Abstract
Background
Pancreatic islet transplantation is currently proven as a promising treatment for type 1 diabetes patients with labile glycemic control and severe hypoglycemia unawareness. Upon islet transplantation, revascularization is essential for proper functioning of the transplanted islets. As IL-6 is important for endothelial cell survival and systemic inflammation related to xenograft, the effect of IL-6 receptor antagonist, tocilizumab on revascularization of the transplanted islets was examined in pig to nonhuman primate islet xenotransplantation model. Also, the endothelial cell origin in a new vessel of the transplanted pig islets was determined.
Methods
Pig islets were isolated from designated pathogen-free (DPF) SNU miniature pigs and transplanted via portal vein into five streptozotocin-induced diabetic monkeys. One group (n=2, basal group) was treated with anti-thymoglobulin (ATG), anti-CD40 antibody (2C10R4), sirolimus and tacrolimus and the other group was additionally given tocilizumab on top of basal immunosuppression (n=3, Tocilizumab group). To confirm IL-6 blocking effect, C-reactive protein (CRP) levels and serum IL-6 concentration were measured. Scheduled biopsy of the margin of the posterior segment right lobe inferior of the liver was performed at three weeks after transplantation to assess the degree of revascularization of the transplanted islets. Immunohistochemical staining using anti-insulin, anti-CD31 antibodies and lectin IB4 was conducted to find the origin of endothelial cells in the islet graft.
Results
CRP significantly increased at 1~2 days after transplantation in Basal group, but not in Tocilizumab group and higher serum IL-6 concentration was measured in latter group, showing the biological potency of tocilizumab. In Basal group, well-developed endothelial cells were observed on the peri- and intra islet area, whereas the number of CD31+ cells in the intra-islet space was significantly reduced in Tocilizumab group. Finally, new endothelial cells in the pig islet graft was positive for CD31, but not for lectin IB4, suggesting that they are originated from the recipient monkey.
Conclusions
our results demonstrated that tocilizumab can delay revascularization of the transplanted islet, although this effect had no significant correlation to the overall islet graft survival. In the pig to NHP islet xenotransplantation model, the endothelial cells from recipient monkey form new blood vessels in and around pig islets.
Keywords: Islet xenotransplantation, Revascularization, IL6 receptor antagonist, Tocilizumab
Introduction
Pancreatic islet transplantation is currently proven as a promising treatment for type 1 diabetes patients with labile glycemia and severe hypoglycemic unawareness. Pancreatic islets are highly vascularized cells within the pancreas and this vasculature is required for functional insulin release and physiological blood glucose control (1, 2). However, during islet isolation, vascular connection between the islet and the surrounding tissues can be severed and revascularization process is required for survival, function and successful engraftment of the islets upon transplantation (3–5). Although both donor and recipient endothelial cells contributed new vasculature after engraftment in islet allo-transplantation setting (6, 7), it is unknown whether porcine endothelial cells in the isolated pig islets have a similar contributing role in new vessel generation.
The revascularization process for re-establishment of the blood supply to the transplanted islets has been demonstrated to occur within several days and are susceptible to hypoxic conditions until revascularization is complete (4, 8, 9). Also, during this period, several pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6 were secreted from both donor islets and recipient cells and they can damage transplanted islets, resulting in reduction of the engrafted islet mass (10–12).
IL-6 is a pleiotropic factor, having good and bad impacts on many physiological processes (13–15). For example, IL-6 has been shown to increase vascular endothelial growth factor and induce angiogenesis in a tumor model (16) and stimulate circulating blood-derived endothelial progenitor cell during angiogenesis in vitro (17). In contrast, IL-6 has been reported to amplify activation of coagulation through up-regulation of tissue factor on innate immune cells and resulted in systemic inflammation in xenograft recipient (SIXR) (18, 19). Therefore, it is intriguing to test whether blockade of IL-6 signaling upon islet xenotransplantation can lead to beneficial or deleterious effect on revascularization of the transplanted islet and final clinical outcome. Therefore, in this study, we investigated the effect of IL-6 receptor antagonist, tocilizumab on revascularization of the transplanted islets in pig to NHP islet xenotransplantation model using anti-CD40 antibody and tacrolimus-based immunosuppression regimen. Also, we examined whether the newly formed blood vessels in the pancreatic islets in the liver were from donor or recipient origin after islet xenotransplantation.
Materials and Methods
Animals
Five rhesus monkeys (Macaca mulatta) were used in this study. All procedures of animal care, treatment and experiments complied with the guidelines set forth in the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publication No. 86-23, revised 2011), and the study was approved by the Seoul National University Institutional Animal Care and Use Committee (IACUC no. 12-0374-C1A1).
