Abstract
Objectives
Paris polyphylla var. yunnanensis (PPVY), a Chinese herb, has long been used for cancer treatment, and its steroidal saponins are suggested to exert an anti-tumor activity, however, the underlying mechanism is incompletely understood and their effect on bladder cancer (BC) remains unknown. The present study is thus designed to address these issues.
Material and Methods
Total steroidal saponins were extracted with ethanol from PPVY and used to treat BC cells (HT1197 and J82 carrying mutant p53). Gene expression was determined using qPCR and immunoblotting and cell cycle analyzed using flow cytometry. DNA damage response activation was assessed using immunofluorescence staining.
Results
PPVY saponins treatment led to dose-dependent declines in the number of both HT1197 and J82 cells with IC50 approximately 1.2 μg/ml, which was coupled with strong growth arrest at G2/M phase and the activation of DNA damage response pathway. Moreover, the clonogenic potential of these cells was severely impaired even in the presence of low concentrations of PPVY saponins. Mechanistically, PPVY saponins induced the degradation of mutant p53 while stimulated CDKN1A gene transcription. Phosphorylated AKT was diminished in PPVY saponin-treated cells, but its specific inhibitor LY294002 exhibited significantly weaker efficacy in inducing CDKN1A expression than did PPVY saponins.
Conclusion
PPVY saponins activate DNA damage response pathway, degrade mutant p53 and stimulate CDKN1A expression, thereby inhibiting BC cell growth. Given their poor absorption via oral administration, PPVY saponins may be applicable for intravesical instillations in BC treatment.
Key Words: Bladder cancer, Cancer treatment, CDKN1A, Paris polyphylla
Introduction
Bladder cancer (BC), derived from the urothelium, is one of the most common urological malignancies in both eastern and western countries, with a global annual incidence rate of 350,000 [1, 2]. The pathogenesis of BC is a multi-step process that involves multiple genetic changes including loss of tumor suppressor genes and activation of oncogenes, and comprehensively molecular dissections of BC has significantly contributed to our understanding of the disease [1]. In spite of this, BC patients exhibit a high risk to recur, and relapsed diseases frequently lead to the treatment resistance [1, 2]. Therefore, it is a demanding task to develop a novel anti-BC strategy.
Paris polyphylla var. yunnanensis (PPVY) is a Chinese herb traditionally used for the treatment of traumatic injuries, infection, hepatopathy and many other diseases. It has been demonstrated that steroidal saponins contained in PPVY exert multiple pharmacological and biological activities [3]. In the last decade, the anti-cancer effect of Paris polyphylla has become increasingly attractive and been intensively explored. Steroidal saponin extracts derived from PPVY inhibit proliferation and survival of in vitro cultured cancer cells, and also showed in vivo therapeutic efficacy in mouse tumor models [4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19]. Mechanistically, PPVY saponins target ERK, AKT, Stat3, NF-κB, EZH2, DNMTs and other signal pathways critical to carcinogenesis [5, 9, 20].
The anti-tumor effect of PPVY saponins has been demonstrated in different types of human malignancies [18], however, it is currently unclear whether BC cells respond to PPVY saponin treatment. Moreover, it remains elusive how exactly PPVY achieves its anti-cancer effect. For instance, a number of studies showed a decline in phosphorylated AKT in PPVY saponin-treated cancer cells, but little is known about its importance in PPVY-mediated inhibition of cancer cell proliferation and/or survival [13, 21, 22]. In addition, poor gastrointestinal absorption of PPVY saponins may significantly limit their future clinical application [23, 24]. However, this problem may be easily avoided via intravesical instillations in BC treatment [25]. With all these in mind, we thus evaluated the effect of PPVY saponins on the growth of BC-derived cancer cells.
Materials and Methods
PPVY Saponin Extraction
The raw herb PPVY was utilized to extract saponins. Dried PPVY was extracted with 60% ethanol under reflux for 2 hours. The extract was filtered, and the extraction was repeated once. The filtrates were then combined, concentrated, and in the process of water precipitation. The extract was at 4°C for 12 hours, then filtered, precipitated, and finally dried into powder. The total saponins in the ethanol extracts were greater than 80%, as determined using an ultraviolet-visible spectrophotometer at 406 nm with perchloric acid as chromogenic reagent.
Cell Lines and Cell Culture
BC-derived cell lines HT1197 and J82 cells were used in the present study. Cells were maintained at 37°C 95%air/5%CO2 in RPMI 1640 medium (Thermo Fisher Scientific, Waltham, MA) containing 10% fetal calf serum, 100 units/ml penicillin, and 2 mM L-glutamine. LY294002 was purchased from Millipore Sigma (St. Louis, MO). Cells were incubated with different concentrations of PPVY saponins or LY294002 for different time periods and then harvested for further analyses.
