Abstract
Peripheral detection of nociceptive and painful stimuli by sensory neurons involves a complex repertoire of molecular detectors and/or transducers on distinct subsets of nerve fibers. The majority of such molecular detectors/transducers belong to the transient receptor potential (TRP) family of cation channels, which comprise both specific receptors for distinct nociceptive stimuli, as well as for multiple stimuli. This chapter discusses the classification, distribution, and functional properties of individual TRP channel types that have been implicated in various nociceptive and/or painful conditions.
1. INTRODUCTION
The neural mechanisms and pathways for the encoding and processing of noxious stimuli, widely termed “nociception,”1 constitute the physiological and/or pathophysiological bases of the somatic and trigeminal sensory system. The nociceptive neurons in different sensory ganglia send peripheral afferents to the somatic, visceral, and trigeminal regions and also connect to the spinal cord (SC) and brain stem, thereby serving as the mediator of sensory signal transmission between the peripheral and central nervous systems (PNS and CNS). These neurons express a variety of receptors and ion channels in the plasma membrane throughout the soma and fibers, in order to detect various noxious stimuli, convert those to electrical signals, and subsequent transmission to the CNS. The transient receptor potential (TRP) family of receptor ion channels constitutes the major class of detectors and transducers in nociceptive neurons. Members of the TRP channel superfamily are Ca2+-permeable, nonselective cation channels found in metazoans and fungi (reviewed in Refs. 2,3). Broadly speaking, they act as primary transducers of multiple noxious stimuli, although this belies the diversity of functions they play in eliciting physiological responses to changes in the environment, encompassing the five traditional somatosensory modalities (vision, hearing, olfaction, taste, and touch), as well as sensing changes in temperature and osmolarity (reviewed in Refs. 3–5). The TRP terminology is derived from the Drosophila mutant of the same name, which displays an abnormally transient response to bright light, a defect in the prototypical channel, and its role in phototransduction.6,7 In the decades since this initial discovery, the superfamily has grown to include 28 members across 6 subfamilies in mammals, categorized as canonical (TRPC), vanilloid (TRPV), ankyrin (TRPA), melastatin (TRPM), polycystin (TRPP), and mucolipin (TRPML; reviewed in Refs. 2,3). To date, members of three of these subfamilies have been implicated in the sensory detection transduction of nociception and pain: TRPV, TRPA, and TRPM (Fig. 1).
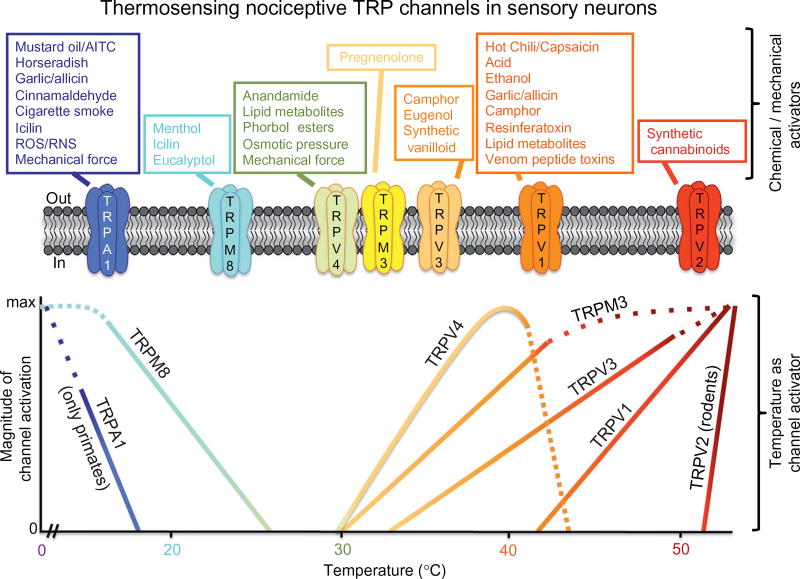
Figure 1.
Classification of thermosensing nociceptive TRP channels in mammalian sensory neurons. The upper row of individual boxes denotes chemical/mechanical activators of marked TRP channels. The lower panel depicts the magnitude of channel activity upon activation by temperature of the independent TRP channels shown.
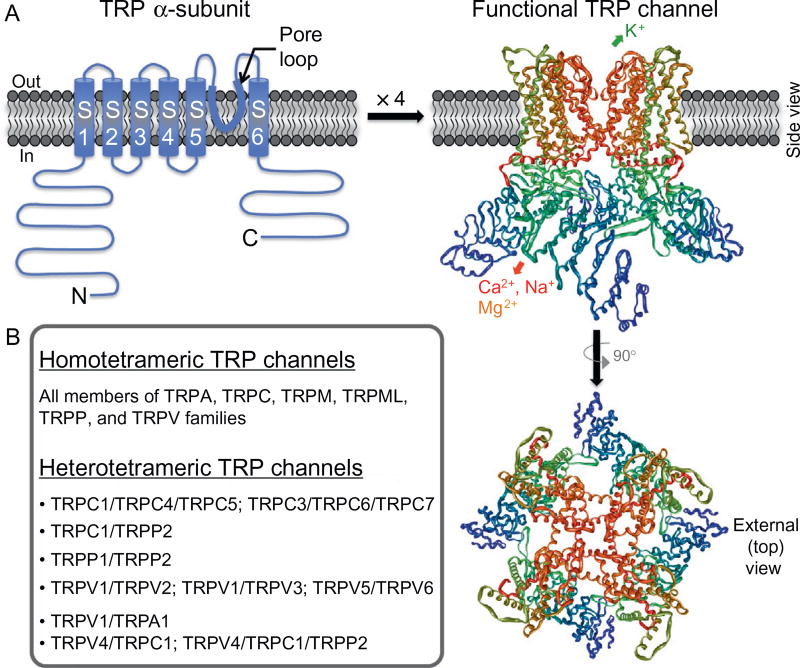
TRP channels exhibit enormous structural and functional diversity, albeit there are several common features that unite them under one family. Functional TRP channels consist of tetramers of 6-transmembrane (6-TM) segment polypeptide subunits, similar to that of the α-subunits of voltage-gated K+ (Kv) channels. A hydrophilic loop between TM segments S5 and S6 of each subunit constitutes the ion conduction pore domain in tetrameric channels (called the pore loop; Fig. 2). The precise positioning of amino acid residues within the pore-loop region dictates the ion selectivity of individual channels for various cations. Although the majority of TRP channels conduct cations in an outwardly rectifying manner, a number of individual channels exhibit linear (e.g., TRPC2 and TRPM2) or inwardly rectifying cation conduction (e.g., TRPV6). TRP channels are generally nonselective cation channels; however, there is considerable variability in divalent cation (Ca2+ and Mg2+) selectivity between channels, with TRPV5, TRPV6, and the majority of TRPC family channels exhibiting very high selectivity for Ca2+ (reviewed in Refs. 3,10,11).
