Abstract
Influenza is a disease of the respiratory system caused by single stranded RNA viruses with varying genotypes. Immunopathogenesis to influenza viruses differs based on virus strain, dose, and mouse strain used in laboratory models. Although effective mucosal immune defenses are important in early host defense against influenza, information on the kinetics of these immune defense mechanisms during the course of influenza infection is limited. We investigated changes to antimicrobial peptides and primary innate immune cells at early time points after infection and compared these variables between two prominent H1N1 influenza A virus (IAV) strains, A/CA/04/2009 and A/PR/08/1934 in C57BL/6 mice. Alveolar and parenchymal macrophage ratios were altered after IAV infection and pro-inflammatory cytokine production in macrophages was induced after IAV infection. Genes encoding antimicrobial peptides, β-defensin (Defb4), bactericidal-permeability increasing protein (Bpifa1), and cathelicidin antimicrobial peptide (Camp), were differentially regulated after IAV infection and the kinetics of Defb4 expression differed in response to each virus strain. Beta-defensin reduced infectivity of A/CA/04/2009 virus but not A/PR/08/1934. Beta defensins also changed the innate immune cell profile wherein mice pre-treated with β-defensin had increased alveolar macrophages and CD103+ dendritic cells, and reduced CD11b+ dendritic cells and neutrophils. In addition to highlighting that immune responses may vary based on influenza virus strain used, our data suggest an important role for antimicrobial peptides in host defense against influenza virus.
Keywords: H1N1 influenza, Defensins, Macrophage, Dendritic Cell, Inflammation
1. Introduction
Influenza is an infectious disease of the pulmonary system caused by viruses of the Orthomyxoviridae family. Influenza A virus (IAV) can undergo changes over a period of time in the form of gradual mutations (antigenic drift) or abrupt changes in surface proteins (antigenic shift) which may render the virus highly infectious and/or transmissible thereby reducing the efficacy of vaccines. IAV infections have resulted in four pandemics since 1900, of which the 1918 pandemic alone claimed over 50 million lives worldwide (Taubenberger and Morens, 2006).
Influenza pathogenesis is a combination of viral virulence and host responses. Early defenses in the lung after virus infection have a strong influence on host protection and antiviral immunity. Primary immune defense strategies in the lungs against environmental pathogens are multilayered, consisting of a physical barrier (epithelial cells), mucosal liquid containing antimicrobial peptides (AMPs), and immune cells (macrophages and neutrophils) (Nicod, 2005). Antimicrobial peptides are an important primary host defense mechanism in the respiratory mucosa and are generally classified by the presence or absence of disulfide bonds. Most AMPs found in the lung mucosa are produced by epithelial and innate immune cells, and their functions are better characterized in defense against bacteria than viruses wherein AMP-induced clumping and trapping of bacteria abrogate their ability to invade host cells (Boyton and Openshaw, 2002). Epithelial AMP expression is regulated by pathogen exposure and innate mediators (Tecle et al., 2010). Defensins, AMPs that contain three disulfide bonds, are produced by epithelial cells and leukocytes in the mucosa (Risso, 2000) and are increased during acute inflammation (Bals et al., 2001). Beta-defensins can activate immature DCs (Biragyn et al., 2002) and inhibit Haemophilus influenzae infections (Moser et al., 2002), and recently shown to inhibit IAV (Zhao et al., 2016). As one of the most abundant proteins in the airways, bactericidal-permeability-increasing (BPI) proteins inhibit bacteria and fungi from colonizing the lungs (Britto and Cohn, 2015; Seshadri et al., 2012). BPI family member SPLUNC can inhibit the growth of biofilm forming bacteria (Gally et al., 2011; Lukinskiene et al., 2011). Cathelicidin antimicrobial peptide (CAMP) deficient mice have increased susceptibility to skin infections (Nizet et al., 2001), and human cathelicidin, LL-37, inhibits IAV infectivity by damaging the viral envelope (Tripathi et al., 2013), and pre-treatment of mice with LL-37 resulted in lower lung viral burden and pro-inflammatory cytokines (Barlow et al., 2011). Since AMPs may have therapeutic potential against viral disease, understanding how they regulate early immune responses is important.
Macrophages play important roles in host defense against inhaled pathogens through phagocytic clearance and production of mediators that enhance immune responses. Numerous studies have established that IAV infects macrophages by binding to lectin-type receptors (Reading et al., 2000; Upham et al., 2010) although effective viral replication does not occur within these cells (Rodgers and Mims, 1981; Tate et. al., 2010). Pro-inflammatory cytokines produced by macrophages during IAV infection (Lee et al., 2012) may promote inflammation and help control viral replication and clearance. IAV can directly impact macrophage function by altering the expression of SOCS-1 and RIG-I and these responses differ by IAV strain (Ramirez-Martinez et al., 2013). Alveolar macrophage depletion in swine prior to IAV infection resulted in blunted adaptive immune responses (Kim et al., 2008), and alveolar macrophages are reduced during IAV infection of mice (Ghoneim et al., 2013). The reduction of macrophage populations in the lungs and associated alterations of functional capacity may significantly impair host defenses.
We hypothesized that mouse-adapted (A/PR/08/1934) and non-adapted (A/CA/04/2009) H1N1 strains of IAV differentially induce primary mucosal defense mechanisms in the lungs. Early markers of inflammation and AMP expression were regulated during IAV infection, and mouse β-defensin 4 (MBD4) had an impact on viral replication and early immune cell regulation. Taken together, our data show that early immune defenses differ by IAV strain and AMPs alter IAV pathogenesis. This has important ramifications both for our understanding of host immunity and for IAV strain selection in immunological studies.
2. Materials and Methods
2.1 Ethics statement
All experiments were approved by the Institutional Animal Use and Care Committees at St. Jude Children's Research Hospital and the University of Tennessee Health Science Center.
2.2 Viruses
A comparison of published protein sequences between the laboratory strain A/PR/08/1934 (PR8) and the 2009 pandemic influenza strain A/CA/04/2009 (CA4) suggested that virus virulence and replication within hosts, and immune responses to these IAV strains may differ (Supplementary Figure 1). The CA4 strain obtained from Dr. Richard Webby at SJCRH was propagated in Madin-Darby canine kidney (MDCK.2, ATCC, Manassas, VA) cells while our PR8 strain was cultured in embryonated chicken eggs. Both strains were sequence verified to be void of any mutations in hemagglutinin and neuraminidase genes. Effects of virus propagation methods have been extensively investigated and are not the focus of this work.