RNA Extraction, Reverse Transcription and Quantitative PCR (qPCR)
Total cellular RNA in cells with different treatments was extracted using the Trizol (Thermal Scientics) according to the manufacturer's protocol. cDNA was synthesized using random primers (N6) (Amersham, Buckinghamshire, UK) and M-MLV reverse transcriptase. qPCR was carried out in an ABI7700 sequence detector (Applied Biosystems, Foster City, CA) using SYBR Green and specific primers. The primer pair for CD-KN1A are: 5′-GCGACTGTGATGCGCTAAT-3′ (Forward) and 5′-TAGGGCTTCCTCTTGGAGAA-3′ (Reverse); the primer pair for β2-microglobulin (β2-M): 5′-GAATTGCTATGTGTCT-GGGT-3′ (Forward) and 5′-CATCTTCAAACCTCCATGATG-3 (Reverse). β2-M expression was used as a control for RNA loading and RT efficiency and amplified. Levels of target CDKN1A mRNA were calculated based on the CT values and normalization of human β2-M expression.
Western Blot Analysis
Proteins were extracted using RIPA Buffer (Thermo Scientific) with 1% Phenylmethanesulfonyl fluoride (Millipore Sigma) and quantified with DC Protein Assay (Bio-Rad). Thirty µg of proteins were separated in Mini-PROTEAN TGX Gels (Bio-Rad) and transferred to PVDF membranes using Trans-Blot Turbo Transfer Pack (Bio-Rad). Membranes were blocked with 5% non-fat milk diluted in TBST, and then incubated with primary antibodies and secondary antibodies prior to imaged with Clarity Max Western ECL Substrate (Bio-Rad, 1705062) and ChemiDoc MP Imaging System (Bio-Rad). Primary antibodies used were: β-actin (Milli-pore Sigma), p53 (Santa Cruz Biotechnology), CDKN1A (Cell Signaling Technology), AKT and pAKT (Cell Signaling Technology), 53BP1, (Bethyl Laboratories) and γ-H2AX (Millipore Sigma).
Colony Formation Assay
The cells were seeded into 6-well plates (1,000 cells/well) and incubated for 10–14 days in the presence or absence of PPVY saponins. Plates were stained with Giemsa and the number of colonies with more than 50 cells was counted.
Flow Cytometry
Cells were treated with PPVY saponins for 24 hours and then fixed with 70% ethanol at 4°C overnight and stained with RNAse A (0.5 µg)-containing Propidium Iodide (50 µg/ml). Cell cycle distribution was determined using flow cytometry with ModFit (BD Biosciences, Franklin Lakes, NJ). The results were analyzed using CellQuest software.
Immunofluorescence
Cells were treated with PPVY saponins for 24 hours. Primary antibodies against 53BP1 were added for 30 minutes at room temperature after blocking steps with background buster. The cells were then incubated with a Cy3 conjugated donkey anti-rabbit antibody (Jackson immunoresearch, West Grove). The nuclei were counterstained with DAPI. The results were analyzed with a Leica TCS SP5 microscope with filters for the detection of DAPI and Cy3.