Figure 2.
Structural features of functional TRP channels. (A) Model of predicted secondary structure topology of a monomeric TRP channel subunit (called α-subunits) on a plasma membrane lipid bilayer. S1–S6 denote transmembrane segments/domains 1 through 6, with a hydrophilic pore loop between S5 and S6 transmembrane segments, and N and C denote cytoplasmic amino- and carboxy-terminals, respectively. Four monomeric α-subunits assemble in a three-dimensional arrangement to make a functional channel, which is shown on the right. The structure was constructed from the PDB structure file obtained from the protein data bank, originally submitted by Refs. 8,9 from their cryoelectron microscopic structure of TRPV1. (B) List of homo- and heterotetrameric TRP channels formed within individual TRP subfamily member channels, as well as in combination of inter-subfamily TRP channel members.
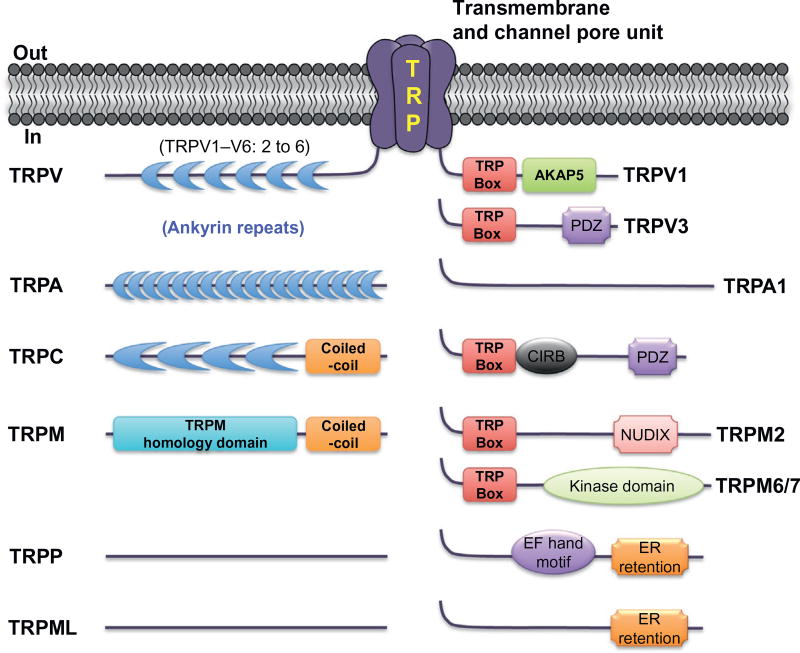
By far the greatest level of sequence diversity among TRP channels has been observed in the cytoplasmic N- and C-termini. The N-terminus often contains ankyrin repeat domains (ARDs; particularly in TRPC, TRPV, and TRPA channels; Fig. 3), the precise role of which in channel assembly and function is not well understood. The so-called TRP box, a 6-residue sequence in the proximal C-terminus, is also a conserved motif among the members of TRPV, TRPC, TRPM, and TRPA families (Fig. 3). Several studies have presented evidence that this region, at least in TRPM and TRPV channels, is crucial for the coupling of ligand sensing to channel gating.12 In addition, there are a number of domains/motifs, such as coiled-coil domain, kinase domain, NUDIX domain, EF hand Ca2+-binding motif, CIRB motif, and PDZ motif in the N- and C-termini of various TRP channels (Fig. 3). Despite the structural resemblance to classical voltage-gated Na+, Ca2+ (Nav, Cav) and Kv channels, TRP channels are only weakly voltage sensitive, presumably due to a relative paucity of positively charged residues in the TM segment S4. However, it has been proposed that modulation of voltage sensitivity of TRPV1 and TRPM8 channels occurs in response to changes in temperature and agonist exposure.13 Such data serve to highlight the broad range of environmental stimuli that are sensed by these channels, and the enormous technical challenge that has been encountered in attempting to understand the function and modulation of these channels.
Figure 3.
Structural–functional features of cytoplasmic regions in TRP channels from individual subfamilies.
Most of the functional TRP channels are homotetramers of an individual channel member, although a number of reports have also suggested the formation of heterotetrameric TRP channels. Several members of TRPC, TRPV, TRPM, and TRPP families form heterotetramers (Fig. 2) with distinct functional properties, when expressed in mammalian cell lines and Xenopus oocytes.11,14–18 However, the existence of such heterotetrameric channels in sensory neurons in vivo and their physiological relevance is elusive. Although activation of nociceptive TRP channels by specific noxious and/or pain-producing stimuli constitutes the principal detection for nociception and pain under physiological conditions, modulation of channel activation/inactivation, intracellular trafficking/targeting, and protein expression is highly critical for nociception and pain under pathophysiological conditions. This chapter presents a summary of the expression, distribution, and structure–function information of key nociceptive TRP channels belonging to TRPV, TRPA, and TRPM subfamilies, as well as their role in various nociception and pain conditions.