Islet transplantation and immunosuppression
Islets were isolated from designated pathogen-free (DPF) Seoul National University (SNU) miniature pigs. Isolated islets were transplanted through the jejunal vein and approached near the portal vein of the streptozotocin (STZ)-induced diabetic rhesus monkeys as previously described (20, 21). For immunosuppression induction, anti-CD40 mAb (2C10R4, NIH NHP Reagent Resource) was infused on -7, -4 days. Sirolimus (Rapamune®, Wyeth) was administrered daily from day -3, and anti-thymocyte globulin (ATG, Thymoglobulin®, Genzyme) was infused on -6, -4, -2 days. Cobra venom factor (CVF) (100 U/kg, Quidel) was administered on day -1 of the transplant. Adalimumab as a TNF-α neutralizing mAb (Humira®, Abbott Laboratories Ltd., Queenborough, UK) was administered subcutaneously 2~3 h before islet infusion. Tocilizumab as an IL-6 antagonist (10 mg/kg, Actemra®, Joongwae pharma, Korea) was infused for 1 hour before islet infusion. After islet transplantation, Anti-CD40 mAb (30~50 mg/kg) was i.v. infused on days 0, 4, 7, 10, 14, 21 of the transplant. Sirolimus and tacrolimus (Advagraf®, Astellas Pharma Korea, Korea) were administered daily. Serum concentration of both drugs was measured in 1 ml of peripheral blood in EDTA-treated tube by liquid chromatography-tandem mass spectrometry weekly. Doses were adjusted to maintain target trough range; sirolimus 3~8 ng/ml and tacrolimus 3~6 ng/ml. Experimental groups were divided into two groups. In control group, two monkeys were treated with basal immunosuppression comprised of anti-thymoglobulin (ATG), anti-CD40 antibody (2C10R4), sirolimus and tacrolimus. In tocilizumab- treated group, three monkeys were treated with basal immunosuppression and tocilizumab as an IL-6 receptor antagonist.
Biopsy and immunohistochemistry
Porcine pancreas, isolated pancreatic islets, and transplanted liver tissue were used for histological experiments. After total pancreatectomy, the margin of the connecting lobe (10 mm) was taken for a sample. Isolated islets were cultured for 1 day or 7 days after isolation, and pre-embedding was performed using fibrin gel (GreenPlast®, Green Cross Corp., Korea). A liver biopsy was performed 20 or 21 days after transplantation. After the general anesthesia, the margin of the posterior segment right lobe inferior was gently grasped and excised about at 10 mm. Bleeding from the biopsy region was controlled with electrocautery and absorbable hemostat (SURGICEL; Ethicon, NJ, USA). Then, the prepared liver samples were immunohistochemically stained with anti-CD31, lectin IB4 and anti-insulin antibodies. For CD31 and insulin staining, the samples were fixed in 4% paraformaldehyde in phosphate buffered saline, and embedded according to routine paraffin embedding protocol. Paraffin-embedded tissues were sectioned for 4 μm using a microtome. Prepared sections were incubated with primary antibody for mouse anti-CD31 (DAKO, Glostrup, Denmark). The sections were incubated with alkaline phosphatase (AP)-conjugated secondary antibody for goat anti-mouse immunoglobulin. Then, the slides were colorized with blue chromogen (Thermo Scientific, MA, USA) for AP. After that, the slides were sequentially incubated with guinea pig anti-insulin antibody (DAKO, Glostrup, Denmark), AP-conjugated goat anti-guinea pig secondary antibody (Abcam, Cambridge, UK) and then treated with red chromogen substrate (Zytomed Systems, Berlin, Germany). After staining procedure, stained slides were dried at 60 °C and mounted with aqueous mounting medium (Thermo Scientific, MA, USA).
To assess the alpha Gal distribution, the slides were incubated with guinea pig anti-insulin antibody (DAKO, Glostrup, Denmark), then followed by secondary antibodies cocktail with fluorescein labeled-lectin IB4 (Fluorescein labeled-Griffonia Simplicifolia Lectin I (GSL I) isolectin B4, Vector, CA, USA) and Alexa fluor 647 goat anti guinea pig IgG (Life Tech. Co., OR, USA). Stained slides were mounted with fluoroshield mounting medium with DAPI (Abcam, Cambridge, UK). The stained samples were observed under an Axio Imager A1 microscope and the micrographs were obtained using an Axio-Vision software (Carl Zeiss AG, Oberkochen, Germany). Semi-quantitative histological analysis was performed. CD31+ cells distributed in the intra-islet and peripheral islet area were counted. They were discriminated between inner region of islet and 30 μm outer region of the islets. Number of islets analyzed was over 10 slides using different area (at least 100 μm interval from previous section) (Supplementary Figure 1).
C Reactive Protein (CRP) measurement
The CRP level was measured with a latex-enhanced turbidimetric immunoassay (Denka Seiken, Tokyo, Japan) using an automated analyzer (Toshiba 200FR; Toshiba, Tokyo, Japan). The detection limit of this assay is 0.1 mg/L. Both intra- and interassay coefficients of variation were < 10%.
Cytokine beads array (CBA)
The cytometric bead array assay was performed to measure peripheral blood IL-6 concentration according to the instructions provided by the manufacturer (BD Biosciences, San Jose CA).
Statistical analysis
The statistical software GRAPHPAD PRISM5 (GraphPad Software, Inc., La Jolla, CA) was used for one way ANOVA test. Data with P values less than 0.05 were considered to be statistically significant.
