Abstract
Background
Adolescent intermittent ethanol (AIE) exposure produces persistent impairments in cholinergic and epigenetic signaling and alters markers of synapses in the hippocampal formation, effects that are thought to drive hippocampal dysfunction in adult rodents. Donepezil (Aricept), a cholinesterase inhibitor, is used clinically to ameliorate memory-related cognitive deficits. Given that donepezil also prevents morphological impairment in preclinical models of neuropsychiatric disorders, we investigated the ability of donepezil to reverse morphological and epigenetic adaptations in the hippocampus of adult rats exposed to AIE. Because of the known relationship between dendritic spine density and morphology with the fragile X mental retardation 1 (Fmr1) gene, we also assessed Fmr1 expression and its epigenetic regulation in hippocampus after AIE and donepezil pre-treatment.
Methods
Adolescent rats were administered intermittent ethanol for 16 days starting on postnatal day 30. Rats were treated with donepezil (2.5mg/kg) once a day for four days starting 20 days after the completion of AIE exposure. Brains were dissected out after the 4th donepezil dose and spine analysis was completed in dentate gyrus granule neurons. A separate cohort of rats, treated identically, was used for molecular studies.
Results
AIE exposure significantly reduced dendritic spine density and altered morphological characteristics of subclasses of dendritic spines. AIE exposure also increased mRNA levels and H3-K27 acetylation occupancy of the Fmr1 gene in hippocampus. Treatment of AIE-exposed adult rats with donepezil reversed both the dendritic spine adaptations and epigenetic modifications and expression of Fmr1.
Conclusions
These findings indicate that AIE produces long-lasting decreases in dendritic spine density and changes in Fmr1 gene expression in the hippocampal formation, suggesting morphological and epigenetic mechanisms underlying previously reported behavioral deficits after AIE. The reversal of these effects by sub-chronic, post-AIE donepezil treatment indicates that these AIE effects can be reversed by upregulating cholinergic function.
Keywords: Adolescent alcohol, Donepezil, Dendritic Spines, Hippocampus, Fmr1, Epigenetics, Histone Acetylation
INTRODUCTION
Alcohol is the world’s most widely used recreational drug, and most people in the U.S. begin use during adolescence or young adulthood. U.S. surveys show that 29% of 12th graders and 42% of college students report having had five or more drinks in a row during the last two weeks (NIAAA, 2006). This prevalence of heavy drinking occurs during a period when the brain is undergoing rapid changes in structure and function that make it vulnerable to negative consequences of alcohol exposure (Dahl, 2004, Monti et al., 2005, Pfefferbaum et al., 2016). Adolescence and young adulthood are distinct developmental periods that involve significant behavioral and neurological changes. This period of late brain development is critically important for an individual’s ability to function effectively in the adult world, extends from the late teens into the mid-twenties, and has become the subject of intense investigation in both humans and animal models (Spear and Swartzwelder, 2014, Brown et al., 2015, Spear, 2000b). In humans and rodents, adolescents and young adults manifest differential responsiveness to acute ethanol (Acheson et al., 1998, Little et al., 1996, Markwiese et al., 1998, Spear, 2000a, White et al., 2002) and are more sensitive to enduring negative effects of repeated ethanol exposure that persist into adulthood (Spear and Swartzwelder, 2014, Brown et al., 2015). It is well known that drinking onset at young ages is strongly associated with alcohol abuse and compromises of cognitive function in adulthood (Dawson et al., 2008, Hanson et al., 2011, Mota et al., 2013, Nguyen-Louie et al., 2016, Sher and Gotham, 1999, Squeglia et al., 2015).
In order to assess the neural mechanisms underlying adolescent intermittent ethanol (AIE) exposure, and possible preventive or ameliorative treatments, rodent AIE models have evolved that approximate the use patterns and blood ethanol levels experienced by adolescent human drinkers (see Spear, 2016, Spear and Swartzwelder, 2014). One of the most robust and reproduced effects of AIE exposure in animal models is a marked reduction of choline acetyltransferase (ChAT) immunoreactivity in the medial septum and vertical limb of the diagonal band of Broca (Ch1–2), which are rich in cholinergic neurons. These reductions persist well into adulthood (Swartzwelder et al., 2015, Boutros et al., 2014, Vetreno et al., 2014), do not appear to occur after comparable ethanol exposure in adulthood (Vetreno et al., 2014), and are associated with specific memory deficits (Swartzwelder et al., 2015). Thus, it appears that AIE results in a chronic deprivation of hippocampal circuits of cholinergic input. Cholinergic neurons in Ch1–2 project diffusely into the hippocampal formation and play an important regulatory and facilitating role in memory-related neuronal function and plasticity (Madison et al., 1987, Mesulam et al., 1983, Smith and Pang, 2005), circuit activity (Dudar, 1977), and synaptic plasticity (Auerbach and Segal, 1994, Fernandez de Sevilla and Buno, 2010, Drever et al., 2011). Importantly, their hippocampally projecting axons increase the expression of acetylcholinesterase during adolescence (Armstrong et al., 1987, Gould et al., 1991), and hippocampal ChAT levels peak during early adolescence and remain stable until early adulthood. In addition, the activity of high-affinity choline transporter increases during adolescence before returning to baseline levels in young adulthood (Zahalka et al., 1993). Thus, the developmental trajectory of basal forebrain cholinergic neurons, which begins prenatally, continues through adolescence, and appears particularly susceptible to disruption by adolescent alcohol exposure.
Donepezil (Aricept) is a selective, reversible cholinesterase inhibitor that is used clinically to ameliorate memory-related cognitive deficits. In animal models, it has been shown to protect against olfactory bulbectomy-induced lesions of cholinergic neurons in Ch1–2 and their behavioral sequelae (Yamamoto et al., 2010), which are similar to the pattern of ChAT immunoreactivity reductions we have observed after AIE (Swartzwelder et al., 2015). Donepezil has also been shown to protect against working memory deficits after saporin injections into Ch1–2 (Cutuli et al., 2013), inflammation-induced, hippocampally-mediated working memory deficits after basal forebrain cholinergic lesions (Field et al., 2012), and hippocampal synaptic and neuronal degeneration in a transgenic mouse model of Alzheimer’s disease (Cavallucci et al., 2013). Interestingly, donepezil has also been shown to reverse aging-related dendritic spine loss in the hippocampus and prefrontal cortex (Alcantara-Gonzalez et al., 2012, Alcantara-Gonzalez et al., 2010). This is a critical finding given that AIE exposure impairs the cholinergic system and alters dendritic spine density and morphology in cortical and subcortical regions (Pandey et al., 2015, Trantham-Davidson et al., 2017, Jury et al., 2017, Risher et al., 2015a, Boutros et al., 2014, Vetreno et al., 2014, Swartzwelder et al., 2015). Taken together, these findings suggest that pharmacological up-regulation of cholinergic function, in animals with deficient cholinergic input to the hippocampus, can reverse or prevent hippocampal deficits associated with diminished cholinergic input. The present experiments were, therefore, designed to assess the effects of AIE on hippocampal dendritic spine density and morphology and the possible ameliorative effects of donepezil on those AIE-induced changes.
In addition to the translational focus of assessing the possible ameliorative effects of donepezil, we also sought to assess a possible mechanism driving the possible effects of AIE on dendritic spine density. Histone acetylation serves as an active epigenetic mark of genomic activity, and changes in the acetylation status regulate gene expression (Pandey et al., 2017, Krishnan et al., 2014). It has been shown that the fragile X mental retardation 1 (Fmr1) gene that encodes fragile X mental retardation protein (FMRP), regulates the morphology of dendritic spines (Grossman et al., 2006a, Grossman et al., 2006b). In addition, deletion of the Fmr1 gene is associated with increased dendritic spine density and cholinergic deficits in hippocampus (Comery et al., 1997, Grossman et al., 2010, Ivanco and Greenough, 2002, Scharkowski et al., 2017, D’Antuono et al., 2003, Scremin et al., 2015). We reported previously that AIE exposure decreases global H3K9 acetylation in the hippocampus due to increased activity of histone deacetylases (HDAC) and decreases neurogenesis markers in adulthood (Sakharkar et al., 2016), and, as noted above, there is evidence of decreased cholinergic input to the hippocampal formation after AIE (Swartzwelder et al., 2015, Boutros et al., 2014, Vetreno et al., 2014). Thus, it is possible that enduring alterations of hippocampal synaptic function after AIE (Risher et al., 2015b, Li et al., 2013, Fleming et al., 2013) could be the result of alterations in dendritic spine density, driven by cholinergic alterations linked to Fmr1 gene expression. To test this hypothesis, we assessed dendritic spine density and Fmr1 gene expression and histone acetylation in the hippocampal formation after AIE, and determined if post-AIE treatment with donepezil can reverse any observed effects.
MATERIALS AND METHODS
Animals and Ethanol Exposure
The procedures in this study were conducted in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and the National Research Council’s Guide for Care and Use of Laboratory Animals. In addition, they were approved by the Durham VA Medical Center and the Duke University Animal Care and Use Committees.
For the dendritic spine studies, twenty-four male Sprague-Dawley rats (Charles River, USA) were housed in pairs in a temperature- and humidity-controlled room. They had ad libitum access to food and water. The rats were delivered to the vivarium on postnatal day (PND)-25 and allowed to acclimatize to the vivarium and handling by technicians for five days on a reverse 12:12-hr light:dark cycle (lights off at 9:00 am) prior to beginning AIE or distilled water exposure on PND-30. Exposure consisted of 10 doses of 5 g/kg ethanol (35% v/v in dH2O at 18.12 mL/kg, VWR, Suwanee, GA, USA) or isovolumetric dH2O administered by intragastric gavage using a 2 days on, 1 day off, 2 days on, 2 days off schedule for 16 days. The range of gavage volumes across the exposure period was 1.1–5.0ml. Exposure was followed by a 20-day period of no treatment. Twenty-one days following exposure, half of the animals in the AIE pretreatment group and half in the control animals were dosed with 2.5 mg/kg of donepezil (1.88 mL/kg in dH2O) and the other half in each pretreatment group received dH2O (1.88 mL/kg) by intragastric gavage once per day for four days. This resulted in four treatment groups. One received water during adolescence and post-treatment (Water/Water), one received AIE during adolescence followed by water post-treatment (AIE/Water), one received water during adolescence followed by donepezil post-treatment (Water/DZ), and one received AIE during adolescence followed by donepezil post-treatment (AIE/DZ).
