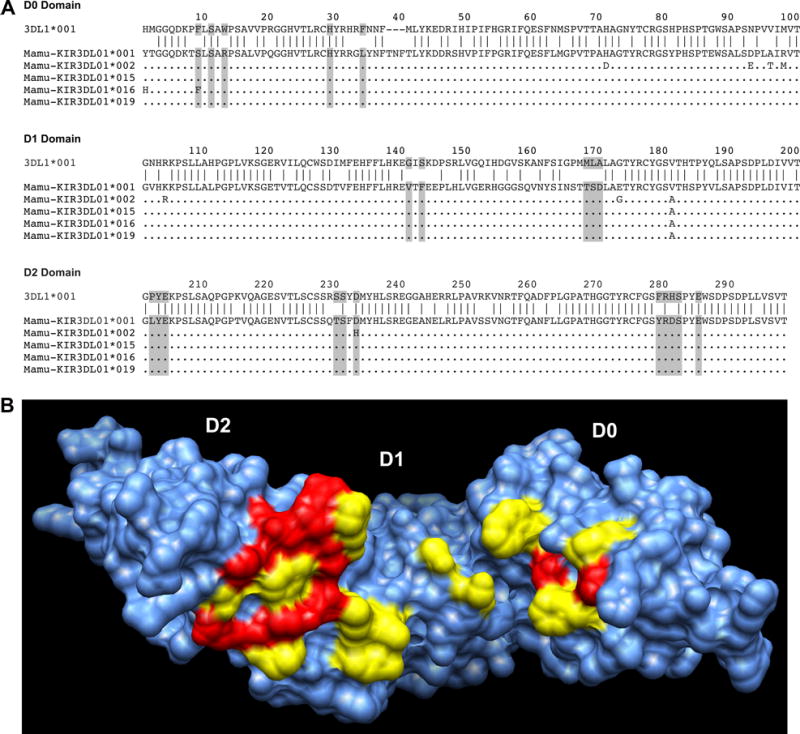
(A) Alignment of five allotypes of Mamu-KIR3DL01 to human KIR3DL1 with residues predicted to contact MHC class I ligands based on the KIR3DL1-HLA-B*57 crystal structure shaded in gray (19, 53). (B) Surface projection of human KIR3DL1 with HLA-B*57-contact residues that are conserved in Mamu-KIR3DL01*001 indicated in red and residues that differ indicated in yellow (53). Copyright 2014. The American Association of Immunologists, Inc.
Mamu-KIR3DL01 is also one of the most polymorphic and commonly expressed KIRs in the rhesus macaque. At least 28 different alleles of Mamu-KIR3DL01 have been identified so far and this gene is present in approximately 85-95% of Indian origin animals (46, 47, 50, 52). The diversity of Mamu-KIR3DL01 therefore mirrors the complexity of the highly polymorphic Mamu-B genes. Moreover, the prevalence of Mamu-KIR3DL01 suggests that its gene product may participate in the regulation of NK cell activity in a majority of animals. Hence, definition of the ligand specificity of Mamu-KIR3DL01, together with the identification of a commercially available antibody that cross-reacts with most Mamu-KIR3DL01 allotypes, is particularly valuable for investigating NK cell responses in the rhesus macaque.
Direct tetramer staining of peripheral blood NK cells also led to the identification of MHC class I ligands for an inhibitory KIR of pigtailed macaques. Mane (Macaca nemestrina)-A1*082 and –A1*084 tetramers folded with SIV peptides stained primary NK cells and transfected cell lines expressing KIR049-4 (66). Although phylogenetic analysis suggests that KIR049-4 is most similar to rhesus macaque Mamu-KIR3DLW03*004, which binds to Mamu-A1*001:01 in a Bw4-dependent manner (77), Mane- A1*082 and –A1*084 both contain a Bw6 motif (66). However, additional characterization with a panel of HLA class I tetramers revealed that KIR049-4 also binds to Bw4, Bw6 and non-Bw4/Bw6 molecules, and that these interactions are sensitive to HLA-bound peptides (66). KIR049-4 therefore exhibits a degenerate, yet peptide-dependent, pattern of MHC class I recognition.
