Abstract
Aquaporin-5 (AQP5) is present on the apical membrane of epithelial cells in various secretory glands as well as on the apical membrane of the airway epithelium, airway submucosal glands, and type 1 pneumocytes, where it can participate in respiratory tract water homeostasis. We examined the effects of cAMP on AQP5 distribution and abundance. When AQP5-expressing mouse lung epithelial cells were treated with cAMP or the β-adrenergic agonist terbutaline, a biphasic AQP5 response was observed. Short term (minutes) exposure to cAMP produced internalization of AQP5 off of the membrane and a decrease in protein abundance. Both of these responses were blocked by inhibition of protein kinase A and the decrease in abundance was blocked by chloroquine, indicating lysosome-mediated degradation. Sustained cAMP exposure (hours) produced an increase in membrane localization and increased abundance; these effects were also blocked by protein kinase A inhibition. The β-adrenergic agonist terbutaline produced changes in AQP5 abundance in mouse trachea and lung, consistent with our findings in cultured epithelial cells. Purified AQP5 protein was phosphorylated by protein kinase A but not protein kinase C or casein kinase II, and aquaporin-5 was phosphorylated in cultured cells after long term (but not short term) exposure to cAMP. These studies indicate that cAMP and β-adrenergic agonists produce distinct short and long term effects on AQP5 distribution and abundance that may contribute to regulation of lung water homeostasis.
Aquaporins are water-specific membrane channel proteins that participate in a wide array of physiologic processes (1). The presence or absence of an aquaporin is the primary determinant of membrane osmotic water permeability. Aquaporin-5 (AQP5)1 is an apical membrane water channel found at several sites in mammals, including corneal and pancreatic epithelium, secretory cells in salivary and lacrimal glands, and airway submucosal glands, bronchial epithelium, and type I pneumocytes of the respiratory tract (2–5). Studies of isolated salivary gland epithelial cells (6, 7) and type I pneumocytes (8) demonstrate that AQP5 expression confers high level membrane water permeability. An in vivo role for AQP5 in water homeostasis is strongly indicated by studies of AQP5-null mice, which have reduced salivary (6, 7), sweat (9), and airway submucosal gland secretions (10) as well as by recent investigation of persons with Sjögren’s syndrome, in whom disrupted AQP5 membrane targeting probably contributes to the clinical hallmarks of dry eyes and dry mouth (11, 12).
Information regarding the regulation of abundance and distribution of AQP5 is relatively limited. Stimuli such as tumor necrosis factor-α (13) and adenoviral infection (14) have been shown to reduce AQP5 expression, whereas hypertonic stress increases AQP5 in cultured lung epithelial cells as well as in lungs and secretory glands of hyperosmolar rats (15). Pilocarpine-stimulated recruitment of AQP5 to the plasma membrane of salivary gland epithelial cells has been proposed (16); however, no redistribution has been observed by direct visualization (17), and little is known about regulation of AQP5 membrane targeting.
The aquaporin to which AQP5 is most closely related genetically is AQP2, located in the collecting duct of the kidney (1). In response to cAMP, either directly or downstream of vasopressin receptor activation, AQP2 is rapidly translocated from an intracellular vesicular pool to the apical plasma membrane, and AQP2 expression is increased (18). Several lines of evidence implicate cAMP in regulation of physiologic processes in the lung. In the alveolus, β-adrenergic agonists increase intracellular cAMP and accelerate alveolar fluid reabsorption, an effect believed to be mediated principally by increased sodium transport (19). In the airways, the effects of β-adrenergic agonists and cAMP on smooth muscle relaxation and on the inflammatory mediators in mucosal secretions in asthmatics are well described (20). cAMP-mediated effects on ciliary beat frequency (21–23) and cystic fibrosis transmembrane conductance regulator activation (24) contribute to regulation of mucociliary transport and the aqueous surface layer, and in animal models prolonged increases in intracellular cAMP increase airway secretions (25). However, little is known about the overall effect of cAMP on transport proteins that may contribute to generation or modulation of the airway surface layer (20).
Yang and colleagues (26) observed cAMP induction of AQP5 mRNA and protein expression in cultured lung epithelial cells after several hours of exposure. We examined the effects of cAMP on AQP5 regulation in a similar manner, but we observed a more complex response. In this study, we describe biphasic effects of cAMP and β-adrenergic agonists on AQP5 protein trafficking and abundance. Short term exposure to cAMP produces internalization of AQP5 off of the plasma membrane and lysosomal degradation of the protein, whereas sustained exposure to cAMP results in AQP5 induction and membrane targeting. These findings indicate that dynamic regulation of AQP5 distribution and abundance by cAMP may contribute to water homeostasis in both the proximal and distal respiratory tracts.
EXPERIMENTAL PROCEDURES
Materials
Electrophoresis reagents were from Bio-Rad. Reagents for enhanced chemiluminescence (ECL+) were from Amersham Biosciences. Bicinchoninic acid (BCA) protein assay kit was from Pierce. Affinity-purified polyclonal antibodies to the carboxyl terminus of rat AQP5 have been described previously (27). Antibodies to the Na,K-ATPase α1 subunit were from Upstate Biotechnology (Lake Placid, NY). Horseradish peroxidase-coupled secondary antibodies specific for rabbit or mouse immunoglobulins were from Amersham Biosciences. MG132 and lactacystin were purchased from Calbiochem-Novabiochem. pCPT-cAMP, chloroquine and H89 were purchased from Sigma. N-octyl-β-d-glucopyranoside (octylglucoside) was obtained from Calbiochem. Escherichia coli total lipid extract acetone/ether preparation was from Avanti Polar Lipids. Nickel-nitrilotriacetic acid agarose was from Qiagen (Valencia, CA). Restriction enzymes were from New England Biolabs. Unless specified, all other reagents were from Sigma.
Cell Culture, Drug Treatments, and Sample Preparation
Mouse lung epithelial cells (MLE-12), a gift from Jeff Whitsett (University of Cincinnati) (28), were grown on untreated culture dishes (Falcon; BD Biosciences) at 37 °C with 5% CO2 in RPMI 1640 (Invitrogen) supplemented with 2 mm l-glutamine, 100 units/ml penicillin, 0.1 mg/ml streptomycin, and 3% fetal bovine serum (Invitrogen). BEAS-2b human lung epithelial cells (American Type Culture Collection) were grown on untreated culture dishes (Falcon; BD Biosciences) at 37 °C with 5%CO2 in equal volumes of Ham’s F12 medium and Dulbecco’s modified Eagle’s medium (low glucose, sodium pyruvate and pyridoxine) (Mediatech, Inc.) supplemented with 2 mm l-glutamine, 10 mm HEPES, pH 7.2, 50 units/ml penicillin, 2 µg/ml gentamicin, and 10% fetal bovine serum (Invitrogen). When specified, medium was made hypertonic by addition of 200 mosm sorbitol. pCPT-cAMP, terbutaline, or forskolin was added for the specified times. Cell harvest was performed as described previously (15).
Transfection
BEAS-2B cells were grown in chamber slides to 50 to 60% confluence and transiently transfected (1 µg/well) with an hemagglutinin-tagged human AQP5 cDNA using FuGENE 6 (1.5 µl; Roche) according to the manufacturer’s recommendations. Human AQP5 was inserted into the EcoRI site in the carboxyl-terminal multicloning site of the EGFP-C3 plasmid (BD Biosciences Clontech). The N-terminal green fluorescent protein was replaced by hemagglutinin using HindIII and AgeI restriction sites.