2. ION CHANNELS IN THE TRPV SUBFAMILY
2.1 Transient receptor potential vanilloid 1
Transient receptor potential vanilloid 1 (TRPV1), also previously known as “vanilloid receptor subtype-1” (VR1), is the most well-characterized TRP channel and the founding member of cloned thermosensitive TRP channels. TRPV1 can be activated by noxious temperatures and acidic pH, as well as by a wide variety of endogenous and exogenous algogenic compounds including capsaicin, the pungent ingredient in hot chili peppers (Fig. 1). Capsaicin’s excitatory effects on neurotransmission, nociception, and pain were well documented before the molecular identity of the receptor was revealed, as far back as the 1960s with most of the work reported in the 1980s and 1990s. In 1997, the molecular signature of the receptor was finally identified by expression cloning of the TRPV1 gene using a cDNA library generated from rodent sensory neurons, and functional identification was performed with dye-based Ca2+ imaging upon capsaicin and heat activation.19 Subsequent characterization revealed that TRPV1 in sensory neurons (and also under heterologous expression) could be activated by multiple noxious stimuli (see Fig. 1 for agonists of TRPV1; reviewed in Ref. 20). Follow-up investigations utilizing in vivo pharmacological approaches and TRPV1 gene knockdown in mice suggested the critical role of this channel in inflammatory pain conditions (reviewed in Ref. 20). This key information, along with the availability and development of specific physicochemical agonists (and antagonists) of TRPV1, led to a massive expansion in studies aimed at characterizing the channel and its role in nociception and various pain states.
2.1.1 Expression and distribution in nervous system
TRPV1 is predominantly expressed in the neurons of the PNS and CNS (reviewed in Ref. 20). In the PNS, TRPV1 is primarily expressed in small- and medium-diameter nociceptive neurons in the dorsal root, nodose, sympathetic, and trigeminal ganglia (DRG, NG, SG, and TG, respectively), such as peptidergic and nonpeptidergic C-fibers, as well as in some Aδ fibers.21–26 TRPV1 expression has also been detected in sensory nerve fibers innervating bladder, lung/airways, and cochlea, where it is involved in bladder function, sensing airway irritants, cough reflex, and hearing.27
In addition to the PNS, TRPV1 is also expressed in different CNS regions, specifically in the laminae I and II (and in the laminae V and X to some extent) of spinal cord (SC) dorsal horn, where it plays an integral role in synaptic transmission/modulation of peripheral nociceptive signal input.28,29 The majority of TRPV1 expressed in SC dorsal horn is presynaptic on the central axon terminals; however, there is also evidence of postsynaptic expression of TRPV1 in some neurons of SC dorsal horn, although their functionality and role in nociception are not understood.30 Presynaptic TRPV1 can act to modulate synaptic transmission in different inflammatory models and conditions.31 Genetically engineered mice with expression of fluorescent reporters, such as eukaryotic yellow fluorescent protein and tdTomato, under the control of TRPV1 promoter led to more convincing information on TRPV1 expression in different regions of the nervous system. Fluorescence labeling was prominent in small- and medium-diameter sensory nerve fibers in the skin, cornea, and bladder; small- and medium-diameter neurons in the DRG and TG; SC dorsal horn and trigeminal tract; and in some restricted regions in the brain, such as the brain stem, nucleus of the solitary tract, nucleus caudalis, nucleus ambiguous, olfactory bulb, and parabrachial nucleus.32,33 Also, some scattered neurons in the cortex exhibited fluorescence labeling, and other major regions in the forebrain, such as hypothalamus and hippocampus, did not show any detectable signal in these mice, suggesting no TRPV1 expression in these brain regions.32,33 In addition to PNS and CNS, TRPV1 mRNA (in some instances functional channels) has also been detected in a number of nonneuronal tissues such as bladder, lungs, keratinocytes, various cell types in dental tissue, fibroblasts, mast cells, hair follicles, blood vessels, testis, and ovary; however, some degree of controversy still remains regarding the extent of expression and function of TRPV1 in these tissues (reviewed in Refs. 20,34).
2.1.2 Structure
Secondary structure prediction of cloned TRPV1 suggested a 6-TM polypeptide, with a hydrophilic pore loop between S5 and S6, as well as tetrameric assembly (Fig. 2) resembling to the α-subunit of Kv channels.19 Subsequently, the first evidence of tetrameric assembly of TRPV1 came from biochemical experiments on heterologously expressed recombinant TRPV1.35 Accurately resolving the structures of complex membrane proteins is technically challenging, and TRP channels have proven no exception. The first structural information on TRPV1 came from the X-ray crystallographic structure of the ARD at the cytoplasmic N-terminus of the channel protein, suggesting six ARDs, and their role in adenosine triphosphate (ATP) and calmodulin (CaM) binding.36 Subsequently, the three-dimensional (3D) structure of purified recombinant TRPV1 channel protein expressed in yeast was determined by using single-particle electron cryo-microscopy (cryo-EM) at a resolution of 19 Å. This structure revealed a tetrameric channel conformation with bulky open basket-like domain formed by the cytoplasmic N- and C-termini, and a compact membrane domain that has several resemblances to the transmembrane domain architectures of Kv channels that were determined by X-ray crystallography.37 More recently, high-resolution cryo-EM allowed the determination of tetrameric TRPV1 structure at a near-atomic resolution, 3.4 Å, exhibiting a four-fold symmetrical TM segments formed by S1–S4 of each subunit that surrounds the central ion conduction pathway constituted by S5, the pore loop, and S6 (Fig. 2).8,9 Structural and functional predictions based on prior evidence were largely confirmed by this new structural model, overall suggesting that TRPV1 does indeed bear a structural resemblance to Kv channels.38 However, unlike Kv channels, the S4 segment does not demonstrate strongly voltage-dependent movement. Rather there appear to be two distinct “gating” regions: the outer one near the pore helix at the inner selectivity filter region of the channel and the inner gate closer to the cytoplasmic side, which upon vanilloid binding leads to shifting of the lower S6 and S4–S5 linker regions to open the channel.9,39
2.1.3 Functional properties of the channel
TRPV1 is the best example of a polymodal receptor channel that can be physicochemically activated by a large number of intracellular and extracellular ligands from both endogenous and exogenous sources (Fig. 1). Endogenous chemical ligands that can directly activate TRPV1 channel include extracellular acidic environment (~pH 6.0 or less),19,24,40 intracellular basic environment (~pH 7.8 or more)41, and several endovanilloids and endocannabinoids that are generated by various lipid metabolism pathways under inflammatory conditions.20,42–44 Most of these are fatty acid derivatives, including amines (anandamide, N-arachidonoyldopamine, N-oleoylethanolamine, N-arachidonolylserine), oxygenated eicosatetraenoic acids (lipoxygenase products and their hydroxylic analogs, prostaglandins and leukotrienes), and lysophosphatidic acid.20,45,46 Reactive oxygen and nitrogen species (ROS and RNS) have also been suggested to activate TRPV1 channel directly, although most of these findings are restricted to recombinant channels expressed in heterologous system, and require further confirmation in native sensory neurons (reviewed in Ref. 47). Noxious temperature (>42 °C) constitutes the endogenous physical/thermal ligand for TRPV1 (reviewed in Ref. 20).