CDKN1A Promoter Activity Assay
The CDKN1A reporter construct contains the human p21 promoter between positions −2,300 and +8 [p21 (−2,300/+8)], as described [26, 27]. Cells were transfected with the CDKN1A promoter plasmid and the luciferase activity was determined using a dual luciferase reporter assay system (Promega, Madison, WI) 48 hours post-transfection. The CDKN1A promoter-driven firefly luciferase activity was normalized to the renilla activity included in the kit.
Results
PPVY Saponin Treatment Induces the Growth Arrest and Inhibits Clonogenic Potential of BC Cells in a Dose-Dependent Manner
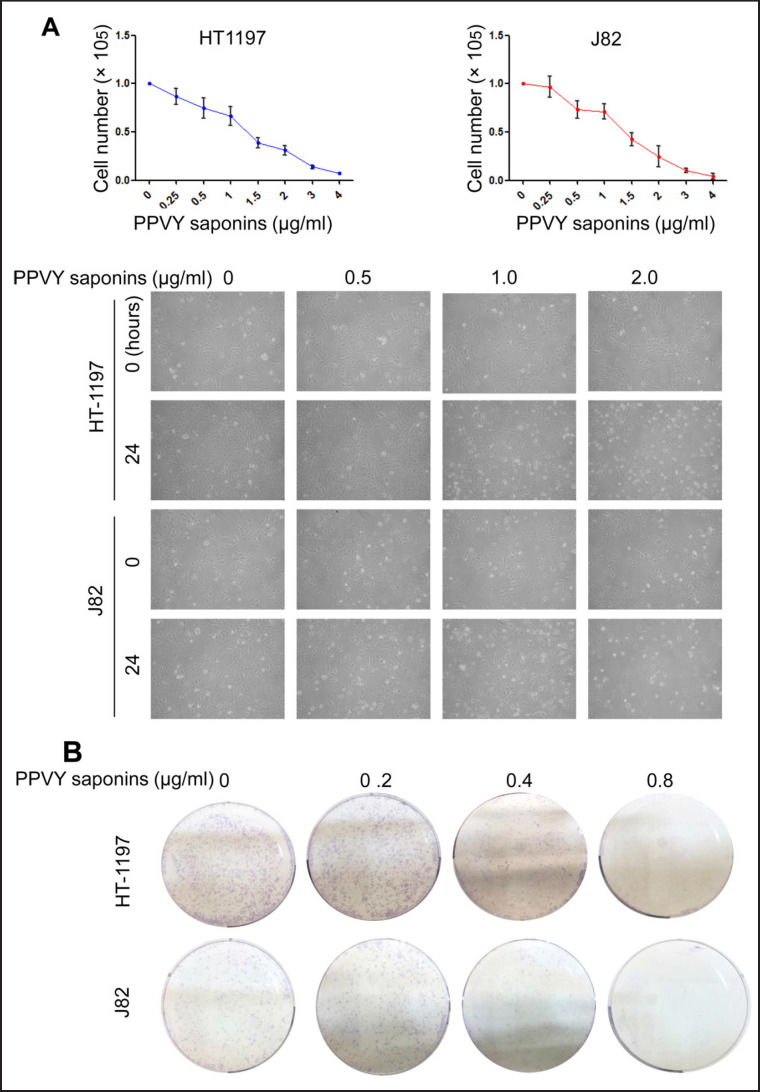
HT1197 and J82 cells were incubated with PPVY saponins at various concentrations (from 0.25 to 4.0 µg/ml) for 24 hours and viable cells then counted using trypan blue exclusion. Both cell lines exhibited similar dose-dependent reduction in cell numbers after their exposure to PPVY saponins. As shown in figure 1A, IC50 for these 2 lines was approximately 1.20 µg/ml, which was comparable to the effect of cisplatin, a widely used chemotherapeutic drug for BC therapy (data not shown). Because the clonogenic ability of tumors reflects their oncogenic capacity to certain extent, we further determined whether PPVY saponins inhibit the colony formation of BC cells. HT1197 and J82 cells were incubated with PPVY saponins at 0.2, 0.4 and 0.8 µg/ml, respectively, and significant reduction in colony numbers derived from these cells was already seen at 0.2 µg/ml, while their clonogenic potential was almost completely abolished at 0.8 µg/ml (fig. 1B). These results collectively suggest a strong inhibition of PPVY saponins on BC cell growth and clonogenic ability.
Fig. 1.
PPVY saponins inhibit BC cell growth and clonogenic ability in a dose-dependent manner. A PPVY saponin treatment leads to significant decline in BC cell numbers. Top panel: HT1197 and J82 cells were incubated with various concentrations of PPVY saponins for 24 hours and counted using trypan blue exclusion. Bottom panel: Representative images of cell exposure to PPVY saponins. B PPVY saponins inhibit the clonogenic potential of BC cells.
PPVY Saponins Block Cell Cycle at G2/M Phase
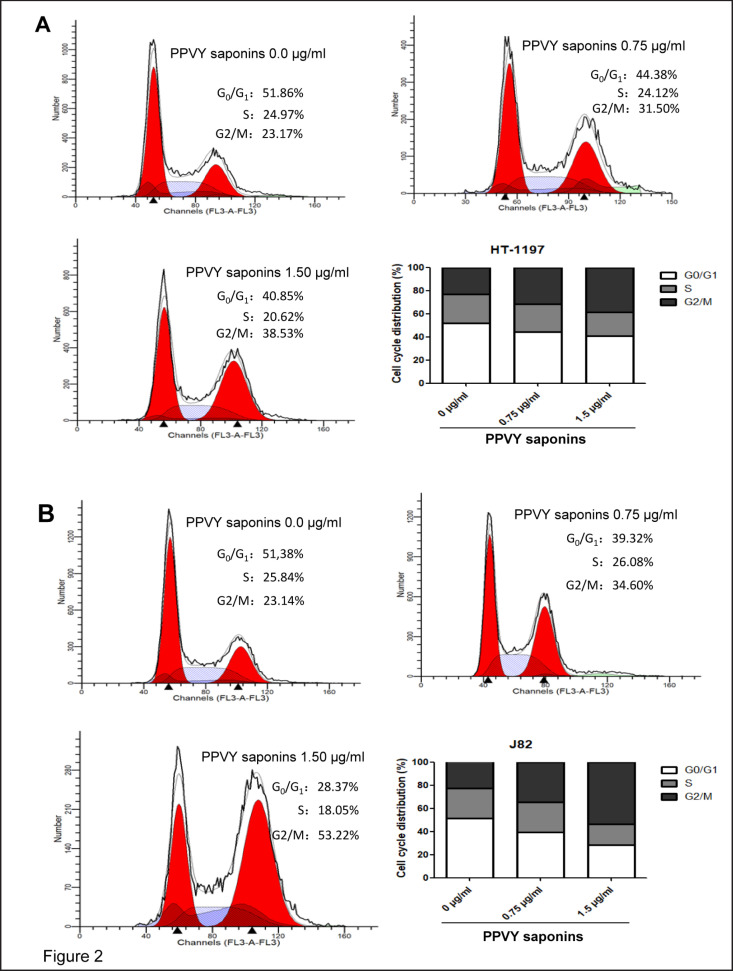
Having observed PPVY-mediated growth inhibition of HT1197 and J82 cells, we sought to determine how it happened. Cell cycle progression was thus analyzed and the obtained results revealed that G2/M arrest occurred in both HT1197 and J82 cells in the presence of PPVY saponins (fig. 2A and B): Cells at G2/M phase reached 38.5 and 53.5% for HT1197 and J82 lines, respectively, when incubated with 1.5 µg/ml PPVY saponins for 24 hours, whereas cells at G2/M were only approximately 23% for control non-treated HT1197 and J82.