The ethanol dose was selected in order to produce blood ethanol concentrations (BECs) consistent with adolescent human BECs during binge drinking episodes (Squeglia et al., 2011). We have found that rats of this age, sex, and strain, receiving 5 g/kg ethanol (i.g.), achieved mean blood ethanol concentrations of 199.7 ± 19.9 mg/dl 60 minutes after the first dose, and 172.8 ± 13.3 mg/dl 60 minutes after the last dose (Risher et al., 2015a). A separate group of 36 rats was treated identically for use in the experiments on mRNA levels and epigenetic modifications of Fmr1, except that after sacrifice hippocampal tissue was flash frozen.
Neuron Labeling Methods
Because the dorsal - but not ventral - dentate gyrus shows a reduction c-Fos activity in adult mice exposed to ethanol during adolescence (Beaudet et al., 2016), analysis of AIE-induced changes in morphology of dendrites and dendritic spines was limited to granule neurons in the dorsal dentate gyrus. Diolistic labeling of slices obtained from fixed brains was used to assess the effects of AIE exposure and donepezil treatment on dendritic spine density and morphology in dorsal dentate gyrus granule neurons (Fig. 1A). Brains were prepared for labeling as described previously (McGuier et al., 2015, Uys et al., 2016). Adult rats (PND-70, n = 6 rats/group) were anesthetized with urethane (1.2 – 1.5 g/kg, IP) three h after the last donepezil dose and were perfused with 0.1 M phosphate buffer (PB) followed by 1.5% paraformaldehyde (PFA) in PB. Brains were blocked and post-fixed in 1.5% PFA for 1 h at room temperature. Brains were washed and shipped to the Medical University of South Carolina overnight in PB. Coronal sections (150 μm thick) were prepared using a vibratome. DiI-coated tungsten particles (1.3 μm diameter) were delivered to the slices using a modified Helio Gene Gun (Bio-Rad; Hercules, CA) fitted with a polycarbonate filter (3.0 μm pore size; BD Biosciences; San Jose, CA). Slices were left overnight at 4°C in PB to allow the DiI to completely diffuse through labeled neurons and sections were post-fixed in 4% PFA for 1 h at room temperature. After mounting with Prolong Gold Antifade mounting media (Life Technologies; Carlsbad, CA), images were acquired (n = 5–6 rats/group; 3 – 6 dendritic sections from each rat; 25–30 sections/treatment group; 111 total sections) on a Zeiss LSM 510 confocal microscope using a 63X oil objective (NA = 1.4). Images of granule neuron dendrites (~60 μm length sections) were collected at a 3X zoom (voxel size: 47 × 47 × 100 nm) and a 4 frame average and were then deconvolved using AutoQuant X (Media Cybernetics; Rockville, MD). Distal sections of granule neuron dendrites (≥ 75 microns from the soma) that receive entorhinal cortex inputs (Witter, 2007) were collected for analysis.
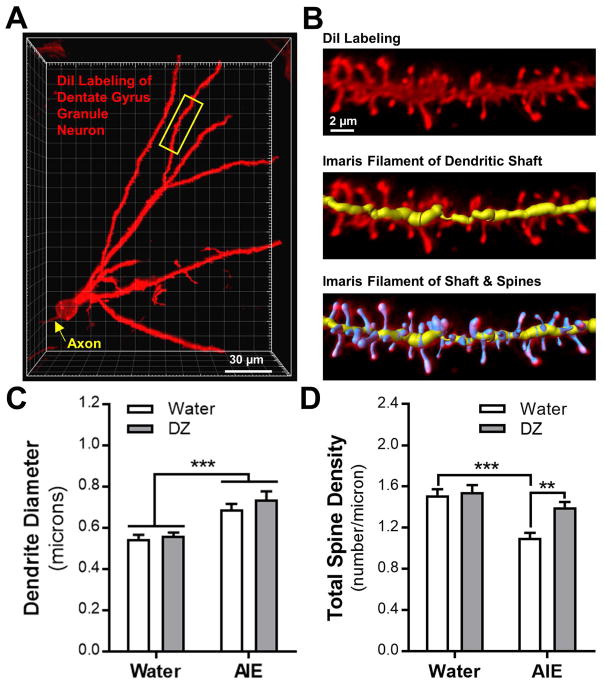
Fig. 1.
Effects of adolescent intermittent ethanol (AIE) exposure and donepezil (DZ) post-treatment on dendrites and total dendritic spine density in adult dentate gyrus granule neurons. (A) Representative 3D image of a DiI labeled granule neuron in the dorsal dentate gyrus. Yellow arrow denotes axon stemming from the soma and yellow box represents a typical section of dendrite used for analysis. (B) Representative labeling of a section of dendrite showing the Imaris filament recreation of the dendritic shaft (shown in yellow) and spines (shown in blue). Quantitation of (C) dendrite diameter (main effect of AIE: F (1,20) = 35.77, ***p < 0.0001) and (D) total dendritic spine density (interaction: F (1,19) = 5.56, p = 0.0293; ***p < 0.001 vs Water/Water; **p < 0.01 vs AIE/Water) in granule neurons from adult rats exposed to AIE and DZ. For all dendrite and dendritic spine analysis, there were n = 5–6 rats/group, n = 3 – 6 dendritic sections/rat, and n = 111 total dendritic sections.
Chronic ethanol exposure during development and adulthood can alter density and morphology of subpopulations of spines (Risher et al., 2015a, McGuier et al., 2015, Uys et al., 2016, Pandey et al., 2015, Trantham-Davidson et al., 2017, Jury et al., 2017, Kroener et al., 2012). Imaris XT (version 8.3.1, Bitplane; Zurich, Switzerland) was used to generate a filament of the dendritic shaft and spines following our routine procedures (Fig. 1B). Dendritic spines (2336 ± 210 spines/treatment group; 389.3 ± 19.0 spines/rat) were classified into 4 categories (stubby, long, filopodia, and mushroom) based on the spine length and the width of the spine head and neck, where L is spine length, HD is spine head diameter, and ND is spine neck diameter. Long spines were identified as having a L ≥ 0.75 μm and < 3 μm, mushroom spines had a L < 3.5 μm, HD > 0.35 μm and a HD > ND, stubby spines had a L < 0.75 μm, and filopodia were identified as having a L ≥ 3 μm. Images were acquired and spines were identified using Imaris software by an experimenter that was blind to the experimental groups. Data on dendritic spine parameters were averaged for each dendritic section and were collated from the Imaris output via a custom script written in Python. Although visual inspection of the classification algorithm clearly identifies spines based upon distinctive morphological characteristics (Uys et al., 2016), some studies have reported a continuum of spine head diameters along the same dendrite (Konur et al., 2003, Wallace and Bear, 2004). Dendritic spine head diameters were parsed into 0.15 μm internals (head diameters ≤ 0.2 μm, 0.21–0.35, 0.36–0.5, etc.) following previously reported methods (Shen et al., 2009, Uys et al., 2016).
Expression of the Fmr1 Gene
Total RNA was extracted from whole hippocampal tissue of Control and AIE exposed animals with or without donepezil treatment using the Qiagen miRNAeasy and DNase kits (Qiagen, Inc., Valencia, CA) as we have described previously (Centanni et al., 2014; Kyzar et al., 2017). Total RNA was then reverse-transcribed at 37°C for 2 hours using the high capacity cDNA RT kit (Life Technologies, Carlsbad, CA). The reaction was stopped by a 5-minute incubation at 85°C. Expression of fragile X mental retardation 1 (Fmr1) (Forward, 5′-AAA GTC CAG AGG GGG ATG GT-3′, Reverse, 5′-TCT CTC CAA ACG CAA CTG GT-3′) was quantified by RT-PCR with SYBR Green mastermix (BioRad, Berkeley, CA, USA). Hypoxanthine phosphoribosyltransferase 1 (Hprt1) (Forward, 5′-TCC TCA GAC CGC TTT TCC CGC-3′, Reverse, 5′-TCA TCA TCA CTA ATC ACG ACG CTG G-3′) was used as the internal control. The Ct value of the Fmr1 gene was corrected with the Ct value of the respective Hprt1 internal standard gene. The ΔΔCt values were calculated for each sample by subtracting the mean ΔCt value of the Water/Water group and the respective fold changes were calculated using the ΔΔCt method (Schmittgen and Livak, 2008, Centanni et al., 2014).
Histone Acetylation of the Fmr1 Gene
Histone H3-K9/14 acetylation (H3-K9/14ac) and H3-K27 acetylation (H3-K27ac) of the Fmr1 gene was measured in the whole hippocampus of control and AIE exposed animals with or without donepezil treatment using chromatin immunoprecipitation (ChIP) assay. The procedure is similar to the methods as described by us earlier (Kyzar et al., 2017). Briefly, hippocampal brain tissue was fixed in methanol-free formaldehyde and DNA sheared by sonication. The resulting DNA-chromatin complex was immunoprecipitated with antibody directed to either H3-K9/14ac (Millipore, 06-599) or H3-K27ac (Cell Signaling, 4353S), and the precipitated DNA was quantified using qPCR with SYBR Green mastermix (BioRad, Berkeley, CA, USA). Primers were designed for the promoter region of the Fmr1 gene (Forward, 5′-CCTCGCTTCCTCCTGTACAA-3′, Reverse, 5′-GCGCAAAGGGTAGTAACAGG-3′) as well as towards a predicted site for the binding if cAMP responsive binding element (CREB) protein in the Fmr1 gene body (Forward, 5′-GCCATTCTGCTTTATTCTAGTTATACTC-3′, Reverse, 5′-AGTCAGAATTTGTGGAAATCTGTTG-3′). The input DNA Ct value was subtracted from the Ct value of each respective sample and the ΔΔCt method (Kyzar et al., 2017, Schmittgen and Livak, 2008, Pandey et al., 2015) was used to determine the fold change of H3-K9/14ac and H3-K27ac levels in the Fmr1 gene. Results are presented as fold change relative to the Water/Water control group.