Immunoblotting
Total protein concentrations of the samples were determined by the BCA assay with bovine serum albumin as the standard. Depending on the experiment, 10–50 µg of total protein in 1.5% (w/v) SDS was loaded per lane and separated on 12% SDS-PAGE gels before transfer to a nitrocellulose membrane. Immunoblotting and blot visualization were performed as described previously (29). Equivalence of samples was confirmed either by Coomassie Brilliant Blue staining of parallel SDS-PAGE gels or Ponceau Red staining (Bio-Rad) of nitrocellulose membranes. Relative band intensities were determined by densitometry using ImageQuant 5.2 densitometry analysis (Amersham Biosciences).
Confocal Immunofluorescence
Confocal laser microscopy (Ultraview; PerkinElmer Life and Analytical Sciences) was performed on cells grown in uncoated chamber slides (Falcon) using antibodies against AQP5 or Na,K-ATPase and goat anti-rabbit secondary antibodies (Alexa 488 or Texas Red; Molecular Probes). Merged images for analysis of double labeling or z-planes were reconstructed by differential interference contrast images.
Surface Biotinylation
Cells were incubated in control medium or medium supplemented with pCPT-cAMP (100 µm, 30 min or 8 h), in the presence or absence of the PKA inhibitor H89. Cell monolayers were washed and a cell impermeable biotin derivative (0.5 mg/ml EZ Link Sulfo-NHS-LC-Biotin; Pierce) was added for 30 min in serum-free control medium or medium supplemented with pCPT-cAMP (100 µm) to biotinylate cell surface proteins. Unreacted biotinylation was quenched by the addition of 50 mm Tris, pH 8.0. Cells were lysed by solubilization buffer (20 mm Tris, pH 8.0, 200 mm NaCl, 1% (v/v) Triton X-100, 1% (w/v) deoxycholate, 0.1% (w/v) SDS, 5 mm EDTA, 5 mm EGTA, and Complete Protease Inhibitor mixture tablet), and immunoprecipitations were performed using streptavidin to precipitate biotinylated proteins. Immunoprecipitates were washed four times in solubilization buffer as described above and eluted in 10 mm Tris, pH 6.8, 3% (w/v) SDS, 10% (v/v) glycerol, 0.01% (w/v) bromphenol blue, and 0.5 M dithiothreitol for 30 min at 37 °C. Changes in the amount of biotinylated AQP5 were assayed by immunoblotting.
Metabolic Labeling of Cells
MLE-12 cells were metabolically labeled with 0.4 mCi/ml 32P in the presence of orthovanadate, in serum-free media using standard conditions (30). Cells were harvested at stated time, and AQP5 was immunoprecipitated as described previously (31). Anti-AQP5 immunoprecipitations were resolved by SDS-PAGE for visualization with autoradiography. Cells were tested in basal conditions and after incubation with pCPT-cAMP (100 µm) to test for inducible phosphorylation.
Animal Protocols
Animal studies were undertaken with protocols approved by the Johns Hopkins School of Medicine Animal Care and Use Committee. Mice (20–30 gm) were given subcutaneous injections of either sterile water (control) or terbutaline (7 or 10 mg/kg). All mice were allowed free access to water and food. Animals were sacrificed by cervical dislocation, and right ventricles were perfused with chilled phosphate buffered saline until free of blood. Tissue samples were removed, frozen on dry ice, and stored at −80 °C for subsequent isolation of membranes as described previously (15, 27). In brief, tissues were homogenized in a Potter-Elvehjem homogenizer on ice in homogenization buffer containing 0.25 m sucrose, 1 mm EDTA, 1 mm sodium azide, and protease inhibitors (Complete protease mixture tablets; Roche Biochemicals). Serial centrifugations were performed to obtain a membrane fraction and crude pellets were solubilized in 1.5% (w/v) SDS, and total protein concentration was measured. SDS-PAGE and immunoblotting were performed as above.
Expression and Purification of AQP5
Human AQP5 was purified from protease-deficient (pep4Δ) Saccharomyces cerevisiae expressing pYES2 (Invitrogen) containing human AQP5 in the multicloning site. Membranes were solubilized in 200 ml of buffer A (100 mm K2HPO4, 10% glycerol, 200 mm NaCl, 5 mm 2-mercaptoethanol, and 3% octyl glucoside (N-octyl-β-d-glucopyranoside; Calbiochem) with protease inhibitors (EDTA-free complete protease inhibitor mixture tablets; Roche Biochemicals)), loaded on a nickel-nitrilotriacetic acid agarose column (Qiagen), washed in 100 volumes of buffer A with 50 mm imidazole, and eluted with buffer A containing 1 m imidazole.
In Vitro Phosphorylation of AQP5
Purified AQP5 protein was reconstituted with Escherichia coli polar lipid extract (Avanti, Alabaster, AL) into proteoliposomes by dialysis at a lipid to protein ratio of ~100:1 overnight at 4 °C against 100 volumes of reconstitution buffer (50 mm MOPS, 150 mm N-methyl-d-glucamine (Calbiochem), 1 mm NaN3 (Sigma), and protease inhibitors (EDTA-free), pH 7.5). AQP5 proteoliposomes were incubated in the presence of catalytic subunits of PKA (1 unit; bovine heart), protein kinase C (PKC, 1 unit; rat brain) and recombinant human casein kinase II (CKII, 1 unit; Calbiochem) with 1 µCi/µl [γ-32P]ATP (Amersham Biosciences) and Buffer A (5 mm NaH2PO4, 1.5 mm CaCl2, 5 mm MgCl2, 1 mm EDTA, and 1 mm DTT) or CKII buffer (50 mm KCl, 20 mm Tris-HCl, and 10 mm MgCl2, pH 7.5). Samples were resolved by gel electrophoresis and exposed to film to assess phosphorylation of AQP5.
AQP5 Proteoliposome Transport Studies
AQP5 proteoliposomes and protein-free control liposomes were analyzed by light scattering using a stopped-flow apparatus (SF-2001; Kin Tek Instruments, University Park, PA) with a dead time of ≤ 1 ms. Osmotic water permeability of proteoliposomes was measured at 4 °C as described previously (32). Second-order rate constants were calculated using the equation L Pf = k/(S/V0) × Vw × Δosm, where Pf is the osmotic water permeability, S/V0 is the vesicle surface area to initial volume ratio, Vw is the partial molar volume of water, and Δosm is the difference in osmolarity between the intravesicular and extravesicular aqueous solutions (usually 300 mosm). Stopped-flow was performed on wild-type and phosphorylated AQP5 to measure water-transport.