Capsaicin, the pungent ingredient of hot chili peppers, is the first known exogenous ligand for TRPV1, and in fact, this knowledge was highly critical for the molecular identification and expression cloning of TRPV1 from mammalian sensory neurons.19 In addition to capsaicin, there are a number of exogenous compounds that activate TRPV1. Other pungent plant products that can activate TRPV1 include piperine, found in black pepper; allicin, found in garlic; and eugenol, found in clove oil (reviewed in Ref. 20). In addition to these compounds, there are also a number of plant and animal toxins that target TRPV1. Resiniferatoxin (RTX), derived from the cactus Euphorbia resinifera, is a highly potent agonist of TRPV1 (3–4 times more than capsaicin).48 There are a number of potent spider toxins that bind and activate TRPV1 including the vanillotoxins (VaTx)-1, 2, 3, and “double-knot toxin” (DkTx).49,50 In addition to these algogenic compounds of plant and animal toxins, TRPV1 can also be activated by ethanol.51
Extensive structure–function studies on recombinant TRPV1 channels have led to the identification of molecular determinants of channel activation by specific ligands. These studies utilized site-directed mutagenesis and chimeric TRPV1/2/M8 channel generation/expression approaches with altered TRPV1 regions and/or single amino acid substitution approaches. Extracellular acidic pH activates TRPV1 by protonation of extracellular residues E600 and E648, whereas intracellular alkalization activates the channel that is dependent on the residue H378.40,41 Capsaicin, RTX and most of the endovanilloids and endocannabinoids, which easily penetrate through the plasma membrane, due to their lipophilic nature, activate the channel from the intracellular side. With the cloning of TRPV1 gene from multiple mammalian species, it was found that the channel in birds is insensitive to capsaicin, whereas the channel in rabbit is mildly sensitive to capsaicin, without any alteration in channel activation by acidic pH and noxious temperature. Based on this information, and subsequent experimentation on mutational/chimeric channels, the capsaicin (and RTX) activation of TRPV1 was localized to regions between the TM S3–S4 segments of the protein, specifically involving residues Y511, S512, M547, and T550.52,53 On the other hand, the toxins VaTx and DkTx activate TRPV1 by directly binding to the external region of the pore loop and lead to prolonged and irreversible channel open durations.49 With the recent unraveling of high-resolution cryo-EM structure of TRPV1, without and with RTX-/DkTx-bound conditions, it became clear that the RTX-binding pocket in the S3–S4 region involves the residues Y511, S512, M547, and T550, and this pocket sits just above the residue E570 in the S4–S5 linker, with proximity to residue L669 in the S6 of neighboring subunit.8 The S4–S5 linker and the nearby proline-valine-proline (PVP) region in the S6 segment of Kv1.2 channel have been established to play the critical role of a channel “gate” in response to voltage-dependent movement of S4 segment.54,55 Therefore, it is suggested that RTX (or capsaicin) binding pulls the S4–S5 linker of one subunit, which then twists the S6 segment of the neighboring subunit to open the lower gate, and subsequently the upper gate of the channel.8 In contrast, the DkTx interacts with TRPV1 at the extracellular pore region by intercalating between the top of the pore helix of one subunit and the outer pore-loop proximal to the S6 segment of the neighboring subunit, thereby locking the channel in the open conformation following activation.8 However, the precise mechanism by which DkTx binding leads to the displacement/opening of both the upper and lower gate of the channel is not well understood from these studies. Regarding heat activation of TRPV1 channels, several studies have convincingly showed that this channel is intrinsically heat sensitive, and increased temperatures shift the voltage dependence of channel activation to physiological membrane potentials, which otherwise do not undergo voltage-dependent activation at these potentials.39,56 Subsequent studies revealed that the pore region is critical to channel activation at noxious temperatures, and prolonged heat activation leads to pore dilation.57 However, the precise structural mechanisms that lead to channel gate opening upon exposure to noxious temperatures still remain elusive. Other intracellular ligands, such as ATP and allicin, are shown to activate the channel by interacting with the ARD in cytoplasmic N-terminus of the channel protein; specifically, ATP intercalates between fingers 1–2 of ARD, whereas allicin interacts with the C157 residue in the same region.36,41
Upon ligand binding (or exposure to noxious temperatures), the channel gate opens, and under physiological conditions, it allows the influx of Na+ and Ca2+ through the central ion conduction pore, with relatively high permeability for Ca2+ (three- to nine-fold).10,19,57,58 The residues D636 in the pore region, and Y671 in the S6 segment have been suggested to be the critical determinants of high relative Ca2+ permeability of TRPV1 channel. Rapid influx of Na+ and Ca2+ through TRPV1 results in depolarization of neuronal plasma membrane potential and opening of Nav/Cav channels, which leads to action potential (AP) firing. Immediately following activation, the TRPV1 channel undergoes desensitization in the presence of the ligand (analogous to voltage-dependent inactivation of Nav/Cav/Kv channels), which results in diminished firing upon prolonged or subsequent TRPV1 activation.19,24,58–60 Desensitization of TRPV1 under physiological conditions is dependent on Ca2+ influx through the channel,58,59 which presumably operates via activation of the protein phosphatase 2B (PP2B), leading to channel dephosphorylation and desensitization.61,62 Additionally, Ca2+/CaM binding and interaction of phosphatidylinositol-bis-phosphate (PIP2) with the N-/C-termini of TRPV1 protein have also shown to be critical structural determinants for channel desensitization.36,63 Although the precise structural mechanisms underlying TRPV1 channel desensitization following activation are not well understood, the residue Y671 in the S6 segment has been proposed to be involved in the structural arrangements in the channel pore that governs channel desensitization.58 In fact, the recently solved high-resolution cryo-EM structure of TRPV1 shows the Y671 residues in the inner pore cavity9; however, the precise structural changes surrounding this residue upon prolonged activation/desensitization awaits further exploration.