Statistical Analysis
Dendritic spine data were analyzed as mixed linear modeling (SAS Proc Mixed) that has been used previously in studies of this type (McGuier et al., 2015, Uys et al., 2016), and was selected because of the capacity to handle unbalanced and complex repeated-measures data and the ability to model the variance and correlation structure of repeated-measures experimental designs (Littell et al., 1998). The dendritic spine variables were nested within animal and were further nested across the sequential slices within rat. All gene expression and epigenetic modification data were analyzed by two-way ANOVAs using GraphPad Prism software (version 6.07; GraphPad Software, Inc., La Jolla, CA). Tukey’s test was used for all post-hoc analyses.
RESULTS
Dendrites and Dendritic Spine Density
AIE-induced changes in morphology of dendrites and dendritic spines was assessed in granule neurons in the dorsal dentate gyrus (n = 6 rats/group, n = 3 – 6 dendritic sections/rat, 25–30 sections/treatment group; and n = 111 total dendritic sections; Fig. 1A,B). Data from one control rat were excluded due to a high degree of variability across section that met statistical significance as an outlier. Consistent with other reports of ‘reactive’ dendrites induced by chronic ethanol exposure (Zhou et al., 2007, Uys et al., 2016), AIE exposure significantly increased dendrite diameter of dentate gyrus granule neurons in adult rats (main effect of AIE: F(1,20) = 35.77, p < 0.0001; Fig. 1C). Donepezil treatment did not affect dendrite diameter in water-treated rats nor did it reverse the increase in dendrite diameter in AIE-treated rats (F(1,20) = 1.26, p = 0.27). A significant AIE X donepezil interaction was observed for total dendritic spine density in AIE exposed and donepezil treated rats (F(1,19) = 5.56, p = 0.029; Fig. 1D). Post-hoc analysis revealed that total spine density on dentate gyrus granule neurons was significantly reduced in AIE exposed rats (p = 0.0004). While treatment with donepezil alone did not affect total spine density in water-treated controls (p = 0.982), donepezil treatment significantly reversed the effects of AIE exposure on total spine density (p = 0.008), thus supporting our general hypothesis that donepezil would reverse the effects of AIE on dendritic spine density.
Spine Subclass Density
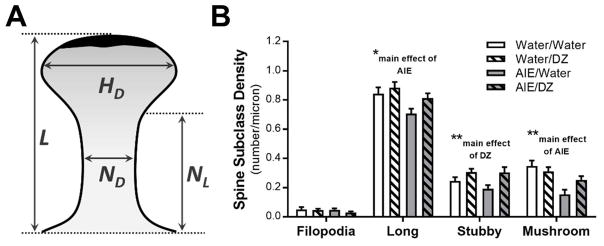
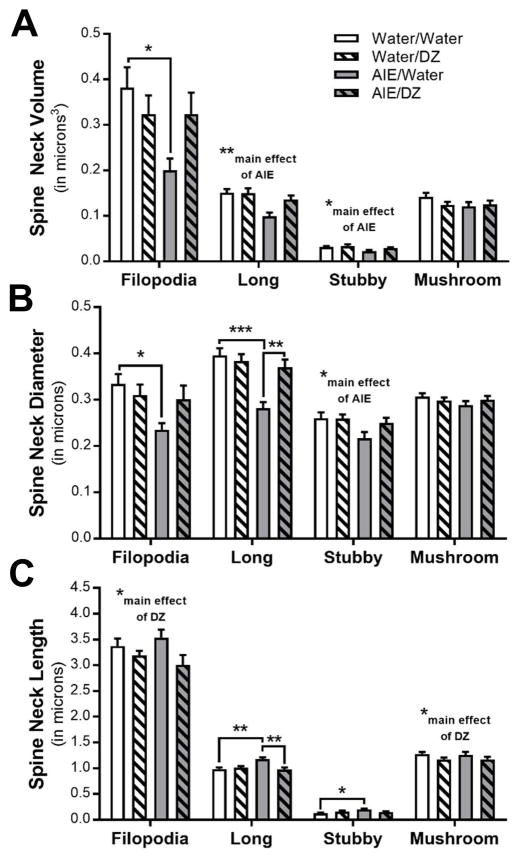
We next determined if AIE exposure affected spine subclasses and if treatment with donepezil was able to reverse those morphological adaptations. Spines were classified into four subclasses (i.e., filopodia, stubby, mushroom, and long) according to their length and head/neck diameters (see Fig. 2A). In addition to analysis of these spine parameters, total spine volume and spine head and neck volume were calculated and analyzed. The results for these analyses are shown in Table 1. AIE exposure significantly reduced the density of long and mushroom shaped spines, and there was a main effect of donepezil on stubby spine density (Fig. 2B).
Fig. 2.
Adaptations in dendritic spine subclasses in adult rats exposed to adolescent intermittent ethanol (AIE) and donepezil (DZ) post-treatment. (A) Schematic illustrating parameters used to classify spines based on their morphological characteristics (HD = head diameter; L = length; ND = neck diameter; NL = neck length). (B) Changes in density of dendritic spine subclasses in AIE and DZ treated rats (Long spines: *p = 0.0131, Water vs AIE; Stubby spines: **p = 0.0056, Water vs DZ; Mushroom spines: **p < 0.001, Water/vs AIE).
Table 1.
Spine subclass statistics (DF = (1,20) for all tests).
| Test | Filopodia | Long | Stubby | Mushroom | |||||
|---|---|---|---|---|---|---|---|---|---|
| F value | P value | F value | P value | F value | P value | F value | P value | ||
| Density | Group | 0.52 | 0.4778 | 7.42 | 0.0131* | 1.00 | 0.3302 | 14.83 | 0.001* |
| Drug | 0.93 | 0.3466 | 3.86 | 0.0633 | 9.62 | 0.0056* | 0.86 | 0.3643 | |
| Interaction | 0.35 | 0.5610 | 0.74 | 0.4006 | 0.92 | 0.3489 | 4.28 | 0.0517 | |
| Length | Group | 0.25 | 0.6216 | 2.32 | 0.1432 | 0.36 | 0.5558 | 0.10 | 0.7549 |
| Drug | 0.5.62 | 0.0279 | 4.18 | 0.0542 | 0.49 | 0.4914 | 3.41 | 0.0795 | |
| Interaction | 0.30 | 0.5904 | 3.75 | 0.0670 | 0.37 | 0.5502 | .20 | 0.6563 | |
| Diameter | Group | 3.15 | 0.0911 | 11.69 | 0.0027 | 11.23 | 0.0032 | 0.50 | 0.4889 |
| Drug | 1.77 | 0.1978 | 8.23 | 0.0095 | 4.83 | 0.0399 | 3.42 | 0.0793 | |
| Interaction | 5.59 | 0.0283 | 10.04 | 0.0048 | 6.79 | 0.0169 | 1.60 | 0.2200 | |
| Head Diameter | Group | 3.45 | 0.0782 | 27.15 | <.0001 | 28.01 | <.0001 | 0.56 | 0.4629 |
| Drug | 0.32 | 0.5786 | 3.52 | 0.0755 | 1.55 | 0.2272 | 1.36 | 0.2574 | |
| Interaction | 6.45 | 0.0195 | 16.81 | <.0006 | 9.7 | 0.0055 | 4.25 | 0.0524 | |
| Volume | Group | 5 | 0.0369 | 16.47 | 0.0006 | 21.8 | <.0001 | 1.53 | 0.2301 |
| Drug | 0.4 | 0.5329 | 4.02 | 0.0586 | 3.98 | 0.0599 | 0.23 | 0.6348 | |
| Interaction | 4.92 | 0.0382 | 5.78 | 0.0260 | 4.61 | 0.0443 | 2.35 | 0.1412 | |
| Head Volume | Group | 3.31 | 0.0839 | 25.16 | <.0001 | 23.24 | <.0001 | 0.4 | 0.5339 |
| Drug | 0.9 | 0.3542 | 2.54 | 0.1270 | 2.3 | 0.1452 | 2.14 | 0.1592 | |
| Interaction | 2.29 | 0.1457 | 13.43 | 0.0015 | 7.81 | 0.0112 | 3.52 | 0.0752 | |
| Neck Length | Group | 0.34 | 0.5674 | 5.85 | 0.0252 | 3.79 | 0.0659 | 0.05 | 0.8281 |
| Drug | 4.55 | 0.0455* | 6.94 | 0.0159 | 0.42 | 0.5235 | 4.48 | 0.0470* | |
| Interaction | 0.48 | 0.49060 | 11.48 | 0.0029 | 6.75 | 0.0172 | 0.05 | 0.8270 | |
| Neck Diameter | Group | 4.54 | 0.0458 | 17.68 | 0.0004 | 4.78 | 0.0408* | 1.16 | 0.2941 |
| Drug | 1.78 | 0.1973 | 6.24 | 0.0214 | 1.85 | 0.1884 | 0.07 | 0.7892 | |
| Interaction | 5.99 | 0.0237 | 11.18 | 0.0032 | 2.01 | 0.1712 | 1.69 | 0.2086 | |
| Neck Volume | Group | 4.75 | 0.0414 | 13.2 | 0.0017* | 6.74 | 0.0173* | 1.46 | 0.2411 |
| Drug | 0.62 | 0.4399 | 3.95 | 0.0609 | 2.33 | 0.1430 | 0.63 | 0.4369 | |
| Interaction | 4.83 | 0.0400 | 4.01 | 0.0588 | 0.67 | 0.4232 | 1.66 | 0.2121 | |
Bold font denotes significant interactions.
denotes significant main effects when interactions did not reach p < 0.05.