RESULTS
cAMP Stimulates AQP5 Redistribution in Lung Epithelial Cells
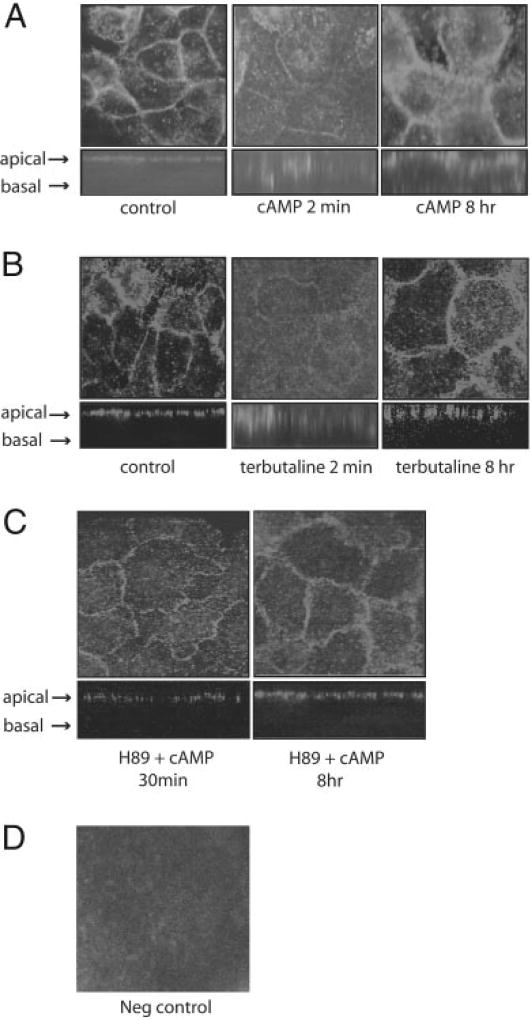
MLE-12 cells spontaneously express AQP5, and the protein is present on the apical membrane as observed in vivo (Fig. 1). To examine the effects of cAMP on AQP5 distribution, MLE-12 cells were incubated with a stable, membrane-permeable cAMP analog, pCPT-cAMP (100 µm). In untreated cells, AQP5 labeling is evident on the apical membrane (Fig. 1A). Labeling along the lateral border in the XY plane is at the superior margin, because we have never observed labeling extending down the lateral membrane in the XZ plane reconstructions. Within 2 min of cAMP stimulation, AQP5 labeling on the membrane was markedly reduced, and increased intracellular labeling was evident (Fig. 1A). After 8 h of stimulation by cAMP, AQP5 labeling of the apical membrane seemed to be greater than that seen at baseline. Incubation of cells with the β-adrenergic agonist terbutaline, which is known to increase intracellular cAMP (33), produced an identical pattern of AQP5 redistribution, with loss of membrane labeling and internalization after short term cAMP exposure, and increased labeling compared with baseline after long term stimulation (Fig. 1B). Forskolin, which activates adenyl cyclase to produce cAMP, produced similar results (data not shown). To examine the effect of cAMP on a different membrane transport protein, we examined the distribution of the α-subunit of the Na,K-ATPase. Short term stimulation with cAMP caused a redistribution of the Na,K-ATPase from its position in the basal membrane in unstimulated cells to an intracellular compartment after cAMP stimulation (Fig. 2).
Fig. 1. Effects of cAMP on AQP5 distribution.
A, MLE-12 cells were incubated in control media or with pCPT-cAMP (100 µm) for 2 min or 8 h. Cells were fixed and permeabilized at the designated times, labeled with anti-AQP5 antibodies and immunofluorescence was performed using confocal microscopy. Images of the xy (top) and xz (bottom) planes are displayed. B, MLE-12 cells were incubated in control media or with terbutaline (100 µm) for the designated times, and examined using confocal microscopy as in A. Both cAMP and terbutaline produced rapid internalization of AQP5 off the cell membrane, whereas prolonged exposure led to increased abundance of AQP5 at the membrane. C, PKA inhibition blocks cAMP-mediated AQP5 redistribution. MLE-12 cells were pre-incubated with the PKA inhibitor H89 (30 µm) then stimulated with pCPT-cAMP (100 µm) for 2 min or 8 h. Anti-AQP5 immunofluorescence revealed that H89 blocked internalization of AQP5 after short term cAMP stimulation and also seemed to limit the increase in AQP5 membrane labeling after long term stimulation. D, negative control. Cells were incubated with pre-immune serum to assess nonspecific binding.
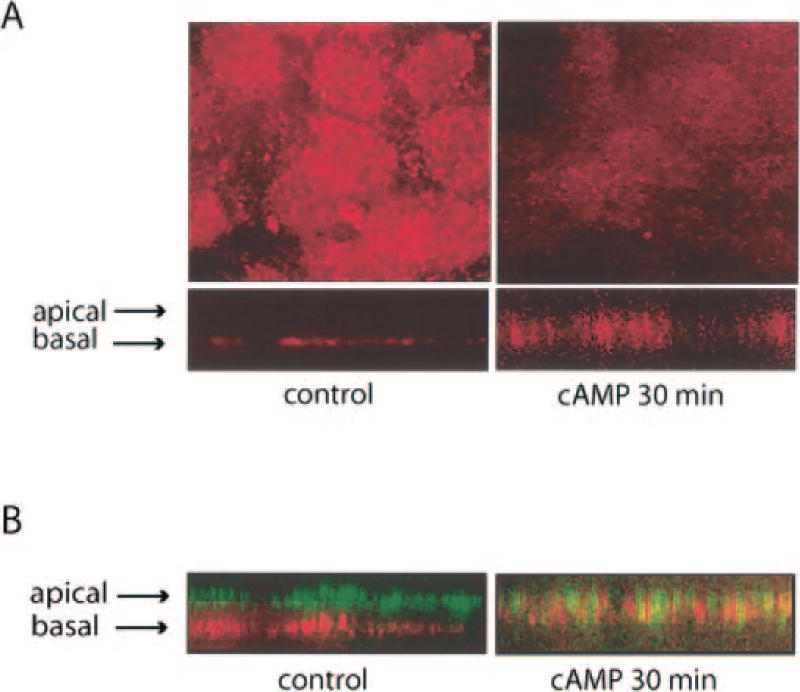
Fig. 2. Effects of cAMP on Na,K-ATPase distribution.
A, MLE-12 cells were incubated in control media or with pCPT-cAMP (100 µm) for 30 min, then fixed and permeabilized for labeling with antibodies to the α1 subunit of the Na,K-ATPase. Immunofluorescence was performed using confocal microscopy and the images of xy (top) and xz (bottom) planes are displayed. B, after incubation with pCPT-cAMP (100 µm; 30 min) cells co-labeled with both anti-AQP5 antibodies (green) and anti-(α1)Na,K-ATPase antibodies (red). cAMP stimulated internalization of both proteins off of their respective membranes. The xz plane is displayed.
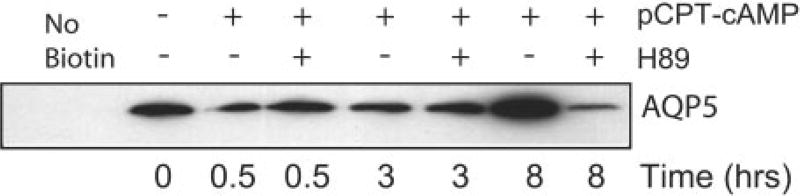
Incubation of cells with the PKA inhibitor H89 blocked the internalization of AQP5 off of the plasma membrane after short term exposure to cAMP (Fig. 1C). In addition, H89 also blocked the apparent increase in plasma membrane AQP5 after stimulation with cAMP for 8 h, suggesting that PKA activation is required for both components of this biphasic response (Fig. 3). The cAMP-mediated redistribution of AQP5 observed with immunofluorescence was confirmed using surface biotinylation. Consistent with the results of immunofluorescence, the amount of AQP5 available at the plasma membrane for binding to biotin was greatly reduced after 30 min of stimulation with pCPT-cAMP (Fig. 3). After 8 h of cAMP exposure, biotinylated AQP5 was increased, indicating an increase in AQP5 at the plasma membrane. PKA inhibition with H89 blocked both the short and long term effects of cAMP on AQP5 distribution (Fig. 3).