Results
Islet transplantation
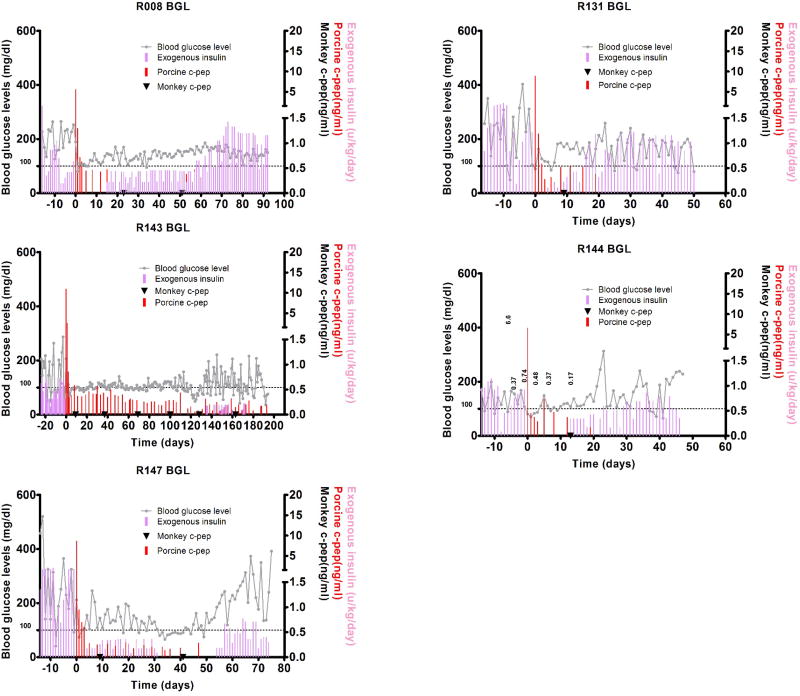
Five STZ-induced diabetic monkeys were transplanted with ~100,000 IEQ/kg (80,645~100,000, mean 93,575 IEQ/kg) adult pig islets isolated from SNU miniature pigs and divided into two groups depending on immunosuppression regimen (Table 1). One group was treated with ATG, anti-CD40 antibody, sirolimus, and tacrolimus (Basal group) and the other group was additionally given IL-6 receptor antagonist, tocilizumab (Tocilizumab group) on top of the basal immunosuppression. All recipients restored normoglycemia shortly after transplantation and controlled blood glucose levels for variable periods. During the normoglycemic period with or without exogenous insulin treatment, porcine C-peptide was detected in serum (Fig. 1).
Table 1.
Immunosuppressive regimens and graft outcomes in the two experimental groups
| Group | Monkey ID | Treatment | Body weight (kg) |
Transplanted Islet mass (IEQ/kg) |
Insulin independence day |
Graft survival# |
Biopsy Date |
|---|---|---|---|---|---|---|---|
| Basal Immunosuppression | R008 | ATG, CVF, Humira, Sirolimus/methylpredinisolone | 4.7 | 87,230 | 14 | >92 | 20 |
| R131 | + anti-CD40 antibody (2C10R4) | 5.3 | 100,000 | 3 | >50 | 20 | |
| + Tacrolimus | |||||||
|
| |||||||
| Tocilizumab group | R143 | ATG, CVF, Humira, Sirolimus/methylpredinisolone | 6.2 | 80,645 | 134 | 176 | 20 |
| R144 | + anti-CD40 antibody (2C10R4) | 6.5 | 100,000 | 11 | 22 | 21 | |
| R147 | + Tacrolimus + Tocilizumab | 5.6 | 100,000 | 3 | 30 | 20 | |
Graft survival day; this was defined as the day on which the serum porcine C-peptide fell <0.15 ng/ml, as measured by ELISA.
Figure 1.
Blood glucose control by the transplanted pig islets using anti-CD40 mAb + tacrolimus-based (Basal group) and anti-CD40 mAb + tacrolimus + tocilizumab-based immunosuppression (Tocilizumab group). Fasting blood glucose levels and porcine and monkey C-peptide were measured in five monkeys that were intraportally transplanted with adult pig islets. In basal group, R008 and R131 monkeys were insulin-independent for 14, and 3 days, respectively (A). In Tocilizumab group, R143, R144 and R147 monkeys were insulin-independent for 134, 11, and 3 days, respectively (B). Fasting blood glucose (grey line), porcine C-peptide (red bar), monkey C-peptide (▼), and exogenous insulin (pink bar) are indicated. The data of R008 and R131 were previously reported.