Simple effect p values from Tukey’s post-hoc tests are reported in the figure legends, when appropriate.
Spine Morphology
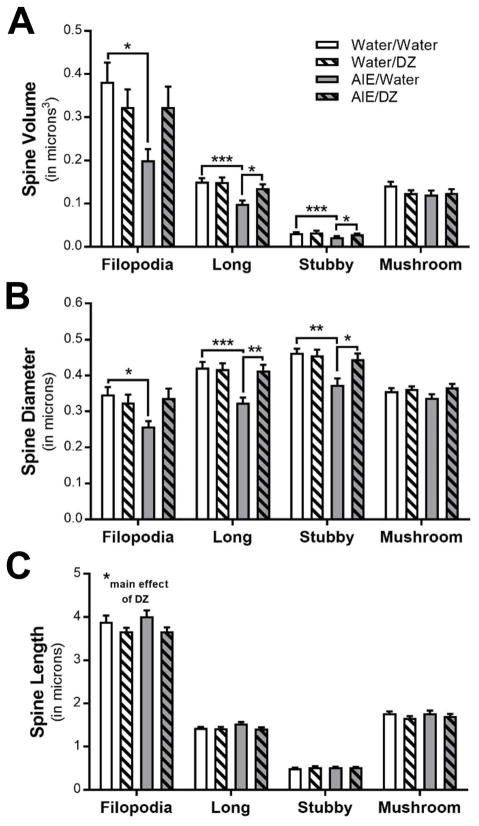
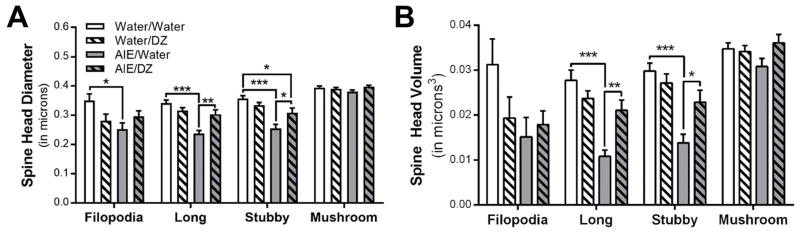
There was a significant reduction in dendritic spine volume and diameter in long and stubby spines and filopodia after AIE, and treatment with donepezil completely reversed this adaptation in long and stubby spines (Fig. 3A,B). The length of dendritic spines within specific subclasses was largely unaffected by AIE or donepezil exposure, with the exception of a minor, but significant decrease in the length of long spines in rats treated with donepezil (Fig. 3C). The head diameter and volume of long and stubby spines were significantly reduced in AIE exposed rats, and these adaptations in the morphology of spine terminal points were reversed by donepezil (Fig. 4A,B). Spine neck volume and diameter were also significantly reduced in long and stubby spines in AIE exposed rats (Fig. 5A,B). Accordingly, spine neck length was increased by AIE in long and stubby spines, and donepezil treatment completely reversed this change in long spines (Fig. 5C). In filopodia, spine head diameter and neck volume and diameter were significantly reduced in AIE-exposed adult rats, but unlike in long spines, donepezil did not reverse these adaptations (Fig. 4 and Fig. 5). Consistent with other studies reporting effects of donepezil on dendritic spines in hippocampus and prefrontal cortex (Alcantara-Gonzalez et al., 2012, Alcantara-Gonzalez et al., 2010), analysis showed main effects of donepezil on stubby spine density (Fig. 2B), filopodia spine length (Fig 3B), and the neck length of mushroom spines and filopodia (Fig. 5C).
Fig. 3.
Morphological adaptations in spine subclass (A) volume (Filopodia: *p = 0.0196, Water/Water vs AIE/Water; Long spines: ***p < 0.0001, Water/Water vs AIE/Water, *p = 0.0313, AIE/Water vs AIE/DZ; Stubby spines: ***p < 0.0001, Water/Water vs AIE/Water, *p = 0.046, AIE/Water vs AIE/DZ), (B) diameter (Filopodia: *p = 0.032, Water/Water vs AIE/Water; Long spines: ***p < 0.001, Water/Water vs AIE/Water; **p < 0.01, AIE/Water vs AIE/DZ; Stubby spines: **p < 0.01, Water/Water vs AIE/Water, *p = 0.0176, AIE/Water vs AIE/DZ), and (C) length in adult rats exposed to AIE and DZ (Filopodia: *p = 0.0279, Water vs DZ).
Fig. 4.
Donepezil reverses AIE-induced morphological adaptations in dendritic spine terminal point (A) diameter (Filopodia: *p = 0.0215, Water/Water vs AIE/Water; Long spines: ***p < 0.0001, Water/Water vs AIE/Water; **p = 0.0029, AIE/Water vs AIE/DZ; Stubby spines: ***p < 0.0001, Water/Water vs AIE/Water, *p = 0.0336, AIE/Water vs AIE/DZ, *p = 0.0456, Water/Water vs AIE/DZ) and (B) volume (Long spines: ***p < 0.0001, Water/Water vs AIE/Water, **p = 0.0088, AIE/Water vs AIE/DZ; Stubby spines: ***p < 0.0001, Water/Water vs AIE/Water, *p = 0.0362, AIE/Water vs AIE/DZ).
Fig. 5.
Morphological characteristics of dendritic spine necks after AIE exposure and donepezil post-treatment. Changes in spine neck (A) volume (Filopodia: *p = 0.0221, Water/Water vs AIE/Water; Long spines: **p = 0.0017, Water vs AIE; Stubby spines: *p = 0.073, Water vs AIE), (B) diameter (Filopodia: *p = 0.0161, Water/Water vs AIE/Water; Long spines: ***p = 0.0001, Water/Water vs AIE/Water; **p = 0.0036, AIE/Water vs AIE/DZ; Stubby spines: *p = 0.031, Water vs AIE), and (C) length (Filopodia: *p = 0.0455, Water vs DZ; Long spines: **p = 0.0025, Water/Water vs AIE/Water; **p = 0.0027, AIE/Water vs AIE/DZ; Stubby spines: *p = 0.0203, Water/Water vs AIE/Water; Mushroom: *p = 0.047, Water vs DZ) in dentate gyrus granule neurons.
As a second level analysis, data were parsed into 0.15 μm intervals according to their spine head diameters following previously reported methods (Uys et al., 2016). AIE exposure significantly increased the number of spines with head diameters ≤ 0.2 μm (interaction: F(1,20) = 11.50, p = 0.0029; post-hoc, p < 0.0001) and reduced the prevalence of spines with head diameters in the 0.36 – 0.5 μm interval (interaction: F(1,20) = 18.73, p = 0.0003; post-hoc, p < 0.0001; Fig. 6). In both intervals, post-AIE treatment with donepezil significantly reversed these effects (≤ 0.2 μm, post-hoc, p = 0.0078; 0.36 – 0.5 μm interval; post-hoc, p = 0.0093). We also observed a small but significant reduction in the prevalence of spine head diameters in the 0.51 – 0.65 μm interval in adult rats exposed to AIE (main effect of AIE: F(1,20) = 15.67, p = 0.0008).
Fig. 6.
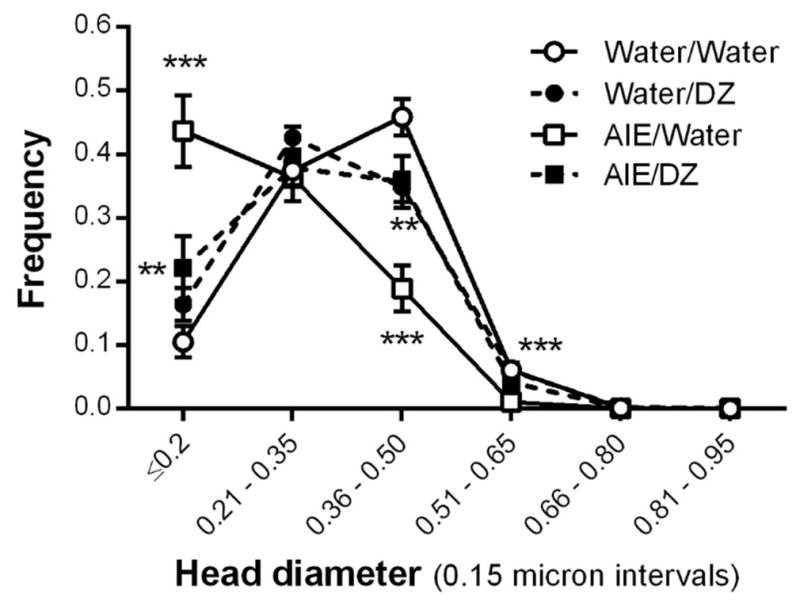
The spine head diameter frequency distribution was significantly changed by AIE exposure and donepezil post-treatment (0.2 μm: ***p < 0.0001, Water/Water vs AIE/Water, **p = 0.0078, AIE/Water vs AIE/DZ; 0.36–0.5 μm: ***p < 0.0001, Water/Water vs AIE/Water, **p = 0.0093, AIE/Water vs AIE/DZ; 0.51–0.65 μm: ***p = 0.0008, Water vs AIE).