2.3 Animals
Seventeen-week-old C57BL/6 female mice from Jackson Laboratories (Bar Harbor, ME) were acclimatized for one week in specific pathogen-free ABSL2 facilities with ad libitum access to food and water in a 12-hour light:dark cycle.
2.4 Influenza model
Both these virus strains can induce severe morbidity and mortality in mice. Since the focus of this study was to investigate innate immune responses to disease, we selected a non-lethal dose of virus to perform the model. Mice were infected intranasally with 1000 TCID50 of either CA4 or PR8 virus in 50 μL. In our hands, C57BL/6 mice infected with CA4 at this dose have a weight loss nadir at ~12-15% while PR8 induces a nadir at ~25-27% and no death (cut off set to 30% weight loss or other signs of severe morbidity). Mice in the mock-treated group were administered PBS in place of virus. All animals were weighed prior to infection and every day thereafter until sacrifice. Animals were euthanized by CO2 asphyxiation followed by cervical dislocation at predetermined time points after infection.
2.5 Sample harvest
Euthanized mice were tracheostomized and bronchoalveolar lavage (BAL) was performed twice with 1 mL of sterile PBS and stored in ice. The middle and accessory lobes of the right lung were snap frozen in liquid nitrogen for RNA analyses. Left lobes were harvest and fixed ex vivo by injecting 1 mL of 10% neutral buffered formalin for histological analysis. Whole lungs were harvested and snap frozen in liquid nitrogen from mice for viral titer determination. All samples were stored at −80°C until use.
2.6 Virus titration
Lungs collected and stored at −80°C were placed in ice and homogenized in the presence of 1 mL of sterile PBS. Homogenates were centrifuged at 600 ×g for 10 mins at 4°C and supernatants were stored in aliquots at −80°C. Thawed aliquots were serially diluted at 1:10 and used to infect MDCK.2 cells grown to confluence in 96-well plates. Cells were washed one hour later and incubated in media containing 1 μg/mL TPCK-trypsin (Worthington Biochemical, Lakewood, NJ) at 37°C/5% CO2. Viral titers were read 72 h later by hemagglutination assay in the presence of 0.5% chicken red blood cells and calculated by the Reed-Muench method.
2.7 BAL cell analysis
BAL contents were centrifuged at 600 ×g for 10 mins at 4°C and supernatants stored at −80°C. BAL cells were re-suspended in 0.2 mL of PBS and cytospun onto a glass slide. Slides were differentially stained (Quik-Dip, Mercedes Medical, Sarasota, FL) for morphometric analyses. Slides were observed under high power magnification (1250×) of a light microscope to enumerate ciliated epithelial cells in five randomly selected fields by an investigator blinded to the study groups.
2.8 Histological analysis
Formalin fixed left lungs were paraffin embedded and 4 μm sections were affixed onto glass slides, de-paraffinized and stained with Hematoxylin and Eosin. Slides were observed under low power magnification (200×) in a light microscope and peribronchial and perivascular inflammation was scored by an investigator blinded to the study groups. Mean values were reported for each group. Photomicrographs were obtained with a camera attached to a Nikon (Eclipse, 50i) light microscope using Nikon Elements software.
2.9 Quantitative real time PCR
Middle and accessory lobes of the right lungs stored at −80°C were used to harvest RNA using TRIzol® (Invitrogen, Grand Island, NY) and chloroform (IBI Scientific, Peosta, IA). One microgram of RNA was converted into cDNA with iScript™ (Bio-Rad, Hercules, CA) and used to quantify changes in gene expression by quantitative real-time PCR with RNA-specific QuantiTect primer assays (Qiagen, Valencia, CA) for Defb4, Bpifa1, and Camp. Data were acquired with the ABI-7500 machine (Applied Biosystems, Carlsbad, CA) and relative fold change in gene expression was determined using the 2−ΔΔCt method normalized to the internal housekeeping gene Hprt.
2.10 ELISA
The amount of IFNγ and TNFα (BD Biosciences, San Jose, CA) in undiluted BAL fluid was measured by ELISA according to manufacturer's recommended protocols.
2.11 Virus infection assays with recombinant beta defensin 4 (MBD4)
Log105 TCID50/mL of each virus strain was incubated for one hour at 37°C with known concentrations of MBD4 recombinant protein (MyBioSource, San Diego, CA) following which a hemagglutination assay was performed to determine the HA titer. Alternatively, virus incubated with MBD4 was used to inoculate confluent MDCK.2 cells and viral titers were determined at 48 hpi.
2.12 Mouse treatment with MBD4
Anesthetized mice were administered 100 μg of MBD4 intranasally in 20 μL and immediately infected with 1000 TCID50 either PR8 or CA4 virus in 50 μL. Samples (BAL and lungs) were harvested for viral titers and immune cell analyses at 48 hpi.
2.13 Flow cytometric analysis
Following BAL, whole lungs were harvested from euthanized mice infected with each strain of virus, or neither at 48 hpi. BAL cells were recovered from centrifugation of BAL at 600 ×g, while immune cells in lung were recovered by gentleMACS (Miltenyi Biotec, San Diego, CA) lung dissociation isolation protocol per manufacturer's guidelines. Cells were enumerated by Trypan Blue dye exclusion and non-specific binding sites were blocked by incubation in human gammaglobulin (Sigma-Aldrich, St. Louis, MO) for 30 minutes in ice. Washed cells were then incubated in fluorescent-tagged antibodies for 30 minutes in ice in the dark. In studies utilizing cells for determination of cytokine production, harvested cells from the BAL or lungs were incubated in protein transport inhibitor (BD Biosciences GolgiPlug) for 6 h, and intracellular staining for cytokines was performed following fixation and permeabilization (BD Biosciences) using fluorescently labeled antibodies against IFNγ and TNFα. Controls included unstained cells, single color and isotype controls. Data were acquired with an LSRFortessa (BD Biosciences, San Jose, CA) and analyzed with FlowJo (Ashland, OR) software. See Supplementary Table for antibodies and Supplemental Figures 2 and 3 for gating strategies.
2.14 Statistical analysis
Each group consisted of at least five animals. The mean and standard deviation were calculated for each group. Studies were repeated for reproducibility and data are representative of one experiment. Data were compared between groups with non-parametric one-way or two-way ANOVA or Student's t-test with alpha set to 0.05. Post-hoc tests were performed and indicated in Figure Legends. Significant (p<0.05) differences were denoted by asterisk (*),delta (Δ), or pound (#) symbols.
