Abstract
Background
Dysregulation of extracellular signal-related kinase (ERK) activity has been potentially implicated in the pathophysiology of autistic disorder (autism). ERK is part of a central intracellular signaling cascade responsible for a myriad of cellular functions. ERK is expressed in peripheral blood lymphocytes, and measurement of activated (phosphorylated) lymphocytic ERK is commonly executed in many areas of medicine. We sought to conduct the first study of ERK activation in humans with autism by utilizing a lymphocytic ERK activation assay. We hypothesized that ERK activation would be enhanced in peripheral blood lymphocytes from persons with autism compared to those of neurotypical control subjects.
Method
We conducted an initial study of peripheral lymphocyte ERK activation in 45 subjects with autism and 26 age- and gender-matched control subjects (total n = 71). ERK activation was measured using a lymphocyte counting method (primary outcome expressed as lymphocytes staining positive for cytosolic phosphorylated ERK divided by total cells counted) and additional Western blot analysis of whole cell phosphorylated ERK adjusted for total ERK present in the lymphocyte lysate sample.
Results
Cytosolic/nuclear localization of pERK activated cells were increased by almost two-fold in the autism subject group compared to matched neurotypical control subjects (cell count ratio of 0.064 ± 0.044 versus 0.034 ± 0.031; p = 0.002). Elevated phosphorylated ERK levels in whole cell lysates also showed increased activated ERK in the autism group compared to controls (n = 54 total) in Western blot analysis.
Conclusions
The results of this first in human ERK activation study are consistent with enhanced peripheral lymphocytic ERK activation in autism, as well as suggesting that cellular compartmentalization of activated ERK may be altered in this disorder. Future work will be required to explore the impact of concomitant medication use and other subject characteristics such as level of cognitive functioning on ERK activation.
Trial Registration
Not applicable.
Keywords: Autistic disorder, Biomarker, Extracellular signal related kinase, Lymphocytes, Neurodevelopmental disorder
1. Introduction
Autism spectrum disorder (ASD) is a developmental disorder characterized by deficits in social communication and interaction, and restricted, repetitive patterns of behavior, interests, or activities. Autism-like behaviors are also observed in Fragile X Syndrome (FXS), which is the most common type of inherited cognitive disability (Mariner et al., 1986). Our goal is to investigate the pathobiochemical pathway(s) responsible for these disorders in order to devise rational-based drug targets and develop useful biomarker(s). We hypothesize that a common cell signaling pathway takes different routes depending on the disease conditions.
The pathophysiology of autism remains poorly understood. As a behaviorally defined disorder with significant phenotypic heterogeneity, success in understanding the cause of illness has remained elusive. Biomarker development in autism, while the focus of significant research, has been met with limited success to date. In this context, we have previously reported higher levels of secreted amyloid-β precursor protein-alpha form (sAPPα) and lower levels of potentially toxic amyloid-β (Aβ) peptide in plasma and brain tissue of children with severe autism (Sokol et al., 2006; Sokol et al., 2011; Lahiri et al., 2013). How sAPPα mediates cell signaling relevant to ASD remains a major unanswered question, and the present work could shed some lights on this knowledge gap in the field.
Communication deficits and repetitive behaviors are seen in autism along with various symptoms that can vary in severity, including seizures and increased anxiety (Maski et al., 2011; Fung and Hardan, 2014). Considering almost innumerable genetic, environmental, or a combination of both factors may contribute to the etiology of a single case of autism spectrum disorder, approaches examining central points of cellular signaling and activity may hold promise to direct efforts towards unifying elements of cellular dysregulation. With these concepts in mind, we focused on study of extracellular signal-related kinase (ERK; also recognized as a mitogen activated kinase or MAP kinase) regulation in autistic disorder. ERK1 (MAPK3) and ERK2 (MAPK1) are central elements of intracellular signaling governing neuronal development (Samuels et al., 2008; Samuels et al., 2009), synaptic plasticity (Kelleher et al., 2004), and memory formation (Cui et al., 2008) which are all functions that are likely dysregulated in autism. ERK1/2 activation has also been implicated in various seizure models (Merlo et al., 2004; Yamagata et al., 2013). An imbalance in optimal ERK1/2 activation may play a role in cognitive function seen in autism-related disorders (Chevere-Torres et al., 2012). ERK1 and ERK2 isoforms exhibit significant functional redundancy and are thought to have resulted from single gene duplication at the onset of vertebrate evolution (Busca et al., 2015). Both exhibit a similar three dimensional structure and are ubiquitously expressed in mammals with similar specific activity (Robbins et al., 1993; Lefloch et al., 2008). Evidence from genetic studies in idiopathic autism, known genetic syndromes associated with autism, and murine models all point to potential aberrant ERK1/2 activity associated with the disorder. Specifically, copy number variation at the human 16p11.2 locus is a common risk variant associated with autism accounting for up to 1% of all cases (Malhotra and Sebat, 2012). The MAPK3 gene which encodes for ERK1 is located in this region. Interestingly, reports on the impact of 16p11.2 deletion on ERK1/2 activity have been conflicting with reports of resultant ERK1/2 up (Pucilowska et al., 2015) or down regulation (Tian et al., 2015).
The developmental syndromes known as RASopathies include neurofibromatosis type 1 (NF1), Noonan syndrome (NS), Costello syndrome (CS), and cardio-facio-cutaneous syndrome (CFC) which are all associated with enhanced Ras/MAPK activity resulting in excessive ERK1/2 activation (phosphorylation; pERK1/2). There are several clinical features that overlap among each syndrome including dysmorphic facial features, short stature, and increased cancer risk (Cizmarova et al., 2015). Recently, a systematic phenotype assessment of the RASopathies noted increased autistic traits in those with a RASopathy compared to non-affected siblings (Adviento et al., 2014).
FXS is a well-established single gene disorder and the leading genetic cause of autism. In both brain samples from patients with FXS and in brain tissue from Fmr1 knockout mice, pERK1/2 is elevated (Michalon et al., 2012; Wang et al., 2012). In Fmr1 knockout mice, treatment with a MEK1/2 inhibitor or lovastatin reduces ERK1/2 phosphorylation and has been associated with phenotypic rescue including reduction in audiogenic seizures (Wang et al., 2012). Lovastatin, an HMG-CoA reductase inhibitor, inhibits Ras-ERK1/2 and prevents the development of seizure-like symptoms in Fmr1−/y mice (Osterweil et al., 2013). Tuberous sclerosis complex (TSC) is an autosomal dominant neurocutaneous and neurodevelopmental disorder caused by the loss of TSC1 or TSC2 suppressor genes which results in enhanced activation of the mammalian target of rapamycin (mTOR) signaling cascade. It is estimated that 50% of persons with TSC meet criteria for autism and/or developmental disability (Curatolo et al., 2008; Jeste et al., 2008). Constituents of the ERK1/2 pathway are overactive in TSC cell lines and in TSC-associated brain lesions further implicating this central signaling cascade in the pathophysiology of autism related disorders (Govindarajan et al., 2003; Ma et al., 2007).