The effect of tocilizumab
Tocilizumab was additionally given in Tocilizumab group compared with Basal group. To test whether tocilizumab has been working in vivotwo independent assays were performed. First, C-reactive protein (CRP), which is promptly induced by IL-6 in the liver was measured. Basal CRP level at pre-transplantation was measured below 0.5 mg/dL in the most cases except R131. However, CRP significantly increased at days 1 and 2 after transplantation in Basal group, but not in Tocilizumab group (Fig. 2A). Second, the cytometric bead array (CBA) assay was performed to measure IL-6 concentration in blood serum. Basal group showed low levels of IL-6 in peripheral blood such that only 27 pg/ml was measured at 4 hrs after transplantation and followed by returning to basal levels. In contrast, IL-6 levels reached to minimum 97 pg/ml and maximum 471 pg/ml, which levels are about 6- to 20-folds higher than Basal group (Fig. 2B). When these values are indicated as area under the curve (AUC) from 0 to 5 days after transplantation, AUC values of Basal group were 36.1 to 43.61, whereas Tocilizumab group was 195.8 to 871.5 (Fig. 2C). Because free form of IL-6 is accumulated in the presence of IL-6 receptor antagonist, tocilizumab, these results showed that tocilizumab is effective for preventing CRP from increase and IL-6 from delivering a signal through IL-6 receptor.
Figure 2.
C-reactive protein (CRP) levels and IL-6 concentration after pig to monkey islet xenotransplantation. (A) Graph showing the CRP levels. CRP significantly increased at 1~2 days in R008 and R131 monkeys after transplantation, whereas the levels remained lower in R143, R144 and R147 monkeys. (B) The concentration of IL-6 in peripheral blood. In Tocilizumab group, IL-6 levels measured at 4 hrs after transplantation are 6 to 20 folds higher than in control group. (C) The serum IL-6 level from day 0 to day 5 after transplantation is presented as Area under the curve (AUC).
CD31 (PECAM) distribution after islet transplantation
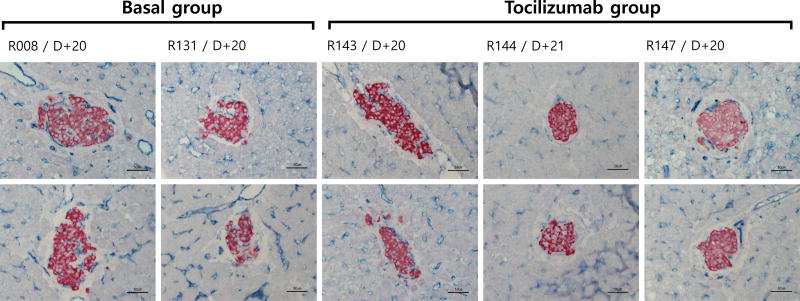
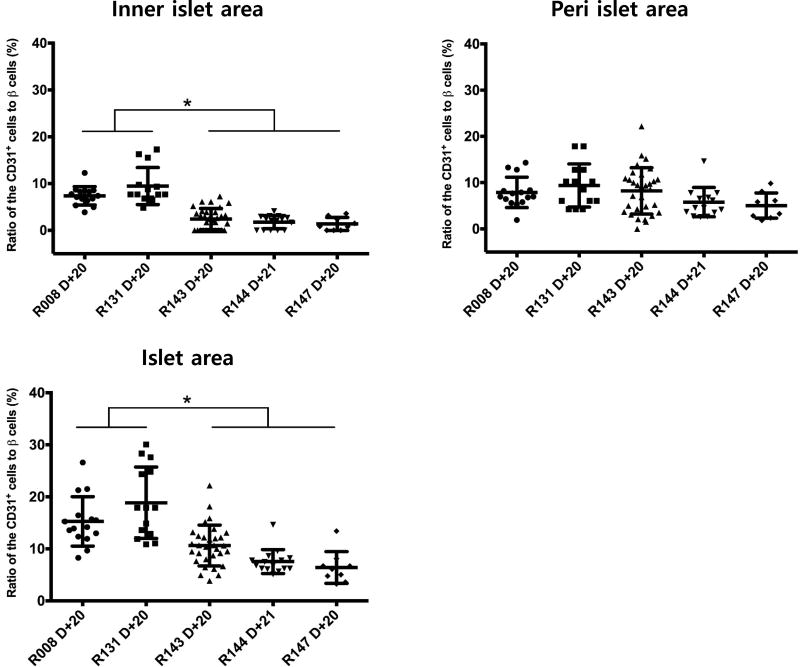
To examine the effect of tocilizumab on revascularization of the transplanted pig islets, scheduled biopsy was performed at 3 weeks after transplantation (20 or 21 days) in both groups. Subsequent immunohistochemical staining showed that the transplanted pig islets and endothelial cells are positively stained with insulin and CD31 antibodies, respectively in the liver of the recipient monkeys. In Basal group, the size of the transplanted islets engrafted in the liver parenchyma ranged 100 to 200 μm in diameter. CD31+ endothelial cells were observed both on the peripheral and intra islet areas, suggesting near total revascularization. In contrast, in Tocilizumab group, the size of the islets was 100 to 150 μm in diameter and intensity of insulin positive beta cells and the extent of CD31+ cell distribution in the intra-islet spaces were significantly lower (Fig. 3). To substantiate this finding in more objective manner, the number of CD31 positive cells either in peri- or intra-islet area and total β-cells were counted (Fig. 4). The ratio of CD31+ cells to total β-cell numbers was significantly higher in Basal than in Tocilizumab group (15.26 ± 4.75 to 18.84 ± 6.87% CD31+ cells in Basal group vs. 6.39 ± 3.05 to 10.62 ± 3.93% CD31+ cells in Tocilizumab group). Collectively, these data confirmed that tocilizumab had negative effect on revascularization of the transplanted islets, delaying endothelial cell ingrowth into the islets.
