Abstract
Background
The incidence of oropharyngeal cancer (OPC) is rising rapidly, with the majority of cases being attributable to human papillomavirus (HPV). Despite the availability of a vaccine, rates of HPV vaccination among Texas youth are low. The healthcare cost of OPC in Texas is unknown. The aims of this study were to estimate the first two years cost of treating new cases of OPC and determine the predictors of OPC treatment cost in Texas.
Methods
This study included a retrospective cohort of 467 Texas patients with commercial insurance claims data with OPC diagnosed from 2011 to 2014 and a control group of 467 non-cancer patients obtained with propensity score matching. Total health care cost during the first two years after the index date was measured. A generalized linear model was used to identify predictors of monthly cost during the two years after the index date.
Results
The mean differential adjusted health care cost for OPC cases was $139,749 in the first two years. The mean adjusted monthly cost in the first two years was $6,693 for cases and $840 for controls. Age, comorbidity, mental health, pre-diagnostic health care cost, and time index were significant predictors of monthly cost.
Conclusions
Medical care cost was about $140,000 in the first two years after diagnosis of OPC among commercially insured patients in Texas.
Impact
The cost estimates provide important parameters for development of decision-analytic models to inform decision-makers about the potential value of initiatives for increasing the HPV immunization rate in the State.
Keywords: health care costs, health expenditures, oropharyngeal neoplasms, oropharynx, insurance claims review
Introduction
The incidence of oropharyngeal cancer (OPC) is rising rapidly in the United States, especially among middle-aged men. Though traditionally thought to be caused by alcohol and tobacco consumption, the proportion of OPC attributable to human papillomavirus (HPV) is increasing. From 2008 through 2012 in the United States, 11,000 new cases of OPC were attributed to HPV annually (1). According to the U.S. Centers for Disease Control and Prevention, OPC is the most common HPV-related cancer (1–3).
The rising incidence of OPC provides a major public health opportunity for primary prevention and approximately 72% are attributable to HPV. The HPV vaccine is highly effective at preventing oncogenic infections and HPV-related premalignancies (4–5). However, the immunization rate in Texas is low, where there is no school-based HPV vaccine requirement. A 2015 survey of adolescents aged 13 to 17 years found approximately 41% of girls and 24% of boys in Texas completed the HPV vaccination series, similar to rates observed in the United States overall (6). Given the cost of increasing the HPV vaccination rate for girls and boys, it is important to consider the potential cost offset of reducing HPV-related cancers in Texas by increasing the vaccine coverage. The primary aims of this study were to estimate the first two years cost of treating new cases of OPC, and identify demographic, comorbidity, and treatment-modality predictors of OPC treatment cost in Texas.
Materials and Methods
Data source
Data were obtained from the 2011–2014 Truven MarketScan Commercial Claims and Encounter Database (CCAE). The database contains enrollment and health care claims data for active employees, early retirees, Consolidated Omnibus Budget Reconciliation Act continues, and dependents insured by employer-sponsored commercial health insurance plans in the United States. The data are collected from employers and health plans and comprise service-level claims for inpatient, outpatient, and emergency room services and outpatient prescription drugs on 160 to 260 million enrollees each year during the study period. Approximately 92% of patients were insured through commercial plans, and 8% were enrolled in a Medicare Supplement plan. The database was fully de-identified conforming to the Health Insurance Portability and Accountability Act of 1996 and was exempt from Institutional Review Board approval.
Identification of cases
All patients at least 18 years of age living in Texas and diagnosed with OPC during the years 2011–2014 were selected. Patients were considered diagnosed with OPC if they had a primary or secondary diagnosis of cancer at one of the following sites indicated by the International Classification of Diseases, 9th revision, Clinical Modification, (ICD-9-CM) code: base of tongue and lingual tonsil (codes 141.0 and 141.6), soft palate and uvula (145.3–145.4), oropharynx (146.0–146.9), pharynx otherwise unspecified, and Waldeyer’s ring (149.0–149.1). Specific attention was given to ensure HPV-related subsites in the oropharynx were included. Patients had at least 1 inpatient service claim or 2 outpatient service claims at least 30 days apart. This requirement of at least 2 outpatient service claims increased the likelihood that only OPC patients with active disease would be selected. The first OPC service date was defined as the index date. Patients had to be continuously enrolled for 6 months before and after the index date. This enrollment requirement allowed assessment of whether any OPC disease code was entered in the database during the 6 months before the index date and assessment of the patient's total health care utilization during the first 6 months after the index date. Cases with total first-year cost above $1,000,000 were excluded.