In the BTBR inbred mouse model of autism, pERK1/2 levels were shown to be increased in the prefrontal cortex (Faridar et al., 2014). Additionally, in this BTBR report pERK1/2 was elevated in lymphocytes which correlated with the cortex findings. In mouse models, there may be a critical developmental period when ERK1/2 dysregulation may result in autistic features. Phospho-blockade of ERK1/2 at postnatal day 6 (P6), but not at P14 leads to the development of autistic-like behaviors in adult mice (Yufune et al., 2015). A conditional ERK2 knockout mouse expresses a phenotype marked by aggressive behavior, reduced social behaviors, and learning deficits(Satoh et al., 2011), which are findings potentially consistent with an autism-like phenotype.
ERK1/2 is expressed in peripheral blood cells including lymphocytes. Analysis of ERK1/2 activation in lymphocytes is well established in the leukemia literature (Balakrishnan et al., 2014; Naci and Aoudjit, 2014; Uzan et al., 2014). Parsing ERK1 and ERK2 activation apart in human biological samples has not to date been reported. ERK requires phosphorylation for full activity and employs phosphatases to regulate signal transduction cascades (Caunt and Keyse, 2013). Activation and inactivation of ERK is influenced by the subcellular localization of the phosphatase (cytoplasm and nuclear compartments) (Owens and Keyse, 2007). Phosphorylation of ERK indicates the translocation of activated ERK into the cytosolic compartment. Given the feasibility to analyze ERK (ERK1 and ERK2 combined) activation in peripheral blood combined with the above evidence indirectly implicating ERK dysregulation in the pathophysiology of autism, we undertook the first known human study to date of ERK activation in autism using peripheral lymphocyte assays. It has been shown that inflammatory responses may lead to homing of lymphocytes to the CNS (Weller, 1996). The brain pathology of children diagnosed with ASD suggests ongoing neuroinflammation in various regions of the brain (Morgan 2010; Tetreault et al., 2012). This connection between neuroinflammation, lymphocyte migration, and the CNS may link the activation of ERK1/2 in the peripheral blood to that of the CNS in many neurological disorders including ASD. We hypothesized that ERK activation visualized as its translocation from the nucleus to the cytoplasm, would be increased in the peripheral lymphocytes of persons with autism compared to age- and gender-matched neurotypical control subjects. Our working hypothesis is based on the findings of potentially enhanced ERK activation in the RASopathies, the BTBR mouse model of ASD, and in FXS.
2. Methods
All subjects were recruited and enrolled at the Christian Sarkine Autism Treatment Center at Riley Hospital for Children between February and June 2012. The project was approved by the Indiana University Institutional Review Board (IRB). Inclusion criteria for persons with autistic disorder included age 5 years or older, a previous professional diagnosis of autism, confirmation of diagnosis by a clinician with expertise in the field (CAE) using a criteria for autistic disorder checklist from the Diagnostic and Statistical Manual for Mental Disorders, 4th Edition Text Revision (DSM-IV TR), and a score on the Social Communication Questionnaire of 15 or greater. Additional subject characterization of the autism subject group included completion of a medical and developmental history, physical examination, the Aberrant Behavior Checklist (ABC), Social Responsiveness Scale (SRS), IQ testing using the Stanford Binet 5th Edition for verbal subjects or the Leiter Revised 2nd Edition for non-verbal subjects, and the Vineland Adaptive Behavior Scales 2nd Editions (VABS II). Neurotypical control group subjects were recruited from flier ads and electronic ad postings within the Indianapolis, Indiana area. Control group subjects completed the SCQ with a required score less than 8 for inclusion. Control group subjects additionally underwent a developmental, psychiatric, and medical history evaluation to confirm no significant history of developmental delays, autism features, or comorbid mental illness. Control group subjects were matched by age (+/− six months) and gender with some controls matching for multiple subjects from the group of persons with autistic disorder. All subjects underwent a basic medical exam, were determined not to be actively ill and to be afebrile.
For the ERK blood lymphocyte analysis, lymphocytes were isolated from blood and used for immunocytochemistry or immunoblotting. Immunocytochemical analysis of ERK or phospho-ERK (p-ERK) expression in lymphocyte samples involves the fixing followed by antibody staining of cells. Immunoblotting, or Western blot analysis allows for semi-quantitative comparison of the protein expression levels of interest.
2.1. Blood collection
The lymphocyte isolation protocol began no longer than 2 h after blood collection. Lymphocytes were isolated from whole blood by centrifuging samples in cell preparation tubes with sodium citrate (BD Vacutainer, Franklin Lakes, NJ) at 1800 g for 20 min at room temperature. After centrifugation, the supernatant, i.e. the lymphocyte layer was transferred to a fresh 15-ml conical polyethylene tube (PET) tube, 1× sterile phosphate-buffered solution (PBS) was added up to 15 ml and this was centrifuged at 400 g for 15 min. Supernatant was aspirated and the pellet was re-suspended in 1 ml sterile PBS. The resuspension was transferred to a 1.5 ml Eppendorf tube and centrifuged at 400 g for 10 min at room temperature. Supernatant was aspirated and pellet resuspended slowly in 500 µl Roswell Park Memorial Institute Medium (RPMI, Corning Cellgro, Manassas, VA) 1640 medium containing 10% fetal bovine serum (FBS, Atlanta Biologicals S11150; Flowery Branch, GA). The lymphocytes/cells in RPMI suspension were plated onto the poly D lysine (PDL)-coated 6-well or 24-well plates (Sigma-Aldrich, St. Louis, MO) and incubated in a sterile cell culture CO2 (5%) incubator at 37 °C. Cells were cultured for 24–36 h for the immunohistochemistry experiments (see Immunocytochemistry). Remaining cell mixture was transferred to an Eppendorf tube and centrifuged at 400 × g for 6 min. Supernatant was removed and the pellet was used to make cell lysate for western blot analysis. Cells were lysed in Mammalian Protein Extraction Reagent (M-PER, Thermo Scientific, Waltham, MA) containing phosphatase inhibitor cocktail (Roche, Indianapolis, IN). The total protein concentration in the cell lysates was measured using the Bradford protein assay (BioRad, Hercules, CA).
2.2. Immunocytochemistry
Isolated lymphocytes in RPMI were plated onto a PDL-coated 24-well plates and cultured at 37 °C for 24–36 h. Conditioned media was removed and cells were fixed by adding 4% paraformaldehyde mixed in phosphate buffer solution (PBS) at room temperature for 12 min. Excess paraformaldehyde solution was aspirated from each well. Fixed cells were washed three times gently with cold PBS, and then permeabilized with 0.12% Triton X-100 solutiondiluted in PBS for 12–15 min at room temperature. Excess Triton-X solution was aspirated and the fixed cells were gently washed three times with chilled PBS to remove all traces of detergent. Cells were blocked with 10% horse serum (HS; diluted in PBS) for 20 min at room temperature. Unbound blocking agent was aspirated. Primary antibody (ERK/MAPK, PhosphoSolutions #500-ERK, Aurora, CO; ERK/MAPK Thr202/Tyr204 PhosphoSolutions #p160-2024, Aurora, CO) diluted 1:250 in 1% HS in PBS was added and allowed to incubate overnight at 4 °C. Primary antibody was removed and cells rinsed three times with cold PBS. Alexa Fluor Probes (Jackson ImmunoResearch, 111-585-144, West Grove, PA) conjugated goat anti rabbit secondary antibody 1:200 in Dulbecco's phosphate-buffered saline (DPBS, 20-031-CV, Corning Cellgro Manassas, VA) was added and incubated for 1 h in the dark. Secondary antibody was removed in the dark and cells were rinsed three times with PBS. Hoechst stain diluted in PBS 1:500 was added into each well. Images including a minimum of 200 lymphocytes per patient sample were captured with the Leica Type 090–135.002 microscope with SPOT RT Diagnostic. Positive control cells from each patient sample were treated with 10 µM tamoxifen with the same ensuing p-ERK and Hoechst staining. The number of p-ERK positive-cells was counted by looking for cytosolic translocation of p-ERK as seen in the tamoxifen-treated positive control cells.