Figure 3.
Insulin and CD31 distribution in the islets transplanted to the liver of recipient monkeys. Liver samples taken at 20 or 21 days after pig islet transplantation was immunostained with anti-insulin and anti-CD31 antibodies. Results show well-engrafted islet β-cells (red) and lining of CD31+ endothelial cells (blue). In Basal group treated with basal immunosuppression, the transplanted islets had well-developed CD31+ cells and these endothelial cells were observed on the peripheral and intra islet areas. By contrast, in Tocilizumab group, intensity of insulin in β-cells and the extent of CD31+ cells in the intra-islet area were significantly lower than in Basal group.
Figure 4.
Histological semi-quantitative analysis of CD31+ cells in pig islet in monkey liver. Semi-quantitative histological analyses of ratio of CD31+ cells either peri- or intra-islet area to total β-cell numbers were conducted. Basal group shows well-developed endothelial cells of the transplanted islets, while in Tocilizumab group, the extent of distribution of CD31+ cells was significantly reduced (*p<0.05).
Endothelial cells origin in the islet grafts
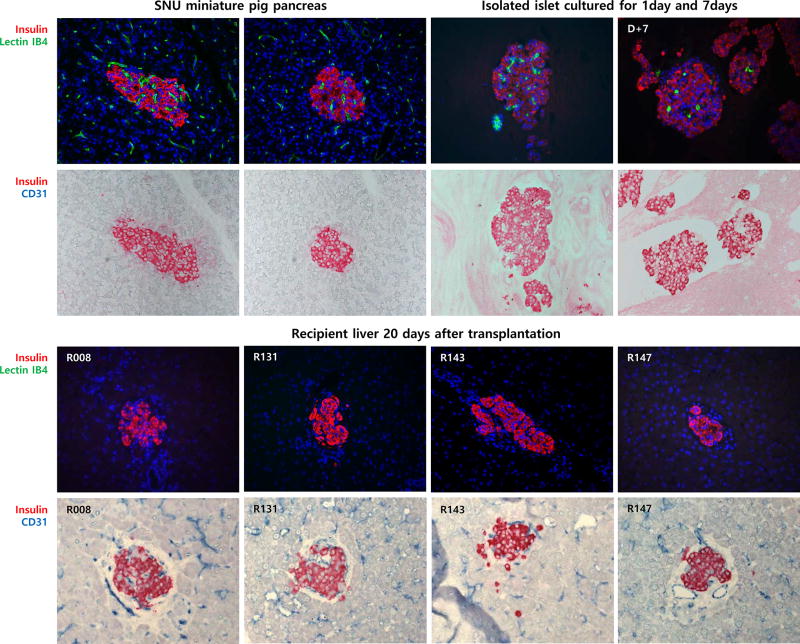
To determine the origin of endothelial cells in the pig islet grafts, alpha Gal and CD31 staining was performed. First, IB4-Lectin, which is specific for alpha Gal moiety was used to detect pig endothelial cells in donor pig pancreas and isolated islet of the SNU miniature pig. As seen in Fig. 5, lectin IB4 distributed throughout the pancreas including islets and exocrine tissues. Of note, lectin IB4 was found in endothelial cells in intra islet area. In case of isolated islet, the lectin distributed in the 1 day- cultured islet similar to in the donor pancreas, but the number of lectin IB4+ cells decreased and the remaining cells were morphologically distinct (rather shrunk) after 7 days of culture. In contrast, IB4 lectin was not detected in the transplanted islets as well as in the liver of the recipient, suggesting that donor pig endothelial cells die out from the islets. Finally, immunohistochemical staining using anti-human CD31 (JC70A) antibody, which has been reported not to react with porcine tissue was used for donor pig pancreas, isolated pig islet and liver biopsy sample. Indeed, this antibody did not bind to porcine endothelial cells, but detected endothelial cells in both pig islet graft and recipient liver. Taken together, this result clearly demonstrated that the origin of endothelial cell in the pig islet grafts was the recipient, but not the donor.
Figure 5.
Insulin, Lectin IB4 and CD31 distribution in pig pancreas, isolated islet and transplanted islet in the recipient monkey liver. In donor pancreas of the SNU miniature pig, Lectin IB4 distributed throughout the pancreas including islets and exocrine tissues. IB4 lectin was detected in endothelial cells in the intra islet area. In the isolated islet, IB4 lectin was detected 1 day after isolation. However, IB4 lectin was significantly reduced in the 7 day-cultured islets after isolation. IB4 Lectin was not found in the transplanted islets as well as in the recipient liver. Human specific-CD31+ cells distributed throughout the recipient liver including transplanted islets, but not in donor pancreas.
Discussion
In the present study, we have shown that IL-6 receptor (R) antagonist, tocilizumab had negative effect on revascularization of the transplanted pig islets, delaying endothelial cells ingrowth into the islets and the endothelial cells in the islet grafts entirely came from the recipient in pig to NHP islet xenotransplantation model.