Selection of controls
The CCAE was searched to identify potential non-cancer controls. Potential controls had to: 1) live in Texas during the study period, 2) be continuously enrolled for 6 months before and after the index date, 3) have no ICD-9-CM code for cancer at any site (140.0–208.9) during the 6 months before the index date, 4) have no diagnosis of recurrent respiratory papillomatosis (ICD-9-CM code 212.1), which is associated with HPV infection, and 5) age within 5 years of age of case. An index date identical to the case index date was randomly assigned to each subject in this initial pool of controls to represent a hypothetical diagnosis date for measuring the cost expected without OPC.
The propensity score was computed from the following covariates: 1) Modified Charlson comorbidity index (CCI) in the 6 months prior to the index date. The modified CCI excluded possible risk factors for OPC, including malignancy, metastatic solid tumor, and chronic pulmonary disease (7). 2) Psychiatric diagnosis groups (PDGs) in the 6 months prior to the index date. PDGs account for subjects’ mental health status and include 12 major psychiatric diagnostic groupings (8). 3) Pre-diagnosis health care cost for the period from 6 months prior to diagnosis to 3 months prior to diagnosis. Costs in the 3 months immediately prior to diagnosis were excluded to avoid including the cost of treating symptoms of an undiagnosed cancer. Therefore, if a patient was diagnosed with OPC on January 7, 2011, the pre-diagnosis-phase cost was defined as the cost from July 7, 2010, through Oct. 6, 2010. 4) Health insurance plan type, 5) Region of Texas (northeast, southeast, or west) categorized using 3-digit zip code (Supplemental Table 1). These regions mainly reflected the populations in and around Dallas (northeast), Houston (southeast), and El Paso (west). CCI and PDG served as indicators of the pre-diagnosis health status for cases and controls. One control was matched to each case using nearest neighbor matching (9). Once individuals were selected as controls for given cases, they were removed from the pool of candidate controls. Matched sets of cases and controls were created with similar demographic, health plan, location, and pre-index-date health status and health care cost.
Analysis of health care cost
The primary outcome measure was the mean differential health care cost for cases in the first two years after the index date with model adjustments. Health care cost was defined as the total gross payments for all inpatient services, outpatient services, and outpatient prescription drugs. The payments included copayments, coinsurance, deductibles, and coordination of benefits and other savings. All dollars were adjusted to year 2015 values using the Consumer Price Index from the U.S. Bureau of Labor Statistics (10). Means and standard deviations were presented to describe the cost distribution. Student t-test was used to identify significant differences between cases and controls. We further analyzed the costs for the treatments relevant to OPC during the first two-years after the index date. The OPC treatments were categorized into three modalities: surgery, radiation therapy (XRT) including intensity-modulated radiotherapy (IMRT), and chemotherapy and were identified by each patient's claims for specific procedure codes (Supplemental Table 2).
Analysis of monthly spending patterns
Adjusted mean monthly payments in the first two years after the index date were estimated for cases and controls. Patients who dis-enrolled during the follow-up period were right censored. Their costs were adjusted for right censoring and skewness by applying a generalized linear model with log link function (11). The log of arithmetic mean monthly cost was modeled (i.e., ln(E(y/x)=Xβ)) during two years, resulting in 24 partitioned monthly cost estimators for each patient. The covariates included sex, age, CCI, PDGs, health insurance plan type, Texas region, pre-diagnosis health care cost, case versus control status, cancer versus no cancer before diagnosis and censored versus non-censored. Subjects’ employment classifications were excluded as more than 50% of data were missing. Each patient had 24-month index partitions and the polynomials up to fifth degree of 24 months (e.g., month1=1, 2, 3, up to 24; month2=1, 4, 9, up to 576; continued up to month5). The model with the smallest Akaike information criterion was selected for cost estimation (12). The significant interaction term for24-month index and case/control was added using forward selection to adjust for different slopes of cost over time between cases and controls. Line graphs were plotted to show the monthly health care spending during the first two years after the index date for cases and controls. All analyses were performed using SAS for Windows, version 9.4 (SAS Inc., Cary, NC).