2.1.4 Modulation of channel expression and function
Activation of TRPV1 in peripheral sensory nerve fibers not only leads to AP firing but also releases of neuropeptides, such as calcitonin gene-related peptide (CGRP) and neurokinins or substance P (SP). Sustained TRPV1 activation in these fibers leads to increased CGRP and SP release, which leads to vasodilatation and activation of a variety of immune and other cell types in the skin, leading to pro-inflammatory mediator release that result in a positive signaling feedback loop causing potentiation of TRPV1 channel activation and nociceptive signaling (reviewed in Ref. 20). At the same time, massive Ca2+ influx resulting from prolonged/episodic TRPV1 activation leads to increased expression of several nociceptive and other genes. Tissue injury and inflammatory conditions have been shown to increase TRPV1 expression in sensory neurons (both at mRNA and protein level), as well as increase/recruitment of functional TRPV1-expressing sensory neurons (reviewed in Ref. 20). More recent studies have also shown that proinflammatory mediators like interleukin-6 induce rapid translation of TRPV1 mRNA at peripheral sensory fibers, thereby increasing the magnitude of nociceptor excitation.64,65 Such modulation of TRPV1 gene transcription and translation has been suggested to underlie enhanced nociceptive/pain sensitivity (mainly thermal) in vivo.
The majority of TRPV1 modulation relates to functional modulation of the channel protein. Cross-sensitization or agonist-induced potentiation of channel activation properties by multiple agonists is one of the hallmarks of functional modulation in TRPV1. Under physiological conditions (~pH 7.3), TRPV1 in sensory neurons is activated at noxious temperatures (>42 °C). The temperature activation threshold of TRPV1 channel drops to ~35–37 °C upon extracellular acidification (~pH 7.0 to 6.5), a pH range that does not induce substantial channel activation at room temperature.24 This phenomenon is critical under tissue injury and inflammation, where there is significant tissue acidosis in the inflamed microenvironment (~pH range 7.2 to 6.4), thereby leading to robust activation of TRPV1 at body temperature.24 Similar cross-sensitization of TRPV1 has also been seen with capsaicin and temperature activation of the channel.24,58 The amino acid residues E648 and Y671 have been identified as the molecular determinants of TRPV1 underlying such cross-sensitization and modulation of channel activation properties.24,58 On the other hand, the channel undergoes diverse and extensive posttranslational modifications that leads to multiple levels of functional modulation TRPV1 that are widely observed under tissue injury, inflammation, and a wide variety of pathological conditions (reviewed in Ref. 20).
A wide variety of pro-inflammatory mediators, cytokines, chemokines, growth factors, pruritogens, ATP, lipid metabolism products, ROS, RNS, neurotransmitters, and neuropeptides are released at elevated levels during injury/inflammation/pathological conditions. These mediators activate their respective receptors expressed on sensory neuron plasma membrane, which mainly belong to the group of G protein-coupled receptors and growth factor tyrosine kinase receptors (TKs). Activation of these receptors leads to a wide variety of intracellular signaling pathways that result in the activation of (1) protein kinases such as protein kinase A (PKA), protein kinase C (PKC), mitogen-activated protein kinases (MAPKs), extracellular signal-regulated kinases, Ca2+–CaM-activated protein kinases (CaMKs), cyclin-dependent kinase-5 (Cdk5), Src kinase, and phosphoinositide kinases (PI3/4/5Ks); (2) phospholipases such as phospholipase C-β (PLC-β) and PLC-γ; and (3) protein phosphatases such as protein phosphatase 1/2A (PP1/2A), PP2B, and protein tyrosine phosphatases. TRPV1 channel activity can be modulated by most of these kinases/phosphatases. TRPV1 protein has been shown to be directly phosphorylated at residues S6, S116, T144, T370, S502, S774, and S820 by PKA; residues S502, T704, and S800 by PKC; residue Y200 by Src; residues S502 and T704 by CaMKII; and residue T407 by Cdk5 (based on rat TRPV1 amino acid sequence).66–73 Phosphorylation of TRPV1 protein by PKA and PKC has been shown to shift the thermal activation of the channel from 43 to 38–33 °C,70,72 thereby opening the channel at body temperature, a mechanism that has been suggested to underlyie inflammatory thermal pain. In addition, TRPV1 protein phosphorylation by PKA and PKC shifts the proton activation of TRPV1 from a pH range of 5.8–6.2 to a pH range of 6.4–7.0, which also serves as a mechanism for nociceptor sensitization during tissue injury/inflammations.60,66,74 On the other hand, phosphorylation of TRPV1 by CaMKII regulates capsaicin and other vanilloid binding to the channel protein.69 In addition to direct modulation of channel activity by PKA/PKC phosphorylation of channel protein, activation of these kinases and p38 MAPK has been shown to upregulate the expression of TRPV1 protein,75 which provides the neurons with a long-term sensitization mechanism. Furthermore, phosphorylation of TRPV1 by the Src-type TK increases the trafficking of TRPV1-containing vesicles to the plasma membrane, thereby providing the neurons with increased numbers of surface channel protein.68,73,76 Collectively, activation of various protein kinases in sensory neurons upon activation of surface receptors for pro-inflammatory mediators, growth factors, and receptors sensitize nociceptors via (1) direct modulation of TRPV1 channel activation at body temperatures and the mildly acidic environment of inflamed/injured tissue, (2) increased number of TRPV1 channels on the plasma membrane, and (3) increased expression of TRPV1 proteins.