Fig. 3. Effects of cAMP on AQP5 surface biotinylation.
Cells were incubated with control media or with pCPT-cAMP (100 µm) for the designated times in the presence or absence of the PKA inhibitor H89 (30 µm), surface biotinylation was performed, and immunoblots of streptavidin immunoprecipitates were probed for AQP5. 30-min exposure to cAMP reduced the abundance of AQP5 at the cell membrane, 8-h exposure increased AQP5 abundance at the membrane, and both effects were blocked by H89.
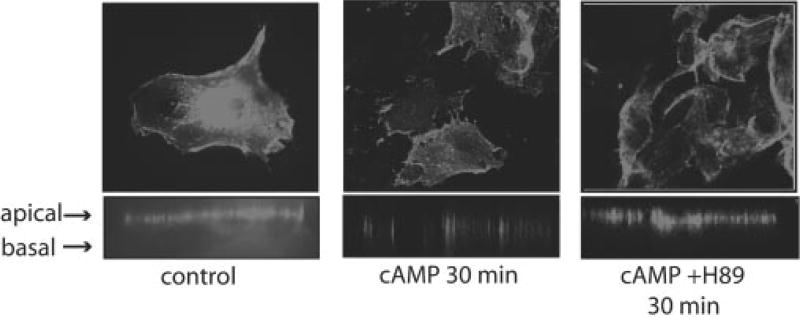
To determine whether cAMP-mediated AQP5 redistribution in mouse lung epithelial cells was conserved in human cells, we transfected Beas-2B cells, a commonly used human lung epithelial cell line, with human AQP5. These cells have no detectable AQP5 protein.2 After transfection, unstimulated Beas-2b cells exhibit apical AQP5 (Fig. 4). After stimulation with cAMP for 30 min, confocal microscopy revealed that AQP5 had been internalized off of the plasma membrane. This effect was blocked by pretreatment with H89.
Fig. 4. cAMP stimulates AQP5 redistribution in human Beas-2b epithelial cells.
Beas-2b cells were transfected with a hemagglutinin-hAQP5 plasmid. Cells were incubated with either control media or media supplemented with pCPT-cAMP (100 µm) in the presence of absence of PKA inhibitor H89 (30 µm) and AQP5 was labeled. Cells were fixed and permeabilized, and immunofluorescence was performed using confocal microscopy. Consistent with the MLE-12 cells, short term incubation with cAMP caused internalization of AQP5 off the cell membrane, which was blocked by PKA inhibition.
cAMP Regulates AQP5 Protein Abundance in Lung Epithelial Cells
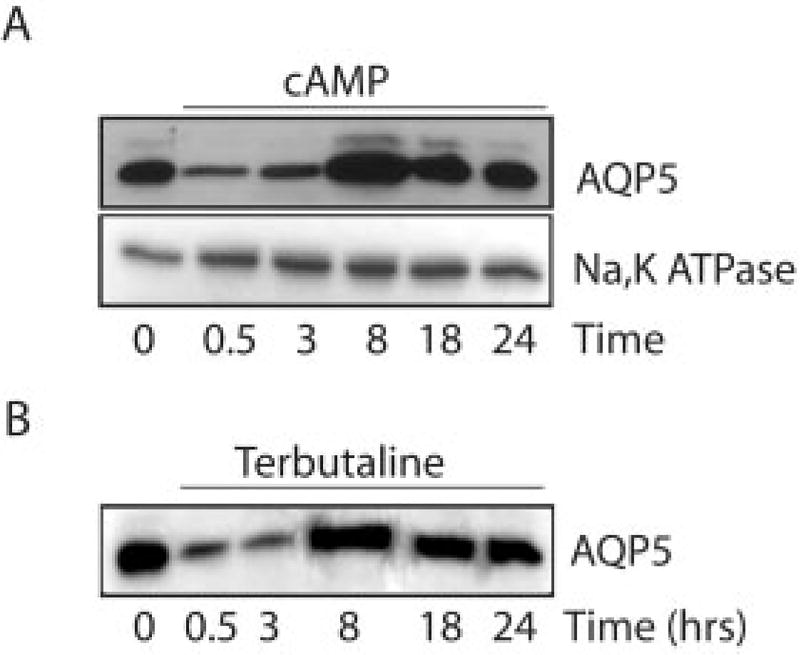
To examine the effects of cAMP on AQP5 protein abundance, MLE-12 cells were stimulated with pCPT-cAMP (100 µm), and cell lysates were probed for AQP5. Similar to its effects on distribution, cAMP stimulation produced biphasic effects on AQP5 protein abundance. Short term exposure to cAMP (30 min) produced a short term decrease in AQP5 protein abundance (Fig. 5A). Exposure to cAMP for 8 h led to a marked increase in protein abundance, which had returned to baseline levels at 24 h (Fig. 5A). In contrast, although it was internalized in response to cAMP (Fig. 2), Na,K-ATPase (α subunit) abundance was not affected by cAMP stimulation (Fig. 5A). A similar pattern of early reduction followed by a later increase in AQP5 was observed after incubation of cells with terbutaline (Fig. 5B).
Fig. 5. Effects of cAMP stimulation on AQP5 abundance.
MLE-12 cells were incubated with control media, pCPT-cAMP (100 µm) (A), or terbutaline (100 µm) (B) and harvested at the specified times. Immunoblots of cell lysates were probed with anti-AQP5 antibodies. cAMP and terbutaline had biphasic effects on AQP5 abundance in cell lysates.
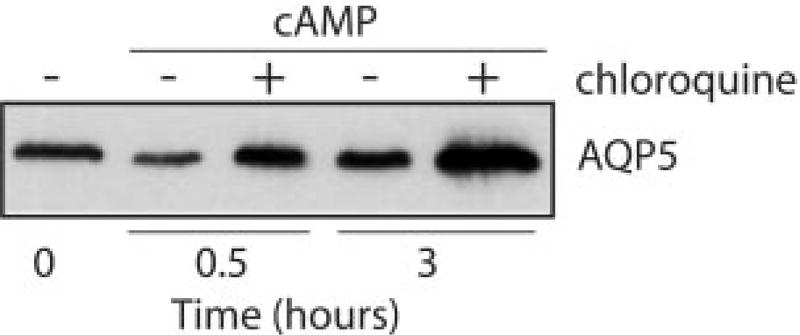
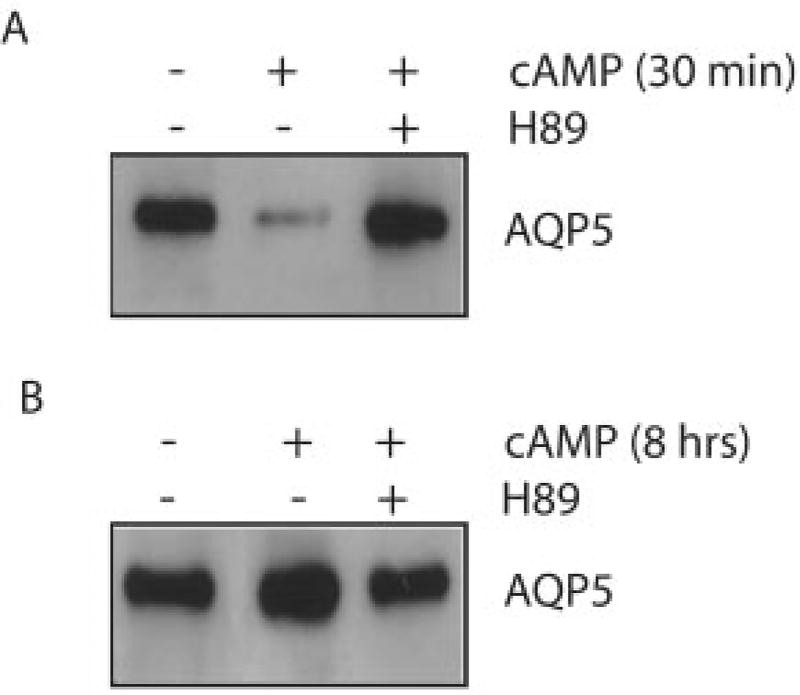
The decrease in AQP5 abundance after short term cAMP stimulation indicated that active protein degradation was probably occurring. We observed previously that a different water channel protein, AQP1, is degraded in proteasomes (31). In contrast to our findings for AQP1, pretreatment of MLE-12 cells with proteasome inhibitors (MG132 or lactacystin) did not block short term AQP5 degradation (data not shown). However, pretreatment with the lysosome inhibitor chloroquine blocked the cAMP-induced decrease in AQP5 protein abundance and produced an overall increase in AQP5 abundance at later times (Fig. 6). In addition, pretreatment of the cells with H89 inhibited both the short term decrease and long term increase in AQP5 protein abundance (Fig. 7), indicating that both responses are PKA-mediated.