These features appear to be shared by other non-human primate KIRs that recognize Bw6 ligands. Similar to KIR049-4, Mamu-KIR3DL05 and a previously described inhibitory KIR of chimpanzees exhibit dual specificity for Bw4 and Bw6 ligands (39, 54, 66, 77). The unusually high avidity of Mamu-KIR3DL05 and KIR049-4 for MHC class I tetramers loaded with specific peptides suggests that Bw6 recognition may involve greater participation of MHC class I bound-peptides (54, 66). These features contrast with the exclusive recognition of Bw4 molecules and a less prominent role for peptides in interactions with human KIR3DL1 and Mamu-KIR3DL01 (15, 53). Thus, although the molecular determinants of Bw6 recognition by non-human primate KIRs have not been defined, Bw6 recognition appears to be associated with more promiscuous MHC class I interactions and a greater dependence on MHC class I-bound peptides.
Macaque KIR-specific reagents
In addition to defining the MHC class I ligands for macaque KIRs, functional studies to assess the role of these receptors in models of infectious disease will require reagents for differentiating these KIRs on primary NK cells. As described above, a few KIR-specific reagents have been identified serendipitously, including Mamu-A1*002 and Mane-A1*082 tetramers, which bind with sufficiently high avidity to stain Mamu-KIR3DL05 and KIR049-4 on primary, unstimulated NK cells (54, 66), and the anti-human KIR2D-specific antibody NKVFS1, which binds to D233 allotypes of Mamu-KIR3DL01 (Table 2) (53). A handful of monoclonal antibodies to rhesus macaque KIRs have also been generated by immunizing mice with soluble KIR-immunoglobulin Fc domain fusion proteins (84). Characterization of these antibodies identified clones with varying specificities. Whereas one clone (1C7) was broadly reactive with all of the Mamu-KIR3D proteins tested, two others (2H5 and 2H9) where more specific. 2H5 bound only to Mamu-KIR3DL05, while 2H9 bound to Mamu-KIR3DLW03 and weakly to Mamu-KIR3DS05 (Table 2) (84). Another clone (2H3) exhibited an intermediate pattern of reactivity against Mamu-KIR3DSW08, -KIR3DS07 and –KIR3DL07. Phenotypic characterization of rhesus macaque lymphocytes using these antibodies confirmed a variegated pattern of KIR expression on NK and T cell subsets similar to the distribution of KIR on human lymphocytes (85).
KIR polymorphisms in SIV pathogenesis
Investigation of the role of KIRs in non-human primate models of infectious disease has so far been limited to studies of SIV pathogenesis in rhesus macaques. One of the first studies to investigate KIR polymorphisms in SIV-infected macaques identified a single nucleotide polymorphism in exon 4, which corresponds to a histidine versus glutamine polymorphism the D1 domain (Q138D) that is over-represented among animals with high viral loads (86). In a cohort of 38 SIV-infected rhesus macaques, the Q138 polymorphism was present in 85% of animals with high viral loads in plasma (>106 viral RNA copies/ml) versus 43% of animals with low plasma viral loads (<105 viral RNA copies/ml) (86). This polymorphism was later shown to be a marker for Mamu-KIR3DL05 (54). It is therefore tempting to speculate that the stabilization of Mamu-KIR3DL05 binding to Mamu-A1*002, or to another MHC class I ligand, by SIV peptides may have facilitated virus replication in some of these animals by suppressing Mamu-KIR3DL05+ NK cell responses.
A more recent study applied next generation sequencing technology to obtain a comprehensive data set for KIR and MHC class I transcripts in 52 SIV-infected rhesus macaques (72). Mamu-KIR3DL02 and –KIR3DSW08 were associated with low set-point viral loads in plasma (<105 viral RNA copies/ml), whereas Mamu-KIR3DS02 was associated with high plasma viral loads (>105 viral RNA copies/ml) (72). Epistatic interactions were also reported for Mamu-KIR3DL05, -KIR3DS05 and –KIR3DL10 in the presence of Mamu-B*012 or in the absence of Mamu-A1*001 (72). This study represents the most comprehensive analysis of KIR and MHC class I associations with viral loads in SIV-infected macaques to date. However, these genetic associations are difficult to interpret due to our limited understanding of the MHC class I ligands for macaque KIRs. Indeed, the ligands for each of the KIRs associated with high or low viral loads in this study remain undefined.