IL-6 is a soluble mediator that has both roles as a pro-inflammatory cytokine and an anti-inflammatory cytokine, including various biological effects on inflammation, immune response, and hematopoiesis (22). Tocilizumab has been known to inhibit IL-6R–mediated signaling which is involved in the elevation of serum IL-6 levels and the reduction C-reactive protein (CRP) levels (23, 24). Recently, it has been reported that IL-6R antagonist has a beneficial effect on artery patch and kidney xenotransplantation model by reducing the inflammatory response (25, 26). In this study, IL-6R blockade using Tocilizumab was incorporated to reduce initial inflammation during early period after islet transplantation. Indeed, CRP as an indicator of inflammation was decreased in tocilizumab treated group. On the other hand, we found that revascularization of the transplanted islet was significantly inhibited by a IL-6R blockade using semi-quantitative histochemical analyses. Therefore, the beneficial effect of IL-6R blockade would be negated by harmful effect on revascularization of the transplanted islets. Consistent with this notion, overall graft survival was not improved by IL-6R blockade.
Islet isolation is an essential procedure for islet transplantation. However, as the isolated islets are completely devascularized because of enzyme digestion during isolation, they are exposed to prolonged ischemia and limited supply of oxygen and nutrients, leading to cell death including apoptosis and/or necrosis, eventually early graft failure (27–30). Thus, revascularization process is necessary for successful islet graft function upon transplantation. Previous report suggested that this process requires several days to 2 weeks if there are no facilitating treatments (3, 30, 31). Therefore, we examined the effect of tocilizumab on revascularization process of the islets transplanted intraportally into liver at 20 days after transplantation.
As IL-6 has been reported to facilitate neovascularization in various models and be essential for liver regeneration, we anticipated that blocking of IL-6 signaling would be detrimental to islet revascularization. Indeed, Tocilizumab group showed significantly reduced number of endothelial cells in the intra islet area. One report showed that the intra-islet endothelial cells play a key role in the process of revascularization (3). Interestingly, although Tocilizumab group showed that revascularization was not complete in intra islet areas, blood glucose levels were still well controlled by porcine insulin from the transplanted islets. It is likely that transplanted islets would sense blood glucose level and secrete insulin extracelluarly and this in turn flow into sinusoid for systemic circulation, even though this mechanism is obviously less efficient as previously suggested (32–34). These studies reported that transplanted islets are dependent on diffusion of nutrients and oxygen from the surrounding tissues and reduction of islet vasculature in the immediate post-transplant period may contribute to the islet loss and their function (31).
When the islets are transplanted, they need to create new vessels for their normal physiologic function. Recent reports indicate that revascularization process in the transplanted islet can be made from recipient endothelial cell (35–39), intra-islet endothelial cells of the donor (31, 40) or chimera from the donor intra-islet endothelial cells and recipient (3, 30). In pig to NHP islet xenotransplantation setting in our study, donor pig endothelial cells were detected in the pig pancreas and isolated islet, but never observed in the graft sites at 20 days after transplantation, and instead, endothelial cells in the islets are only positive for human-specific CD31 antibody, but not for alpha Gal-specific lectin. This result clearly demonstrated that recipient monkey endothelial cells formed new blood vessels in the transplanted islets. It remains to be determined how monkey endothelial cells within pig islets can communicate beyond species difference.
In conclusion, our results demonstrated that tocilizumab can delay revascularization of the transplanted islet, although this effect had no significant correlation to the overall islet graft survival. Finally, in the pig to NHP islet xenotransplantation model, recipient monkey endothelial form new blood vessels in and around pig islets.
Supplementary Material
Acknowledgments
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health & Welfare, Republic of Korea (Project No. HI13C0954). Anti-CD40 antibodies used in these studies were provided by the NIH Nonhuman Primate Reagent Resource (R24 RR016001, and NIAID contract HHSN 2722000900037C).
Footnotes
Author contributions:
Byoung-Hoon Min participated in the research design, the performance of the research and the writing of the paper.
Jun-Seop Shin participated in the research design, the performance of the research and the writing of the paper.
Jong-Min Kim participated in the research design, the performance of the research, and data analysis.
Seong-Jun Kang, Hyun-Je Kim, Il-hee Yoon, Su-Kyoung Park, Ji-won Choi and Min-Seok Lee participated in the performance of the research.
Chung-Gyu Park directed the project and participated in research design, writing and approval of the article.