Fig. 2.
PPVY saponins block cell cycle at G2/M phase. HT1197 and J82 cells were treated with 2 different concentrations of PPVY saponins (0.75 and 1.5 µg/ml, respectively) and cell cycle was analyzed using flow cytometry. A and B: Cell cycle distribution of HT1197 and J82 cells, respectively.
PPVY Saponins Activate the DNA Damage Response Pathway
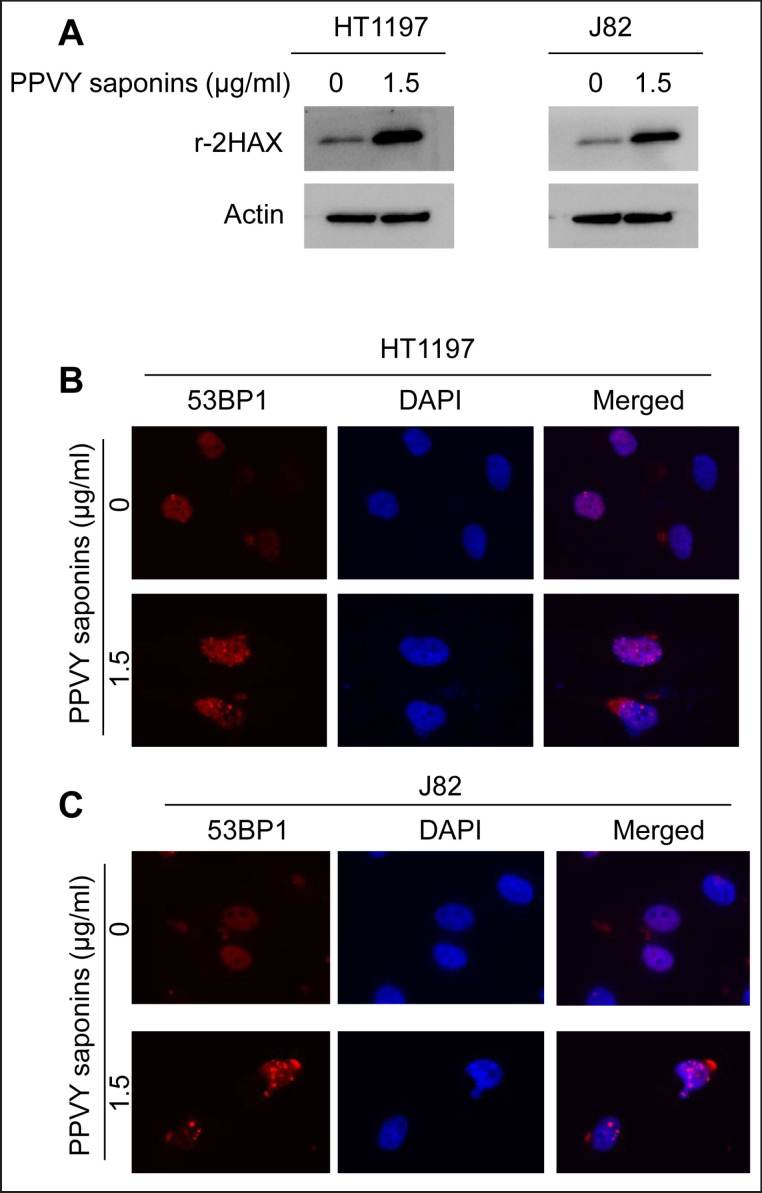
Cell cycle arrest at G2/M indicates that DNA damage takes place and its repair is required before cells progress into next cycle. To probe whether this is the case in PPVY-treated BC cells, we analyzed the expression of γ-H2AX, a DNA damage response marker. As expected, the exposure of HT1197 and J82 cells to PPVY saponins significantly increased the γ-H2AX protein level (fig. 3A). We further examined the formation of DNA damage foci by assessing another DNA damage response marker 53BP1. 53BP1 foci were readily detected in PPVY saponin-treated cells but not in control cells by using immunofluorescent staining (fig. 3B and C). The observed activation of the DNA damage response pathway indicates that PPVY saponins induce DNA damage in BC cells.
Fig. 3.
PPVY saponins activate the DNA damage response pathway. A γ-H2AX expression is significantly up-regulated in PPVY saponin-treated BC cells. HT1197 and J82 cells were treated with PPVY saponins for 24 hours and then analyzed for γ-H2AX using immunoblotting. (B and C) 53BP1 focal formation in PPVY saponin-treated BC cells. HT1197 (B) and J82 (C) cells were treated with PPVY saponins for 24 hours and then analyzed for 53BP1 foci using immunofluorescent staining.