AIE and Epigenetic Regulation of Fmr1 expression
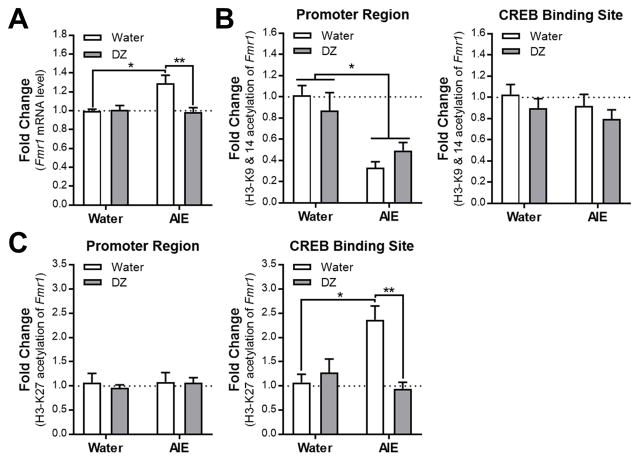
The next set of studies examined if AIE altered Fmr1 expression in hippocampus, and if AIE-induced changes were reversed by donepezil treatment. We further examined if the expression of Fmr1 gene is regulated by histone acetylation mechanisms in the hippocampus in adulthood, after AIE. We found that AIE exposure significantly increased the Fmr1 expression in the hippocampus and this effect was completely normalized by donepezil treatment (interaction: F(1,23) = 6.813, p = 0.0156; n = 5–8/group; Fig. 7A). These results support our hypothesis that AIE may modulate Fmr1 expression via cholinergic mechanisms. Next, we determined the occupancy of H3K9/14ac and H3K27ac of the Fmr1 promoter and CREB binding site of the Fmr1 gene body using ChIP assay. H3K9/14ac levels at the promoter of the Fmr1 gene were significantly decreased in adulthood after AIE, an effect that was not modulated by donepezil treatment (main effect of AIE: F(1,23) = 28.46, p < 0.0001; n = 6–8/group; Fig. 7B). These epigenetic modifications were not changed at the CREB binding site of Fmr1 gene body either by AIE or donepezil treatment (main effect of AIE: (F(1,24) = 1.152, p = 0.294); main effect of DZ: (F(1,24) = 1.671, p = 0.208); n = 6–8/group; Fig. 7B). Interestingly, H3K27ac occupancy at the promoter of Fmr1 gene is not changed by AIE or donepezil treatment (main effect of AIE: (F(1,24) = 0.157, p = 0.695); main effect of DZ: (F(1,24) = 0.189, p = 0.668); n = 6–8/group; Fig. 7C), whereas its occupancy at the CREB binding site of the Fmr1 gene was significantly increased by AIE, which was normalized by donepezil treatment in adulthood (interaction: F(1,24) = 12.77, p = 0.0015; n = 6–8/group; Fig. 7C). These findings suggest a novel regulatory site within the Fmr1 gene that is modulated by H3K27 active mark during AIE exposure as well as donepezil treatment. Furthermore, these novel data suggest the possibility that AIE-induced increased expression of Fmr1 may be regulated by H3K27 acetylation of the CREB binding site within the Fmr1 gene body but not by changes in H3K9/14 acetylation of promoter of the gene.
Fig. 7.
Effects of AIE and donepezil treatment on Fmr1 gene expression and its regulation by histone modifications (H3K9/14 and H3K27 acetylation) in the hippocampus in adulthood. (A) Fmr1 gene expression data is represented as fold changes in mRNA levels. (*p = 0.012, Water/Water vs AIE/Water; **p = 0.0047, AIE/Water vs AIE/DZ). (B,C) ChIP data is represented as fold changes in the occupancy of H3K9/14 and H3K27 acetylation of both promoter and gene body of Fmr1 (CREB binding site: *p = 0.027, Water/Water vs AIE/Water; **p = 0.0004, AIE/Water vs AIE/DZ). All values mean ± SEM and derived from n = 5–8/group.
DISCUSSION
The major finding in this manuscript is that donepezil treatment reversed several adaptations in dendritic spines and genetic and epigenetic modifications of Fmr1 in hippocampus of adult rats exposed to alcohol during adolescence. To our knowledge, this is the first report to show pharmacological reversal of morphological and molecular adaptations produced by AIE. Previous studies have shown that AIE reduces ChAT immunoreactivity in areas Ch1–2 that send cholinergic projections to the hippocampal formation (Swartzwelder et al., 2015, Boutros et al., 2014, Vetreno et al., 2014). Thus, the present findings indicate that targeting this impaired cholinergic system with a clinically used cholinesterase inhibitor is a viable treatment approach to ameliorate hippocampal deficits induced by alcohol exposure during adolescence. In addition, we found that AIE alters Fmr1 transcript levels and histone acetylation at the promoter region and CREB binding site of the gene, in whole hippocampal tissue. We previously reported that FMRP regulates chronic alcohol-induced homeostatic plasticity in hippocampus (Spencer et al., 2016), and a recent study demonstrated that synthesis of new GABAB receptors, which modulate hippocampal local circuit function (Mott et al., 1990), requires FMRP (Wolfe et al., 2016). Thus, the present findings further indicate that FMRP is a critical protein mediating alcohol actions, and add to a growing literature that AIE induces epigenetic modifications in cortical and subcortical regions (Kokare et al., 2017, Sakharkar et al., 2016, Pandey et al., 2017, Pandey et al., 2015, Trantham-Davidson et al., 2017).
The effects of adolescent alcohol exposure on dendritic spines are complex and differ depending on brain region and time since last exposure (Pandey et al., 2015, Trantham-Davidson et al., 2017, Jury et al., 2017). In a previous study, we reported that while AIE exposure does not alter total spine density in CA1 pyramidal neurons, though the prevalence of long spines and filopodia were significantly increased (Risher et al., 2015a). Here, we found that AIE exposure enlarged dendritic shafts, which is consistent with reactive dendrites (Zhou et al., 2007, Uys et al., 2016), and decreased total spine density in dentate gyrus granule neurons. The latter finding is similar to a previous publication that reported reductions in granule neuron spine density when alcohol exposure began in late adolescence (Golub et al., 2015). The present analysis of spine subclasses showed that AIE exposure reduced the density of long and mushroom-shaped spines, and detailed analysis of the morphological characteristics of spine subclasses revealed reductions in overall spine size and spine head size, as measured by both volume and diameter. Moreover, AIE exposure altered characteristics of dendritic spine necks. This is an intriguing finding because spine necks act as diffusion barriers that isolate spino-somatic voltage signals and biochemically compartmentalize spine terminal points from dendritic shafts (Tonnesen et al., 2014, Jayant et al., 2017). Although density was reduced only among long and mushroom spines, AIE exposure impacted the morphological characteristics of all four spine subclasses in the dentate gyrus. This detailed morphological analysis of dendritic spines adds to the growing evidence that AIE exposure has an enduring detrimental impact on hippocampal structure and function (Broadwater et al., 2014, Swartzwelder et al., 2015, Li et al., 2013, Risher et al., 2013, Fleming et al., 2013).
The present findings suggest that donepezil, which is used clinically to enhance the hypo-functioning cholinergic system in Alzheimer’s disease patients, represents a possible pharmacotherapy to reverse AIE-induced deficits in cholinergic signaling. Moreover, donepezil treatment increases dendritic spine density and dendritic arborization in hippocampal neurons (Alcantara-Gonzalez et al., 2012, Alcantara-Gonzalez et al., 2010), underscoring the possibility that cholinergic modulation of spine density may be an important component of functional hippocampal activity. Donepezil also enhances vesicular acetylcholine transporter immunoreactive boutons in a rat model of cholinergic degeneration in nucleus basalis (Ch-4) projection neurons (Ginestet et al., 2007), a region that has also been shown to have reduced ChAT immunoreactivity after AIE (Swartzwelder et al., 2015). Here, donepezil reversed AIE-induced changes in total spine density in dentate granule neurons, while also reversing morphological changes in spine subclass volume and size. In control rats, donepezil had few and modest effects on spine subclass density and morphological characteristics, likely due to the relatively short treatment period. In contrast, its ability to reverse AIE-induced morphological adaptations was quite robust, as a complete reversal of deficits was observed in many of the spine variables that were measured, including parameters of spine heads and necks. Thus, these finding demonstrate that targeting the cholinergic system can quickly reverse morphological abnormalities emerging from adolescent alcohol exposure, while leaving uncompromised spine features intact.
In addition to the spine changes, another major finding reported in this manuscript is that AIE exposure increased Fmr1 transcript levels in hippocampus. Similar to many of the morphological changes, the enhanced expression of Fmr1 levels observed in AIE-exposed rats was reversed by donepezil treatment. Fmr1 knockout mice have higher dendritic spine density in dentate gyrus granule neurons with a higher proportion of long, thin spines (Grossman et al., 2010). Spine density gradually increases in granule neurons before reaching adult levels between PND-30 and PND-60 (Grossman et al., 2010), and recent evidence has shown that FMRP controls spine stabilization during development (Cruz-Martin et al., 2010). It is noteworthy that the increase in Fmr1 expression and parallel reduction in spine density (and long spines, in particular) in the AIE-exposed rats is opposite of what is observed when Fmr1 is deleted (Grossman et al., 2010). Although speculative, these data suggest that the spine changes observed in rats with a history of AIE may be mediated through elevated FMRP signaling. Since spines in granule neurons are still reaching maturation during AIE exposure, Fmr1 expression adaptations represent a possible mechanism that regulates morphological abnormalities. This hypothesis is supported by evidence that donepezil reverses both the spine changes and the elevation in Fmr1 expression in AIE-exposed rats. It is important, however, that these data be interpreted cautiously because the Fmr1 measurements were made in whole hippocampal tissue whereas spines were assessed only on dentate granule cells. Still, we believe that the data on Fmr1 gene expression are valuable in this context because they lead suggest the intriguing hypothesis that the spine changes may be related to changes in Fmr1 expression. Thus, they lay the groundwork for future studies that can address the specific hippocampal nuclei hypothesis.