References
- 1.Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, et al. Role of VEGF-A in vascularization of pancreatic islets. Current biology : CB. 2003;13:1070–1074. doi: 10.1016/s0960-9822(03)00378-6. [DOI] [PubMed] [Google Scholar]
- 2.Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, et al. Pancreatic islet production of vascular endothelial growth factor--a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55:2974–2985. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- 3.Pepper AR, Gala-Lopez B, Ziff O, Shapiro AM. Revascularization of transplanted pancreatic islets and role of the transplantation site. Clinical & developmental immunology. 2013;2013:352315. doi: 10.1155/2013/352315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Toro-Arreola A, Robles-Murillo AK, Daneri-Navarro A, Rivas-Carrillo JD. The role of endothelial cells on islet function and revascularization after islet transplantation. Organogenesis. 2016;12:28–32. doi: 10.1080/15476278.2016.1165378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 6.Contreras JL, Smyth CA, Eckstein C, Bilbao G, Thompson JA, Young CJ, et al. Peripheral mobilization of recipient bone marrow-derived endothelial progenitor cells enhances pancreatic islet revascularization and engraftment after intraportal transplantation. Surgery. 2003;134:390–398. doi: 10.1067/msy.2003.250. [DOI] [PubMed] [Google Scholar]
- 7.Miller R, Cirulli V, Diaferia GR, Ninniri S, Hardiman G, Torbett BE, et al. Switching-on survival and repair response programs in islet transplants by bone marrow-derived vasculogenic cells. Diabetes. 2008;57:2402–2412. doi: 10.2337/db08-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlsson PO, Palm F, Andersson A, Liss P. Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes. 2001;50:489–495. doi: 10.2337/diabetes.50.3.489. [DOI] [PubMed] [Google Scholar]
- 9.Mattsson G, Jansson L, Carlsson PO. Decreased vascular density in mouse pancreatic islets after transplantation. Diabetes. 2002;51:1362–1366. doi: 10.2337/diabetes.51.5.1362. [DOI] [PubMed] [Google Scholar]
- 10.Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. Journal of leukocyte biology. 2005;77:587–597. doi: 10.1189/jlb.1104649. [DOI] [PubMed] [Google Scholar]
- 11.Bottino R, Fernandez LA, Ricordi C, Lehmann R, Tsan MF, Oliver R, et al. Transplantation of allogeneic islets of Langerhans in the rat liver: effects of macrophage depletion on graft survival and microenvironment activation. Diabetes. 1998;47:316–323. doi: 10.2337/diabetes.47.3.316. [DOI] [PubMed] [Google Scholar]
- 12.Bennet W, Groth CG, Larsson R, Nilsson B, Korsgren O. Isolated human islets trigger an instant blood mediated inflammatory reaction: implications for intraportal islet transplantation as a treatment for patients with type 1 diabetes. Upsala journal of medical sciences. 2000;105:125–133. doi: 10.1517/03009734000000059. [DOI] [PubMed] [Google Scholar]
- 13.Schett G, Elewaut D, McInnes IB, Dayer JM, Neurath MF. How cytokine networks fuel inflammation: Toward a cytokine-based disease taxonomy. Nature medicine. 2013;19:822–824. doi: 10.1038/nm.3260. [DOI] [PubMed] [Google Scholar]
- 14.Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. The Journal of clinical investigation. 2011;121:3375–3383. doi: 10.1172/JCI57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraakman MJ, Kammoun HL, Allen TL, Deswaerte V, Henstridge DC, Estevez E, et al. Blocking IL-6 trans-signaling prevents high-fat diet-induced adipose tissue macrophage recruitment but does not improve insulin resistance. Cell metabolism. 2015;21:403–416. doi: 10.1016/j.cmet.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Crowther M, Brown NJ, Bishop ET, Lewis CE. Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. Journal of leukocyte biology. 2001;70:478–490. [PubMed] [Google Scholar]
- 17.Fan Y, Ye J, Shen F, Zhu Y, Yeghiazarians Y, Zhu W, et al. Interleukin-6 stimulates circulating blood-derived endothelial progenitor cell angiogenesis in vitro. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2008;28:90–98. doi: 10.1038/sj.jcbfm.9600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ezzelarab MB, Cooper DK. Systemic inflammation in xenograft recipients (SIXR): A new paradigm in pig-to-primate xenotransplantation? International journal of surgery. 2015;23:301–305. doi: 10.1016/j.ijsu.2015.07.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ezzelarab MB, Ekser B, Azimzadeh A, Lin CC, Zhao Y, Rodriguez R, et al. Systemic inflammation in xenograft recipients precedes activation of coagulation. Xenotransplantation. 2015;22:32–47. doi: 10.1111/xen.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin J, Kim J, Kim J, Min B, Kim Y, Kim H, et al. Long-Term Control of Diabetes in Immunosuppressed Nonhuman Primates (NHP) by the Transplantation of Adult Porcine Islets. American Journal of Transplantation. 2015;15:2837–2850. doi: 10.1111/ajt.13345. [DOI] [PubMed] [Google Scholar]