Results
Characteristics of the cases and controls are summarized in Table 1. A total of 467 Texas patients were identified who had a primary or secondary diagnosis of OPC during the study period (Supplemental Figure 1). One of the patients in the case group had payments exceeding $1,000,000 and was excluded. The propensity score was not significantly different between cases (0.00218±0.00124) and controls (0.00237±0.00172) (p-value=0.05). Supplemental Figure 2 shows the propensity score distributions. The mean age of the OPC patients was 54.62 years. Most patients were located in southeast Texas (n = 219; 46.90%). Approximately half were covered by a preferred provider organization health insurance plan. Baseline characteristics were not significantly different between cases and controls except for follow-up duration and employment classification.
Table 1.
Characteristics of OPC patients and matched controls
| Characteristic | Cases | Controls | P |
|---|---|---|---|
| Number of patients | 467 | 467 | |
| Age, mean (SD), years | 54.62 (7.38) | 54.26 (7.47) | 0.459 |
| Sex, no. (%) | |||
| Male | 382 (81.80) | 382 (81.80) | Matched |
| Female | 85 (18.20) | 85 (18.20) | |
| Follow-up, mean (SD), years | 1.51 (0.80) | 1.73 (0.91) | <.001 |
| Employee classification, no. (%) | |||
| Salary | 78(16.70) | 125(26.77) | <.001 |
| Hourly | 97(20.77) | 113(24.2) | |
| Other | 29(6.21) | 38(8.14) | |
| Missing/unknown | 263(56.32) | 191(40.9) | |
| Union/Non-union, no. (%) | |||
| Union | 39(8.35) | 52(11.13) | <.001 |
| Non-union | 138(29.55) | 171(36.62) | |
| Other | 27(5.78) | 53(11.35) | |
| Missing/unknown | 263(56.32) | 191(40.9) | |
| Employment status, no. (%) | <.001 | ||
| Active full time | 177 (37.90) | 267 (57.17) | |
| Active part time or seasonal | 3 (0.64) | 5 (1.07) | |
| Retired | 39(8.35) | 42(8.99) | |
| COBRA(continue)/Long-term disability/Surviving spouse/depend | 6(1.28) | 1(0.21) | |
| Other/unknown | 242 (51.82) | 152 (32.55) | |
| Plan type, no. (%) | 0.571 | ||
| Comprehensive | 9 (1.93) | 13 (2.78) | |
| EPO/missing | 22 (4.71) | 15 (3.21) | |
| HMO/POS/POS with capitation | 68 (14.56) | 78 (16.70) | |
| PPO | 338 (72.38) | 329 (70.45) | |
| CDHP/HDHP | 30 (6.42) | 32 (6.85) | |
| Region, no. (%) | 0.481 | ||
| Southeast | 219 (46.90) | 230 (49.25) | |
| Northeast | 201 (43.04) | 200 (42.83) | |
| West | 47 (10.06) | 37 (7.92) | |
| CCI, mean (SD) a | 0.49 (1.00) | 0.45 (0.96) | 0.594 |
| PDGs, mean (SD)a,b | 0.09 (0.30) | 0.07 (0.28) | 0.373 |
| Health care cost for 3 months before OPC diagnosis, mean (SD), USD c | 3,205 (9,748) | 2,220 (10,254) | 0.133 |
Abbreviations: CCI, Charlson comorbidity index; CDHP, consumer-driven health plan; COBRA, Consolidated Omnibus Budget Reconciliation Act; EPO, exclusive provider organization; HDHP, high-deductible health plan; HMO, health maintenance organization; OPC, oropharyngeal cancer; PDGs, psychiatric diagnosis groups; POS, point of service plan; PPO, preferred provider organization; USD, United States dollars.
Measured 6 months before the index date.
The score indicated the average number of mental psychiatric diagnostic disease groupings among our case and control group
The cost incurred during the 3 months immediately prior to the index diagnosis date was excluded to avoid including the cost of treating symptoms of an undiagnosed cancer.