3. Results
Influenza viruses infect host cells and replicate rapidly causing disease that manifests partially due to direct cellular damage and partially due to the host's immune response to replicating virus. Immediate host immune responses to IAV infection are important to neutralize and limit the viral burden. These responses may differ based on genetic differences between H1N1 IAV strains (Supplemental Fig 1). Therefore, we investigated the innate immune responses against two H1N1 IAV strains, the mouse-adapted laboratory strain PR8 and a clinical strain from the last pandemic, CA4.
3.1 PR8 induced rapid weight loss despite similar replication kinetics
Weight loss is a marker of influenza morbidity in mice. While both IAV strains caused weight loss at the dose used for infection, changes in weight loss kinetics were apparent early during infection after PR8 (Fig 1A) although viral replication kinetics were similar (Fig 1B).
Figure 1. Host morbidity and viral replication in the lungs were induced by influenza A virus.
At an infectious dose of 1000 TCID50, PR8 induced significantly more weight loss starting early in the infection compared to CA4 (A). Both viruses replicated efficiently at the same rate in the lungs of infected mice appearing to reach the plateau at 120 hpi (B and C). Data are represented as the mean and standard deviation with n=10 animals per timepoint and are representative of two independent experiments. Asterisks (*) represent p<0.05 by two-way ANOVA and Sidak's multiple comparisons test.
3.2 Kinetics of pulmonary inflammation differed between PR8 and CA4 viruses
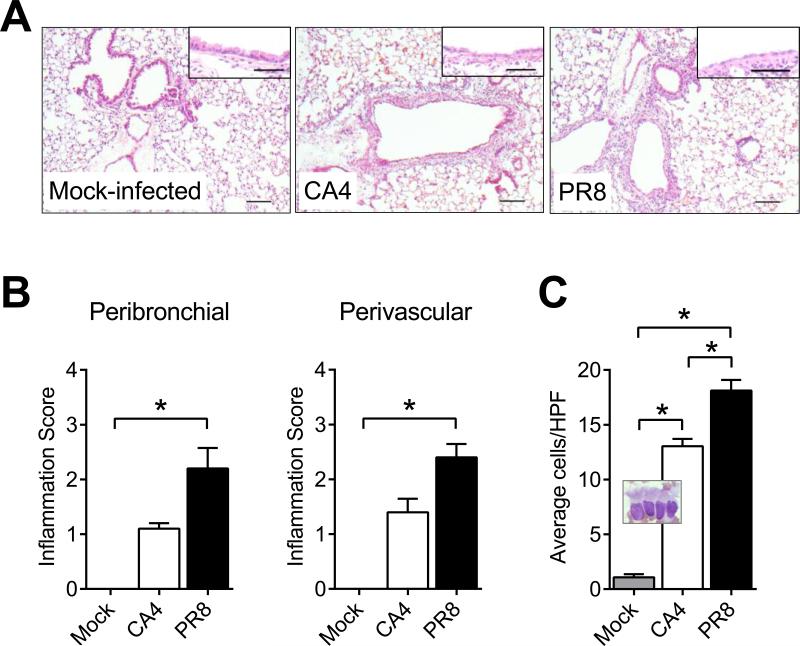
We used histopathology to determine the structural changes induced by the viruses during infection. Representative images of left lung sections stained with H&E and observed by light microscopy and semi-quantitative analyses thereof at 48 hpi are shown in Figure 2. Peribronchial and perivascular inflammatory foci were visible as early as 3 hpi (data not shown), prominent at 48 hpi (Fig 2A), and severe by 120 hpi (data not shown). Early peribronchovascular inflammation in response to PR8 was more robust than that induced by CA4 (Fig 2B). Damage to the bronchial epithelial cells was apparent after infection (insets, Fig 2A) and the number of ciliated epithelia in the airways increased with significantly more cells shed after PR8 infection (Fig 2C).
Figure 2. Inflammatory cell recruitment into the airways differed between the two virus strains.
The presence of inflammatory cells around the airways was observed as early as 48 hours after infection with each virus as was damage to the bronchial epithelia (insets) compared to naïve controls (A). Peribronchovascular inflammation scores were significantly higher in mice infected with PR8 (B). Ciliated epithelia shed into the airways were visualized on cytospin (inset) and enumerated in 5 randomly selected fields per mouse and there were more shed epithelia in the PR8 infected mice (C). Data are represented as the mean and standard deviation with n=5 animals at 48 hpi and are representative of two independent experiments. Asterisks (*) represent p<0.05 by nonparametric one-way ANOVA and Dunn's multiple comparisons test. Scale bar = 100 μm
3.3 Macrophage responses differed by IAV strain
Since macrophage populations are dynamically regulated during virus infection (Ghoneim et al., 2013), we aimed to determine if the intracellular cytokine profile also differed between the alveolar and parenchymal macrophages during IAV infection (Fig 3). Live singlets in each compartment were gated on Mac3 as a pan-macrophage marker prior to identification of populations based on CD11b and CD11c (Supplemental Figure 2). Macrophage populations changed in the BAL and lungs after infection (Fig 3A and D). The percentages of alveolar (CD11b−CD11c+) and parenchymal (CD11b+CD11c−) macrophages were calculated in the Mac3+ cells in the BAL and lungs to determine changes in the ratio of these two populations. Alveolar macrophages dominate the airways and lungs in mock-treated mice while these cells are decreased in both compartments as parenchymal macrophages increase after infection (Fig 3B and E). Both types of macrophages in the airways produced pro-inflammatory cytokines, TNFα than IFNγ, while macrophages in the mock-treated animals did not have measurable levels of these cytokines (Fig 3C). However, IFNγ production was significantly lower in alveolar macrophages after PR8 infection compared to these cells in mice infected with CA4 (Fig 3C). Unlike in the BAL, alveolar macrophages in the lungs did not alter IFNγ and TNFα production in response to IAV. Parenchymal macrophages produced less IFNγ after infection although TNFα production remained unaffected by infection (Fig 3F).
Figure 3. Macrophage populations changed in response to virus.
Live singlets were pre-gated on expression of Mac3 as a pan-macrophage marker and then by subtype of macrophage (A). Virus infection resulted in a change in ratio of macrophages wherein CD11b−CD11c+ cells decreased and CD11b+CD11c− population increased in the in the bronchoalveolar lavage (BAL) compared to mock-infected controls (B). Both populations of macrophages in the BAL produced IFNγ and TNFα in response to infection (C). While the total number of live CD11b−CD11c+ cells decreased in the lung after infection, the number of CD11b+CD11c− increased (D). Similar to the BAL compartment, the ratio of macrophage subtypes changed in the lungs wherein CD11b-CD11c+ macrophages decreased while the CD11b+CD11c− population increased after infection compared to mock-infected controls (E). Cytokine production in these cells was similar to that in naïve animals except IFNγ production which was significantly reduced in the CD11b+CD11c− population after infection (D). Data are represented as the mean and standard deviation with n=10 animals at 48 hpi. Asterisks (*) represent p<0.05 by nonparametric one-way ANOVA and Dunn's multiple comparisons test. Please see Supplemental Figure 2 for detailed gating strategy.