Viral load differences in SIV-infected macaques have also been attributed to variation in the copy number of activating Mamu-KIR3DS genes (87). Using a quantitative real-time PCR assay, the number of Mamu-KIR3DS genes in rhesus macaques was found to vary from 0 to 12 (median 4.11) copies per diploid genome (87). Evaluation of a cohort of 57 SIV-infected rhesus macaques for plasma viral load associations with Mamu-KIR3DS copy number variation (CNV) reported that significantly lower peak viral loads were associated with high Mamu-KIR3DS CNV, but only for animals that were negative for Mamu-A1*001 and positive for restrictive TRIM5 alleles (87). Regardless of Mamu-A1*001 or TRIM5 genotype, no significant differences were detectable in set-point viral loads during chronic infection. Although Mamu-A1*001 has been associated with lower set-point viral loads in SIV-infected animals, this MHC class I allele has no effect on peak viremia during acute infection (88). It is therefore unclear how it could have masked the effects of Mamu-KIR3DS CNV on peak viral loads. It is also unclear how polymorphisms in the TRIM5 gene, which encodes a post-entry block to virus infection with little or no effect on the strain of SIV used in this study (89, 90), could have modified the effects of Mamu-KIR3DS. Given that the conclusions of this study are based on comparisons of smaller groups of animals after parsing according to Mamu-A1*001 and TRIM5 genotype, which were not corrected for multiple comparisons, the reported association of Mamu-KIR3DS CNV with peak viral loads is not very robust and potentially reflects a statistical anomaly.
Concluding remarks
Macaques afford a potentially powerful model for the functional study of KIRs and NK cells. Genetic characterization of macaque KIRs has defined the most common macaque KIR genes and created a framework within which to describe macaque KIR genetics. The most striking feature of macaque KIRs is the expansion of the lineage II loci, where we observe ten Mamu-KIR3DL and nine Mamu-KIR3DS genes. Recent studies have begun to define the MHC class I ligands of macaque KIRs; however, more work is needed. Current evidence suggests that while Bw4 and Bw6 epitopes are important for KIR-MHC class I interactions in macaques, these epitopes do not necessarily delineate ligands. Evidence suggests that at least some macaque KIRs may have more promiscuous MHC class I binding profiles, and that some of these interactions are highly dependent on MHC class I-bound peptides. MHC class I tetramers have proven useful in the study of macaque KIRs, and in fact the ligands for two KIRs were identified from unusual patterns of tetramer staining. Even so, reagents are not available for differentiating most KIRs on primary macaque lymphocytes. Thus, while the identification of a handful of MHC class I ligands for macaque KIRs has opened the door for animal studies to address the role of these receptors in shaping immune responses, continued definition of the ligands for macaque KIRs, together with development of additional macaque KIR-specific antibodies, is needed to take full advantage of the potential of macaques for investigating the influence of KIR and MHC class I immunogenetics in models of human disease.
Acknowledgments
This work was supported by Public Health Service Grants AI095098, AI098485 and OD011106. DTE is an Elizabeth Glaser Scientist of the Elizabeth Glaser Pediatric AIDS Foundation.