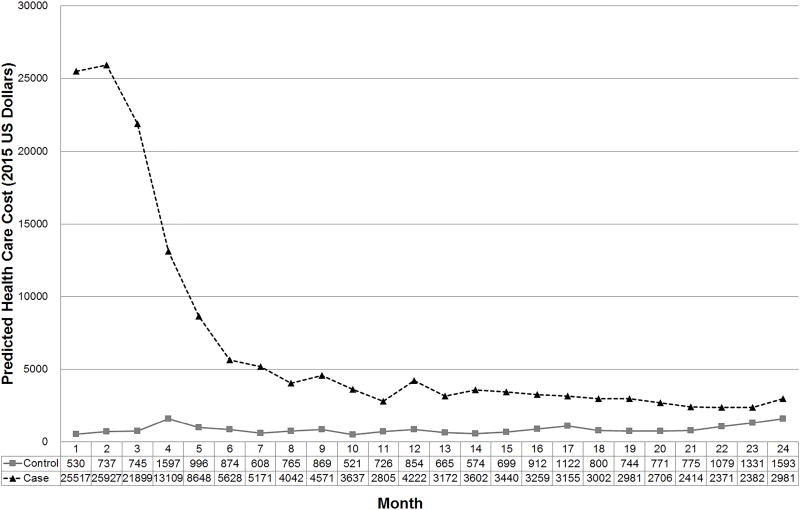
Expenditures for OPC patients and controls are summarized in Table 2. The unadjusted observed mean total health care expenditures during the first two years for cases and controls were $134,454 and $13,693, respectively (P< 0.001). Supplemental Figure 3 shows the unadjusted costs for the pre- and post-index date periods between cases and controls. On average, the unadjusted out-of-pocket payment for cases and controls were $2,825 and $1,192 (P< 0.001). For OPC patients, mean unadjusted cost in the first two years was $106,604 for outpatient service, $24,341 for inpatient services, and $3,550 for outpatient prescription drugs. There were 7 deaths among the cases and 1 death among the controls reported in the healthcare claims data. Deaths not reported in the claims data were unavailable. Table 3 shows the mean cost of specific services for OPC patients who received them during the first two years following the index diagnosis date. Seventy-three percent of patients were treated with XRT costing an average of $50,362. Of this 73 percent of patients, 96 percent received IMRT. Mean cost was $8,320 for the 50 percent of patients treated with surgery and $3,277 for the 61 percent of patients treated with chemotherapy. Supplemental Table 3 shows the number of days from the index date to the initiation of the first modality of therapy. The significant predictors of monthly cost over the first two years were age, CCI, PDGs, pre-diagnosis health care cost, case versus control status, polynomial of fifth degree of months since index date and the interaction term of 24-month index and case/control. (Supplemental Table 4). The adjusted mean total health care costs in the first two years for cases and controls were $160,639 and $20,890, respectively (mean differential cost, $139,749; P< 0.001) (Table 2). The adjusted monthly cost during the first two years for the cases and controls were $6,693 and $870 (Figure 1). Supplemental Figures 4 and 5 show histograms of the adjusted 1-year cost and 2-year cost distributions, respectively.
Table 2.
Unadjusteda and adjusted cost during the first two years after the index date for OPC patients and matched controls
| Mean expenditure in 2015 US Dollars (SD) | ||||
|---|---|---|---|---|
| Type of cost | Cases | Controls | Difference | P |
| First two years(unadjusted) | ||||
| Total | 134,454(108,635) | 13,693(38,306) | 120,802(81,472) | <.001 |
| Inpatient services | 24,341(48,972) | 5,031(29,550) | 19,310(40,444) | <.001 |
| Outpatient services | 106,604(82,821) | 5,738(12,293) | 100,866(59,275) | <.001 |
| Outpatient prescription drugs | 3,550(5,183) | 2,924(6,661) | 626(5,968) | 0.11 |
| First two years(adjusted) | ||||
| Total | 163.639(114,893) | 20,890(53,543) | 139,749(89631) | <.001 |
Unadjusted is the observed cost with no regression model estimate of cost for months not enrolled.
Abbreviation: SD, standard deviation.
Table 3.
The two-year mean cost after index date by treatment
| Treatment | N(%) | Mean Cost in 2015 US Dollars per person (SD) |
|---|---|---|
| Surgery | 233 (50%) | 8,320 (15,111) |
| Inpatient | 90 (19%) | 17,440 (19,030) |
| Outpatient | 190 (41%) | 1,942 (2,641) |
| Radiation-XRT (Including IMRT) | 341 (73%) | 50,362 (28,928) |
| Inpatient | 14 (3%) | 1,305 (1,369) |
| Outpatient | 341 (73%) | 50,308 (28,938) |
| Chemotherapy | 284 (61%) | 3,277 (2,822) |
| Inpatient | 0 (0%) | NA |
| Outpatient | 284 (61%) | 3,277 (2,822) |
Abbreviation: SD: standard deviation, XRT, radiation therapy; IMRT: intensity-modulated radiotherapy
Figure 1.