3.4 Antimicrobial peptides were differentially expressed in response to the PR8 and CA4 viruses
Differences in the inflammatory profile between the two IAV strains tested may be influenced by primary mucosal defenses in the host. The airway surface is bathed in mucosal liquids which contain AMPs that can initiate an inflammatory cascade. Therefore, we measured changes in AMP gene expression in the lungs. Influenza virus infection led to differential expression of AMPs in the lungs with variations between the two IAV strains (Fig 4). Mouse β-defensin-4 gene (Defb4) was regulated in response to both viruses in a bimodal pattern compared to mock-treated mice although not all peaks reached statistical significance. Gene expression of Bpifa1 peaked at 3 h after CA4 infection, and was significantly downregulated at late time points. Bpifa1 expression pattern was slightly different after PR8 infection wherein it was significantly increased over baseline at 1 hpi, and remained elevated until it was significantly downregulated at 120 hpi. Camp expression was upregulated in response to both strains of IAV, but was significantly higher in CA4 at 3 hpi compared to PR8.
Figure 4. Early mucosal immune defense genes were upregulated after influenza A virus infection.
Defb4 expression had a bimodal pattern with early expression in response to CA4 and delayed expression after PR8. Additionally, Defb4 was undetectable at some timepoints after both strains (ND, not detected). More Bpifa1 was expressed in response to CA4 compared to PR8, but this gene was significantly downregulated at later timepoints after both viruses. While elevated in expression compared to naïve controls, Camp levels were more stable after PR8 compared to CA4 peaking at 120 hpi. An additional peak in Camp expression occurred after CA4 at 3 hpi. Graphs represent the mean and standard error of the mean of n=5 mice per timepoint each with a technical replicate and are representative of two independent experiments. Dotted lines represent the mean in mock-infected controls. Delta (Δ) symbols represent p<0.05 compared to mock-infected control and asterisks (*) represent p<0.05 when CA4 and PR8 were compared by two-way ANOVA with Sidak's multiple comparisons test.
3.5 Beta-defensin 4 may enhance antiviral host protection in the lungs
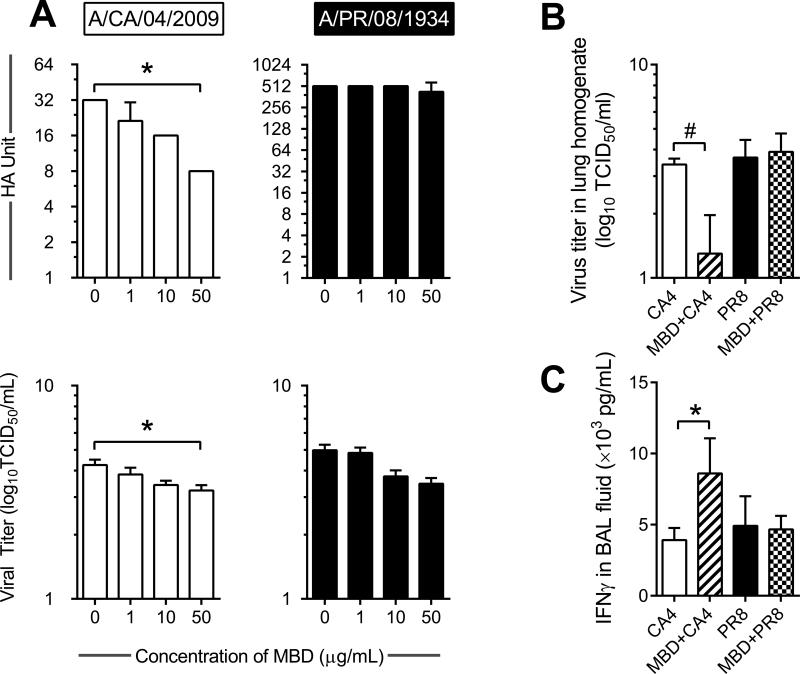
Although AMPs are commonly considered in defense against bacteria rather than viruses, since we noted differential expression of common AMPs in response to influenza virus infection (Fig 4) we hypothesized that these peptides played an immunoregulatory role during virus infection. We selected to investigate antiviral properties of MBD4 as the gene expression profile was dynamically regulated in response to each IAV strain. We incubated each virus in the presence of varying concentrations of biologically active MBD4 and performed hemagglutination assays to determine if MBD4 had an impact on hemagglutinin. The hemagglutinin titer of CA4 decreased in a dose dependent manner while that of PR8 did not change (Fig 5A). Virus pre-incubated in MBD4 led to a reduction in infectivity of both IAV strains although statistical significance was only achieved for CA4 incubated in 50 μg/mL of MBD4 (Fig 5A). Administration of recombinant MBD4 into animals immediately prior to virus infection resulted in a significant reduction in the viral burden in CA4 but not PR8 (Fig 5B). The IFNγ concentration in the BAL fluid of mice that received MBD4 prior to CA4 increased with MBD4 treatment (Fig 5C) while TNFα levels did not change remaining at approximately 12 ng/mL in all groups (data not shown).
Figure 5. Mouse β-defensin 4 (MBD4) may negatively impact virus infectivity.
Incubation of Incubation of CA4 in MBD4 caused a reduction in hemagglutinin (HA) and virus titer 48 hpi after infection although virus infectivity of PR8 was unaffected (A). Mice treated with MBD4 immediately prior to virus infection had reduced CA4 lung viral load at 48 hpi while viral burden in PR8 infected mice was not affected by MBD4 treatment(B). The amount of IFNγ in the bronchoalveolar lavage (BAL) fluid also increased in MBD4 treated mice infected with CA4 (C). Data are represented as the mean and standard deviation of n=4-5 wells/mice per group. Asterisks (*) represent p<0.05 by nonparametric one-way ANOVA and Dunn's multiple comparisons test, while # represents p<0.05 by Mann-Whitney test when data were compared to that of untreated controls.