PPVY Saponin Treatment Leads to Mutant p53 Degradation While Up-Regulation of CDKN1A Expression
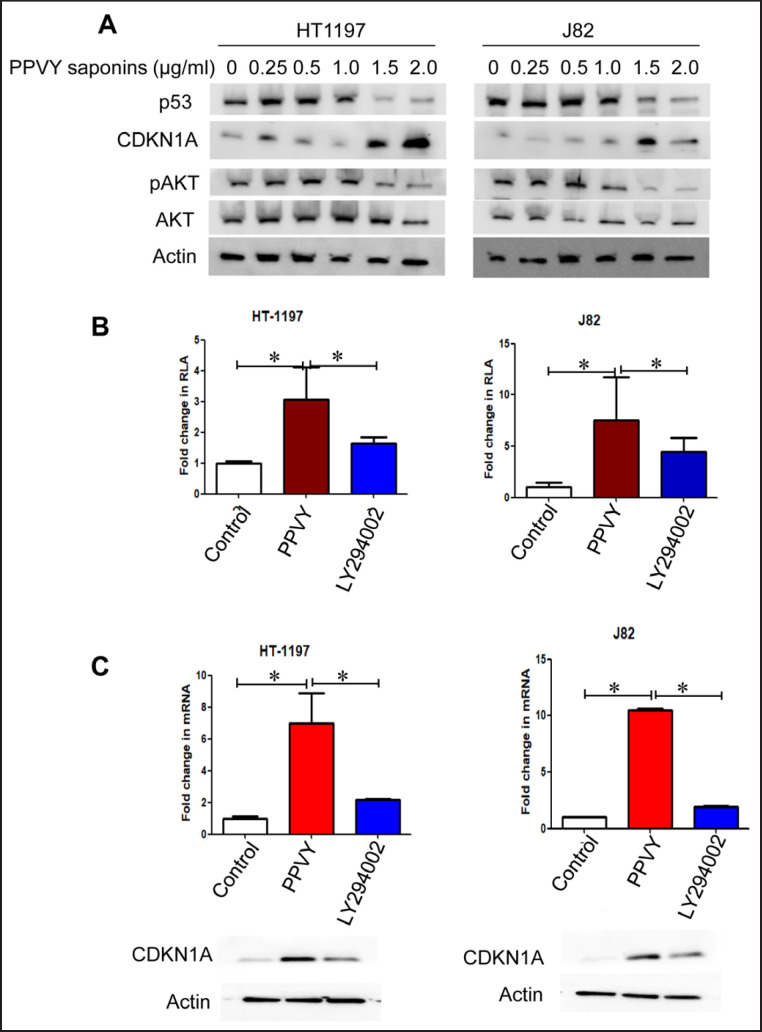
The tumor suppressor p53 plays a critical role in DNA damage response, but it is known that both HT1197 and J82 cells harbor mutant p53 [28]. Nevertheless, we determined mutant p53 expression in these 2 cell lines. As expected, both cell lines expressed high basal levels of p53, however, we surprisingly found that PPVY saponin treatment dose-dependently inhibited p53 protein expression (fig. 4A). In sharp contrast, a robust up-regulation of CD-KN1A (the p53 target gene) expression was observed in PPVY-treated cells (fig. 4A).
Fig. 4.
PPVY saponin treatment triggers diminished mutant p53 and pAKT levels while promotes CDKN1A expression. A The mutant p53 and phosphorylated AKT were diminished while CDKN1A expression up-regulated in PPVY saponin-treated BC cells. HT1197 and J82 cells were incubated with different concentrations of PPVY saponins for 24 hours and then analyzed for expression of mutant p53, pAKT and CDKN1A using immunoblotting. B The CDKN1A promoter activity is enhanced by PPVY saponins or the AKT inhibitor LY294002. HT1197 and J82 cells were transfected with the CDKN1A promoter-containing plasmid and then incubated with either PPVY saponins or LY294002. The promoter activity was determined 48 hours post-transfection. C PPVY saponins induce CDKN1A expression much stronger than does LY294002. HT1197 and J82 cells were incubated with either PPVY saponins or LY294002, and analyzed for CDKN1A mRNA and protein expression using qPCR and immunoblotting, respectively. * indicates statistically significant difference.
PPVY Saponins Stimulate CDKN1A Gene Transcription
We further sought to determine how PPVY saponins promoted CDKN1A expression. For this purpose, the luciferase reporter containing a full-length of the CD-KN1A promoter was transfected into BC cells and cells were then incubated with 1.5 µg/ml PPVY saponins for 48 hours. A robust increase in the CDKN1A promoter activity was seen in cells treated with PPVY saponins (fig. 4B). Consistently, CDKN1A mRNA expression was significantly up-regulated in those same cells (fig. 4C). These findings reveal that PPVY saponin-mediated induction of CDKN1A expression occurs at the transcriptional level.
Because previous studies showed that PPVY saponins inhibited the PI3C/AKT signaling, while AKT represses CDKN1A expression. Therefore, we further compared their effect on CDKN1A transcription between PPVY saponins and the PI3C/AKT specific inhibitor LY294002. LY294002 treatment indeed enhanced CDKN1A promoter activity and mRNA/protein expression (fig. 4B and C), but with much weaker stimulatory effect compared to PPVY saponins.