References
- 1.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 2.Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5:201–214. doi: 10.1038/nri1570. [DOI] [PubMed] [Google Scholar]
- 3.Kim S, et al. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc Natl Acad Sci U S A. 2008;105:3053–3058. doi: 10.1073/pnas.0712229105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yokoyama WM, Kim S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol Rev. 2006;214:143–154. doi: 10.1111/j.1600-065X.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 5.Valiante NM, et al. Functionally and structurally distinct NK cell receptor repertoires in the peripheral blood of two human donors. Immunity. 1997;7:739–751. doi: 10.1016/s1074-7613(00)80393-3. [DOI] [PubMed] [Google Scholar]
- 6.Huard B, Fruh K. A role for MHC class I down-regulation in NK cell lysis of herpes virus-infected cells. Eur J Immunol. 2000;30:509–515. doi: 10.1002/1521-4141(200002)30:2<509::AID-IMMU509>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 7.Falk CS, Mach M, Schendel DJ, Weiss EH, Hilgert I, Hahn G. NK cell activity during human cytomegalovirus infection is dominated by US2-11-mediated HLA class I down-regulation. J Immunol. 2002;169:3257–3266. doi: 10.4049/jimmunol.169.6.3257. [DOI] [PubMed] [Google Scholar]
- 8.Cohen G, et al. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–671. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard JM. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 10.Collins K, Chen B, Kalams S, Walker B, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 11.Porgador A, Mandelboim O, Restifo NP, Strominger JL. Natural killer cell lines kill autologous beta2-microglobulin-deficient melanoma cells: implications for cancer immunotherapy. Proc Natl Acad Sci U S A. 1997;94:13140–13145. doi: 10.1073/pnas.94.24.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alter G, et al. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J Exp Med. 2007;204:3027–3036. doi: 10.1084/jem.20070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrington M, Norman P. The KIR Gene Cluster. National Center for Biotechnology Information (US); 2003. p. 48. [Google Scholar]
- 14.Ayers J, Cresswell P. HLA-B specificities and w4, w6 specificities are on the same polypeptide. Eur J Immunol. 1976;6:794–799. doi: 10.1002/eji.1830061108. [DOI] [PubMed] [Google Scholar]
- 15.Gumperz JE, Litwin V, Phillips JH, Lanier LL, Parham P. The Bw4 public epitope of HLA-B molecules confers reactivity with natural killer cell clones that express NKB1, a putative HLA receptor. J Exp Med. 1995;181:1133–1144. doi: 10.1084/jem.181.3.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winter CC, Gumperz JE, Parham P, Long EO, Wagtmann N. Direct binding and functional transfer of NK cell inhibitory receptors reveal novel patterns of HLA-C allotype recognition. J Immunol. 1998;161:571–577. [PubMed] [Google Scholar]
- 17.Boyington JC, Motyka SA, Schuck P, Brooks AG, Sun PD. Crystal structure of an NK cell immunoglobulin-like receptor in complex with its class I MHC. Nature. 2000;405:537–543. doi: 10.1038/35014520. [DOI] [PubMed] [Google Scholar]
- 18.Fan QR, Long EO, Wiley DC. Crystal structure of the human natural killer cell inhibitory receptor KIR2DL1-HLA-Cw4 complex. Nat Immunol. 2001;2:452–460. doi: 10.1038/87766. [DOI] [PubMed] [Google Scholar]
- 19.Vivian JP, et al. Killer cell immunoglobulin-like receptor 3DL1-mediated recognition of human leukocyte antigen B. Nature. 2011;479:401–405. doi: 10.1038/nature10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malnati MS, et al. Peptide specificity in the recognition of MHC class I by natural killer cell clones. Science. 1995;267:1016–1018. doi: 10.1126/science.7863326. [DOI] [PubMed] [Google Scholar]
- 21.Peruzzi M, Parker KC, Long EO, Malnati MS. Peptide sequence requirements for the recognition of HLA-B*2705 by specific natural killer cells. J Immunol. 1996;157:3350–3356. [PubMed] [Google Scholar]