In addition, while these findings implicate FMRP signaling as a possible molecular mechanism for regulation of the spine adaptations in AIE-treated rats, we also provide evidence that this may occur via changes in histone acetylation of the Fmr1 gene specifically at the CREB binding site. A recent study has reported that AIE exposure reduced markers of neurogenesis and increased cellular toxicity of immature neurons in dentate gyrus (Broadwater et al., 2014). Moreover, markers of neurogenesis and spine density were reduced in newborn granule neurons in a late adolescent alcohol exposure model (Golub et al., 2015). Interestingly, donepezil treatment in adult rats elevated hippocampal neurogenesis and enhanced CREB phosphorylation (Kotani et al., 2008), providing evidence that activating cholinergic systems enhances neurogenesis via CREB signaling. In the present study, we observed elevated H3-K27 acetylation at the CREB binding site of Fmr1 in AIE-exposed rats, an effect that was completely reversed by donepezil treatment. These results show that the CREB binding site of the Fmr1 gene is sensitive to AIE-induced H3-K27 acetylation. These interactions are consistent with previous evidence linking functional coupling of CREB and FMRP proteins (Hwu et al., 1997, Wang et al., 2012, Smith et al., 2006). Furthermore, increased binding of H3-K27 acetylated proteins at the CREB binding site may change the conformation of the gene and allow gene expression to occur. We observed that the AIE-induced increase in Fmr1 expression paralleled the increase in H3-K27 acetylation of CREB binding site of Fmr1 gene in the hippocampus after AIE in adulthood. We previously reported that global H3-K9 acetylation is decreased by AIE in the hippocampus in adulthood (Sakharkar et al., 2016). Here, we observed that H3-K9/14 acetylation is decreased at the promoter but not at the CREB binding site of Fmr1 in the hippocampus after AIE in adulthood. Interestingly, this histone modification is not sensitive to donepezil treatment and appears to not be regulating the Fmr1 expression. Together, these data provide evidence for the interesting mechanism that the CREB binding site of the Fmr1 gene is susceptible to epigenetic modifications that possibly regulate its expression after AIE in adulthood and this is modulated by donepezil treatment.
In recent years it has become clear that in both humans (see Brown et al., 2015) and animal models (Spear, 2016, Spear and Swartzwelder, 2014), adolescence is a period during which individuals are highly sensitive to enduring negative effects of repeated ethanol exposure, which have been described across multiple domains of brain and behavioral function. The present findings add to this timely and important literature in both translational and mechanistic ways. The reductions of dendritic spine density and Fmr1 expression in the hippocampal formation indicate enduring AIE effects on both morphological and molecular endpoints in this structure, which is involved in both cognitive and affective functioning. That both of these effects were ameliorated by sub-chronic donepezil well after the AIE exposure suggests 1) that certain AIE effects are pharmacologically reversible and 2) that these specific effects may be driven, in part, by the depletion of cholinergic input to the hippocampal formation (Swartzwelder et al., 2015, Boutros et al., 2014, Vetreno et al., 2014). Thus, the present findings identify both neuromorphological and gene expression changes that suggest specific mechanisms whereby AIE may alter hippocampal function, and an anti-cholinesterase agent in clinical use (donepezil) that reverses those effects in adulthood, long after the last ethanol dose. These findings also point toward specific epigenetic mechanisms that may underlie AIE-induced pathophysiology and a specific class of therapeutic agents that may be capable of reversing it. Importantly, AIE has been shown to reduce ChAT immunoreactivity not only in regions that project to the hippocampal formation, but also to the amygdala and frontal cortex, both of which have been implicated in mediating the enduring effects of AIE. Thus, further studies of those regions may reveal similar possible mechanisms and therapeutic opportunities.
Acknowledgments
This research was supported by the NIAAA NADIA U24-AA024603 (PJM); P50AA022538, UO1AA-019971, and U24AA-024605 (SCP); U01-AA019925 (HSS) and by VA Senior Research Career Scientist awards to SCP and HSS.
Footnotes
CONFLICT OF INTEREST: The authors have no conflicts of interest.
References
- ACHESON SK, STEIN RM, SWARTZWELDER HS. Impairment of semantic and figural memory by acute ethanol: age-dependent effects. Alcohol Clin Exp Res. 1998;22:1437–42. doi: 10.1111/j.1530-0277.1998.tb03932.x. [DOI] [PubMed] [Google Scholar]
- ALCANTARA-GONZALEZ F, JUAREZ I, SOLIS O, MARTINEZ-TELLEZ I, CAMACHO-ABREGO I, MASLIAH E, MENA R, FLORES G. Enhanced dendritic spine number of neurons of the prefrontal cortex, hippocampus, and nucleus accumbens in old rats after chronic donepezil administration. Synapse. 2010;64:786–93. doi: 10.1002/syn.20787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALCANTARA-GONZALEZ F, MENDOZA-PEREZ CR, ZARAGOZA N, JUAREZ I, ARROYO-GARCIA LE, GAMBOA C, DE LA CRUZ F, ZAMUDIO S, GARCIA-DOLORES F, FLORES G. Combined administration of cerebrolysin and donepezil induces plastic changes in prefrontal cortex in aged mice. Synapse. 2012;66:938–49. doi: 10.1002/syn.21588. [DOI] [PubMed] [Google Scholar]
- ARMSTRONG DM, BRUCE G, HERSH LB, GAGE FH. Development of cholinergic neurons in the septal/diagonal band complex of the rat. Brain Res. 1987;433:249–56. doi: 10.1016/0165-3806(87)90028-9. [DOI] [PubMed] [Google Scholar]
- AUERBACH JM, SEGAL M. A novel cholinergic induction of long-term potentiation in rat hippocampus. J Neurophysiol. 1994;72:2034–40. doi: 10.1152/jn.1994.72.4.2034. [DOI] [PubMed] [Google Scholar]
- BEAUDET G, VALABLE S, BOURGINE J, LELONG-BOULOUARD V, LANFUMEY L, FRERET T, BOULOUARD M, PAIZANIS E. Long-Lasting Effects of Chronic Intermittent Alcohol Exposure in Adolescent Mice on Object Recognition and Hippocampal Neuronal Activity. Alcohol Clin Exp Res. 2016;40:2591–2603. doi: 10.1111/acer.13256. [DOI] [PubMed] [Google Scholar]
- BOUTROS N, SEMENOVA S, LIU W, CREWS FT, MARKOU A. Adolescent intermittent ethanol exposure is associated with increased risky choice and decreased dopaminergic and cholinergic neuron markers in adult rats. Int J Neuropsychopharmacol. 2014:18. doi: 10.1093/ijnp/pyu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROADWATER MA, LIU W, CREWS FT, SPEAR LP. Persistent loss of hippocampal neurogenesis and increased cell death following adolescent, but not adult, chronic ethanol exposure. Dev Neurosci. 2014;36:297–305. doi: 10.1159/000362874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN SA, BRUMBACK T, TOMLINSON K, CUMMINS K, THOMPSON WK, NAGEL BJ, DE BELLIS MD, HOOPER SR, CLARK DB, CHUNG T, HASLER BP, COLRAIN IM, BAKER FC, PROUTY D, PFEFFERBAUM A, SULLIVAN EV, POHL KM, ROHLFING T, NICHOLS BN, CHU W, TAPERT SF. The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): A Multisite Study of Adolescent Development and Substance Use. J Stud Alcohol Drugs. 2015;76:895–908. doi: 10.15288/jsad.2015.76.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAVALLUCCI V, BERRETTA N, NOBILI A, NISTICO R, MERCURI NB, D’AMELIO M. Calcineurin inhibition rescues early synaptic plasticity deficits in a mouse model of Alzheimer’s disease. Neuromolecular Med. 2013;15:541–8. doi: 10.1007/s12017-013-8241-2. [DOI] [PubMed] [Google Scholar]
- CENTANNI SW, TEPPEN T, RISHER ML, FLEMING RL, MOSS JL, ACHESON SK, MULHOLLAND PJ, PANDEY SC, CHANDLER LJ, SWARTZWELDER HS. Adolescent alcohol exposure alters GABAA receptor subunit expression in adult hippocampus. Alcohol Clin Exp Res. 2014;38:2800–8. doi: 10.1111/acer.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMERY TA, HARRIS JB, WILLEMS PJ, OOSTRA BA, IRWIN SA, WEILER IJ, GREENOUGH WT. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci U S A. 1997;94:5401–4. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRUZ-MARTIN A, CRESPO M, PORTERA-CAILLIAU C. Delayed stabilization of dendritic spines in fragile X mice. J Neurosci. 2010;30:7793–803. doi: 10.1523/JNEUROSCI.0577-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUTULI D, DE BARTOLO P, CAPORALI P, TARTAGLIONE AM, ODDI D, D’AMATO FR, NOBILI A, D’AMELIO M, PETROSINI L. Neuroprotective effects of donepezil against cholinergic depletion. Alzheimers Res Ther. 2013;5:50. doi: 10.1186/alzrt215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’ANTUONO M, MERLO D, AVOLI M. Involvement of cholinergic and gabaergic systems in the fragile X knockout mice. Neuroscience. 2003;119:9–13. doi: 10.1016/s0306-4522(03)00103-9. [DOI] [PubMed] [Google Scholar]