In addition to phosphorylation-dependent modulation, PLC regulation of TRPV1 channel activity has also been suggested to provide a molecular mechanism underlying nociceptor sensitization, although conflicting observations have been reported in this regard.77–79 PLC has been shown to both activate and desensitize TRPV1. On one hand, PLC activation results in hydrolysis of PIP2 to inositol triphosphate and diacylglycerol, and then later activates PKC to sensitize TRPV1 (reviewed in Ref. 20). On the other hand, it has been shown that PIP2 constitutively binds to TRPV1 protein in the plasma membrane to inhibit channel opening, and hydrolysis of PIP2 by PLC releases the channel from constitutive inhibition, thereby enhancing channel opening.77 In addition, results from other studies have shown that plasma membrane PIP2 is necessary for TRPV1 channel activation.78 One major reason for these discrepancies could be the use of different cell types, conditions, and test parameters used therein, as well as the possible involvement of specific PLC isoforms in individual cell types,79 but as a result a definitive understanding of PIP2 regulation of TRPV1 is currently lacking.
2.1.5 Channel desensitization
Under physiological conditions, activation of TRPV1 causes a Ca2+-dependent desensitization of channel activation. This is an important physiological property of TRPV1 and is critical to the efficacy of TRPV1 agonists to induce pain. Removal of extracellular Ca2+ reduces desensitization elicited by capsaicin, but does not completely abolish desensitization58,59; a small part of TRPV1 desensitization is therefore Ca2+ independent. It has been suggested that Ca2+ entering into neurons via TRPV1 channels presumably binds to CaM, and this complex activates the protein phosphatase calcineurin (or PP2B), which dephosphorylates the channel protein, resulting in channel desensitization.62 CaM has also been reported to physically interact with the cytoplasmic C-terminus of the channel protein, thereby regulating Ca2+-dependent desensitization of TRPV1; however, it primarily affects desensitization to short/repeated agonist applications, but not to prolonged agonist applications.63,80 Phosphorylation by PKA has been shown to reduce the Ca2+-dependent desensitization of TRPV1.66,74 In addition to Ca2+/CaM, PIP2 has also been implicated in TRPV1 channel desensitization, although it remains a highly debated matter. Finally, long-term desensitization due to repeated activation of TRPV1 can activate endocytosis and liposomal degradation of the channel.81
2.1.6 Involvement in pain conditions
TRPV1 is involved or proposed to be involved in a wide variety of pain conditions including migraine, dental pain, cancer pain, inflammatory pain, neuropathic pain, visceral pain, and osteoarthritis. This section briefly describes the suspected role of TRPV1 in these disease states. The first and foremost evidence of the role of TRPV1 in peripheral pain processing came from observations on the attenuation of inflammatory thermal hyperalgesia behaviors in rodents injected with capsazepine, a competitive vanilloid antagonist of TRPV1 channel. Furthermore, mice lacking functional TRPV1 (Trpv1−/− mice) exhibited no change in response to noxious temperatures, but they did show dramatic attenuation of thermal hyperalgesia elicited by a number of inflammatory mediators.82,83 These observations not only prompted the development of small-molecule antagonists of TRPV1 as new-generation efficacious analgesics but also made it possible to thorough investigate the critical role of TRPV1 in the detection/transduction of pain in several pathologies.
One of the best-studied pain modalities using animal models is inflammatory pain. With the strong evidence from Trpv1−/− mice exhibiting a lack of inflammatory thermal hyperalgesia, various rodent models of inflammatory pain, including the widely used complete Freund’s adjuvant (CFA)-induced inflammation, were subsequently utilized to determine the role of TRPV1 therein.82 Similarly, studies on Trpv1−/− mice revealed its significant role in neuropathic pain conditions, including diabetic neuropathy (reviewed in Refs. 20,84).
In animal models, both genetic ablation and pharmacological blockade of TRPV1 can reduce arthritis-like symptoms.85,86 Additionally, a variant of TRPV1 has been correlated with increased risk of knee osteoarthritis, as evident from observations on increased expression of TRPV1 in knee synovium in patients with osteoarthritis.86,87 Furthermore, pharmacological evidence also suggests the critical role of TRPV1 in arthritis and/or joint pain. Topical capsaicin creams have long been used to alleviate arthritis-like joint pain, and this treatment works by desensitizing and/or excitotoxic degeneration of TRPV1-positive nociceptive terminals (reviewed in Refs. 20,84). Additionally, Trpv1−/− mice exhibit no edema or hypersensitivity following joint inflammation, another piece of evidence suggesting the involvement of TRPV1 in the pathogenesis of arthritis-like inflammatory conditions.85 Altogether, this supports a potentially critical role for TRPV1 in arthritis and joint pain conditions, and that both TRPV1 agonism and antagonism presumably constitute analgesic targets for these conditions.