Discussion
The results presented here demonstrate that PPVY saponins robustly inhibited BC cell growth by blocking the cell cycle at G2/M phase. The concentration of PPVY for IC50 was 2.0 µg/ml during a 24-hour culture period, which is comparable with the effect of cisplatin, a widely used chemotherapeutic drug for the treatment of BC and other types of cancer. Mechanistically, we observed that PPVY saponins activated the DNA damage response pathway, induced the mutant p53 degradation whereas stimulated the transcription of the CDKN1A gene.
One of the key findings in the present study is the PPVY saponin-mediated inhibition of the mutant p53. The tumor suppressor p53 is mutated in more than 50% human malignancies, which inactivates its function, thereby promoting cancer development and progression [29]. However, the recently accumulated evidence has suggested that the mutant p53 acquires gain-of-function, and thereby plays a more active role in oncogenesis [29, 30, 31, 32]. For instance, ETS2 transcription factor tethers mutant rather than wide type 53 to the promoters of its numerous targets and therefore assists mtp53 in the activation of genes critical for oncogenesis [30, 31]. In addition, mutant p53 was also shown to involve the up-regulation of both nucleotide de novo synthesis and nucleoside salvage pathways for cancer cell proliferation [31]. Given the findings above, targeting mutant p53 has been suggested as a novel anti-cancer strategy and obtained results are promising. Based on our present result, PPVY saponins exhibits a strong effect on mutant p53 degradation, and further investigations are required to define the underlying mechanism and to explore their values in targeting mutant p53 for cancer therapy.
Our current finding also reveals a strongly stimulatory effect of PPVY saponins on CDKN1A transcription. p53 is a master regulator of the CDKN1A expression [26], however, this activity is clearly p53-independent in the present setting, because HT1197 and J82 cells both carry mutant p53 [28], and moreover, CDKN1A and mutant p53 expression was disassociated in PPVY-treated cells. The PIC3/AKT signalling is known to inhibit CDKN1A expression [33], while PPVY saponins were previously observed to inhibit phosphorylated AKT in different types of cancer cells [13, 21]. Therefore, it is likely that the PPVY-mediated AKT inhibition derepresses CD-KN1A expression. We similarly observed the diminished phosphorylated AKT in the treated BC cells, however, the specific AKT inhibitor LY294002 did facilitate CD-KN1A transcription and up-regulate its expression, but with a much weaker effect than PPVY saponins. Likely, PPVY saponins induce CDKN1A expression via multiple mechanisms. For instance, the activation of DNA damage response pathway may also stimulate CDKN1A expression in PPVY-treated BC cells.
The up-regulation of γ-H2AX expression and 53BP1 focal formation, together with G2/M arrest in PPVY-treated BC cells demonstrates that DNA damage occurs in these cells. It is currently unclear how PPVY saponins result in DNA damage, and which kinds of damage are induced. PPVY saponins were previously shown to stimulate the production of reactive oxygen species [22]. Thus, further studies are required to define the underlying mechanism, and especially to probe the relationship between DNA damage and PPVY-mediated reactive oxygen species production.
A poor gastrointestinal absorption of PPVY saponins [23, 24] makes their oral administration less efficient for cancer treatment, while intravenous injection is inconvenient and costive, and importantly, may cause complications. All these disadvantages likely limit their clinical application. However, intravesical instillations are routinely performed to treat BC patients, and directly local administration of PPVY saponins via this approach provides a good solution.
In summary, here we show that PPVY saponins mediate potent inhibition of BC cell growth by triggering DNA damage, blocking cells at G2/M phase, targeting mutant p53 for degradation and stimulating CDKN1A expression. Further studies are required to define these activities in detail using different BC model systems, and more importantly, the therapeutic potential of PPVY saponins via intravesical instillations should be investigated and evaluated.
Acknowledgement
We thank Dr. SH Juan (Taipei Medical University, Taiwan) for the CDKN1A promoter construct. The study was supported by grants from Natural Science Foundation of China (81041065, 81702538), Shandong Provincial Natural Science Foundation, China (2016ZDJS07A09), the Swedish Cancer Society, the Swedish Research Council, Cancer Society in Stockholm, and Karolinska Institutet.