Predicted monthly cost for the two years between case and control
Discussion
Management of OPC resulted in a mean differential health care cost of $139,749 in 2015 USD in the first two years after diagnosis. To our knowledge, this is the first population-based estimate of OPC-specific health care cost in Texas. It provides information for state-level modeling and policy analysis of public decisions regarding investment in HPV-related cancer prevention.
An estimated 72% of OPC cases are attributable to HPV (3). There is currently no screening paradigm for OPC, and with few symptoms in early stages, the disease often presents at an advanced stage (13). The latency of HPV-induced OPC is long, and is often diagnosed in the fifth or sixth decade of life (13). Given these characteristics, immunization against HPV is an effective strategy to reduce the morbidity and economic cost of OPC. The U.S. Centers for Disease Control and Prevention recommend routine HPV vaccination for girls and boys (14). The quadrivalent HPV vaccine is covered under the Affordable Care Act for boys and girls in the recommended age range, and most plans are expected to cover the 9-valent vaccine in 2017 (15–16). Despite federal program and policy support, states like Texas have low immunization rates and may benefit from investment in initiatives to promote HPV immunization (6). To guide such decision making, the potential economic and health benefits of these investments need to be estimated at the state level.
Ours is not the first economic analysis of the potential benefits of HPV immunization, but we believe it is the first analysis to provide information specifically about OPC in Texas. The leading economic decision analytic models of HPV immunization rely on dated cost estimates for OPC. In these studies, a variety of methods were applied to identify the cases, estimate the index diagnosis date, and adjust for non-cancer costs, non-normal cost distribution, and censoring. The Elbasha and Dasbach (17) model utilized cost estimates by Hu and Goldie (18) that were rough estimates based on a synthesis of the literature, secondary data, and scenario analysis. The model developed by Brisson et al. (19) also applied cost-per-case data for OPC from work by Hu and Goldie (18). More recent studies have applied SEER-Medicare, state Medicaid, and commercial insurance claims data but considered oral cavity cancer (which is unrelated to HPV) and pharyngeal cancers (which include both HPV-related and non-HPV-related cancers) together (20–22). Etiology and presentation of OPC differ widely from oral cavity cancer, resulting in major differences in treatment and long-term outcomes (23–24). HPV is estimated to be causally involved in 72% of OPCs compared to fewer than 10% of oral cavity cancers in North America (3, 25–27). Currently, there is no distinction in HPV-status available in diagnostic codes. Therefore, our study attempted to estimated cost of HPV-related OPC through careful selection of HPV-relation OPC sites. Prior studies have tended to underestimate the population-attributable fraction of HPV for OPC. The majority of OPCs present at late stages, requiring extensive treatment; the health care cost of OPC may be underestimated when OPC is combined with oral cavity cancer (13, 28).
The mean differential two-year health care cost of OPC in our study, $139,749, is higher than in previous studies. The differential cost estimate for 1-year after diagnosis was $115,350 (Supplemental Table 5). Much of this cost can be attributed to outpatient treatment with radiation therapy. We found that more than 70% of patients received radiotherapy and most of them were treated in outpatient settings. With the introduction of intensity-modulated radiation therapy (IMRT), its application in the head and neck has grown from 1% in 2000 to 46% in 2005, and was found to be 70% in our study (29). Rafzar et al. (30) found that mean cost of patients undergoing treatment with IMRT was $101,099, significantly higher compared to patients undergoing traditional radiation therapy. This change in treatment paradigm may explain the differences in the high outpatient costs found in our study. Many prior estimates of cost pre-date or occur during this treatment transition (18, 20–21) Additionally, previous estimates may vary depending on inclusion of non-OPC cancer types and study population. Using 1995–2003 Medi-Cal data, Epstein et al. (21) found a median cost of oral cavity and pharyngeal cancer of $33,358 (2015 USD) in the first year after diagnosis. Hollenbeak et al. (20) used inverse probability weighting to adjust for censoring in SEER-Medicare data and found a 5-year cumulative cost of pharyngeal cancer of $48,544 (2015 USD). Hu and Goldie (18) used SEER registry and Medicare data to identify the mean cost per OPC case as $42,534 (2015 USD). However, HPV-associated OPC has been found to represent 72% of all OPCs rather than 11%, the proportion used in their estimates (3, 18). The conclusions derived from Medicare cannot be generalized to the overall population. Furthermore, those estimates were derived from public payment rates paid by state and federal governments, which may underestimate the economic burden of disease (31). Jacobsen et al. (22) found that for patients with commercial insurance, the mean cost estimates for OPC were $87,445 (2015 USD), double that of the cost in Medicare cases. This study also included patients with salivary gland and oral cavity cancer, which may have lowered mean cost.