Images were captured using SPOT Basic microimaging software and saved for scoring using Adobe Photoshop. Pseudocolor was added to each image and only cells exhibiting translocation from the nucleus to the cytosol were counted as activated. These cells were generally seen has having an empty or hollow area in the cell due to lack of staining in this region. These cells were counted using the count feature in Adobe Photoshop (Ray et al., 2014).
2.3. Immunoblotting
Protein estimation from the Bradford assay was used to load 10 µg of total protein in each lane of a 26-lane 10% Criterion gel (BioRad). Laemmli Sample buffer and water were used to bring each sample up to 30 µl total. Samples were denatured at 95 °C for 5 min using a thermocycler. Running buffer (XT-MOPS, BioRad) was added to a cassette before loading wells with sample. Protein standard (BioRad #161–0374) was loaded in one lane of the gel as a marker. Human fetal neuron (HFN) lysate (Ray et al., 2014) and human brain lysate (Long et al., 2014) were also loaded as positive controls for p-ERK expression. Gel was run at 200 V for 1.5 h, and transfer was performed using the Invitrogen iBlot system. Dry transfer was performed for 7 min and the polyvinylidene difluoride (PVDF) blot was stained with Ponceau Stain (BioRad) for 10 min to observe effective protein transfer afterwards, then de-stained with 5% acetic acid solution. The blot was rinsed in 1× Tris-Buffered Saline (TBS) and then blocked with 5% milk in Tris-Buffered Saline and Tween 20 (TBST) for 1 h at room temperature. This was discarded and the blot was probed with p-ERK primary antibody (same as used in IHC) diluted in 5% milk in TBST overnight at 4 °C. The following day, primary antibody was removed, and the blot was washed in TBST 3 times for 10-min each. The blot was placed in goat-anti-rabbit secondary antibody (Thermo #31460) diluted in 5% milk in TBST for 1 h at room temperature. The blot was then rinsed in TBST 3 times for 10 min each. After rinsing, 1:1 Enhanced Chemiluminescence (ECL) reagent (GE) was added to the PDVF membrane and incubated at room temperature for 1 min. Film was developed at 5-min and 10-min. Blot was stripped using stripping buffer (Thermo Restore PLUS) for 8 min, then rinsed in TBST for 5 min. The blot was then blocked in 5% milk in TBST for 1 h and reprobed with the secondary antibody to ensure proper stripping. After washing three times, the blot was re-probed with pan-ERK (1:1000; same as IHC) primary antibody overnight at 4 °C. Goatanti-rabbit was also used at the secondary antibody in this case. Blot was rinsed and film was developed as described above. Gel densitometry was performed using ImageJ software. A phospho-to-pan-ERK ratio was determined for each sample and plotted. Please see Alley et al., 2010 for a review of the immunoblotting methodology (Alley et al., 2010).
2.4. Statistics
Our primary analysis was a comparison of lymphocyte ERK activation ratio (number of cytosolic positive/nuclear negative p-ERK lymphocytes divided by total number of lymphocytes counted) between the autistic disorder and neurotypical control groups. Independent sample t-tests were utilized to compare group ERK activation ratios, total number of cells counted and number of ERK activated cells. We additionally compared subject group ages, gender, and SCQ scores using t tests and Chi Squared testing when appropriate. For the confirmatory Western blot statistical analysis, group p-ERK values adjusted for total ERK were compared using an independent sample t-test. All molecular ERK analyses were done blinded to group assignment. Given the pilot nature of this work and single primary analysis of two groups, we did not Bonferroni correct for multiple primary comparisons.
Finally, we conducted an exploratory Pearson Correlation analysis to look for any potential relationships between cellular ERK activation ratios and various subject characteristics in the autistic disorder subject group. A two-tailed bivariate Pearson Correlation Coefficient was generated for relationships between subject cell count ERK activation ratio and age, ABC subscale scores, SRS total raw score, Vineland Adaptive Behavior composite score, IQ and SCQ score.
3. Results and discussion
A total of 71 persons enrolled in this project during the five month recruitment period. All subjects were Caucasian. This included 45 persons with autistic disorder and 26 age- and gender-matched neurotypical control subjects who completed the project. The groups were well matched with similar age and gender ratio as noted in Table 1. The SCQ scores between the groups were consistent with study inclusion criteria. Additional characterization of the autistic disorder group in Table 2 notes a mean level of mild developmental disability in the subjects with autism combined with SRS and ABC mean scores consistent with autism population means. Overall, these values are consistent with our sample of persons with autism being generally representative of the Caucasian population of persons with autistic disorder. We additionally present concomitant medication use in the autistic disorder subject group given the high rates of medication use in this population (see Table 3; 42 of 45 (93%) subjects with autism were taking at least one psychotropic drug).
Table 1.
Characterization of autistic disorder (N = 45) versus neurotypical control subjects (N = 26).
| Features | Autistic disorder | Neurotypical controls | P value |
|---|---|---|---|
| Age | 13.8 ± 9.2 years (range 5–52 years) | 14.6 ± 9.4 years (range 5–52 years) | 0.71 |
| Gender | 37 male/8 female (82% male) | 21 male/5 female (81% males) | 0.88 |
| SCQ score | 23.6 ± 5.3 | 2.1 ± 3.4 | <0.0001 |
P determined by independent samples t-tests or Chi Squared.
SCQ = Social Communication Questionnaire.
Table 2.
Characterization of subjects with autistic disorder.
| SRS total raw score | 109.9 ± 25.9 |
| ABC irritability Subscale | 16.8 ± 11.3 |
| ABC social withdrawal subscale | 11.2 ± 7.8 |
| ABC stereotypy subscale | 7.7 ± 4.8 |
| ABC hyperactivity subscale | 19.9 ± 12.5 |
| ABC inappropriate speech subscale | 4.9 ± 3.9 |
| SRS total raw score | 109.9 ± 25.9 |
| Full scale IQ | 63.2 ± 21.6 |
| Vineland adaptive behavior composite score | 61.1 ± 18.3 |
All values reported as mean ± standard deviation.
N = 45 for all measures except IQ testing N = 32. IQ measured by Stanford Binet 5th Edition, verbal subjects and Lieter Revised for non-verbal subjects.
SRS = Social Responsiveness Scale, ABC = Aberrant Behavior Checklist.
Table 3.