- 22.Zappacosta F, Borrego F, Brooks AG, Parker KC, Coligan JE. Peptides isolated from HLA-Cw*0304 confer different degrees of protection from natural killer cell-mediated lysis. Proc Natl Acad Sci U S A. 1997;94:6313–6318. doi: 10.1073/pnas.94.12.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajagopalan S, Long EO. The direct binding of a p58 killer cell inhibitory receptor to human histocompatibility leukocyte antigen (HLA)-Cw4 exhibits peptide selectivity. J Exp Med. 1997;185:1523–1528. doi: 10.1084/jem.185.8.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansasuta P, et al. Recognition of HLA-A3 and HLA-A11 by KIR3DL2 is peptide-specific. Eur J Immunol. 2004;34:1673–1679. doi: 10.1002/eji.200425089. [DOI] [PubMed] [Google Scholar]
- 25.Thananchai H, et al. Allele-specific and peptide-dependent interactions between KIR3DL1 and HLA-A and HLA-B. J Immunol. 2007;178:33–37. doi: 10.4049/jimmunol.178.1.33. [DOI] [PubMed] [Google Scholar]
- 26.Martin MP, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–434. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 27.Martin MP, et al. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet. 2007;39:733–740. doi: 10.1038/ng2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khakoo SI, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 29.Carrington M, et al. Hierarchy of resistance to cervical neoplasia mediated by combinations of killer immunoglobulin-like receptor and human leukocyte antigen loci. J Exp Med. 2005;201:1069–1075. doi: 10.1084/jem.20042158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen C, et al. Activating KIR genes are associated with CMV reactivation and survival after non-T-cell depleted HLA-identical sibling bone marrow transplantation for malignant disorders. Bone Marrow Transplant. 2006;38:437–444. doi: 10.1038/sj.bmt.1705468. [DOI] [PubMed] [Google Scholar]
- 31.Cooley S, et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009;113:726–732. doi: 10.1182/blood-2008-07-171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooley S, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116:2411–2419. doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delgado DC, et al. Genotypes of NK cell KIR receptors, their ligands, and Fcgamma receptors in the response of neuroblastoma patients to Hu14.18-IL2 immunotherapy. Cancer Res. 2010;70:9554–9561. doi: 10.1158/0008-5472.CAN-10-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong S, et al. Maternal uterine NK cell-activating receptor KIR2DS1 enhances placentation. J Clin Invest. 2013;123:4264–4272. doi: 10.1172/JCI68991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakimuli A, et al. A KIR B centromeric region present in Africans but not Europeans protects pregnant women from pre-eclampsia. Proc Natl Acad Sci U S A. 2015;112:845–850. doi: 10.1073/pnas.1413453112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokoyama WM, Kehn PJ, Cohen DI, Shevach EM. Chromosomal location of the Ly-49 (A1, YE1/48) multigene family, genetic association with the NK1.1 antigen. J Immunol. 1990;145:2353–2358. [PubMed] [Google Scholar]
- 37.Abi-Rached L, et al. A small, variable, and irregular killer cell Ig-like receptor locus accompanies the absence of MHC-C and MHC-G in gibbons. J Immunol. 2010;184:1379–1391. doi: 10.4049/jimmunol.0903016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guethlein LA, Flodin LR, Adams EJ, Parham P. NK cell receptors of the orangutan (Pongo pymaeus): A pivotal species for tracking the coevolution of killer cell Ig-like receptors with MHC-C. J Immunol. 2002;169:220–229. doi: 10.4049/jimmunol.169.1.220. [DOI] [PubMed] [Google Scholar]
- 39.Khakoo SI, et al. Rapid evolution of NK cell receptor systems demonstrated by comparison of chimpanzee and humans. Immunity. 2000;12:687–698. doi: 10.1016/s1074-7613(00)80219-8. [DOI] [PubMed] [Google Scholar]