Fig. 6. cAMP-induced AQP5 degradation occurs in lysosomes.
MLE-12 cells were incubated in control or pCPT-cAMP-supplemented media (100 µm) in the presence of absence of the lysosome inhibitor chloroquine (100 µm), and harvested at the designated times for anti-AQP5 immunoblots of cell lysates. Chloroquine blocked the short term cAMP-induced reduction in AQP5 abundance.
Fig. 7. PKA inhibition blocks cAMP-induced changes in AQP5 abundance.
Cells were incubated with control media or media containing pCPT-cAMP (100 µm) in the presence or absence of H89 (30 µm), and harvested after 30 min or 8 h. Immunoblots of cell lysates were examined for AQP5 abundance. PKA inhibition blocked both the short term decrease and long term increase in AQP5 abundance.
Terbutaline Regulates AQP5 Abundance in Vivo
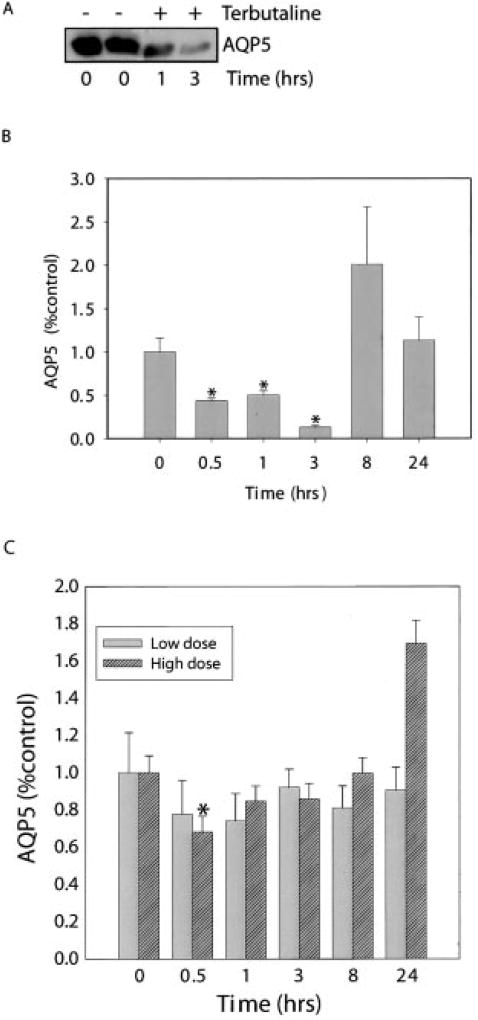
To determine whether terbutaline might alter AQP5 abundance in vivo, mice were injected subcutaneously with sterile water or terbutaline, followed by harvest of the trachea and lungs at intervals. A single injection of terbutaline (7 mg/kg) produced a decrease in AQP5 abundance in the trachea within 1 h that was sustained at 3 h (Fig. 9, A and B). Eight hours after terbutaline injection, there was a non-significant trend toward increased AQP5 expression in the trachea. 7 mg/kg of terbutaline did not alter AQP5 abundance in the peripheral lung (Fig. 8C); however, a single injection of 10 mg/kg produced a 40% reduction in AQP5 in peripheral lung after 30 min, followed by a trend toward an increase in AQP5 at 24 h, consistent with potential regulation of AQP5 by β-adrenergic receptor activation in vivo.
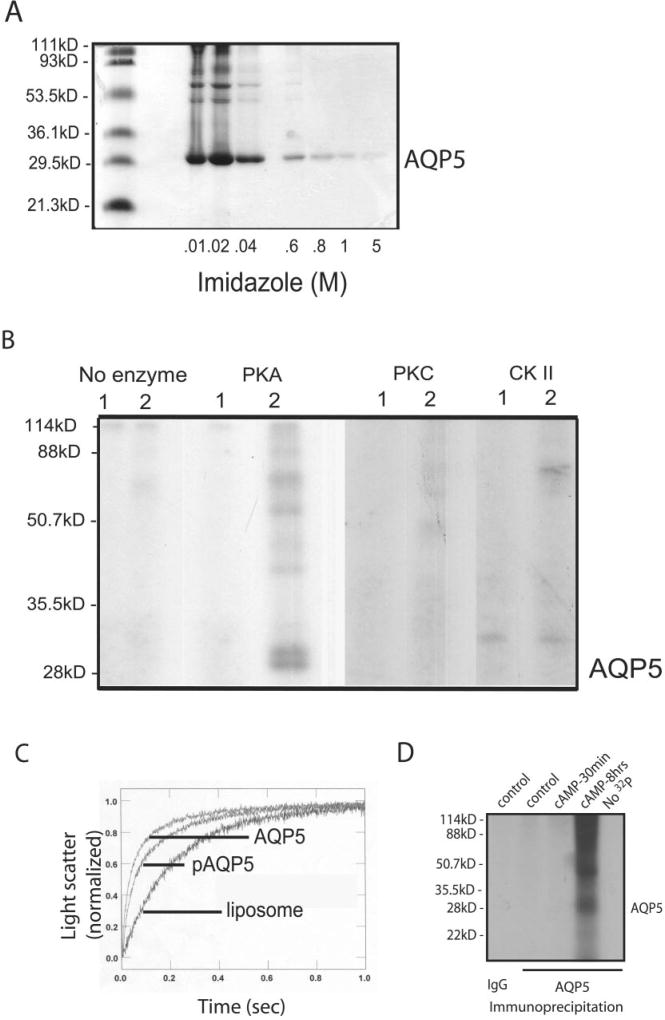
Fig. 9. Phosphorylation of AQP5.