Concomitant psychotropic medication use in group with autistic disorder.
| Drug | Number of subjects |
|---|---|
| Risperidone | 12 |
| Guanfacine | 11 |
| Paliperidone | 7 |
| Aripiprazole | 6 |
| Melatonin | 4 |
| Sertraline | 4 |
| Ziprasidone | 4 |
| Atomoxetine | 3 |
| Fluoxetine | 3 |
| Dextroamphetamine | 2 |
| Lithium | 2 |
| Methylphenidate | 2 |
| Mirtazapine | 2 |
| Mixed Amphetamine Salts | 2 |
| Quetiapine | 2 |
| Trazodone | 2 |
| Valproic acid | 2 |
| Clonazepam | 1 |
| Clozapine | 1 |
| Duloxetine | 1 |
| Escitalopram | 1 |
| Fluvoxamine | 1 |
| Lamotrigine | 1 |
| Paroxetine | 1 |
| Olanzapine | 1 |
| Topiramate | 1 |
42/45 Subjects with Autistic Disorder were taking psychotropic medication.
Mean # meds = 1.8 ± 1.0 meds.
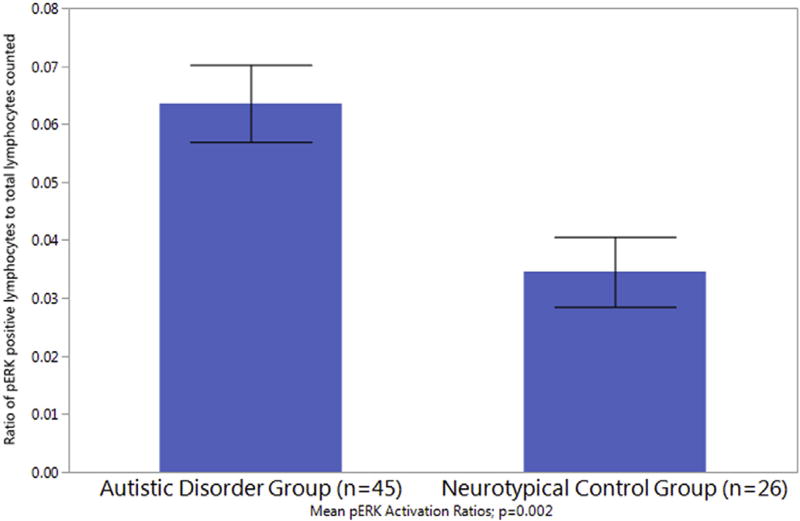
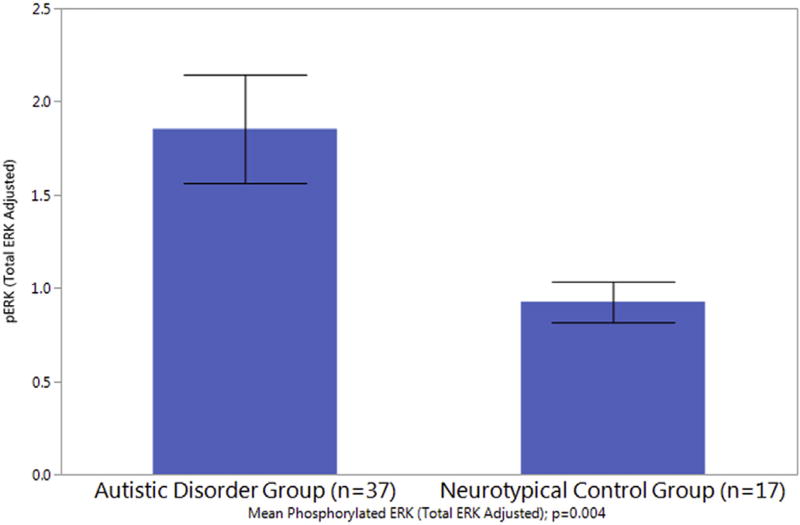
Lymphocytes from all subjects were analyzed using the compartmental lymphocyte counting method. Fifty four subjects had evaluable samples remaining for Western blot analysis. The unstimulated cell count ratio (cytosolic positive/nucleus negative pERK lymphocytes counted, divided by the total number of cells counted) was significantly higher in the autistic disorder group compared to controls (see Table 4 and Fig. 1; ratio of 0.064 ± 0.044 versus 0.034 ± 0.031, p = 0.002). Consistent with this primary ratio finding, despite a trend towards more cells counted in the neurotypical control group, more cells staining positive for cytosolic positive/nucleus negative ERK phosphorylation (activation) were noted in the autistic disorder subject group (p = 0.045). Additionally, whole cell pERK Western Blotting analysis demonstrated an increased phosphorylated to total ERK ratio in the autistic disorder group (n = 37) compared to neurotypical controls (n = 17; see Fig. 2; p = 0.004). The subjects whose samples were included in the Western Blotting analysis did not differ in demographic characteristics compared to the larger group in the lymphocyte counting analysis. Our Western blotting analysis included the subset of participants whose samples had sufficient usable lymphocyte material for processes. Our exploratory Pearson correlation analysis yielded no significant correlations between lymphocyte counted ERK activation ratio and various subject characteristics including age, IQ, ABC, Vineland, and SCQ scores in the autistic disorder subject group.
Table 4.
ERK Activation analysis.
| Autistic disorder (N = 45) ±SD | Neurotypical controls (N = 26) ±SD | P value | |
|---|---|---|---|
| Total cells counted | 407.3 ± 189.3 | 480.6 ± 207.1 | 0.15 |
| Cytosolic pERK cells counted | 23.7 ± 16.9 | 15.8 ± 14.7 | 0.045 |
| Ratio of cytosolic pERK positive to total cells counteda | 0.064 ± 0.044 | 0.034 ± 0.031 | 0.002 |
Fig. 1.
Lymphocyte Counting Phosphorylated ERK Analysis. Phosphorylated-ERK positive cells were identified and counted as activated based on the cytosolic translocation of p-ERK in the cell. The ratio of pERK positive lymphoctyes to total lymphocytes counted is significantly increased in the autistic disorder group as compared to the control group.
Fig. 2.
Increased ERK activation (phosphorylation) in autistic subjects. Densitometry (Image J) of Western blot shows a significant increase in pERK expression adjusted for total ERK in the autistic disorder group. Representative Western blot image shows respective p-ERK and total ERK expression of 11 subjects.
In this first preliminary report on blood lymphocytic ERK activation (phosphorylation) in humans with autistic disorder, our finding is consistent with genetic, murine, and human postmortem findings in the field that have potentially implicated ERK dysregulation in the pathophysiology of the disorder. Enhanced lymphocytic activation in autism may be interpreted as a non-specific sign of excessive cellular activity. Given the fine-tuned nature and central location of ERK activity in cells, this perturbation of ERK activation may potentially have significant impact on cellular growth, signaling, and senescence.
While the strengths of this report include confirmation of ERK dysregulation by two independent methodologies conducted blinded to group assignment, the results of this report must be taken in the context of several limitations of the analysis. First, the sample sizes are not sufficiently large enough to conduct effective analyses of potential patient subgroups such as ERK activation analysis within, for example, age, gender, concomitant medication status, or IQ defined subgroups. Furthermore, since limited to the Caucasian population, it cannot conclusively establish the role of ERK/p-ERK in the ASD seen in other ethnic groups. Given these limitations, it is not possible to understand potential subject characteristics that may be associated with aberrant ERK activation. Additionally, our limited number of control subjects was based on the maximum number of controls we could recruit within a six month study period. This may have further limited our ability to understand factors that may have predicted ERK activation rates.