In our study, the health care cost of OPC was highest in the first 6 months, and outpatient services accounted for the bulk of the cost. A previous study also showed that most of the cost for OPC was incurred in the first year following the diagnosis (22). Furthermore, previous studies, showed that outpatient services accounted for the greatest portion of utilization and cost (22, 32). This cost pattern is consistent with the National Comprehensive Cancer Network practice guidelines for head and neck cancer, which recommend a history and physical examination every 1 to 3 months in the first year after the initial treatment (33). Future studies are needed to define and quantify the long-term posttreatment management of OPC to better understand the lifetime cost of this disease.
Our finding that patients’ prediagnosis co-morbidities were significantly associated with two year cost of OPC treatment is consistent with findings of previous studies (20, 21). The mean CCI of our case population was lower than that in previous studies (21, 22). We excluded other cancer and chronic pulmonary diseases from the OPC cancer risk factors when calculating the CCI score. This exclusion allowed us to identify a general population for the control group that was used to estimate the counter-factual of cost incurred without OPC.
Use of retrospective administrative claims data over a 4-year period for one state limit the generalizability of our findings. Costs associated with lost earnings due to morbidity and premature mortality were not available, precluding a full societal cost estimate. Propensity score matching reduces observable differences between cases and controls but does not correct for unobservable differences that may affect costs. Whether people were salaried, in a union, or working full time were significantly different between cases and controls in table 1, but most of these variables had nearly 50 percent missing data. Cases experienced statistically significant shorter follow-up time compared to controls, 1.51 years compared to 1.73 years. This difference did not affect the predicted stable cost trend in both groups following the initial 6 month treatment for the cases. Data were not available for the stage at diagnosis, deaths outside the hospital, and other covariates, which precluded analysis of the variation in cost based on provider characteristics and patients’ socioeconomic status, race, ethnicity, specific geographic location, and vital status. Previous authors have used patients’ 5-digit zip code to link to census data as a proxy for socioeconomic status (22). This information was not available based on our agreement with Truven. Laboratory results to identify HPV-related cancer were not available in the claims data (34). However, epidemiologic studies demonstrate that approximately72% of OPC is HPV-related (3).
Strengths of the study include use of comprehensive longitudinal up-to-date health care claims data along with methods to measure the differential cost due to OPC, while adjusting for the cost of unrelated health care, right censoring, and non-normally distributed cost data. Careful selection of OPC-specific ICD-9-CM codes ensured that patients with head and neck cancers from other sites were excluded from the analysis. We included only cancer sites in the oropharynx that are known to be HPV-related, in contrast to other studies that included oral cavity sites, which may have resulted in underestimation of cost associated with treating HPV-related cancer (16, 18–20, 31).
Conclusion
Medical care cost was about $140,000 in the first two years after diagnosis of OPC among commercially insured patients in Texas. The cost estimates provide important parameters for development of decision-analytic models to inform decision-makers about the potential value of alternative strategies for increasing the HPV immunization rate in the State.
Supplementary Material
Acknowledgments
Financial Support: This study was supported by the Christopher and Susan Damico Chair in Viral Associated Malignancies (E.M. Sturgis), the Stiefel Oropharyngeal Research Fund (E.M. Sturgis), and the Moon Shots Program of The University of Texas MD Anderson Cancer Center (E.M. Sturgis).