- DAHL RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- DAWSON DA, LI TK, GRANT BF. A prospective study of risk drinking: at risk for what? Drug Alcohol Depend. 2008;95:62–72. doi: 10.1016/j.drugalcdep.2007.12.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DREVER BD, RIEDEL G, PLATT B. The cholinergic system and hippocampal plasticity. Behav Brain Res. 2011;221:505–14. doi: 10.1016/j.bbr.2010.11.037. [DOI] [PubMed] [Google Scholar]
- DUDAR JD. The role of the septal nuclei in the release of acetyl-choline from the rabbit cerebral cortex and dorsal hippocampus and the effect of atropine. Brain Res. 1977;129:237–46. doi: 10.1016/0006-8993(77)90004-x. [DOI] [PubMed] [Google Scholar]
- FERNANDEZ DE SEVILLA D, BUNO W. The muscarinic long-term enhancement of NMDA and AMPA receptor-mediated transmission at Schaffer collateral synapses develop through different intracellular mechanisms. J Neurosci. 2010;30:11032–42. doi: 10.1523/JNEUROSCI.1848-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIELD RH, GOSSEN A, CUNNINGHAM C. Prior pathology in the basal forebrain cholinergic system predisposes to inflammation-induced working memory deficits: reconciling inflammatory and cholinergic hypotheses of delirium. J Neurosci. 2012;32:6288–94. doi: 10.1523/JNEUROSCI.4673-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEMING RL, LI Q, RISHER ML, SEXTON HG, MOORE SD, WILSON WA, ACHESON SK, SWARTZWELDER HS. Binge-pattern ethanol exposure during adolescence, but not adulthood, causes persistent changes in GABAA receptor-mediated tonic inhibition in dentate granule cells. Alcohol Clin Exp Res. 2013;37:1154–60. doi: 10.1111/acer.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINESTET L, FERRARIO JE, RAISMAN-VOZARI R, HIRSCH EC, DEBEIR T. Donepezil induces a cholinergic sprouting in basocortical degeneration. J Neurochem. 2007;102:434–40. doi: 10.1111/j.1471-4159.2007.04497.x. [DOI] [PubMed] [Google Scholar]
- GOLUB HM, ZHOU QG, ZUCKER H, MCMULLEN MR, KOKIKO-COCHRAN ON, RO EJ, NAGY LE, SUH H. Chronic Alcohol Exposure is Associated with Decreased Neurogenesis, Aberrant Integration of Newborn Neurons, and Cognitive Dysfunction in Female Mice. Alcohol Clin Exp Res. 2015;39:1967–77. doi: 10.1111/acer.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOULD E, WOOLF NJ, BUTCHER LL. Postnatal development of cholinergic neurons in the rat: I. Forebrain. Brain Res Bull. 1991;27:767–89. doi: 10.1016/0361-9230(91)90209-3. [DOI] [PubMed] [Google Scholar]
- GROSSMAN AW, ALDRIDGE GM, LEE KJ, ZEMAN MK, JUN CS, AZAM HS, ARII T, IMOTO K, GREENOUGH WT, RHYU IJ. Developmental characteristics of dendritic spines in the dentate gyrus of Fmr1 knockout mice. Brain Res. 2010;1355:221–7. doi: 10.1016/j.brainres.2010.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSSMAN AW, ALDRIDGE GM, WEILER IJ, GREENOUGH WT. Local protein synthesis and spine morphogenesis: Fragile X syndrome and beyond. J Neurosci. 2006a;26:7151–5. doi: 10.1523/JNEUROSCI.1790-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSSMAN AW, ELISSEOU NM, MCKINNEY BC, GREENOUGH WT. Hippocampal pyramidal cells in adult Fmr1 knockout mice exhibit an immature-appearing profile of dendritic spines. Brain Res. 2006b;1084:158–64. doi: 10.1016/j.brainres.2006.02.044. [DOI] [PubMed] [Google Scholar]
- HANSON KL, CUMMINS K, TAPERT SF, BROWN SA. Changes in neuropsychological functioning over 10 years following adolescent substance abuse treatment. Psychol Addict Behav. 2011;25:127–42. doi: 10.1037/a0022350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HWU WL, WANG TR, LEE YM. FMR1 enhancer is regulated by cAMP through a cAMP-responsive element. DNA Cell Biol. 1997;16:449–53. doi: 10.1089/dna.1997.16.449. [DOI] [PubMed] [Google Scholar]
- IVANCO TL, GREENOUGH WT. Altered mossy fiber distributions in adult Fmr1 (FVB) knockout mice. Hippocampus. 2002;12:47–54. doi: 10.1002/hipo.10004. [DOI] [PubMed] [Google Scholar]
- JAYANT K, HIRTZ JJ, PLANTE IJ, TSAI DM, DE BOER WD, SEMONCHE A, PETERKA DS, OWEN JS, SAHIN O, SHEPARD KL, YUSTE R. Targeted intracellular voltage recordings from dendritic spines using quantum-dot-coated nanopipettes. Nat Nanotechnol. 2017;12:335–342. doi: 10.1038/nnano.2016.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JURY NJ, POLLACK GA, WARD MJ, BEZEK JL, NG AJ, PINARD CR, BERGSTROM HC, HOLMES A. Chronic Ethanol During Adolescence Impacts Corticolimbic Dendritic Spines and Behavior. Alcohol Clin Exp Res. 2017;41:1298–1308. doi: 10.1111/acer.13422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOKARE DM, KYZAR EJ, ZHANG H, SAKHARKAR AJ, PANDEY SC. Adolescent alcohol exposure-induced changes in alpha-melanocyte stimulating hormone and neuropeptide Y pathways via histone acetylation in the brain during adulthood. Int J Neuropsychopharmacol. 2017 doi: 10.1093/ijnp/pyx041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONUR S, RABINOWITZ D, FENSTERMAKER VL, YUSTE R. Systematic regulation of spine sizes and densities in pyramidal neurons. J Neurobiol. 2003;56:95–112. doi: 10.1002/neu.10229. [DOI] [PubMed] [Google Scholar]
- KOTANI S, YAMAUCHI T, TERAMOTO T, OGURA H. Donepezil, an acetylcholinesterase inhibitor, enhances adult hippocampal neurogenesis. Chem Biol Interact. 2008;175:227–30. doi: 10.1016/j.cbi.2008.04.004. [DOI] [PubMed] [Google Scholar]
- KRISHNAN HR, SAKHARKAR AJ, TEPPEN TL, BERKEL TD, PANDEY SC. The epigenetic landscape of alcoholism. Int Rev Neurobiol. 2014;115:75–116. doi: 10.1016/B978-0-12-801311-3.00003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KROENER S, MULHOLLAND PJ, NEW NN, GASS JT, BECKER HC, CHANDLER LJ. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One. 2012;7:e37541. doi: 10.1371/journal.pone.0037541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KYZAR EJ, ZHANG H, SAKHARKAR AJ, PANDEY SC. Adolescent alcohol exposure alters lysine demethylase 1 (LSD1) expression and histone methylation in the amygdala during adulthood. Addict Biol. 2017;22:1191–1204. doi: 10.1111/adb.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI Q, FLEMING RL, ACHESON SK, MADISON RD, MOORE SD, RISHER ML, WILSON WA, SWARTZWELDER HS. Long-term modulation of A-type K(+) conductances in hippocampal CA1 interneurons in rats after chronic intermittent ethanol exposure during adolescence or adulthood. Alcohol Clin Exp Res. 2013;37:2074–85. doi: 10.1111/acer.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTELL RC, HENRY PR, AMMERMAN CB. Statistical analysis of repeated measures data using SAS procedures. Journal of Animal Science. 1998;76:1216– 1231. doi: 10.2527/1998.7641216x. [DOI] [PubMed] [Google Scholar]
- LITTLE PJ, KUHN CM, WILSON WA, SWARTZWELDER HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20:1346–51. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- MADISON DV, LANCASTER B, NICOLL RA. Voltage clamp analysis of cholinergic action in the hippocampus. J Neurosci. 1987;7:733–41. doi: 10.1523/JNEUROSCI.07-03-00733.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKWIESE BJ, ACHESON SK, LEVIN ED, WILSON WA, SWARTZWELDER HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–21. [PubMed] [Google Scholar]
- MCGUIER NS, PADULA AE, LOPEZ MF, WOODWARD JJ, MULHOLLAND PJ. Withdrawal from chronic intermittent alcohol exposure increases dendritic spine density in the lateral orbitofrontal cortex of mice. Alcohol. 2015;49:21–7. doi: 10.1016/j.alcohol.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MESULAM MM, MUFSON EJ, WAINER BH, LEVEY AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1–Ch6) Neuroscience. 1983;10:1185–201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- MONTI PM, MIRANDA R, JR, NIXON K, SHER KJ, SWARTZWELDER HS, TAPERT SF, WHITE A, CREWS FT. Adolescence: booze, brains, and behavior. Alcohol Clin Exp Res. 2005;29:207–20. doi: 10.1097/01.alc.0000153551.11000.f3. [DOI] [PubMed] [Google Scholar]
- MOTA N, PARADA M, CREGO A, DOALLO S, CAAMANO-ISORNA F, RODRIGUEZ HOLGUIN S, CADAVEIRA F, CORRAL M. Binge drinking trajectory and neuropsychological functioning among university students: a longitudinal study. Drug Alcohol Depend. 2013;133:108–14. doi: 10.1016/j.drugalcdep.2013.05.024. [DOI] [PubMed] [Google Scholar]
- MOTT DD, LEWIS DV, FERRARI CM, WILSON WA, SWARTZWELDER HS. Baclofen facilitates the development of long-term potentiation in the rat dentate gyrus. Neurosci Lett. 1990;113:222–6. doi: 10.1016/0304-3940(90)90307-u. [DOI] [PubMed] [Google Scholar]
- NGUYEN-LOUIE TT, TRACAS A, SQUEGLIA LM, MATT GE, EBERSON-SHUMATE S, TAPERT SF. Learning and Memory in Adolescent Moderate, Binge, and Extreme-Binge Drinkers. Alcohol Clin Exp Res. 2016;40:1895–904. doi: 10.1111/acer.13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA. Why do adolescents drink, what are their risks, and how can underage drinking be prevented? Alcohol Alert. 2006;67:1–7. [Google Scholar]