- 40.Sambrook JG, et al. Single haplotype analysis demonstrates rapid evolution of the killer immunoglobulin-like receptor (KIR) loci in primates. Genome Res. 2005;15:25–35. doi: 10.1101/gr.2381205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hatziioannou T, Evans DT. Animal models for HIV/AIDS research. Nature reviews Microbiology. 2012;10:852–867. doi: 10.1038/nrmicro2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lockridge KM, Sequar G, Zhou SS, Yue Y, Mandell CP, Barry PA. Pathogenesis of experimental rhesus cytomegalovirus infection. J Virol. 1999;73:9576–9583. doi: 10.1128/jvi.73.11.9576-9583.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moghaddam A, Rosenzweig M, Lee-Parritz D, Annis B, Johnson RP, Wang F. An animal model for acute and persistent Epstein-Barr virus infection. Science. 1997;276:2030–2033. doi: 10.1126/science.276.5321.2030. [DOI] [PubMed] [Google Scholar]
- 44.Desrosiers RC, et al. A herpesvirus of rhesus monkeys related to the human Kaposi’s sarcoma-associated herpesvirus. J Virol. 1997;71:9764–9769. doi: 10.1128/jvi.71.12.9764-9769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hershberger KL, Shyam R, Miura A, Letvin NL. Diversity of the killer cell Ig-like receptors of rhesus monkeys. J Immunol. 2001;166:4380–4390. doi: 10.4049/jimmunol.166.7.4380. [DOI] [PubMed] [Google Scholar]
- 46.Blokhuis JH, Wiel MKvd, Doxiadis GG, Bontrop RE. The mosaic of KIR haplotypes in rhesus macaques. Immunogenetics. 2010;62:295–306. doi: 10.1007/s00251-010-0434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blokhuis JH, van der Wiel MK, Doxiadis GG, Bontrop RE. The extreme plasticity of killer cell Ig-like receptor (KIR) haplotypes differentiates rhesus macaques from humans. Eur J Immunol. 2011;41:2719–2728. doi: 10.1002/eji.201141621. [DOI] [PubMed] [Google Scholar]
- 48.Blokhuis JH, Doxiadis GGM, Bontrop RE. A splice mutation converts an inhibitory killer cell Ig-like receptor into an activating one. Mol Immunol. 2009;46:640–648. doi: 10.1016/j.molimm.2008.08.270. [DOI] [PubMed] [Google Scholar]
- 49.Bimber BN, Moreland AJ, Wiseman RW, Hughes AL, O’Connor DH. Complete characterization of killer Ig-like receptor (KIR) haplotypes in Mauritian cynomolgus macaques: Novel insights into nonhuman primate KIR gene content and organization. J Immunol. 2008;181:6301–6308. doi: 10.4049/jimmunol.181.9.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moreland AJ, et al. Characterization of killer immunoglobulin-like receptor genetics and comprehensive genotyping by pyrosequencing in rhesus macaques. BMC genomics. 2011;12:295. doi: 10.1186/1471-2164-12-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robinson J, Mistry K, McWilliam H, Lopez R, Marsh SGE. IPD-the immuo polymorphism database. Nucleic Acids Res. 2010;38:D863–D869. doi: 10.1093/nar/gkp879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kruse PH, Rosner C, Walter L. Characterization of rhesus macaque KIR genotypes and haplotypes. Immunogenetics. 2010;62:281–293. doi: 10.1007/s00251-010-0433-4. [DOI] [PubMed] [Google Scholar]
- 53.Schafer JL, et al. KIR3DL01 recognition of Bw4 ligands in the rhesus macaque: maintenance of Bw4 specificity since the divergence of apes and Old World monkeys. J Immunol. 2014;192:1907–1917. doi: 10.4049/jimmunol.1302883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Colantonio AD, et al. KIR polymorphisms modulate peptide-dependent binding to an MHC class I ligand with a Bw6 motif. PLoS Pathog. 2011;7:e1001316. doi: 10.1371/journal.ppat.1001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parham P, Moffett A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat Rev Immunol. 2013;13:133–144. doi: 10.1038/nri3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rajalingam R, Parham P, Abi-Rached L. Domain shuffling has been the main mechanism forming new hominoid killer cell Ig-like receptors. J Immunol. 2004;172:356–369. doi: 10.4049/jimmunol.172.1.356. [DOI] [PubMed] [Google Scholar]
- 57.Guethlein LA, Older Aguilar AM, Abi-Rached L, Parham P. Evolution of killer cell Ig-like receptor (KIR) genes: definition of an orangutan KIR haplotype reveals expansion of lineage III KIR associated with the emergence of MHC-C. J Immunol. 2007;179:491–504. doi: 10.4049/jimmunol.179.1.491. [DOI] [PubMed] [Google Scholar]