The potential impact of concomitant medication use may be a potential confounder to our ERK activation analyses. Limited data exists describing the impact of psychotropic drugs on ERK activation (phosphorylation). Our autistic disorder group is enriched for use of antipsychotics, alpha 2 agonists, selective serotonin reuptake inhibitors (SSRIs), and stimulants. There is no available data describing psychotropic drug impact on lymphocytic ERK activation. In vitro and murine data describing antipsychotic ERK activation effects have included reports of risperidone-associated desensitization to ERK activation (Clarke et al., 2013), aripiprazole-associated suppression of ERK activation (Ishii et al., 2010), and quetiapine having no impact on ERK activation (Pereira et al., 2014). The alpha2-agonist literature regarding ERK activation is very limited with one report of the alpha2-agonist xylazine being associated with reduction in ERK activation in rat brain (Peng et al., 1998). In the dopamine transporter knockout mouse model of hyperactivity, both stimulant (methylphenidate and mixed amphetamine salts) and fluoxetine (SSRI) treatment were associated with inhibition of ERK signaling in brain (Beaulieu et al., 2006). Overall, it is difficult given review of the available literature to attribute our excessive lymphocytic ERK activation finding in those with autism to psychotropic drug use alone. Future study enriching enrollment for persons with autism and no concomitant medication use will help address this potential confounder.
Our analysis does not control for intellectual disability between comparison groups. Given this, it is not possible to exactly attribute the ERK dysregulation noted to the diagnosis of autistic disorder versus being a feature potentially shared among a broader group of persons with intellectual disability with or without autism. While we did not note a correlation of ERK activation values and IQ that could have signaled greater likelihood of an intellectual disability effect on ERK activity, it still may be possible that ERK dysregulation is driven more by the presence of intellectual disability versus the presence of autistic disorder alone. Additionally, our autistic disorder diagnoses were not validated using research administration of gold standard measures such as the Autism Diagnostic Observation Schedule or the Autism Diagnostic Interview-Revised. Future larger-scale work should likely utilize one or both of these measures to verify diagnosis.
Given the ubiquitous nature of ERK signaling across cell types and organ systems in mammals, equating ERK dysregulation in one cell type or organ system with similar activity in other systems has not been well explored. Inflammatory responses seen in the brains of ASD patients link lymphocyte migration to the CNS to neuroinflammation and the activation of ERK in the peripheral blood (Morgan et al., 2010; Tetreault et al., 2012). ERK dysregulation in lymphocytes may provide some insight into the role of ERK activation in ASD severity or detection. While our methodology utilizes a readily available biological sample that can be accessed across a wide functioning range of persons with autism, we cannot be sure that enhanced ERK activation in peripheral lymphocytes equates with ERK dysregulation in brain where we would expect molecular perturbation to result in an autism phenotype. Despite this, in recent study in the knockout mouse model of FXS, excessive ERK activation was noted in both brain and blood cell samples showing that some correlation may exist (Deacon et al., 2015).
Despite these limitations, the concept that ERK activation may be dysregulated in persons with autistic disorder presents a potential molecular target of treatment and a molecular means to match potential treatments with those within the autism umbrella who may best respond to a specific intervention. As an example, phosphorylated ERK levels are currently in study in oncology as a predictor of targeted treatment response (Branca et al., 2004; Campbell et al., 2009; Li and Yang, 2009; Zhang et al., 2009; Matsubara et al., 2010). Future work in autism will require larger sample sizes, use of likely multiple control groups controlling for age, gender, and IQ, and enrollment of some concomitant medication free affected subjects with autism. It will also be necessary in the future to work towards understanding what upstream mediators of ERK activation may be responsible for ERK dysregulation. Furthermore, analysis of which downstream effectors of ERK activation may show dysregulation secondary to aberrant ERK activation will help foster future understanding of the impact of aberrant ERK activity.
We are tempted to discuss the above results with other recent biomarker studies in a broader context. Neurodevelopmental disorders (ASD and FXS) are the opposite end of late-life neurodegenerative disorders, such as Alzheimer's disease (AD), which is the most common form of age-related dementia (Maloney and Lahiri, 2016). There are distinct brain abnormalities as well as cellular and neurochemical pathways found in ASD, FXS and AD subjects. In this context, we have recently proposed that anabolic excess may result in a gain of function overgrowth associated with elements of neurodevelopmental conditions, while catabolic excess would be associated with neurodegeneration (Lahiri et al., 2013). Higher levels of sAPPα and lower levels of a potentially toxic Aβ peptide are observed in plasma and brain tissue of children with severe autism. The sAPPα results have been replicated by an independent laboratory (Bailey et al., 2008; Bailey et al., 2013). Previously, we found increased sAPPα, APP, and Aβ plasma markers in children with FXS compared to youth with ASD (Erickson et al., 2014). The present work takes our sAPPα forward to link ERK-mediated cell signaling relevant to ASD. Interestingly, sAPPα activity is required to promote neurite outgrowth in neural stem cell-derived neurons via activation of the MAPK pathway (Gakhar-Koppole et al., 2008). Indeed, direct stimulation of AMPA receptor increases non-amyloidogenic α-secretase-mediated APP processing and inhibits Aβ generation. APP processing was blocked by the matrix metalloproteinase inhibitor TAPI-1 but was only partially dependent on Ca(2+) influx and ERK activity (Hoey et al., 2013). Another group elucidated a potential pathway for sAPPα signaling through MAP kinase activation in iPSC of adult progenitor cells of ectodermal and mesodermal origin. Importantly, sAPPα operates independently of the prominent proliferation factors epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF), but in association with ERK signaling and MAP-kinase signaling pathways (Demars et al., 2011). Even in non-neuronal cells, sAPP activates microglia via JNK and p38-MAPK. Further, sAPP activates the ERK, JNK and p38 classes of MAP kinases but that only JNK and p38-MAPK are critical for activation of microglia by sAPPα, a process that compromises neuronal function and survival (Bodles and Barger, 2005).
Taken together, studying the ERK signaling pathway in autism assumes significance as it integrates ERK activation with an important putative biomarker, such as sAPPα. The overall rationale for selecting the ERK signaling pathway (this manuscript) and its integration with other biomarker-related studies (e.g., APP pathway metabolites) in autism and FXS provides a necessary conceptual framework and feasible experimental platform that warrant further studies.
4. Conclusions
This initial study of peripheral lymphocytic ERK activation in humans with autistic disorder compared to neurotypical control subjects noted increased ERK activation (phosphorylation) in subjects with autism. Future work focused on understanding potential ERK dysregulation in autism is warranted and holds promise to further elucidate the pathophysiology of the disorder. Such work will also be essential to developing the knowledge base necessary to potentially utilize ERK activation as a useful peripheral biomarker capable of tracking the progression of disease, identification of environmental triggers, and even predicting treatment response. Future larger-scale analysis of ERK activation in autism will potentially need to control in the analysis for key potential confounding factors including, but not limited to, subject age and cognitive functioning level.