- PANDEY SC, KYZAR EJ, ZHANG H. Epigenetic basis of the dark side of alcohol addiction. Neuropharmacology. 2017;122:74–84. doi: 10.1016/j.neuropharm.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANDEY SC, SAKHARKAR AJ, TANG L, ZHANG H. Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood. Neurobiol Dis. 2015;82:607–619. doi: 10.1016/j.nbd.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFEFFERBAUM A, ROHLFING T, POHL KM, LANE B, CHU W, KWON D, NOLAN NICHOLS B, BROWN SA, TAPERT SF, CUMMINS K, THOMPSON WK, BRUMBACK T, MELOY MJ, JERNIGAN TL, DALE A, COLRAIN IM, BAKER FC, PROUTY D, DE BELLIS MD, VOYVODIC JT, CLARK DB, LUNA B, CHUNG T, NAGEL BJ, SULLIVAN EV. Adolescent Development of Cortical and White Matter Structure in the NCANDA Sample: Role of Sex, Ethnicity, Puberty, and Alcohol Drinking. Cereb Cortex. 2016;26:4101–21. doi: 10.1093/cercor/bhv205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RISHER ML, FLEMING RL, BOUTROS N, SEMENOVA S, WILSON WA, LEVIN ED, MARKOU A, SWARTZWELDER HS, ACHESON SK. Long-term effects of chronic intermittent ethanol exposure in adolescent and adult rats: radial-arm maze performance and operant food reinforced responding. PLoS One. 2013;8:e62940. doi: 10.1371/journal.pone.0062940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RISHER ML, FLEMING RL, RISHER WC, MILLER KM, KLEIN RC, WILLS T, ACHESON SK, MOORE SD, WILSON WA, EROGLU C, SWARTZWELDER HS. Adolescent intermittent alcohol exposure: persistence of structural and functional hippocampal abnormalities into adulthood. Alcohol Clin Exp Res. 2015a;39:989–97. doi: 10.1111/acer.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RISHER ML, SEXTON HG, RISHER WC, WILSON WA, FLEMING RL, MADISON RD, MOORE SD, EROGLU C, SWARTZWELDER HS. Adolescent Intermittent Alcohol Exposure: Dysregulation of Thrombospondins and Synapse Formation are Associated with Decreased Neuronal Density in the Adult Hippocampus. Alcohol Clin Exp Res. 2015b;39:2403–13. doi: 10.1111/acer.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKHARKAR AJ, VETRENO RP, ZHANG H, KOKARE DM, CREWS FT, PANDEY SC. A role for histone acetylation mechanisms in adolescent alcohol exposure-induced deficits in hippocampal brain-derived neurotrophic factor expression and neurogenesis markers in adulthood. Brain Struct Funct. 2016;221:4691–4703. doi: 10.1007/s00429-016-1196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHARKOWSKI F, FROTSCHER M, LUTZ D, KORTE M, MICHAELSEN-PREUSSE K. Altered Connectivity and Synapse Maturation of the Hippocampal Mossy Fiber Pathway in a Mouse Model of the Fragile X Syndrome. Cereb Cortex. 2017 doi: 10.1093/cercor/bhw408. [DOI] [PubMed] [Google Scholar]
- SCHMITTGEN TD, LIVAK KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- SCREMIN OU, ROCH M, NORMAN KM, DJAZAYERI S, LIU YY. Brain acetylcholine and choline concentrations and dynamics in a murine model of the Fragile X syndrome: age, sex and region-specific changes. Neuroscience. 2015;301:520–8. doi: 10.1016/j.neuroscience.2015.06.036. [DOI] [PubMed] [Google Scholar]
- SHEN HW, TODA S, MOUSSAWI K, BOUKNIGHT A, ZAHM DS, KALIVAS PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009;29:2876–84. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHER KJ, GOTHAM HJ. Pathological alcohol involvement: a developmental disorder of young adulthood. Dev Psychopathol. 1999;11:933–56. doi: 10.1017/s0954579499002394. [DOI] [PubMed] [Google Scholar]
- SMITH HR, PANG KC. Orexin-saporin lesions of the medial septum impair spatial memory. Neuroscience. 2005;132:261–71. doi: 10.1016/j.neuroscience.2004.12.037. [DOI] [PubMed] [Google Scholar]
- SMITH KT, NICHOLLS RD, REINES D. The gene encoding the fragile X RNA-binding protein is controlled by nuclear respiratory factor 2 and the CREB family of transcription factors. Nucleic Acids Res. 2006;34:1205–15. doi: 10.1093/nar/gkj521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPEAR L. Modeling adolescent development and alcohol use in animals. Alcohol Res Health. 2000a;24:115–23. [PMC free article] [PubMed] [Google Scholar]
- SPEAR LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000b;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- SPEAR LP. Consequences of adolescent use of alcohol and other drugs: Studies using rodent models. Neurosci Biobehav Rev. 2016;70:228–243. doi: 10.1016/j.neubiorev.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPEAR LP, SWARTZWELDER HS. Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: a mini-review. Neurosci Biobehav Rev. 2014;45:1–8. doi: 10.1016/j.neubiorev.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPENCER KB, MULHOLLAND PJ, CHANDLER LJ. FMRP Mediates Chronic Ethanol-Induced Changes in NMDA, Kv4.2, and KChIP3 Expression in the Hippocampus. Alcohol Clin Exp Res. 2016;40:1251–61. doi: 10.1111/acer.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SQUEGLIA LM, SCHWEINSBURG AD, PULIDO C, TAPERT SF. Adolescent binge drinking linked to abnormal spatial working memory brain activation: differential gender effects. Alcohol Clin Exp Res. 2011;35:1831–41. doi: 10.1111/j.1530-0277.2011.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SQUEGLIA LM, TAPERT SF, SULLIVAN EV, JACOBUS J, MELOY MJ, ROHLFING T, PFEFFERBAUM A. Brain development in heavy-drinking adolescents. Am J Psychiatry. 2015;172:531–42. doi: 10.1176/appi.ajp.2015.14101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWARTZWELDER HS, ACHESON SK, MILLER KM, SEXTON HG, LIU W, CREWS FT, RISHER ML. Adolescent Intermittent Alcohol Exposure: Deficits in Object Recognition Memory and Forebrain Cholinergic Markers. PLoS One. 2015;10:e0140042. doi: 10.1371/journal.pone.0140042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONNESEN J, KATONA G, ROZSA B, NAGERL UV. Spine neck plasticity regulates compartmentalization of synapses. Nat Neurosci. 2014;17:678–85. doi: 10.1038/nn.3682. [DOI] [PubMed] [Google Scholar]
- TRANTHAM-DAVIDSON H, CENTANNI SW, GARR SC, NEW NN, MULHOLLAND PJ, GASS JT, GLOVER EJ, FLORESCO SB, CREWS FT, KRISHNAN HR, PANDEY SC, CHANDLER LJ. Binge-Like Alcohol Exposure During Adolescence Disrupts Dopaminergic Neurotransmission in the Adult Prelimbic Cortex. Neuropsychopharmacology. 2017;42:1024–1036. doi: 10.1038/npp.2016.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UYS JD, MCGUIER NS, GASS JT, GRIFFIN WC, 3RD, BALL LE, MULHOLLAND PJ. Chronic intermittent ethanol exposure and withdrawal leads to adaptations in nucleus accumbens core postsynaptic density proteome and dendritic spines. Addict Biol. 2016;21:560–74. doi: 10.1111/adb.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VETRENO RP, BROADWATER M, LIU W, SPEAR LP, CREWS FT. Adolescent, but not adult, binge ethanol exposure leads to persistent global reductions of choline acetyltransferase expressing neurons in brain. PLoS One. 2014;9:e113421. doi: 10.1371/journal.pone.0113421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLACE W, BEAR MF. A morphological correlate of synaptic scaling in visual cortex. J Neurosci. 2004;24:6928–38. doi: 10.1523/JNEUROSCI.1110-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG H, MORISHITA Y, MIURA D, NARANJO JR, KIDA S, ZHUO M. Roles of CREB in the regulation of FMRP by group I metabotropic glutamate receptors in cingulate cortex. Mol Brain. 2012;5:27. doi: 10.1186/1756-6606-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE AM, TRUESDALE MC, BAE JG, AHMAD S, WILSON WA, BEST PJ, SWARTZWELDER HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73:673–7. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- WITTER MP. The perforant path: projections from the entorhinal cortex to the dentate gyrus. Prog Brain Res. 2007;163:43–61. doi: 10.1016/S0079-6123(07)63003-9. [DOI] [PubMed] [Google Scholar]
- WOLFE SA, WORKMAN ER, HEANEY CF, NIERE F, NAMJOSHI S, CACHEAUX LP, FARRIS SP, DREW MR, ZEMELMAN BV, HARRIS RA, RAAB-GRAHAM KF. FMRP regulates an ethanol-dependent shift in GABABR function and expression with rapid antidepressant properties. Nat Commun. 2016;7:12867. doi: 10.1038/ncomms12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAMOTO Y, SHIODA N, HAN F, MORIGUCHI S, FUKUNAGA K. Donepezil-induced neuroprotection of acetylcholinergic neurons in olfactory bulbectomized mice. Yakugaku Zasshi. 2010;130:717–21. doi: 10.1248/yakushi.130.717. [DOI] [PubMed] [Google Scholar]
- ZAHALKA EA, SEIDLER FJ, LAPPI SE, YANAI J, SLOTKIN TA. Differential development of cholinergic nerve terminal markers in rat brain regions: implications for nerve terminal density, impulse activity and specific gene expression. Brain Res. 1993;601:221–9. doi: 10.1016/0006-8993(93)91714-4. [DOI] [PubMed] [Google Scholar]
- ZHOU FC, ANTHONY B, DUNN KW, LINDQUIST WB, XU ZC, DENG P. Chronic alcohol drinking alters neuronal dendritic spines in the brain reward center nucleus accumbens. Brain Res. 2007;1134:148–61. doi: 10.1016/j.brainres.2006.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]