References
- 1.Viens LJ, Henley SJ, Watson M, Markowitz LE, Thomas CC, Thompson TD, et al. Human Papillomavirus-Associated Cancers - United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2016;65(26):661–666. doi: 10.15585/mmwr.mm6526a1. [DOI] [PubMed] [Google Scholar]
- 2.Chesson HW, Ekwueme DU, Saraiya M, Watson M, Lowy DR, Markowitz LE. Estimates of the Annual Direct Medical Costs of the Prevention and Treatment of Disease Associated with Human Papillomavirus in the United States. Vaccine. 2012;30:6016–6019. doi: 10.1016/j.vaccine.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, et al. Efficacy of a Bivalent L1 Virus-like Particle Vaccine in Prevention of Infection with Human Papillomavirus Types 16 and 18 in Young Women: A Randomised Controlled Trial. Lancet. 2004;364(9447):1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 5.Apter D, Wheeler CM, Paavonen J, Castellsague X, Garland SM, Skinner SR, et al. Efficacy of Human Papillomavirus 16 and 18 (HPV-16/18) AS04-Adjuvanted Vaccine Against Cervical Infection and Precancer in Young Women: Final Event-Driven Analysis of the Randomized, Double-Blind PATRICIA Trial. Clin Vaccine Immunol. 2015;22(4):361–373. doi: 10.1128/CVI.00591-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reagan-Steiner SYD, Jeyarajah J, Elam-Evans LD, Singleton JA, Curtis CR, MacNeil J, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years - United States, 2015. MMWR Morb Mortality Wkly Rep. 2016;65:850–858. doi: 10.15585/mmwr.mm6533a4. [DOI] [PubMed] [Google Scholar]
- 7.D'Hoore W, Bouckaert A, Tilquin C. Practical Considerations on the Use of the Charlson Comorbidity Index with Administrative Data Bases. J Clin Epidemiol. 1996;49:1429–1233. doi: 10.1016/s0895-4356(96)00271-5. [DOI] [PubMed] [Google Scholar]
- 8.Ashcraft MLF, Fries BE, Nerenz DR, Falcon SP, Sujan V, Lee CZ, et al. A Psychiatric Patient Classification System: An Alternative to Diagnosis-Related Groups. Med Care. 1989;27:543–557. doi: 10.1097/00005650-198905000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Stuart EA. Matching Methods for Causal Inference: A Review and a Look Forward. StatSci. 2010;25:1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.United States Department of Labor. [accessed July 20, 2016];Consumer Price Index-Medical Care. Available from URL: http://data.bls.gov/cgi-bin/surveymost?cu.
- 11.Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. 2. New York: Oxford University Press; 2014. Analyzing cost. [Google Scholar]
- 12.Yamaoka K, Nakagawa T, Uno T. Application of Akaike's Information Criterion (AIC) in the Evaluation of Linear Pharmacokinetic Equations. J Pharmacokinet Biopharm. 1978;6:165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]
- 13.Dahlstrom KR, Calzada G, Hanby JD, Garden AS, Glisson BS, Li G, et al. An Evolution in Demographics, Treatment, and Outcomes of Oropharyngeal Cancer at a Major Cancer Center: a Staging System in Need of Repair. Cancer. 2013;119:81–89. doi: 10.1002/cncr.27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, et al. Human Papillomavirus Vaccination: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2014;63:1–30. [PubMed] [Google Scholar]
- 15.Kaiser Family Foundation. [accessed July 18, 2016];Preventative Services Covered by Private Health Plans Under the ACA. Available from URL: http://kff.org/health-reform/fact-sheet/preventive-services-covered-by-private-health-plans/
- 16.Kaiser Family Foundation. [accessed July 18, 2016];The HPV Vaccine: Access and Use in the U.S. Available from URL: http://files.kff.org/attachment/fact-sheet-the-hpv-vaccine-access-and-use-in-the-u-s.