- 58.LaBonte ML, Hershberger KL, Korber B, Letvin NL. The KIR and CD94/NKG2 families of molecules in the rhesus monkey. Immunol Rev. 2001;183:25–40. doi: 10.1034/j.1600-065x.2001.1830103.x. [DOI] [PubMed] [Google Scholar]
- 59.Maxwell LD, Wallace A, Middleton D, Curran MD. A common KIR2DS4 deletion variant in the human that predicts a soluble KIR molecule analogous to the KIR1D molecule observed in the rhesus monkey. Tissue Antigens. 2002;60:254–258. doi: 10.1034/j.1399-0039.2002.600307.x. [DOI] [PubMed] [Google Scholar]
- 60.Ponte M, et al. Inhibitory receptors sensing HLA-G1 molecules in pregnancy: decidua-associated natural killer cells express LIR-1 and CD94/NKG2A and acquire p49, an HLA-G1-specific receptor. Proc Natl Acad Sci U S A. 1999;96:5674–5679. doi: 10.1073/pnas.96.10.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rajagopalan S, Long EO. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J Exp Med. 1999;189:1093–1100. doi: 10.1084/jem.189.7.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boyson JE, Iwanaga KK, Golos TG, Watkins DI. Identification of the rhesus monkey HLA-G ortholog. Mamu-G is a pseudogene. J Immunol. 1996;157:5428–5437. [PubMed] [Google Scholar]
- 63.Boyson JE, Iwanaga KK, Golos TG, Watkins DI. Identification of a novel MHC class I gene, Mamu-AG, expressed in the placenta of a primate with an inactivated G locus. J Immunol. 1997;159:3311–3321. [PubMed] [Google Scholar]
- 64.Palacios C, Cuervo LC, Cadavid LF. Evolutionary patterns of killer cell Ig-like receptor genes in Old World monkeys. Gene. 2011;474:39–51. doi: 10.1016/j.gene.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 65.Boyson JE, et al. The MHC class I genes of the rhesus monkey: Different evolutionary histories of the MHC class I and II genes in primates. J Immunol. 1996;156:4656–4665. [PubMed] [Google Scholar]
- 66.Maloveste SM, et al. Degenerate recognition of MHC class I molecules with Bw4 and Bw6 motifs by a killer cell Ig-like receptor 3DL expressed by macaque NK cells. J Immunol. 2012;189:4338–4348. doi: 10.4049/jimmunol.1201360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parham P, et al. Primate-specific regulation of natural killer cells. J Med Primatol. 2010;39:194–212. doi: 10.1111/j.1600-0684.2010.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kikuchi-Maki A, Catina TL, Campbell KS. Cutting edge: KIR2DL4 transduces signals into human NK cells through association with the Fc receptor gamma protein. J Immunol. 2005;174:3859–3863. doi: 10.4049/jimmunol.174.7.3859. [DOI] [PubMed] [Google Scholar]
- 69.Alter G, et al. HLA class I subtype-dependent expansion of KIR3DS1+ and KIR3DL1+ NK cells during acute human immunodeficiency virus type 1 infection. J Virol. 2009;83:6798–6805. doi: 10.1128/JVI.00256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carr WH, Rosen DB, Arase H, Nixon DF, Michaelsson J, Lanier LL. Cutting edge: KIR3DS1, a gene implicated in resistance to progression to AIDS, encodes a DAP12-associated receptor expressed on NK cells that triggers NK cell activation. J Immunol. 2007;178:647–651. doi: 10.4049/jimmunol.178.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parham P, Norman PJ, Abi-Rached L, Guethlein LA. Variable NK cell receptors exemplified by human KIR3DL1/S1. J Immunol. 2011;187:11–19. doi: 10.4049/jimmunol.0902332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Albrecht C, Malzahn D, Brameier M, Hermes M, Ansari AA, Walter L. Progression to AIDS in SIV-Infected Rhesus Macaques is Associated with Distinct KIR and MHC class I Polymorphisms and NK Cell Dysfunction. Frontiers in immunology. 2014;5:600. doi: 10.3389/fimmu.2014.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morales JC, Melnick DJ. Phylogenetic relationships of the macaques (Ceropithecidae: Macaca), as revealed by high resolution restriction site mapping of mitochondrial ribosomal gens. J Hum Evol. 1998;34:1–23. doi: 10.1006/jhev.1997.0171. [DOI] [PubMed] [Google Scholar]
- 74.Sussman RW, Tattersall I. Distribution, abundance, and putative ecological strategy of Macaca fascicularis on the island of Mauritius, soutwestern Indian Ocean. Folia Primatol (Basel) 1986;46:28–43. [Google Scholar]