Supplementary Material
Acknowledgments
We sincerely thank the technical assistance from LaTosha Phelps and Nipun Chopra (Indiana University School of Medicine, Indianapolis). Funding for this study was provided by an Indiana University Funding Opportunities for Research Commercialization and Economic Success (FORCES) grant to Dr. Erickson. DKL is also partially supported by grants from the National Institute on Aging, NIH (R01AG051086–01 and R21 1R21AG047447-01 awards); Indiana Clinical and Translational Sciences Institute (ICTSI) (Ul1 TR001108); and ISDH Spinal Cord and Brain Injury Board (ISDH 13609).
Conflict of interest
Drs. Erickson and Lahiri are co-inventors on intellectual property held by Indiana University describing techniques to aid the diagnosis of autism spectrum disorder. Dr. Erickson receives/received research grant support from the National Institutes of Health, the US Department of Defense, the John Merck Fund, the US Centers for Disease Control, the National Fragile X Foundation, FRAXA, Indiana University, Cincinnati Children's Hospital Medical Center, the Fragile X Alliance of Ohio, the Roche Group, Neuren Pharmaceuticals, Novartis, Seaside Therapeutics, Stemina, the Simons Foundation, Autism Speaks, Riovant. Dr. Erickson is a consultant to and holds equity interest in Confluence Pharmaceuticals. Dr. Erickson is past or present consultant to the Roche Group, Novartis, Alcobra, Fulcrum, Neurotrope.
Source of funding
Funding for this study was provided by an Indiana University Funding Opportunities for Research Commercialization and Economic Success (FORCES) grant to Dr. Erickson. Indiana University did not exercise any editorial content control over the content of this manuscript. The FORCES funding was essentially unrestricted focused on assessing ERK activation in the peripheral blood of persons with autism. Dr. Lahiri thanks support from the National Institute on Aging, NIH (R01AG051086, R21AG4687100, P30AG010133), Indianan Clinical and Translational Sciences Institute (ICTSI), and ISDH Spinal Cord and Brain Injury Board.
Abbreviations
- ABC
Aberrant Behavior Checklist
- ERK
Extracellular signal-related kinase
- SCQ
Social communication questionnaire
- SRS
Social responsiveness scale
- VABS
Vineland adaptive behavior scale
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jpsychires.2016.09.003.
Authors’ contributions
CE participated in the design of the study, was the primary writer of the manuscript, and participated directly in running study participants. LW and RS revised the manuscript draft and participated directly in running study participants. EP and TLS participated in study analysis including aspects of statistical analysis and revised the draft. BR participated in all aspects of the molecular assays utilized in the manuscript and revised the manuscript. BLB participated in Western blot analysis, immunocytochemistry, scoring of ERK-activated cells, and revised the manuscript. DL participated in conceptualization and design of the experiments, participated in the statistical analysis, was responsible for all aspects of the molecular assays, and revised the manuscript. All authors read and approved the manuscript.
References
- Adviento B, Corbin IL, Widjaja F, Desachy G, Enrique N, Rosser T, Risi S, Marco EJ, Hendren RL, Bearden CE, Rauen KA, Weiss LA. Autism traits in the RASopathies. J. Med. Genet. 2014;51(1):10–20. doi: 10.1136/jmedgenet-2013-101951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alley GM, Bailey JA, Chen D, Ray B, Puli LK, Tanila H, Banerjee PK, Lahiri DK. Memantine lowers amyloid-beta peptide levels in neuronal cultures and in APP/PS1 transgenic mice. J. Neurosci. Res. 2010;88(1):143–154. doi: 10.1002/jnr.22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey AR, Giunta BN, Obregon D, Nikolic WV, Tian J, Sanberg CD, Sutton DT, Tan J. Peripheral biomarkers in Autism: secreted amyloid precursor protein-alpha as a probable key player in early diagnosis. Int. J. Clin. Exp. Med. 2008;1(4):338–344. [PMC free article] [PubMed] [Google Scholar]
- Bailey AR, Hou H, Song M, Obregon DF, Portis S, Barger S, Shytle D, Stock S, Mori T, Sanberg PG, Murphy T, Tan J. GFAP expression and social deficits in transgenic mice overexpressing human sAPPalpha. Glia. 2013;61(9):1556–1569. doi: 10.1002/glia.22544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan K, Burger JA, Fu M, Doifode T, Wierda WG, Gandhi V. Regulation of Mcl-1 expression in context to bone marrow stromal microenvironment in chronic lymphocytic leukemia. Neoplasia. 2014;16(12):1036–1046. doi: 10.1016/j.neo.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Paradoxical striatal cellular signaling responses to psychostimulants in hyperactive mice. J. Biol. Chem. 2006;281(43):32072–32080. doi: 10.1074/jbc.M606062200. [DOI] [PubMed] [Google Scholar]
- Bodles AM, Barger SW. Secreted beta-amyloid precursor protein activates microglia via JNK and p38-MAPK. Neurobiol. Aging. 2005;26(1):9–16. doi: 10.1016/j.neurobiolaging.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Branca M, Ciotti M, Santini D, Bonito LD, Benedetto A, Giorgi C, Paba P, Favalli C, Costa S, Agarossi A, Alderisio M, Syrjanen K, Group HP-PIS. Activation of the ERK/MAP kinase pathway in cervical intraepithelial neoplasia is related to grade of the lesion but not to high-risk human papillomavirus, virus clearance, or prognosis in cervical cancer. Am. J. Clin. Pathol. 2004;122(6):902–911. doi: 10.1309/VQXF-T880-JXC7-QD2W. [DOI] [PubMed] [Google Scholar]
- Busca R, Christen R, Lovern M, Clifford AM, Yue JX, Goss GG, Pouyssegur J, Lenormand P. ERK1 and ERK2 present functional redundancy in tetra-pods despite higher evolution rate of ERK1. BMC Evol. Biol. 2015;15:179. doi: 10.1186/s12862-015-0450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L, Nuttall R, Griffiths D, Gumbleton M. Activated extracellular signal-regulated kinase is an independent prognostic factor in clinically confined renal cell carcinoma. Cancer. 2009;115(15):3457–3467. doi: 10.1002/cncr.24389. [DOI] [PubMed] [Google Scholar]
- Caunt CJ, Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs): shaping the outcome of MAP kinase signalling. FEBS J. 2013;280(2):489–504. doi: 10.1111/j.1742-4658.2012.08716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizmarova M, Hlinkova K, Bertok S, Kotnik P, Duba HC, Bertalan R, Polockova K, Kostalova L, Pribilincova Z, Hlavata A, Kovacs L, Ilencikova D. New Mutations associated with rasopathies in a central european population and genotype-phenotype correlations. Ann. Hum. Genet. 2015;80(1):50–62. doi: 10.1111/ahg.12140. [DOI] [PubMed] [Google Scholar]