A, Coomassie showing purified AQP5 eluted by imidazole. B, purified AQP5 protein was reconstituted into liposomes and incubated with [γ-32P]ATP in the absence or presence of PKA, PKC, or CKII; (1) control liposomes; (2) AQP5-containing proteoliposomes. Samples were resolved by gel electrophoresis and exposed to film to assess phosphorylation. Only PKA produced AQP5 phosphorylation; higher MW bands consistent with oligomers of AQP5 can be seen. Labeling observed in the CKII lanes is at a higher MW than AQP5 and is consistent with autophosphorylation of CKII (50). C, control and AQP5-containing proteoliposomes were examined for water permeability by stopped flow. AQP5 was highly water permeable compared with control liposomes. Water permeability of phosphorylated AQP5 was not statistically different from nonphosphorylated AQP5. D, MLE-12 cells were incubated with 32P for 30 min, exposed to control media or media containing pCPT-cAMP (100 µm), and harvested at the specified times. Control immunoprecipitates (IgG) or anti-AQP5 immunoprecipitates were resolved by gel electrophoresis and exposed to film for assessment of phosphorylated proteins.
Fig. 8. Terbutaline alters AQP5 abundance in mouse trachea and lung.
A, mice were injected with terbutaline (7 mg/kg; subcutaneous) or sterile water (100 µl) and tracheas were harvested for analysis of AQP5 expression by immunoblot. B, anti-AQP5 immunoblots from animals treated as in A were analyzed by densitometry. Results are expressed as percentage baseline (time 0) (n = 4 per group). C, mice were injected subcutaneously with 7 mg/kg (low dose) or 10 mg/kg (high dose) terbutaline. Lungs were harvested at the designated times for preparation of lung membranes, and anti-AQP5 immunoblots were analyzed by densitometry. Results are expressed as percentage of baseline (time 0) control (n = 4 per group). *, p < 0.05.
AQP5 Can Be Phosphorylated by PKA
Our observations indicate cAMP- and PKA-mediated changes in AQP5 trafficking and abundance; however, phosphorylation of AQP5 has never been demonstrated. To assess this possibility, human AQP5 was over-expressed in yeast, purified (Fig. 9A), and reconstituted into proteoliposomes. Analysis of the AQP5 primary sequence (Scansite 2.0) revealed putative phosphorylation consensus sites for PKA, PKC, and CKII that are accessible on the cytoplasmic face. When proteoliposomes containing human AQP5 were incubated with each of these enzymes, PKA produced phosphorylation of AQP5, whereas PKC and CKII had no effect (Fig. 9B). The phosphorylated AQP5 monomer (~27 kDa) appeared as a doublet (confirmed by repeated studies); however, the reason for the doublet is not yet known, although a similar pattern is occasionally observed on standard protein immunoblots (see Fig. 5A). To determine whether phosphorylation altered the water permeability of AQP5, permeability of non-phosphorylated and phosphorylated human AQP5 were compared using stopped flow (Fig. 9C). As expected, purified AQP5 was highly water permeable. After phosphorylation by PKA, AQP5 permeability was not statistically different. Although purified AQP5 can be phosphorylated, it remained unknown whether AQP5 is phosphorylated in cells under the conditions described above. To assess protein phosphorylation after cAMP stimulation of cultured cells, cells were metabolically labeled with 32P and exposed to cAMP for varying times, and AQP5 immunoprecipitates were examined by autoradiography. It is noteworthy that, after short term cAMP stimulation, no phosphorylation of AQP5 was observed; however, 8-h stimulation with cAMP produced phosphorylation of AQP5 in MLE-12 cells (Fig. 9D). The phosphorylated AQP5 monomer (~27 kDa) appeared as a doublet on repeated experiments, a pattern we also see occasionally on standard immunoblots (see Fig. 5A).
DISCUSSION
Regulation of water homeostasis is fundamental to the physiology of both the proximal and distal respiratory tracts. Several determinants of fluid flux in the respiratory tract have been identified; however, the molecular mechanisms regulating respiratory tract water homeostasis remain only partially defined. In this study, we examined the effects of the cAMP-PKA signaling pathway on the distribution and abundance of AQP5 in lung epithelial cells and have uncovered surprising complexity.
The best-defined example of cAMP regulation of a water channel protein is that of AQP2. AQP2 resides in vesicles in renal collecting duct principal cells (18). Minutes after vasopressin binds its receptor on principal cells, intracellular cAMP increases, serine 256 on AQP2 is phosphorylated by PKA, AQP2-containing vesicles are translocated to the apical plasma membrane, and the collecting duct becomes highly water permeable (34–36). In parallel, vasopressin and elevated cAMP stimulates transcriptional activation of the AQP2 gene and increased abundance of the protein (37, 38).
Within the aquaporin family, AQP5 is most similar genetically to AQP2. Perhaps as expected by extrapolations from AQP2, and consistent with the findings of Yang and colleagues (26), we observed that exposure of lung epithelial cells for several hours to cAMP, the β2-adrenergic agonist terbutaline (which binds its receptor and increases intracellular cAMP), or forskolin (which activates adenylate cyclase directly to increase cAMP) led to increased AQP5 labeling on the apical membrane and increased AQP5 protein abundance. Both of these effects were blocked by inhibition of PKA activation.
In marked contrast, our observations of distinct short term effects of cAMP on AQP5 were completely unexpected and have not been described. Immunofluorescence revealed that within minutes of addition of cAMP, terbutaline, or forskolin to MLE-12 cells, AQP5 was internalized from the plasma membrane, a result confirmed by examination of changes in surface biotinylation of AQP5 after cAMP treatment. When AQP5 was transfected into a commonly used human lung airway epithelial cell line (Beas-2B), we observed similar apical membrane labeling at baseline, with rapid internalization of AQP5 in response to cAMP. This finding indicates not only that internalization of AQP5 acutely in response to cAMP is conserved in both mouse and human cells but also that it is present in alveolar (MLE-12) and airway (Beas-2B) type cells. In the study by Yang and colleagues (26), AQP5 was predominantly intracellular at baseline, and after 24 h of cAMP stimulation, membrane labeling was observed. The reason for the discrepancy with our findings is not apparent. Both our group and theirs used MLE-12 cells from the same source and grown under the same conditions. We uniformly observe predominantly membrane labeling of AQP5 at baseline, consistent with in vivo observations in rat (3) and human lung (4).
In addition to altering protein distribution, cAMP markedly decreased AQP5 abundance within 30 min of exposure. Both the redistribution and degradation of AQP5 were blocked by PKA inhibition. The reduction in AQP5 was also blocked by chloroquine, indicating that after internalization, AQP5 is targeted to lysosomes for degradation. We were unable to detect AQP5 in the culture medium after cAMP stimulation by either immunoprecipitation of AQP5 or trichloroacetic acid precipitation of the medium; however, we cannot completely eliminate the possibility that some fraction of the apical AQP5 is released into the medium rather than being internalized, as has been shown for AQP2 after relief from vasopressin stimulation (3). It is noteworthy that we also observed short term reductions in AQP5 abundance in mouse trachea and peripheral lung after subcutaneous administration of terbutaline, followed over several hours by an increase in AQP5, indicating that this pattern of cAMP-mediated regulation of AQP5 expression is not an isolated in vitro phenomenon.