- 75.Budde ML, Wiseman RW, Karl JA, Hanczaruk B, Simen BB, O’Connor DH. Characterization of Mauritian cynomolgus macaque major histocompatibility complex class I haplotypes by high-resolution pyrosequencing. Immunogenetics. 2010;62:773–780. doi: 10.1007/s00251-010-0481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wiseman RW, Karl JA, Bohn PS, Nimityongskul FA, Starrett GJ, O’Connor DH. Haplessly hoping: macaque major histocompatibility complex made easy. ILAR journal/National Research Council, Institute of Laboratory Animal Resources. 2013;54:196–210. doi: 10.1093/ilar/ilt036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosner C, Kruse PH, Hermes M, Otto N, Walter L. Rhesus macaque inhibitory and activating KIR3D interact with Mamu-A-encoded ligands. J Immunol. 2011;186:2156–2163. doi: 10.4049/jimmunol.1002634. [DOI] [PubMed] [Google Scholar]
- 78.Biassoni R, et al. Role of amino acid position 70 in the binding affinity of p50.1 and p58.1 receptors for HLA-Cw4 molecules. Eur J Immunol. 1997;27:3095–3099. doi: 10.1002/eji.1830271203. [DOI] [PubMed] [Google Scholar]
- 79.Graef T, et al. KIR2DS4 is a product of gene conversion with KIR3DL2 that introduced specificity for HLA-A*11 while diminishing avidity for HLA-C. J Exp Med. 2009;206:2557–2572. doi: 10.1084/jem.20091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaizu M, et al. Molecular typing of major histocompatibility complex class I alleles in the Indian rhesus macaque which restrict SIV CD8+ T cell epitopes. Immunogenetics. 2007;59:693–703. doi: 10.1007/s00251-007-0233-7. [DOI] [PubMed] [Google Scholar]
- 81.Loffredo JT, et al. Idenification of seventeen new simian immunodeficiency virus-derived CD8+ T cell epitopes restricted by the high frequency molecule, Mamu-A*02, and potential escape from CTL recognition. J Immunol. 2004;173:5064–5076. doi: 10.4049/jimmunol.173.8.5064. [DOI] [PubMed] [Google Scholar]
- 82.Alter G, et al. HIV-1 adaptation to NK-cell-mediated immune pressure. Nature. 2011;476:96–100. doi: 10.1038/nature10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rhesus Macaque Genome S, et al. Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316:222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- 84.Hermes M, Weil S, Groth A, Dressel R, Koch J, Walter L. Characterisation of mouse monoclonal antibodies against rhesus macaque killer immunoglobulin-like receptors KIR3D. Immunogenetics. 2012;64:845–848. doi: 10.1007/s00251-012-0640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hermes M, Albrecht C, Schrod A, Brameier M, Walter L. Expression patterns of killer cell immunoglobulin-like receptors (KIR) of NK-cell and T-cell subsets in Old World monkeys. PLoS One. 2013;8:e64936. doi: 10.1371/journal.pone.0064936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bostik P, et al. Decreased NK cell frequency and function is associated with increased risk of KIR3DL allele polymorphism in simian immunodeficiency virus-infected rhesus macaques with high viral loads. J Immunol. 2009;182:3638–3649. doi: 10.4049/jimmunol.0803580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hellmann I, Lim SY, Gelman RS, Letvin NL. Association of activating KIR copy number variation of NK cells with containment of SIV replication in rhesus monkeys. PLoS Pathog. 2011;7:e1002436. doi: 10.1371/journal.ppat.1002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mothe BR, et al. Expression of the major histocompatibility complex class I molecule Mamu-A*01 is associated with control of simian immunodeficiency virus SIVmac239 replication. J Virol. 2003;77:2736–2740. doi: 10.1128/JVI.77.4.2736-2740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- 90.Kirmaier A, et al. TRIM5 suppresses cross-species transmission of a primate immunodeficiency virus and selects for emergence of resistant variants in the new species. PLoS Biology. 2010;8:e1000462. doi: 10.1371/journal.pbio.1000462. [DOI] [PMC free article] [PubMed] [Google Scholar]