3.6 Beta-defensin treatment altered the innate immune cell profile after IAV infection
Pulmonary inflammation that results from IAV infection was similar between the two strains. We aimed to determine whether the innate immune cell profile was affected by MBD4 pre-treatment prior to IAV infection. The gating strategy published in detail by Misharin et al. (Misharin et al., 2013) was adapted to determine the numbers of macrophages (alveolar and insterstitial), dendritic cells (CD103+ and CD11b+), and neutrophils in the BAL/airways and lungs of virus-infected mice (Supplementary Fig 3). While the number of cells in the airways and lungs increased in response to infection, pre-treatment with MBD trended toward reduced inflammation in response to both strains (Fig 6). Alveolar macrophages (AM) were reduced in the airways and lungs after virus infection (Fig 6), as noted before and corroborating a previous finding utilizing different gating strategies to quantify this population (Ghoneim et al., 2013). Pre-treatment with MBD4 led to the maintenance of the AM population in the lungs after virus infection (Fig 6). Interstitial macrophage (IM) population remained unaltered in the airways after IAV infection although MBD4 treated mice infected with CA4 had significantly less IMs in the airways compared to untreated mice. Lung IMs after MBD4 treatment were similar to that in mock-infected controls, but were significantly reduced when compared to levels in IAV-infected mice (Fig 6). Dendritic cell (DC) populations differed between the BAL and lungs wherein CD103+ DCs in the airways were not affected by infection while those in the lungs were reduced in response to CA4 infection. The population of CD11b+ DCs increased in the airways after CA4 but remained unaltered after PR8 and MBD treatment. The percentage of CD11b+ DCs decreased in the lungs of IAV-infected mice that were pre-treated with MBD (Fig 6). Neutrophils (Neuts) increased in the airways after IAV infection and these cells were reduced in both niches after MBD4 treatment (Fig 6).
Figure 6. Macrophage and dendritic cell populations were altered after β-defensin treatment during virus infection.
Mouse β-defensin 4 (MBD4) treatment reduced the total number of cells in the bronchoalveolar lavage (BAL) and lungs after infection. Both strains of influenza virus led to a reduction in the alveolar macrophage (AM) populations which was reversed by MBD. The virus-induced increase in interstitial macrophages (IM) did not occur in MBD-treated mice. The number of CD103+ dendritic cells (DCs) did not change in the airways either during virus infection alone of MBD treatment, although these cells increased in the lungs of MBD-treated mice infected with CA4. The number of CD11b+ DCs was reduced in the lungs after MBD as did neutrophils in both compartments. Graphs represent the mean and standard deviation of n=5 mice/group and are representative of two independent experiments. Asterisks (*) represent p<0.05 by nonparametric one-way ANOVA and Dunn's multiple comparisons test. Please see Supplemental Figure 3 for detailed gating strategy.
4. Discussion
Influenza viruses adapt to the host and may become more or less pathogenic. Common H1N1 influenza strains used in laboratory research include PR8 and CA4. However, these two strains differ in genetic makeup and host impact. In this study, we showed that PR8 and CA4 differed in induction of primary host defenses and morbidity despite having equivalent viral load in the lungs. Our data add novel information by comparing two frequently used H1N1 strains of IAV and exploring immune defenses at early time points after infection in the context of AMPs.
The bronchial epithelium is multifunctional and plays a vital role in lung innate immunity as reviewed in detail by Vareille et al (Vareille et al., 2011), and penetrance of this barrier defense by targeting epithelial junctional proteins (Golebiewski et al., 2011) is indicative of influenza virus virulence. Airway epithelial cells are potent producers of cytokines and chemokines that lead to airway inflammation and stimulation of resident immune cells after IAV infection (Tate et. al., 2011). Primary bronchial epithelial cells have been noted to have different inflammatory profiles and kinetics depending on the virus strain used (Gerlach et al., 2013; Ioannidis et al., 2012). Epithelial cells that were found in the BAL compartment at early time points (1, 3, 9, 24, and 48 h) after IAV appeared “healthy.” However, PR8 antigen is present in the bronchial epithelia by 24 hpi (Tate et al., 2011) and becasue we did not determine if shed epithelia were undergoing apoptosis or anoikis, it is possible that PR8 may have induced changes in epithelial cells more rapidly than CA4. Since bronchial epithelia are a main source of AMPs that were differentially regulated during IAV infection, it may be interesting to explore molecular characteristics and proteomics of shed epithelia to understand how alterations in the bronchial epithelia due to sloughing may impact early activation of other immune mechanisms.
Upon penetration of the epithelial barrier, IAV encounters alveolar macrophages in the airways and interstitial macrophages in the lung parenchyma. Macrophage responses to IAV include pro-inflammatory cytokine production, oxidative burst, and efferocytosis (Oslund and Baumgarth, 2011). Macrophage depletion during IAV infection leads to increased viral load, severe morbidity, and mortality (Purnama et al., 2014; Schneider et al., 2014; Tate et al., 2010; Wijburg et al., 1997) emphasizing the importance of appropriate macrophage availability and function in host defense against influenza. Characterization of macrophage subpopulations in the lungs during influenza is limited in the literature, and variations in classifications used to define subpopulations in addition to the harvest techniques can alter results. Alveolar and interstitial macrophages are dynamically regulated during influenza (Ghoneim et al., 2013). Using alternative markers and gating strategies, we confirmed these findings and also that macrophage subpopulation kinetics differed by IAV strain. Macrophage cytokine production in response to IAV requires viral replication and PR8 does not efficiently infect macrophages (Reading et al., 2010; Tate et al., 2010). Therefore, changes in macrophage populations are likely a result of differences in immune activation of epithelial cells by each IAV strain used. Decreased IFN signaling leads to neutrophil recruitment into the lungs (Seo et al., 2011), and IFNγ treatment early during IAV infection leads to better outcome (Weiss et al., 2010). Therefore, it is possible that antiviral defenses in the lungs were delayed after PR8 due to reduced IFNγ production in resident and recruited macrophages.
Reduction in tissue virus burden late in the infection course occurs as a result of an effectively activated adaptive immune response. A well-orchestrated CD8+ T cell response depends on the functions of antigen presenting cells, wherein mice that lack CD103+ DCs have reduced anti-influenza CD8+ T cells (GeurtsvanKessel et al., 2008; Helft et al., 2012). Of the DC subtypes, CD103+ DCs do not support IAV replication and are efficient at cross-presentation and activation of CD8+ T cells against IAV (Helft et al., 2012). A reduction in the number of CD103+ DCs in the lungs after IAV may suggest their migration to the draining lymph nodes, and it is worth noting that there were significantly less CD103+ DCs in the lungs after CA4 compared to the more pathogenic strain PR8. In contrast, CD11b+ DCs support virus replication (Hao et al., 2008; Helft et al., 2012), and increase in the lungs and draining lymph nodes after IAV infection (Helft et al., 2012). Our findings were somewhat different from Helft et al. (Helft et al., 2012), wherein a decrease in the CD103+ DCs occurred in response to CA4 but not PR8 and the CD11b+ DCs only increased in the BAL compartment after CA4. These variations may be due to viral strains and infectious doses used.