We examined cAMP effects on the α-subunit of the Na,K-ATPase simply as a control. Like AQP5, the α-subunit was rapidly internalized from the basolateral membrane in MLE cells in response to cAMP; however, in contrast to AQP5, the protein was not degraded. Endocytosis of the Na, K-ATPase α-subunit as a means of regulating its activity has been reported; however, PKC-mediated phosphorylation seems to be required (39, 40). Inhibition of Na,K-ATPase activity by PKA has been described previously (41, 42); however, no effect on distribution was included. The effects of PKA on the Na,K-ATPase α-subunit may be cell type-specific, and interactions between PKA- and PKC-induced phosphorylation have been described previously (43, 44); the relevant mechanisms in MLE-12 cells have not been elucidated.
We provide the first evidence that we are aware of that AQP5 can be directly phosphorylated, a potential mechanism relevant to our observations of AQP5 redistribution and degradation. Whereas the long term effects of cAMP may be mediated by direct phosphorylation, the short term changes apparently do not require AQP5 phosphorylation. However, both the short and long term responses are PKA-mediated. It is possible that short term PKA stimulation facilitates phosphorylation of an AQP5 binding partner that mediates protein trafficking and subsequent changes in abundance.
Dynamic targeting of AQP5 on and off of the plasma membrane as a means of regulating water permeability has been proposed, but direct evidence that it does or can occur is limited. Ishikawa and colleagues (45, 46) examined rat salivary glands in response to secretagogues such as pilocarpine and reported that stimulation of secretion was accompanied by redistribution of AQP5 into a membrane fraction; no direct visualization was reported (16). Gresz and colleagues recently reported direct visualization of AQP5 distribution in rat salivary glands by immunofluorescence and immunoelectron microscopy and observed no change in AQP5 distribution in response to stimulation with pilocarpine or epinephrine (47). Mistargeting of AQP5 in salivary and lacrimal glands has been reported in humans with Sjögren’s syndrome (11, 12); however, the mechanism of the trafficking defect, and its potential relationship to physiologic regulation of distribution, is undefined.
The physiologic implications of cAMP-mediated regulation of AQP5 are unclear, particularly given the biphasic effects we have observed. Although it is known that AQP5 plays a fundamental role in regulation of airway submucosal gland secretion (10), the contribution of AQP5 in the apical membrane of the surface epithelium to the generation or modulation of the airway surface layer has not been defined. Molecular details of chloride and sodium transport, mediated by the cystic fibrosis transmembrane conductance regulator and epithelial Na channel, respectively, have been described primarily, however, in the context of cystic fibrosis (48). It is well established that in persons with asthma, β-adrenergic agonists trigger relaxation of airway smooth muscle and may impact the inflammatory cell infiltrate in the airways; however, their contribution to regulation of the overall fluid secretion in the airways is unclear. Dynamic regulation of AQP5 by cAMP is likely to contribute to changes in airway water homeostasis in both of these contexts.
Likewise, β-adrenergic agonists are known to stimulate sodium transport and fluid clearance in the alveolar epithelium. It is interesting that AQP5-null mice were not found to have different isoosmolar fluid clearance compared with wild-type mice when treated with β-adrenergic agonists (49), a result suggesting that AQP5 did not participate in isoosmolar fluid clearance. That may indeed be correct; however, the overall interpretation of this process may be more complex if the short term response to terbutaline includes internalization of both AQP5 and the α-1 subunit of the Na,K-ATPase. High resolution immunoelectron microscopy is being pursued to confirm this redistribution in type I pneumocytes in mice.
Dynamic regulation of AQP5 distribution and abundance by cAMP provides a potential mechanism for short and long term regulation of membrane water permeability in AQP5 expressing tissues. Further dissection of both the mechanisms and physiologic implications of this regulation may provide insights into novel targets for therapy in a variety of conditions.
Acknowledgments
We thank Peter Agre and David Kozono for general comments and technical assistance.
Footnotes
This work was supported by NHLBI, National Institutes of Health Grants F32-HL074515 (National Research Service Award) (to V. S.) and R01-HL70217 and by American Heart Association Grant-in-aid AHA-0255234N (to L. S. K.).
The abbreviations used are: AQP, aquaporin; pCPT-cAMP, 8-(4-chlorophenylthio)adenosine 3′,5′-cyclic monophosphate; PKA, protein kinase A; PKC, protein kinase C; CKII, casein kinase II; MOPS, 3-(N-morpholino) propanesulfonic acid.
L. King and V. Sidhaye, unpublished observations.
References
- 1.King LS, Kozono D, Agre P. Nat. Rev. Mol. Cell. Biol. 2004;5:687–698. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- 2.Raina S, Preston GM, Guggino WB, Agre P. J. Biol. Chem. 1995;270:1908–1912. doi: 10.1074/jbc.270.4.1908. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen S, King LS, Christensen BM, Agre P. Am. J. Physiol. 1997;273:C1549–C1561. doi: 10.1152/ajpcell.1997.273.5.C1549. [DOI] [PubMed] [Google Scholar]
- 4.Kreda SM, Gynn MC, Fenstermacher DA, Boucher RC, Gabriel SE. Am. J. Respir. Cell Mol. Biol. 2001;24:224–234. doi: 10.1165/ajrcmb.24.3.4367. [DOI] [PubMed] [Google Scholar]
- 5.Burghardt B, Elkaer ML, Kwon TH, Racz GZ, Varga G, Steward MC, Nielsen S. Gut. 2003;52:1008–1016. doi: 10.1136/gut.52.7.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krane CM, Melvin JE, Nguyen HV, Richardson L, Towne JE, Doetschman T, Menon AG. J. Biol. Chem. 2001;276:23413–23420. doi: 10.1074/jbc.M008760200. [DOI] [PubMed] [Google Scholar]