TRPV1 has long been suspected to be involved in bone cancer pain, and in fact, pharmacological inhibition of TRPV1 significantly decreased pain associated with an animal model of primary bone cancer.88,89 There are a number of different mediators found in the bone tumor microenvironment including prostaglandins, bradykinin, and nerve growth factor (NGF), which could presumably sensitize TRPV1 through posttranslational modifications, thereby leading to nociceptor sensitization.89 In addition to these mediators, the acidic tumor microenvironment has also been suggested to enhance the proton activation of TRPV1, thereby providing yet another TRPV1-dependent mechanism for constitutive nociceptor sensitization and pain in bone cancers.89
The pathophysiology of migraine is not well understood, but a number of studies suggest the involvement of TRPV1. Anandamide, an endogenous endocannabinoid, can activate TRPV1 and increase CGRP release, leading to neurogenic inflammation and vasodilation, which are key elements to migraine.90 Additionally, alcohol is known to increase migraine symptoms, and ethanol has been shown to both directly activate and potentiate the activation of TRPV1 channel.91–93 Such activation/potentiation of TRPV1 could also lead to nociception as well as further release of CGRP to potentiate migraine symptoms.
TRPV1 activation at noxious temperatures and the ability of different inflammatory mediators to reduce the thermal activation of the channel at temperatures below body temperature also suggest its involvement in dental pain conditions. TRPV1 is highly expressed in a large percentage (45–85%) of primary sensory afferents that innervate the tooth pulp.94,95 Recent studies have demonstrated that TRPV1 expression is upregulated in a rat model of pulpitis induced by lipopolysaccharide (LPS).96 While the mechanistic evidence is weak, TRPV1 still remains an attractive target for pharmaceutical intervention of dental pain.97
Both pharmacological and genetic inhibition of TRPV1 has been shown to exhibit decreased responses to colorectal distention in mice before and after inflammation.98 Additional studies have shown that TRPV1 inhibition can reduce such disease severity in animal models, and evidence suggests that it may play a critical role in both the initiation and the maintenance of visceral hypersensitivity after injury.99 In patients with colorectal disease, TRPV1 expression correlates positively with clinical score of severity of colorectal function.100 In support of these observations, Trpv1−/− mice exhibit reduced visceral pain, including reduced mechanical hypersensitivity in models of cystitis101 and inflammatory bowel disease.102,103 Additionally, in Trpv1−/− mice increased number and levels of inflammatory markers have been detected after bowel inflammation as compared to wild-type controls, which further complicates understanding of the precise role of TRPV1 in inflammatory bowel conditions.104,105 TRPV1-positive sensory nerve fibers also innervate the bladder and are important for the enhancement of bladder contractility after inflammation.106–108 In this context, ablation of TRPV1-positive neurons in mice with RTX can decrease bladder contractions in animals with bladder inflammation.106,109 Altogether, this indicates that both TRPV1 agonists and/or antagonists may be useful in treating specific types of inflammatory bowel diseases, interstitial cystitis, and painful bladder syndromes.
2.1.7 Involvement in other physiological and pathological conditions
In addition to its critical role in nociception, TRPV1 has been suspected to play a role in the regulation of body temperature. Initial observations from Trpv1−/− mice showing no alteration in body temperature as compared to their wild-type littermates suggested that TRPV1 might not be involved in body temperature regulation.82,83 However, subsequent reports showed that systemic administration of small-molecule antagonists of TRPV1 leads to induction of transient hyperthermia,110 which suggests a critical role for this channel in body temperature regulation. Lack of such observations in Trpv1−/− mice could be attributed to some as yet unidentified compensatory mechanisms that are introduced under genetic knockout of TRPV1. Peripheral TRPV1 expressed in sensory afferents was thought to be the critical component of peripheral detection of noxious temperatures. However, ablation of TRPV1-expressing cells in the SC dorsal horn with high dose of intrathecal capsaicin leads to a complete loss of responsiveness to noxious heat, suggesting the critical role of spinal TRPV1 in the detection of noxious temperatures.21 Although TRPV1 is predominantly expressed in peripheral sensory afferents and SC dorsal horn, expression of TRPV1 mRNA and protein has also been reported in the brain and brain stem. In fact, the critical role of TRPV1 in thermoregulation has been suggested to be mediated in part by TRPV1 in the brain stem. Whether TRPV1 in brain plays any role in nociceptive recognition is yet to be elucidated. TRPV1 mRNA and/or protein is expressed in periaqueductal gray, insular cortex, somatosensory cortex, hippocampus, thalamus, cortex, and amygdala region of brain (reviewed in Ref. 111). The function of TRPV1 in brain neurons is somewhat varied depending on the brain regions studied, with several reports showing its involvement in long-term potentiation, long-term depression, and synaptic plasticity (reviewed in Ref. 111).
TRPV1 has also been reported to be expressed in several nonneuronal tissues and has therefore been implicated in a number of nonpain-related pathologies, including diseases of the respiratory tract, cancer, and diabetes. TRPV1 is highly expressed in sensory neurons innervating the airways, and evidence suggests that it is involved in the cough reflex. Furthermore, antagonism of TRPV1 has been shown to reduce cough in some animal models.112–115 TRPV1 protein expression is also upregulated in asthma and gastroesophageal reflux disease.113,114 Part of the mechanism behind the activation of TRPV1 in the cough reflex may be due to the activation of protease-activated receptor-2 (PAR-2), which can be activated by extracellular proteases released during inflammation. Activation of PAR-2 has been shown to sensitize TRPV1 channel activity,116,117 leading to nociceptor sensitization. TRPV1 also appears to be important for certain types of cancers, specifically one report showing that increased consumption of hot chilies (capsaicin) could lead to higher rates of stomach cancer.114 TRPV1 has also been suggested to be involved in diabetes; specifically, Trpv1−/− mice exhibit increased sensitivity to insulin.118 Current evidence suggests that TRPV1 activation plays a protective role in type I diabetes, although another study suggested that channel activation could be detrimental in type 2 diabetes conditions.114 Therefore, further in-depth research is needed to better understand the role of TRPV1 in multiple disease conditions, which could warrant the utilization of TRPV1 antagonists not only for treating painful conditions but also for the treatment of other pathological conditions such as asthma, pancreatitis, cancers, and diabetes.