References
- 1.Griffiths TR. Current perspectives in bladder cancer management. Int J Clin Pract. 2013;67:435–448. doi: 10.1111/ijcp.12075. [DOI] [PubMed] [Google Scholar]
- 2.Wang K, Liu T, Liu C, Meng Y, Yuan X, Liu L, Ge N, Liu J, Wang C, Ren H, Yan K, Hu S, Xu Z, Fan Y, Xu D. TERT promoter mutations and TERT mRNA but not FGFR3 mutations are urinary biomarkers in Han Chinese patients with urothelial bladder cancer. Oncologist. 2015;20:263–269. doi: 10.1634/theoncologist.2014-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu X, Wang L, Wang H, Dai Y, Ye WC, Li YL. Steroidal saponins from Paris polyphylla var. yunnanensis. Phytochemistry. 2012;81:133–143. doi: 10.1016/j.phytochem.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 4.He H, Sun YP, Zheng L, Yue ZG. Steroidal saponins from Paris polyphylla induce apoptotic cell death and autophagy in A549 human lung cancer cells. Asian Pac J Cancer Prev. 2015;16:1169–1173. doi: 10.7314/apjcp.2015.16.3.1169. [DOI] [PubMed] [Google Scholar]
- 5.He H, Zheng L, Sun YP, Zhang GW, Yue ZG. Steroidal saponins from Paris polyphylla suppress adhesion, migration and invasion of human lung cancer A549 cells via down-regulating MMP-2 and MMP-9. Asian Pac J Cancer Prev. 2014;15:10911–10916. doi: 10.7314/apjcp.2014.15.24.10911. [DOI] [PubMed] [Google Scholar]
- 6.Ke JY, Zhang W, Gong RS, Cen WJ, Huang HQ, Li YR, Kong WD, Jiang JW. A monomer purified from Paris polyphylla (PP-22) triggers S and G2/M phase arrest and apoptosis in human tongue squamous cell carcinoma SCC-15 by activating the p38/cdc25/cdc2 and caspase 8/caspase 3 pathways. Tumour Biol. 2016;37:14863–14872. doi: 10.1007/s13277-016-5376-4. [DOI] [PubMed] [Google Scholar]
- 7.Lee MS, Yuet-Wa JC, Kong SK, Yu B, Eng-Choon VO, Nai-Ching HW, Chung-Wai TM, Fung KP. Effects of polyphyllin D, a steroidal saponin in Paris polyphylla, in growth inhibition of human breast cancer cells and in xenograft. Cancer Biol Ther. 2005;4:1248–1254. doi: 10.4161/cbt.4.11.2136. [DOI] [PubMed] [Google Scholar]
- 8.Li FR, Jiao P, Yao ST, Sang H, Qin SC, Zhang W, Zhang YB, Gao LL. Paris polyphylla Smith extract induces apoptosis and activates cancer suppressor gene connexin26 expression. Asian Pac J Cancer Prev. 2012;13:205–209. doi: 10.7314/apjcp.2012.13.1.205. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Wu J, Zheng F, Tang Q, Wu W, Hann SS. Inhibition of EZH2 via activation of SAPK/ JNK and reduction of p65 and DNMT1 as a novel mechanism in inhibition of human lung cancer cells by polyphyllin I. J Exp Clin Cancer Res. 2016;35:112. doi: 10.1186/s13046-016-0388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Man S, Li J, Zhang Y, Meng X, Gao W. Inhibition of diethylnitrosamine-induced liver cancer in rats by Rhizoma paridis saponin. Environ Toxicol Pharmacol. 2016;46:103–109. doi: 10.1016/j.etap.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, Gao W, Man S, Zhang Y, Li H, Wu S, Zhang J, Liu C. Synergistic effects of Rhizoma Paridis and Rhizoma Curcuma longa on different animal tumor models. Environ Toxicol Pharmacol. 2014;38:31–40. doi: 10.1016/j.etap.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Li H, Fan Y, Liu Y, Man S, Yu P, Gao W. Combination treatment with Rhizoma Paridis and Rhizoma Curcuma longa extracts and 10-hydroxycamptothecin enhances the antitumor effect in H22 tumor model by increasing the plasma concentration. Biomed Pharmacother. 2016;83:627–634. doi: 10.1016/j.biopha.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 13.Man S, Chai H, Cui J, Yao J, Ma L, Gao W. Antitumor and anti-metastatic mechanisms of Rhizoma paridis saponins in Lewis mice. Environ Toxicol. 2018;33:149–155. doi: 10.1002/tox.22501. [DOI] [PubMed] [Google Scholar]
- 14.Man S, Fan W, Liu Z, Gao W, Li Y, Zhang L, Liu C. Antitumor pathway of Rhizoma Paridis Saponins based on the metabolic regulatory network alterations in H22 hepatocarcinoma mice. Steroids. 2014;84:17–21. doi: 10.1016/j.steroids.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Man S, Gao W, Zhang Y, Huang L, Liu C. Identification of chemical constituents in Rhizoma Paridis Saponins and their oral administration in rat plasma by UPLC/Q-TOF/ MS. Biomed Chromatogr. 2011;25:712–719. doi: 10.1002/bmc.1507. [DOI] [PubMed] [Google Scholar]
- 16.Man S, Gao W, Zhang Y, Yan L, Ma C, Liu C, Huang L. Antitumor and antimetastatic activities of Rhizoma Paridis saponins. Steroids. 2009;74:1051–1056. doi: 10.1016/j.steroids.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Man S, Li J, Qiu P, Liu J, Liu Z, Ma L, Gao W. Inhibition of lung cancer in diethylnitrosamine-induced mice by Rhizoma paridis saponins. Mol Carcinog. 2017;56:1405–1413. doi: 10.1002/mc.22601. [DOI] [PubMed] [Google Scholar]