- 21.Shin JS, Min BH, Kim JM, Kim JS, Yoon IH, Kim HJ, et al. Failure of transplantation tolerance induction by autologous regulatory T cells in the pig-to-non-human primate islet xenotransplantation model. Xenotransplantation. 2016;23:300–309. doi: 10.1111/xen.12246. [DOI] [PubMed] [Google Scholar]
- 22.Nishimoto N, Kishimoto T. Interleukin 6: from bench to bedside. Nat Clin Pract Rheum. 2006;2:619–626. doi: 10.1038/ncprheum0338. [DOI] [PubMed] [Google Scholar]
- 23.Nishimoto N, Kishimoto T. Humanized antihuman IL-6 receptor antibody, tocilizumab. Handb Exp Pharmacol. 2008:151–160. doi: 10.1007/978-3-540-73259-4_7. [DOI] [PubMed] [Google Scholar]
- 24.Mihara M, Kasutani K, Okazaki M, Nakamura A, Kawai S, Sugimoto M, et al. Tocilizumab inhibits signal transduction mediated by both mIL-6R and sIL-6R, but not by the receptors of other members of IL-6 cytokine family. Int Immunopharmacol. 2005;5:1731–1740. doi: 10.1016/j.intimp.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Iwase H, Liu H, Wijkstrom M, Zhou H, Singh J, Hara H, et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation. 2015;22:302–309. doi: 10.1111/xen.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwase H, Ekser B, Zhou HD, Liu H, Satyananda V, Humar SR, et al. Further evidence for sustained systemic inflammation in xenograft recipients (SIXR) Xenotransplantation. 2015;22:399–405. doi: 10.1111/xen.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emamaullee JA, Shapiro AM. Interventional strategies to prevent beta-cell apoptosis in islet transplantation. Diabetes. 2006;55:1907–1914. doi: 10.2337/db05-1254. [DOI] [PubMed] [Google Scholar]
- 28.Wittig C, Laschke MW, Scheuer C, Menger MD. Incorporation of bone marrow cells in pancreatic pseudoislets improves posttransplant vascularization and endocrine function. Plos One. 2013;8:e69975. doi: 10.1371/journal.pone.0069975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brennan DC, Kopetskie HA, Sayre PH, Alejandro R, Cagliero E, Shapiro AM, et al. Long-Term Follow-Up of the Edmonton Protocol of Islet Transplantation in the United States. Am J Transplant. 2016;16:509–517. doi: 10.1111/ajt.13458. [DOI] [PubMed] [Google Scholar]
- 30.Pepper AR, Gala-Lopez B, Pawlick R, Merani S, Kin T, Shapiro AM. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nature biotechnology. 2015;33:518–523. doi: 10.1038/nbt.3211. [DOI] [PubMed] [Google Scholar]
- 31.Brissova M, Fowler M, Wiebe P, Shostak A, Shiota M, Radhika A, et al. Intraislet endothelial cells contribute to revascularization of transplanted pancreatic islets. Diabetes. 2004;53:1318–1325. doi: 10.2337/diabetes.53.5.1318. [DOI] [PubMed] [Google Scholar]
- 32.Davalli AM, Ogawa Y, Scaglia L, Wu YJ, Hollister J, Bonner-Weir S, et al. Function, mass, and replication of porcine and rat islets transplanted into diabetic nude mice. Diabetes. 1995;44:104–111. doi: 10.2337/diab.44.1.104. [DOI] [PubMed] [Google Scholar]
- 33.Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner-Weir S, Weir GC. Vulnerability of islets in the immediate posttransplantation period. Dynamic changes in structure and function. Diabetes. 1996;45:1161–1167. doi: 10.2337/diab.45.9.1161. [DOI] [PubMed] [Google Scholar]
- 34.Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner-Weir S, Weir GC. Early changes in syngeneic islet grafts: effect of recipient's metabolic control on graft outcome. Transplantation proceedings. 1995;27:3238–3239. [PubMed] [Google Scholar]
- 35.Jansson L, Carlsson PO. Graft vascular function after transplantation of pancreatic islets. Diabetologia. 2002;45:749–763. doi: 10.1007/s00125-002-0827-4. [DOI] [PubMed] [Google Scholar]
- 36.Menger MD, Yamauchi J, Vollmar B. Revascularization and microcirculation of freely grafted islets of Langerhans. World journal of surgery. 2001;25:509–515. doi: 10.1007/s002680020345. [DOI] [PubMed] [Google Scholar]
- 37.Vajkoczy P, Olofsson AM, Lehr HA, Leiderer R, Hammersen F, Arfors KE, et al. Histogenesis and ultrastructure of pancreatic islet graft microvasculature. Evidence for graft revascularization by endothelial cells of host origin. The American journal of pathology. 1995;146:1397–1405. [PMC free article] [PubMed] [Google Scholar]
- 38.Carlsson PO, Palm F, Mattsson G. Low revascularization of experimentally transplanted human pancreatic islets. The Journal of clinical endocrinology and metabolism. 2002;87:5418–5423. doi: 10.1210/jc.2002-020728. [DOI] [PubMed] [Google Scholar]
- 39.Hirshberg B, Mog S, Patterson N, Leconte J, Harlan DM. Histopathological study of intrahepatic islets transplanted in the nonhuman primate model using edmonton protocol immunosuppression. J Clin Endocrinol Metab. 2002;87:5424–5429. doi: 10.1210/jc.2002-020684. [DOI] [PubMed] [Google Scholar]
- 40.Nyqvist D, Kohler M, Wahlstedt H, Berggren PO. Donor islet endothelial cells participate in formation of functional vessels within pancreatic islet grafts. Diabetes. 2005;54:2287–2293. doi: 10.2337/diabetes.54.8.2287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.