Epithelia and macrophages produce AMPs in the lungs. While AMPs are generally considered important in responses to bacteria, AMPs may also serve as antiviral immune mediators (Klotman and Chang, 2006). BPIFA1 is downregulated 48 h after IAV infection in tracheal epithelia and tracheal cells from bpifa1−/− mice have increased viral replication and cell death (Akram et al., 2015). Differences in Bpifa1 levels after IAV infection in our study suggests that early expression may be necessary in host defense and its reduction may be associated with damage to the epithelia. Human LL-37 was recently shown to have direct antiviral properties against respiratory syncytial virus and mouse CAMP reduced viral infectivity (Currie et al., 2016). While we did not use CAMP as a therapeutic in this study, we noted increased Camp expression in response to IAV that remained elevated throughout the infection perhaps indicative of a role for CAMP in antiviral responses to IAV. Human β-defensins are differentially expressed in health and disease (Singh et al., 1998), and MBD expression did not correlate with viral titers in a mouse model of influenza (Chong et al., 2008) suggesting that AMP expression is tightly regulated in disease and may be reflective of damage to the epithelia, inflammation, and immunoregulation, and host-pathogen interactions. We selected to investigate properties of MBD4 here as it had the most dynamic changes in gene expression in our model of IAV infection.
The bimodal expression pattern of Defb4 gene (albeit not statistically significant) suggests altered availability of β-defensins during the course of infection. A recent study elegantly demonstrated that a short peptide from MBD4 was able to reduce viral infectivity and counter morbidity of various lethal respiratory viruses included IAV (Zhao et al., 2016). Using recombinant MBD4 we found that MBD4 reduced viral infectivity and had a direct role on innate immune cell profile after IAV infections. Although epithelial cells of the respiratory tract are the main source of AMPs, resident and recruited leukocytes also contribute to local production during inflammation (Oppenheim et al., 2003). Therefore, changes in the expression dynamics of these AMPs after IAV may indicate changes in resident/recruited immune cells in the lungs. Human AMs produce β-defensins (Duits et al., 2002), and β-defensins can attract macrophages and DCs (Rohrl et al., 2010; Soruri et al., 2007), and promote a pro-inflammatory phenotype (Barabas et al., 2013). We noted a reduction in AMs in our study after IAV, and MBD4 treatment-induced increase in AMs only had a positive impact on CA4-infected mice wherein viral titers were lower. Blunting AMs can increase pathogenesis of H3N2 IAV but has no impact on PR8 (Tate et al., 2010). It would be of interest to explore whether MBD-mediated changes in macrophage populations occur as a result of monocyte recruitment or prevention/promotion of apoptosis. MBD-induced differences in the DC population may be due to their migration into draining lymph nodes, which may be beneficial in host defense. In addition to alterations in the myeloid cells, MBD4 treatment resulted in a significant reduction in the number of neutrophils in the airways and lungs. Neutrophils have been shown to have both beneficial and detrimental effects on the host during influenza (Sakai et al., 2000; Tate et al., 2009; Tate et al., 2011). Neutrophils were significantly reduced after MBD4 treatment in mice infected with both IAV strains although viral load was only reduced in the CA4-infected mice. Furthermore, considering the changes to the inflammatory profile and viral load after MBD4 treatment, it is possible that inflammation and viral burden may not be correlative on all occasions. Future studies are aimed at delineating a role for these AMPs in the late phase of IAV replication kinetics.
Variations in influenza pathogenesis in animal models can occur based on the mouse strain, virus strain, virus culture methods, virus dose, and volume of inoculum, among other factors. As shown in this study, a breakdown in immune homeostasis occurs very quickly after virus infection wherein AMPs are rapidly regulated and innate immune profile is altered. These defenses may control the initial pathogen burden and trigger subsequent immune responses. Primary innate immune defense molecules are important when investigating the immunopathogenesis of influenza virus infections and should be considered for further investigation as therapeutic targets.
Supplementary Material
Highlights.
Antimicrobial peptides may modulate host defense against influenza virus infections
Beta-defensin pretreatment leads to a reduction in pandemic H1N1 virus burden in the lungs.
Beta-defensin pretreatmentalters the innate immune profile in response to H1N1 influenza virus.
Acknowledgements
The authors would like to thank Mr. Arthur Covington at St. Jude Children's Research Hospital, and Mr. Ghana Gurung at the University of Tennessee Health Science Center for animal husbandry and care, and the Veterinary Histopathology Core at St. Jude Children's Research Hospital. The authors thank Mr. Panduka Nagahawatte and Dr. Jyoti Batra at the University of Tennessee Health Science Center for help with cytospin preparations and for genetic comparison between virus strains respectively. AES wishes to thank Dr. Jonathan McCullers for his mentorship and support. This project was partly funded through the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, under Contract number HHSN266200700005C, Centers of Excellence in Influenza Research and Surveillance (CEIRS), N01 7005C (McCullers) and the Le Bonheur Junior Faculty Grant (AES).
Funding Sources: NIAID CEIRS N01 7005 C (McCullers); Le Bonheur Children's Hospital Junior Faculty Award and Le Bonheur Foundation Bea Gerber Award (Samarasinghe).