- Clarke WP, Chavera TA, Silva M, Sullivan LC, Berg KA. Signalling profile differences: paliperidone versus risperidone. Br. J. Pharmacol. 2013;170(3):532–545. doi: 10.1111/bph.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Costa RM, Murphy GG, Elgersma Y, Zhu Y, Gutmann DH, Parada LF, Mody I, Silva AJ. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell. 2008;135(3):549–560. doi: 10.1016/j.cell.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372(9639):657–668. doi: 10.1016/S0140-6736(08)61279-9. [DOI] [PubMed] [Google Scholar]
- Chévere-Torres I, Kaphzan H, Bhattacharya A, Kang A, Maki JM, Gambello MJ, Arbiser JL, Santini E, Klann E. Metabotropic glutamate receptor-dependent long-term depression is impaired due to elevated ERK signaling in the ΔRG mouse model of tuberous sclerosis complex. Neurobiol. Dis. 2012 Mar.45(3):1101–1110. doi: 10.1016/j.nbd.2011.12.028. http://dx.doi.org/10.1016/j.nbd.2011.12.028 [Epub 2011 Dec 16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RM, Glass L, Snape M, Hurley MJ, Altimiras FJ, Biekofsky RR, Cogram P. NNZ-2566, a novel analog of (1–3) IGF-1, as a potential therapeutic agent for fragile X syndrome. Neuromolecular Med. 2015;17(1):71–82. doi: 10.1007/s12017-015-8341-2. [DOI] [PubMed] [Google Scholar]
- Demars MP, Bartholomew A, Strakova Z, Lazarov O. Soluble amyloid precursor protein: a novel proliferation factor of adult progenitor cells of ectodermal and mesodermal origin. Stem Cell Res. Ther. 2011;2(4):36. doi: 10.1186/scrt77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson C, Ray B, Maloney B, Wink LK, Bowers K, Schaefer TL, McDougle C, Sokol DK, Lahiri D. Impact of acamprosate on plasma amyloid-β precursor protein in youth: a pilot analysis in fragile X syndrome-associated and idiopathic autism spectrum disorder suggests a pharmacodynamic protein marker. J. Psychiatri. Res. 2014 Dec.59:220–228. doi: 10.1016/j.jpsychires.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faridar A, Jones-Davis D, Rider E, Li J, Gobius I, Morcom L, Richards LJ, Sen S, Sherr EH. Mapk/Erk activation in an animal model of social deficits shows a possible link to autism. Mol. Autism. 2014;5:57. doi: 10.1186/2040-2392-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung LK, Hardan AY. Autism in DSM-5 under the microscope: implications to patients, families, clinicians, and researchers. Asian J. Psychiatr. 2014;11:93–97. doi: 10.1016/j.ajp.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Gakhar-Koppole N, Hundeshagen P, Mandl C, Weyer SW, Allinquant B, Muller U, Ciccolini F. Activity requires soluble amyloid precursor protein alpha to promote neurite outgrowth in neural stem cell-derived neurons via activation of the MAPK pathway. Eur. J. Neurosci. 2008;28(5):871–882. doi: 10.1111/j.1460-9568.2008.06398.x. [DOI] [PubMed] [Google Scholar]
- Govindarajan B, Mizesko MC, Miller MS, Onda H, Nunnelley M, Casper K, Brat D, Cohen C, Arbiser JL. Tuberous sclerosis-associated neoplasms express activated p42/44 mitogen-activated protein (MAP) kinase, and inhibition of MAP kinase signaling results in decreased in vivo tumor growth. Clin. Cancer Res. 2003;9(9):3469–3475. [PubMed] [Google Scholar]
- Hoey SE, Buonocore F, Cox CJ, Hammond VJ, Perkinton MS, Williams RJ. AMPA receptor activation promotes non-amyloidogenic amyloid precursor protein processing and suppresses neuronal amyloid-beta production. PLoS One. 2013;8(10):e78155. doi: 10.1371/journal.pone.0078155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii D, Matsuzawa D, Kanahara N, Matsuda S, Sutoh C, Ohtsuka H, Nakazawa K, Kohno M, Hashimoto K, Iyo M, Shimizu E. Effects of aripiprazole on MK-801-induced prepulse inhibition deficits and mitogen-activated protein kinase signal transduction pathway. Neurosci. Lett. 2010;471(1):53–57. doi: 10.1016/j.neulet.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Jeste SS, Sahin M, Bolton P, Ploubidis GB, Humphrey A. Characterization of autism in young children with tuberous sclerosis complex. J. Child. Neurol. 2008;23(5):520–525. doi: 10.1177/0883073807309788. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116(3):467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Sokol DK, Erickson C, Ray B, Ho CY, Maloney B. Autism as early neurodevelopmental disorder: evidence for an sAPPalpha-mediated anabolic pathway. Front. Cell Neurosci. 2013;7:94. doi: 10.3389/fncel.2013.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefloch R, Pouyssegur J, Lenormand P. Single and combined silencing of ERK1 and ERK2 reveals their positive contribution to growth signaling depending on their expression levels. Mol. Cell Biol. 2008;28(1):511–527. doi: 10.1128/MCB.00800-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Yang Z. Expression of phospho-ERK1/2 and PI3-K in benign and malignant gallbladder lesions and its clinical and pathological correlations. J. Exp. Clin. Cancer Res. 2009;28:65. doi: 10.1186/1756-9966-28-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JM, Ray B, Lahiri DK. MicroRNA-339-5p down-regulates protein expression of beta-site amyloid precursor protein-cleaving enzyme 1 (BACE1) in human primary brain cultures and is reduced in brain tissue specimens of Alzheimer disease subjects. J. Biol. Chem. 2014;289(8):5184–5198. doi: 10.1074/jbc.M113.518241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Teruya-Feldstein J, Bonner P, Bernardi R, Franz DN, Witte D, Cordon-Cardo C, Pandolfi PP. Identification of S664 TSC2 phosphorylation as a marker for extracellular signal-regulated kinase mediated mTOR activation in tuberous sclerosis and human cancer. Cancer Res. 2007;67(15):7106–7112. doi: 10.1158/0008-5472.CAN-06-4798. [DOI] [PubMed] [Google Scholar]
- Malhotra D, Sebat J. CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell. 2012;148(6):1223–1241. doi: 10.1016/j.cell.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney B, Lahiri DK. Epigenetics of dementia: understanding the disease as a transformation rather than a state. Lancet Neurol. 2016;15(7):760–774. doi: 10.1016/S1474-4422(16)00065-X. [DOI] [PubMed] [Google Scholar]
- Mariner R, Jackson AW, 3rd, Levitas A, Hagerman RJ, Braden M, McBogg PM, Smith AC, Berry R. Autism, mental retardation, and chromosomal abnormalities. J Autism Dev. Disord. 1986 Dec.16(4):425–440. doi: 10.1007/BF01531709. [DOI] [PubMed] [Google Scholar]