- 18.Negi JS, Bisht VK, Bhandari AK, Bhatt VP, Singh P, Singh N. Paris polyphylla: chemical and biological prospectives. Anticancer Agents Med Chem. 2014;14:833–839. doi: 10.2174/1871520614666140611101040. [DOI] [PubMed] [Google Scholar]
- 19.Xiao X, Bai P, Bui Nguyen TM, Xiao J, Liu S, Yang G, Hu L, Chen X, Zhang X, Liu J, Wang H. The antitumoral effect of Paris Saponin I associated with the induction of apoptosis through the mitochondrial pathway. Mol Cancer Ther. 2009;8:1179–1188. doi: 10.1158/1535-7163.MCT-08-0939. [DOI] [PubMed] [Google Scholar]
- 20.Xiang S, Zou P, Tang Q, Zheng F, Wu J, Chen Z, Hann SS. HOTAIR-mediated reciprocal regulation of EZH2 and DNMT1 contribute to polyphyllin I-inhibited growth of castration-resistant prostate cancer cells in vitro and in vivo. Biochim Biophys Acta. 2017;1862:589–599. doi: 10.1016/j.bbagen.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Xie ZZ, Li MM, Deng PF, Wang S, Wang L, Lu XP, Hu LB, Chen Z, Jie HY, Wang YF, Liu XX, Liu Z. Paris saponin-induced autophagy promotes breast cancer cell apoptosis via the Akt/mTOR signaling pathway. Chem Biol Interact. 2017;264:1–9. doi: 10.1016/j.cbi.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Yu S, Wang L, Cao Z, Gong D, Liang Q, Chen H, Fu H, Wang W, Tang X, Xie Z, He Y, Peng C, Li Y. Anticancer effect of Polyphyllin Iota in colorectal cancer cells through ROS-dependent autophagy and G2/M arrest mechanisms. Nat Prod Res. 2017;16:1–4. doi: 10.1080/14786419.2017.1353512. [DOI] [PubMed] [Google Scholar]
- 23.Yi X, Lin L, Cao F. The analysis of Paris polyphylla-derived saponins I, II, VI, VII and hyperoside in Chonglou Keganjiaonang. Chin J Exp Trad Med Formul. 2013:58–60. [Google Scholar]
- 24.Pan M, Lu W, Cao Z. Pharmacokenetics of Paris polyphylla-derived saponins in rat palsma using UPLC-MS/MS. J Hubei Univ Med. 2017;36:404–410. [Google Scholar]
- 25.Campodonico F, Di Stasi S, Lev GM, Terrone C, Bongiovanni L, Mattioli F, Pagliarulo V, Introini C. Intravesical chemotherapy and chemohyperthermia in non-muscle-invasive bladder cancer; an overview on drug administration technologies and pharmacokinetics. Curr Drug Metab. 2017;18:657–665. doi: 10.2174/1389200218666170427092421. [DOI] [PubMed] [Google Scholar]
- 26.Pang PH, Lin YH, Lee YH, Hou HH, Hsu SP, Juan SH. Molecular mechanisms of p21 and p27 induction by 3-methylcholanthrene, an aryl-hydrocarbon receptor agonist, involved in antiproliferation of human umbilical vascular endothelial cells. J Cell Physiol. 2008;215:161–171. doi: 10.1002/jcp.21299. [DOI] [PubMed] [Google Scholar]
- 27.Zeng J, Ge Z, Wang L, Li Q, Wang N, Björkholm M, Jia J, Xu D. The histone demethylase RBP2 is overexpressed in gastric cancer and its inhibition triggers senescence of cancer cells. Gastroenterology. 2010;138:981–992. doi: 10.1053/j.gastro.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez-Carbayo M, Socci ND, Chary-tonowicz E, Lu M, Prystowsky M, Childs G, Cordon-Cardo C. Molecular profiling of bladder cancer using cDNA microarrays: defining histogenesis and biological phenotypes. Cancer Res. 2002;62:6973–6980. [PubMed] [Google Scholar]
- 29.Bykov VJN, Eriksson SE, Bianchi J, Wiman KG. Targeting mutant p53 for efficient cancer therapy. Nat Rev Cancer. 2017;18:89–102. doi: 10.1038/nrc.2017.109. [DOI] [PubMed] [Google Scholar]
- 30.Haupt S, Raghu D, Haupt Y. Mutant p53 drives cancer by subverting multiple tumor suppression pathways. Front Oncol. 2016;6:12. doi: 10.3389/fonc.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kollareddy M, Dimitrova E, Vallabhaneni KC, Chan A, Le T, Chauhan KM, Carrero ZI, Ramakrishnan G, Watabe K, Haupt Y, Haupt S, Pochampally R, Boss GR, Romero DG, Radu CG, Martinez LA. Regulation of nucleotide metabolism by mutant p53 contributes to its gain-of-function activities. Nat Commun. 2015;6:7389. doi: 10.1038/ncomms8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu J, Sammons MA, Donahue G, Dou Z, Vedadi M, Getlik M, Barsyte-Lovejoy D, Al-awar R, Katona BW, Shilatifard A, Huang J, Hua X, Arrowsmith CH, Berger SL. Gain-of-function p53 mutants co-opt chromatin pathways to drive cancer growth. Nature. 2015;525:206–211. doi: 10.1038/nature15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Worby CA, Dixon JE. Pten. Annu Rev Biochem. 2014;83:641–669. doi: 10.1146/annurev-biochem-082411-113907. [DOI] [PubMed] [Google Scholar]