- 17.Elbasha EH, Dasbach EJ. Impact of Vaccinating Boys and Men against HPV in the United States. Vaccine. 2010;28:6858–6867. doi: 10.1016/j.vaccine.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 18.Hu D, Goldie S. The Economic Burden of Noncervical Human Papillomavirus Disease in the United States. Am J Obstet Gynecol. 2008;198:500 e501–507. doi: 10.1016/j.ajog.2008.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brisson M, Laprise JF, Chesson HW, Drolet M, Malagon T, Boily MC, et al. Health and Economic Impact of Switching from a 4-Valent to a 9-Valent HPV Vaccination Program in the United States. J Natl Cancer Inst. 2016;108(1) doi: 10.1093/jnci/djv282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollenbeak CS, Kulaylat AN, Mackley H, Koch W, Schaefer EW, Goldenberg D. Determinants of Medicare Costs for Elderly Patients With Oral Cavity and Pharyngeal Cancers. JAMA Otol Head Neck Surg. 2015;141:628–635. doi: 10.1001/jamaoto.2015.0940. [DOI] [PubMed] [Google Scholar]
- 21.Epstein JD, Knight TK, Epstein JB, Bride MA, Nichol MB. Cost of Care for Early- and Late-Stage Oral and Pharyngeal Cancer in the California Medicaid Population. Head Neck. 2008;30:178–186. doi: 10.1002/hed.20670. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson JJ, Epstein JB, Eichmiller FC, Gibson TB, Carls GS, Vogtmann E, et al. The Cost Burden of Oral, Oral Pharyngeal, and Salivary Gland Cancers in Three Groups: Commercial Insurance, Medicare, and Medicaid. Head Neck Oncol. 2012;4:15. doi: 10.1186/1758-3284-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belcher R, Hayes K, Fedewa S, Chen AY. Current Treatment of Head and Neck Squamous Cell Cancer. J Surg Oncol. 2014;110:551–574. doi: 10.1002/jso.23724. [DOI] [PubMed] [Google Scholar]
- 24.Bessell A, Glenny AM, Furness S, Clarkson JE, Oliver R, Conway DI, et al. Interventions for the Treatment of Oral and Oropharyngeal Cancers: Surgical Treatment. Cochrane Database Syst Rev. 2011;(9) doi: 10.1002/14651858.CD006205.pub3. CD006205. [DOI] [PubMed] [Google Scholar]
- 25.Saraiya M, Unger ER, Thompson TD, Lynch CF, Hernandez BY, Lyu CW, et al. US Assessment of HPV Types in Cancers: Implications for Current and 9-Valent HPV Vaccines. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv086. djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lingen MW, Xiao W, Schmitt A, Jiang B, Pickard R, Kreinbrink P, et al. Low Etiologic Fraction for High-Risk Human Papillomavirus in Oral Cavity Squamous Cell Carcinomas. Oral Oncol. 2013;49:1–8. doi: 10.1016/j.oraloncology.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Castellsague X, Alemany L, Quer M, Halec G, Quiros B, Tous S, et al. HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3680 Patients. J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djv403. djv403. [DOI] [PubMed] [Google Scholar]
- 28.Borggreven PA, Aaronson NK, Verdonck-de Leeuw IM, Muller MJ, Heiligers ML, Bree R, et al. Quality of Life after Surgical Treatment for Oral and Oropharyngeal Cancer: a Prospective Longitudinal Assessment of Patients Reconstructed by a Microvascular Flap. Oral Oncol. 2007;43:1034–1042. doi: 10.1016/j.oraloncology.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 29.Guadagnolo BA, Liu CC, Cormier JN, Du XL. Evaluation of Trends in the Use of Intensity-Modulated Radiotherapy for Head and Neck Cancer from 2000 through 2005: Socioeconomic Disparity and Geographic Variation in a Large Population-Based Cohort. Cancer. 2010;116(14):3505–3512. doi: 10.1002/cncr.25205. [DOI] [PubMed] [Google Scholar]
- 30.Razfar A, Mundi J, Grogan T, Lee S, Elashoff D, Abemayor E, et al. IMRT for Head and Neck Cancer: Cost Implications. Am J Otolaryngol. 2016;37(6):479–483. doi: 10.1016/j.amjoto.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 31.Coughlan D, Frick KD. Economic Impact of Human Papillomavirus-Associated Head and Neck Cancers in the United States. Otolaryngol Clin North Am. 2012;45(4):899–917. doi: 10.1016/j.otc.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Lang K, Menzin J, Earle CC, Jacobson J, Hsu MA. The Economic Cost of Squamous Cell Cancer of the Head and Neck: Findings from Linked SEER-Medicare Data. Arch Otolaryngol Head Neck Surg. 2004;130:1269–1275. doi: 10.1001/archotol.130.11.1269. [DOI] [PubMed] [Google Scholar]
- 33.National Comprehensive Cancer Network. [accessed October 26, 2016];Head and Neck Cancer (Version 2.2016) Available at URL: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf.
- 34.Ekwueme DU, Chesson HW, Zhang KB, Balamurugan A. Years of Potential Life Lost and Productivity Costs Because of Cancer Mortality and for Specific Cancer Sites Where Human Papillomavirus May Be a Risk Factor for Carcinogenesis-United States, 2003. Cancer. 2008;113:2936–2945. doi: 10.1002/cncr.23761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.