- Maski KP, Jeste SS, Spence SJ. Common neurological co-morbidities in autism spectrum disorders. Curr. Opin. Pediatr. 2011;23(6):609–615. doi: 10.1097/MOP.0b013e32834c9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara D, Ishikawa S, Oguni S, Aburatani H, Fukayama M, Niki T. Molecular predictors of sensitivity to the MET inhibitor PHA665752 in lung carcinoma cells. J. Thorac. Oncol. 2010;5(9):1317–1324. doi: 10.1097/JTO.0b013e3181e2a409. [DOI] [PubMed] [Google Scholar]
- Merlo D, Cifelli P, Cicconi S, Tancredi V, Avoli M. 4-Aminopyridineinduced epileptogenesis depends on activation of mitogen-activated protein kinase ERK. J. Neurochem. 2004;89(3):654–659. doi: 10.1111/j.1471-4159.2004.02382.x. [DOI] [PubMed] [Google Scholar]
- Michalon A, Sidorov M, Ballard TM, Ozmen L, Spooren W, Wettstein JG, Jaeschke G, Bear MF, Lindemann L. Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron. 2012;74(1):49–56. doi: 10.1016/j.neuron.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, Courchesne E, Everall IP. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol. Psychiatry. 2010;68(4):368–376. doi: 10.1016/j.biopsych.2010.05.024. [DOI] [PubMed] [Google Scholar]
- Naci D, Aoudjit F. Alpha2beta1 integrin promotes T cell survival and migration through the concomitant activation of ERK/Mcl-1 and p38 MAPK pathways. Cell Signal. 2014;26(9):2008–2015. doi: 10.1016/j.cellsig.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Osterweil EK, Chuang SC, Chubykin AA, Sidorov M, Bianchi R, Wong RK, Bear MF. Lovastatin corrects excess protein synthesis and prevents epileptogenesis in a mouse model of fragile X syndrome. Neuron. 2013;77(2):243–250. doi: 10.1016/j.neuron.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26(22):3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- Peng M, Li Y, Luo Z, Liu C, Laties AM, Wen R. Alpha2-adrenergic agonists selectively activate extracellular signal-regulated kinases in Muller cells in vivo. Invest Ophthalmol. Vis. Sci. 1998;39(9):1721–1726. [PubMed] [Google Scholar]
- Pereira A, Zhang B, Malcolm P, Sugiharto-Winarno A, Sundram S. Quetiapine and aripiprazole signal differently to ERK, p90RSK and c-Fos in mouse frontal cortex and striatum: role of the EGF receptor. BMC Neurosci. 2014;15:30. doi: 10.1186/1471-2202-15-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucilowska J, Vithayathil J, Tavares EJ, Kelly C, Karlo JC, Landreth GE. The 16p11.2 deletion mouse model of autism exhibits altered cortical progenitor proliferation and brain cytoarchitecture linked to the ERK MAPK pathway. J. Neurosci. 2015;35(7):3190–3200. doi: 10.1523/JNEUROSCI.4864-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B, Chopra N, Long JM, Lahiri DK. Human primary mixed brain cultures: preparation, differentiation, characterization and application to neuroscience research. Mol. Brain. 2014;7:63. doi: 10.1186/s13041-014-0063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins DJ, Zhen E, Owaki H, Vanderbilt CA, Ebert D, Geppert TD, Cobb MH. Regulation and properties of extracellular signal-regulated protein kinases 1 and 2 in vitro. J. Biol. Chem. 1993;268(7):5097–5106. [PubMed] [Google Scholar]
- Samuels IS, Karlo JC, Faruzzi AN, Pickering K, Herrup K, Sweatt JD, Saitta SC, Landreth GE. Deletion of ERK2 mitogen-activated protein kinase identifies its key roles in cortical neurogenesis and cognitive function. J. Neurosci. 2008;28(27):6983–6995. doi: 10.1523/JNEUROSCI.0679-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels IS, Saitta SC, Landreth GE. MAP'ing CNS development and cognition: an ERKsome process. Neuron. 2009;61(2):160–167. doi: 10.1016/j.neuron.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh Y, Endo S, Nakata T, Kobayashi Y, Yamada K, Ikeda T, Takeuchi A, Hiramoto T, Watanabe Y, Kazama T. ERK2 contributes to the control of social behaviors in mice. J. Neurosci. 2011;31(33):11953–11967. doi: 10.1523/JNEUROSCI.2349-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol DK, Chen D, Farlow MR, Dunn DW, Maloney B, Zimmer JA, Lahiri DK. High levels of Alzheimer beta-amyloid precursor protein (APP) in children with severely autistic behavior and aggression. J. Child. Neurol. 2006;21(6):444–449. doi: 10.1177/08830738060210062201. [DOI] [PubMed] [Google Scholar]
- Sokol DK, Maloney B, Long JM, Ray B, Lahiri DK. Autism, Alzheimer disease, and fragile X: APP, FMRP, and mGluR5 are molecular links. Neurology. 2011;76(15):1344–1352. doi: 10.1212/WNL.0b013e3182166dc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetreault NA, Hakeem AY, Jiang S, Williams BA, Allman E, Wold BJ, Allman JM. Microglia in the cerebral cortex in autism. J. Autism Dev. Disord. 2012 Dec.42(12):2569–2584. doi: 10.1007/s10803-012-1513-0. http://dx.doi.org/10.1007/s10803-012-1513-0. [DOI] [PubMed] [Google Scholar]
- Tian D, Stoppel LJ, Heynen AJ, Lindemann L, Jaeschke G, Mills AA, Bear MF. Contribution of mGluR5 to pathophysiology in a mouse model of human chromosome 16p11.2 microdeletion. Nat. Neurosci. 2015;18(2):182–184. doi: 10.1038/nn.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzan B, Poglio S, Gerby B, Wu CL, Gross J, Armstrong F, Calvo J, Cahu X, Deswarte C, Dumont F, Passaro D, Besnard-Guerin C, Leblanc T, Baruchel A, Landman-Parker J, Ballerini P, Baud V, Ghysdael J, Baleydier F, Porteu F, Pflumio F. Interleukin-18 produced by bone marrow-derived stromal cells supports T-cell acute leukaemia progression. EMBO Mol. Med. 2014;6(6):821–834. doi: 10.1002/emmm.201303286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Snape M, Klann E, Stone JG, Singh A, Petersen RB, Castellani RJ, Casadesus G, Smith MA, Zhu X. Activation of the extracellular signal-regulated kinase pathway contributes to the behavioral deficit of fragile x-syndrome. J. Neurochem. 2012;121(4):672–679. doi: 10.1111/j.1471-4159.2012.07722.x. [DOI] [PubMed] [Google Scholar]
- Yamagata Y, Kaneko K, Kase D, Ishihara H, Nairn AC, Obata K, Imoto K. Regulation of ERK1/2 mitogen-activated protein kinase by NMDA-receptor-induced seizure activity in cortical slices. Brain Res. 2013;1507:1–10. doi: 10.1016/j.brainres.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yufune S, Satoh Y, Takamatsu I, Ohta H, Kobayashi Y, Takaenoki Y, Pages G, Pouyssegur J, Endo S, Kazama T. Transient blockade of ERK phosphorylation in the critical period causes autistic phenotypes as an adult in mice. Sci. Rep. 2015;5:10252. doi: 10.1038/srep10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhou X, Shen H, Wang D, Wang Y. Phosphorylated ERK is a potential predictor of sensitivity to sorafenib when treating hepatocellular carcinoma: evidence from an in vitro study. BMC Med. 2009;7:41. doi: 10.1186/1741-7015-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.