- 7.Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. J. Biol. Chem. 1999;274:20071–20074. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- 8.Dobbs LG, Gonzalez R, Matthay MA, Carter EP, Allen L, Verkman AS. Proc. Natl. Acad. Sci. U. S. A. 1998;95:2991–2996. doi: 10.1073/pnas.95.6.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nejsum LN, Kwon TH, Jensen UB, Fumagalli O, Frokiaer J, Krane CM, Menon AG, King LS, Agre PC, Nielsen S. Proc. Natl. Acad. Sci. U. S. A. 2002;99:511–516. doi: 10.1073/pnas.012588099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song Y, Verkman AS. J. Biol. Chem. 2001;276:41288–41292. doi: 10.1074/jbc.M107257200. [DOI] [PubMed] [Google Scholar]
- 11.Tsubota K, Hirai S, King LS, Agre P, Ishida N. Lancet. 2001;357:688–689. doi: 10.1016/S0140-6736(00)04140-4. [DOI] [PubMed] [Google Scholar]
- 12.Steinfeld S, Cogan E, King LS, Agre P, Kiss R, Delporte C. Lab. Investig. 2001;81:143–148. doi: 10.1038/labinvest.3780221. [DOI] [PubMed] [Google Scholar]
- 13.Towne JE, Krane CM, Bachurski CJ, Menon AG. J. Biol. Chem. 2001;276:18657–18664. doi: 10.1074/jbc.M100322200. [DOI] [PubMed] [Google Scholar]
- 14.Towne JE, Harrod KS, Krane CM, Menon AG. Am. J. Respir. Cell Mol. Biol. 2000;22:34–44. doi: 10.1165/ajrcmb.22.1.3818. [DOI] [PubMed] [Google Scholar]
- 15.Hoffert JD, Leitch V, Agre P, King LS. J. Biol. Chem. 2000;275:9070–9077. doi: 10.1074/jbc.275.12.9070. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa Y, Skowronski MT, Inoue N, Ishida H. Biochem. Biophys. Res. Commun. 1999;265:94–100. doi: 10.1006/bbrc.1999.1630. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa Y, Eguchi T, Skowronski MT, Ishida H. Biochem. Biophys. Res. Commun. 1998;245:835–840. doi: 10.1006/bbrc.1998.8395. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Physiol. Rev. 2002;82:205–244. doi: 10.1152/physrev.00024.2001. [DOI] [PubMed] [Google Scholar]
- 19.Matthay MA, Clerici C, Saumon G. J Appl. Physiol. 2002;93:1533–1541. doi: 10.1152/japplphysiol.01210.2001. [DOI] [PubMed] [Google Scholar]
- 20.Salathe M. J. Allergy Clin. Immunol. 2002;110:S275–S281. doi: 10.1067/mai.2002.129412. [DOI] [PubMed] [Google Scholar]
- 21.Sanderson MJ, Dirksen ER. Am. Rev. Respir. Dis. 1989;139:432–440. doi: 10.1164/ajrccm/139.2.432. [DOI] [PubMed] [Google Scholar]
- 22.Lansley AB, Sanderson MJ, Dirksen ER. Am. J. Physiol. 1992;263:L232–L242. doi: 10.1152/ajplung.1992.263.2.L232. [DOI] [PubMed] [Google Scholar]
- 23.Sanderson MJ, Lansley AB, Dirksen ER. Chest. 1992;101:69S–71S. doi: 10.1378/chest.101.3_supplement.69s. [DOI] [PubMed] [Google Scholar]
- 24.Huang P, Lazarowski ER, Tarran R, Milgram SL, Boucher RC, Stutts MJ. Proc. Natl. Acad. Sci. U. S. A. 2001;98:14120–14125. doi: 10.1073/pnas.241318498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bredenbroker D, Dyarmand D, Meingast U, Fehmann HC, Staats P, Von Wichert P, Wagner U. Eur. J. Pharmacol. 2001;411:319–325. doi: 10.1016/s0014-2999(00)00918-3. [DOI] [PubMed] [Google Scholar]
- 26.Yang F, Kawedia JD, Menon AG. J. Biol. Chem. 2003;278:32173–32180. doi: 10.1074/jbc.M305149200. [DOI] [PubMed] [Google Scholar]
- 27.King LS, Nielsen S, Agre P. Am. J. Physiol. 1997;273:C1541–C1548. doi: 10.1152/ajpcell.1997.273.5.C1541. [DOI] [PubMed] [Google Scholar]
- 28.Wikenheiser KA, Vorbroker DK, Rice WR, Clark JC, Bachurski CJ, Oie HK, Whitsett JA. Proc. Natl. Acad. Sci. U. S. A. 1993;90:11029–11033. doi: 10.1073/pnas.90.23.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis JQ, Bennett V. J. Biol. Chem. 1984;259:13550–13559. [PubMed] [Google Scholar]
- 30.Moore DBS. Current Protocols in Molecular Biology. Vol. 3. John Wiley & Sons; New York: 2004. pp. 18.2.1–18.2.8. [Google Scholar]
- 31.Leitch V, Agre P, King LS. Proc. Natl. Acad. Sci. U. S. A. 2001;98:2894–2898. doi: 10.1073/pnas.041616498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saparov SM, Kozono D, Rothe U, Agre P, Pohl P. J. Biol. Chem. 2001;276:31515–31520. doi: 10.1074/jbc.M104267200. [DOI] [PubMed] [Google Scholar]
- 33.Scott MG, Swan C, Jobson TM, Rees S, Hall IP. Br. J. Pharmacol. 1999;128:721–729. doi: 10.1038/sj.bjp.0702829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. J. Biol. Chem. 1998;273:4296–4299. doi: 10.1074/jbc.273.8.4296. [DOI] [PubMed] [Google Scholar]
- 35.Katsura T, Gustafson CE, Ausiello DA, Brown D. Am. J. Physiol. 1997;272:F817–F822. [PubMed] [Google Scholar]
- 36.Marples D, Schroer TA, Ahrens N, Taylor A, Knepper MA, Nielsen S. Am. J. Physiol. 1998;274:F384–F394. doi: 10.1152/ajprenal.1998.274.2.F384. [DOI] [PubMed] [Google Scholar]
- 37.Ecelbarger CA, Nielsen S, Olson BR, Murase T, Baker EA, Knepper MA, Verbalis JG. J. Clin. Investig. 1997;99:1852–1863. doi: 10.1172/JCI119352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yasui M, Zelenin SM, Celsi G, Aperia A. Am. J. Physiol. 1997;272:F443–F450. doi: 10.1152/ajprenal.1997.272.4.F443. [DOI] [PubMed] [Google Scholar]
- 39.Chibalin AV, Katz AI, Berggren PO, Bertorello AM. Am. J. Physiol. 1997;273:C1458–C1465. doi: 10.1152/ajpcell.1997.273.5.C1458. [DOI] [PubMed] [Google Scholar]
- 40.Chibalin AV, Pedemonte CH, Katz AI, Feraille E, Berggren PO, Bertorello AM. J. Biol. Chem. 1998;273:8814–8819. doi: 10.1074/jbc.273.15.8814. [DOI] [PubMed] [Google Scholar]
- 41.Satoh T, Cohen HT, Katz AI. J. Clin. Investig. 1992;89:1496–1500. doi: 10.1172/JCI115740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Satoh T, Cohen HT, Katz AI. Am. J. Physiol. 1993;265:F399–F405. doi: 10.1152/ajprenal.1993.265.3.F399. [DOI] [PubMed] [Google Scholar]
- 43.Feschenko MS, Stevenson E, Sweadner KJ. J. Biol. Chem. 2000;275:34693–34700. doi: 10.1074/jbc.M005869200. [DOI] [PubMed] [Google Scholar]
- 44.Cheng XJ, Hoog JO, Nairn AC, Greengard P, Aperia A. Am. J. Physiol. 1997;273:C1981–1986. doi: 10.1152/ajpcell.1997.273.6.C1981. [DOI] [PubMed] [Google Scholar]
- 45.Ishikawa Y, Iida H, Ishida H. Mol. Pharmacol. 2002;61:1423–1434. doi: 10.1124/mol.61.6.1423. [DOI] [PubMed] [Google Scholar]
- 46.Ishikawa Y, Skowronski MT, Ishida H. FEBS Lett. 2000;477:253–257. doi: 10.1016/s0014-5793(00)01763-4. [DOI] [PubMed] [Google Scholar]
- 47.Gresz V, Kwon TH, Gong H, Agre P, Steward MC, King LS, Nielsen S. Am. J. Physiol. 2004;287:G151–G161. doi: 10.1152/ajpgi.00480.2003. [DOI] [PubMed] [Google Scholar]
- 48.Boucher RC. Pfluegers Arch. Eur. J. Physiol. 2003;445:495–498. doi: 10.1007/s00424-002-0955-1. [DOI] [PubMed] [Google Scholar]
- 49.Ma T, Fukuda N, Song Y, Matthay MA, Verkman AS. J. Clin. Investig. 2000;105:93–100. doi: 10.1172/JCI8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pyerin W. Adv. Enzyme Regul. 1994;34:225–246. doi: 10.1016/0065-2571(94)90018-3. [DOI] [PubMed] [Google Scholar]