Abbreviations
- CA4
A/CA/04/2009
- PR8
A/PR/08/1934
- MBD
mouse beta-defensin
- hpi
hours post infection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akram K, Moyo N, Tompkins M, Tripp R, Bingle L, Stewart J, Bingle C. An innate defence role for BPIFA1/SPLUNC1 against influenza A virus infection. Eur Res J. 2015;46:OA1791. [Google Scholar]
- Bals R, Weiner DJ, Meegalla RL, Accurso F, Wilson JM. Salt-independent abnormality of antimicrobial activity in cystic fibrosis airway surface fluid. Am J Resp Cell Mol. 2001;25:21–25. doi: 10.1165/ajrcmb.25.1.4436. [DOI] [PubMed] [Google Scholar]
- Barabas N, Rohrl J, Holler E, Hehlgans T. Beta-defensins activate macrophages and synergize in pro-inflammatory cytokine expression induced by TLR ligands. Immunobiology. 2013;218:1005–1011. doi: 10.1016/j.imbio.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Barlow PG, Svoboda P, Mackellar A, Nash AA, York IA, Pohl J, Davidson DJ, Donis RO. Antiviral Activity and Increased Host Defense against Influenza Infection Elicited by the Human Cathelicidin LL-37. PloS One. 2011;6 doi: 10.1371/journal.pone.0025333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, Shirakawa AK, Farber JM, Segal DM, Oppenheim JJ, Kwak LW. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298:1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- Boyton RJ, Openshaw PJ. Pulmonary defences to acute respiratory infection. British Med Bull. 2002;61:1–12. doi: 10.1093/bmb/61.1.1. [DOI] [PubMed] [Google Scholar]
- Britto CJ, Cohn L. Bactericidal/Permeability-increasing protein fold-containing family member A1 in airway host protection and respiratory disease. Am J Resp Cell Mol. 2015;52:525–534. doi: 10.1165/rcmb.2014-0297RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong KT, Thangavel RR, Tang XH. Enhanced expression of murine beta-defensins (MBD-1,-2,-3, and -4) in upper and lower airway mucosa of influenza virus infected mice. Virology. 2008;380:136–143. doi: 10.1016/j.virol.2008.07.024. [DOI] [PubMed] [Google Scholar]
- Currie SM, Findlay EG, McFarlane AJ, Fitch PM, Bottcher B, Colegrave N, Paras A, Jozwik A, Chiu A, Schwarze J, Davidson DJ. Cathelicidins have direct antiviral activity against respiratory syncytial virus in vitro and protective function in vivo in mice and humans. J Immunol. 2016;196:2699–2710. doi: 10.4049/jimmunol.1502478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duits LA, Ravensbergen B, Rademaker M, Hiemstra PS, Nibbering PH. Expression of beta-defensin 1 and 2 mRNA by human monocytes, macrophages and dendritic cells. Immunology. 2002;106:517–525. doi: 10.1046/j.1365-2567.2002.01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gally F, Di YP, Smith SK, Minor MN, Liu Y, Bratton DL, Frasch SC, Michels NM, Case SR, Chu HW. SPLUNC1 promotes lung innate defense against Mycoplasma pneumoniae infection in mice. Am J Path. 2011;178:2159–2167. doi: 10.1016/j.ajpath.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach RL, Camp JV, Chu YK, Jonsson CB. Early host responses of seasonal and pandemic influenza A viruses in primary well-differentiated human lung epithelial cells. PloS One. 2013;8:e78912. doi: 10.1371/journal.pone.0078912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GeurtsvanKessel CH, Willart MA, van Rijt LS, Muskens F, Kool M, Baas C, Thielemans K, Bennett C, Clausen BE, Hoogsteden HC, Osterhaus AD, Rimmelzwaan GF, Lambrecht BN. Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells. J Exp Med. 2008;205:1621–1634. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoneim HE, Thomas PG, McCullers JA. Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections. J Immunol. 2013;191:1250–1259. doi: 10.4049/jimmunol.1300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golebiewski L, Liu H, Javier RT, Rice AP. The avian influenza virus NS1 ESEV PDZ binding motif associates with Dlg1 and Scribble to disrupt cellular tight junctions. J Virol. 2011;85:10639–10648. doi: 10.1128/JVI.05070-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao XL, Kim TS, Braciale TJ. Differential response of respiratory dendritic cell subsets to influenza virus infection. J Virol. 2008;82:4908–4919. doi: 10.1128/JVI.02367-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helft J, Manicassamy B, Guermonprez P, Hashimoto D, Silvin A, Agudo J, Brown BD, Schmolke M, Miller JC, Leboeuf M, Murphy KM, Garcia-Sastre A, Merad M. Cross-presenting CD103(+) dendritic cells are protected from influenza virus infection. J Clinical Invest. 2012;122:4037–4047. doi: 10.1172/JCI60659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis I, McNally B, Willette M, Peeples ME, Chaussabel D, Durbin JE, Ramilo O, Mejias A, Flano E. Plasticity and virus specificity of the airway epithelial cell immune response during respiratory virus infection. J Virol. 2012;86:5422–5436. doi: 10.1128/JVI.06757-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HM, Lee YW, Lee KJ, Kim HS, Cho SW, van Rooijen N, Guan Y, Seo SH. Alveolar macrophages are indispensable for controlling influenza viruses in lungs of pigs. J Virol. 2008;82:4265–4274. doi: 10.1128/JVI.02602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotman ME, Chang TL. Defensins in innate antiviral immunity. Nat Rev Immunol. 2006;6:447–456. doi: 10.1038/nri1860. [DOI] [PubMed] [Google Scholar]
- Lee SM, Dutry I, Peiris JS. Editorial: macrophage heterogeneity and responses to influenza virus infection. J Leukocyte Biol. 2012;92:1–4. doi: 10.1189/jlb.0312130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukinskiene L, Liu Y, Reynolds SD, Steele C, Stripp BR, Leikauf GD, Kolls JK, Di YP. Antimicrobial activity of PLUNC protects against Pseudomonas aeruginosa infection. J Immunol. 2011;187:382–390. doi: 10.4049/jimmunol.1001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am J Resp Cell Mol. 2013;49:503–510. doi: 10.1165/rcmb.2013-0086MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser C, Weiner DJ, Lysenko E, Bals R, Weiser JN, Wilson JM. beta-Defensin 1 contributes to pulmonary innate immunity in mice. Infect Immun. 2002;70:3068–3072. doi: 10.1128/IAI.70.6.3068-3072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicod LP. Lung defences: an overview. Eur Respir Rev. 2005;14:45–50. [Google Scholar]
- Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- Oppenheim JJ, Biragyn A, Kwak LW, Yang D. Roles of antimicrobial peptides such as defensins in innate and adaptive immunity. Ann Rheum Dis. 2003;62:17–21. doi: 10.1136/ard.62.suppl_2.ii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslund KL, Baumgarth N. Influenza-induced innate immunity: regulators of viral replication, respiratory tract pathology & adaptive immunity. Future Virol. 2011;6:951–962. doi: 10.2217/fvl.11.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnama C, Ng SL, Tetlak P, Setiagani YA, Kandasamy M, Baalasubramanian S, Karjalainen K, Ruedl C. Transient ablation of alveolar macrophages leads to massive pathology of influenza infection without affecting cellular adaptive immunity. Eur J Immunol. 2014;44:2003–2012. doi: 10.1002/eji.201344359. [DOI] [PubMed] [Google Scholar]
- Ramirez-Martinez G, Cruz-Lagunas A, Jimenez-Alvarez L, Espinosa E, Ortiz-Quintero B, Santos-Mendoza T, Herrera MT, Canche-Pool E, Mendoza C, Banales JL, Garcia-Moreno SA, Moran J, Cabello C, Orozco L, Aguilar-Delfin I, Hidalgo-Miranda A, Romero S, Suratt BT, Selman M, Zuniga J. Seasonal and pandemic influenza H1N1 viruses induce differential expression of SOCS-1 and RIG-I genes and cytokine/chemokine production in macrophages. Cytokine. 2013;62:151–159. doi: 10.1016/j.cyto.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading PC, Miller JL, Anders EM. Involvement of the mannose receptor in infection of macrophages by influenza virus. J Virol. 2000;74:5190–5197. doi: 10.1128/jvi.74.11.5190-5197.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading PC, Whitney PG, Pickett DL, Tate MD, Brooks AG. Influenza viruses differ in ability to infect macrophages and to induce a local inflammatory response following intraperitoneal injection of mice. Immunol Cell Biol. 2010;88:641–650. doi: 10.1038/icb.2010.11. [DOI] [PubMed] [Google Scholar]
- Risso A. Leukocyte antimicrobial peptides: multifunctional effector molecules of innate immunity. J Leukocyte Biol. 2000;68:785–792. [PubMed] [Google Scholar]
- Rodgers B, Mims CA. Interaction of influenza virus with mouse macrophages. Infect Immun. 1981;31:751–757. doi: 10.1128/iai.31.2.751-757.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrl J, Yang D, Oppenheim JJ, Hehlgans T. Human beta-Defensin 2 and 3 and Their Mouse Orthologs Induce Chemotaxis through Interaction with CCR2. J Immunol. 2010;184:6688–6694. doi: 10.4049/jimmunol.0903984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai S, Kawamata H, Mantani N, Kogure T, Shimada Y, Terasawa K, Sakai T, Imanishi N, Ochiai H. Therapeutic effect of anti-macrophage inflammatory protein 2 antibody on influenza virus-induced pneumonia in mice. J Virol. 2000;74:2472–2476. doi: 10.1128/jvi.74.5.2472-2476.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, Nobs SP, Heer AK, Kurrer M, Klinke G, van Rooijen N, Vogel J, Kopf M. Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS Path. 2014;10:e1004053. doi: 10.1371/journal.ppat.1004053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo SU, Kwon HJ, Ko HJ, Byun YH, Seong BL, Uematsu S, Akira S, Kweon MN. Type I interferon signaling regulates Ly6C(hi) monocytes and neutrophils during acute viral pneumonia in mice. PLoS Path. 2011;7:e1001304. doi: 10.1371/journal.ppat.1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S, Lin DC, Rosati M, Carter RG, Norton JE, Suh L, Kato A, Chandra RK, Harris KE, Chu HW, Peters AT, Tan BK, Conley DB, Grammer LC, Kern RC, Schleimer RP. Reduced expression of antimicrobial PLUNC proteins in nasal polyp tissues of patients with chronic rhinosinusitis. Allergy. 2012;67:920–928. doi: 10.1111/j.1398-9995.2012.02848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PK, Jia HP, Wiles K, Hesselberth J, Liu LD, Conway BAD, Greenberg EP, Valore EV, Welsh MJ, Ganz T, Tack BF, McCray PB. Production of beta-defensins by human airway epithelia. P Natl Acad Sci USA. 1998;95:14961–14966. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soruri A, Grigat J, Forssmann U, Riggert J, Zwirner J. beta-defensins chemoattract macrophages and mast cells but not lymphocytes and dendritic cells: CCR6 is not involved. Eur J Immunol. 2007;37:2474–2486. doi: 10.1002/eji.200737292. [DOI] [PubMed] [Google Scholar]
- Tate MD, Deng YM, Jones JE, Anderson GP, Brooks AG, Reading PC. Neutrophils ameliorate lung injury and the development of severe disease during influenza infection. J Immunol. 2009;183:7441–7450. doi: 10.4049/jimmunol.0902497. [DOI] [PubMed] [Google Scholar]
- Tate MD, Pickett DL, van Rooijen N, Brooks AG, Reading PC. The critical role of airway macrophages in modulating disease severity during influenza virus infection of mice. J Virol. 2010;84:7569–7580. doi: 10.1128/JVI.00291-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate MD, Schilter HC, Brooks AG, Reading PC. Responses of mouse airway epithelial cells and alveolar macrophages to virulent and avirulent strains of influenza A virus. Viral Immunol. 2011;24:77–88. doi: 10.1089/vim.2010.0118. [DOI] [PubMed] [Google Scholar]
- Tate MD, Ioannidis LJ, Croker B, Brown LE, Brooks AG, Reading PC. The role of neutrophils during mild and severe influenza virus infections of mice. PloS One. 2011;6:e17618. doi: 10.1371/journal.pone.0017618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis. 2006;12:15–22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecle T, Tripathi S, Hartshorn KL. Review: Defensins and cathelicidins in lung immunity. Innate Immun. 2010;16:151–159. doi: 10.1177/1753425910365734. [DOI] [PubMed] [Google Scholar]
- Tripathi S, Tecle T, Verma A, Crouch E, White M, Hartshorn KL. The human cathelicidin LL-37 inhibits influenza A viruses through a mechanism distinct from that of surfactant protein D or defensins. J Gen Virol. 2013;94:40–49. doi: 10.1099/vir.0.045013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upham JP, Pickett D, Irimura T, Anders EM, Reading PC. Macrophage receptors for influenza A virus: role of the macrophage galactose-type lectin and mannose receptor in viral entry. J Virol. 2010;84:3730–3737. doi: 10.1128/JVI.02148-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vareille M, Kieninger E, Edwards MR, Regamey N. The airway epithelium: soldier in the fight against respiratory viruses. Clin Microbiol Rev. 2011;24:210–229. doi: 10.1128/CMR.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss ID, Wald O, Wald H, Beider K, Abraham M, Galum E, Nagler A, Peled A. IFN-[.gamma]treatment at early stages of influenza virus infection protects mice from death in a NK cell-dependent manner. J Interferon Cytokine Res. 2010;30:439–449. doi: 10.1089/jir.2009.0084. [DOI] [PubMed] [Google Scholar]
- Wijburg OL, DiNatale S, Vadolas J, van Rooijen N, Strugnell RA. Alveolar macrophages regulate the induction of primary cytotoxic T-lymphocyte responses during influenza virus infection. J Virol. 1997;71:9450–9457. doi: 10.1128/jvi.71.12.9450-9457.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.