2.1.8 Drug development targeting TRPV1
Since its discovery, TRPV1 has been at the forefront of pharmaceutical developments as an attractive analgesic drug target. Predominant expression of TRPV1 in sensory neurons is advantageous because an antagonist could theoretically inhibit pain without producing significant central side effects. Unfortunately, most of the first-generation small-molecule antagonists developed against TRPV1 failed at different stages of preclinical trials, mainly due to their hyperthermia-inducing effects.20,110,119 These results in fact led to the further understanding of the role of TRPV1 in body temperature regulation, which is thought to be mediated by the channels expressed in the SC and brain stem.110 The second-generation TRPV1 antagonists have attempted to target different modalities of channel activation, mainly avoiding the blockade of heat activation and focusing on inhibiting the proton activation of the channel.120 At least one such drug is currently being investigated for its efficacy in advanced clinical trial phases as a new-generation analgesic.
On the other hand, TRPV1 agonists have also shown great promise in the treatment of moderate to severe pain. By rapidly and strongly activating TRPV1, an agonist can cause fast and prolonged channel desensitization, and/or eventual degeneration/death of nociceptive fibers due to excitotoxicity. This is the same rationale behind the use of topical capsaicin creams for the treatment of arthritis pain but has also been extended to more severe cancer pain. Studies using intrathecal injection ofRTXshowed that it can decrease pain behaviors in dogs with osteosarcoma, and in this line preliminary studies in human patients with bone cancer pain have been promising. However, the results from experiments on humans also showed that RTX does not work in all pain conditions; for example, intravesicular injection of RTX was not effective at relieving symptoms of pain associated with interstitial cystitis.121–123 Overall, the debate continues over the use of agonists or antagonists of TRPV1 as effective pain-relieving mechanism; however, this channel still remains a hot spot for drug discovery in pain.
2.2 Transient receptor potential vanilloid 2, 3, and 4
The TRPV2 channel was identified/cloned soon after the cloning of TRPV1 and was primarily characterized as a molecular transducer of higher noxious temperatures (>52 °C) in rodent sensory neurons.46,124 TRPV2 is expressed in mechano- and thermoresponsive neurons in the DRG and TG, and SC, as well as in a number of nonneuronal cells, including immune cells.46,124 In addition to higher noxious temperatures, certain synthetic cannabinoids, such as cannabidiol, can activate heterologously expressed recombinant TRPV2 channels (Fig. 1), although specific physiological and natural chemical agonist(s) of this channel still remains to be identified.46 TRPV2 shares high sequence similarity to TRPV1 (50% identical) and forms functional homotetrameric channels, as well as heterotetrameric channels with TRPV1, conducting both Ca2+ and Na+ (at a ratio of 3:1).124 Although some modulation of TRPV2 channel expression and/or function by PKA has been shown in immune cells,125 and TRPV2’s role in macrophage function, phagocytosis,126 so far no reports have shown its direct involvement in any painful conditions. Furthermore, mice lacking functional TRPV2 display normal thermal and mechanical nociceptive behaviors,127 which is suggestive of no significant contribution of this channel to pathological pain/nociception conditions.
The TRPV3 channel was cloned, based on sequence homology cloning strategy utilizing the sequence data for other TRPV channels (TRPV1, TRPV2, TRPV5, TRPV6), simultaneously from human sensory neurons and from rodent keratinocytes.128,129 Accordingly, its expression has been detected in human DRG and TG neurons, SC, brain, and keratinocytes, although some controversy exists regarding its expression in both rodent PNS and CNS neurons.46,128–130 As opposed to the previous two TRPV channels, TRPV3 can be activated at warm temperatures, ranging from 32 to 40 °C, and by a number of chemical ligands such as camphor and the pungent ingredient of clove, eugenol (Fig. 1), as well as by a number of synthetic vanilloid compounds. TRPV3 shares 40% sequence similarity with TRPV1 and forms functional homotetrameric channels, as well as heterotetrameric channels with TRPV1, conducting both Ca2+ and Na+ with high relative Ca2+ permeability.130 TRPV3 channel activity can also be enhanced by inflammatory signaling, lipid metabolites, and PKC,46,130 which could contribute to the development of inflammatory pain. Due to species differences in the expression, tissue distribution, and mode of activation of TRPV3, precise identification of its role in pain and pathological nociception remains controversial and unclear. However, small-molecule antagonists of TRPV3 have been shown to provide some attenuation of inflammatory pain, skin hypersensitivity, and itch conditions.46,130 Therefore, further in-depth studies, taking into account the species differences in the expression, distribution, and functional properties of TRPV3, are required to define its role in pain and nociception.
The TRPV4 channel was one of the first channels identified as the molecular detector of osmotic changes, pressure, and shear stress, both in neurons and in muscles.84,131,132 Following cloning and characterization, TRPV4 was found to be expressed ubiquitously and contributes to intracellular Ca2+ signaling, transduction of osmotic and mechanical pressure, temperature sensing to some extent, cell volume regulation, and maintenance of energy homeostasis.84,131,133 In line with the expression and function of TRPV4 in the sensory neurons of both DRG and TG, the heterologously expressed recombinant channel can be activated by increasing temperatures (≥33 °C), polyunsaturated fatty acids (PUFAs) such as arachidonic acid metabolites, exogenous chemical ligands including synthetic phorbol esters such as 4α-phorbol 12,13-didecanoate, and plant extract bisandrographolide A.84,134 Activation of TRPV4 leads to the influx of Ca2+ and Na+ (at a ratio of 2:1) in sensory neurons, leading to membrane depolarization and subsequent AP firing.3,84 Like other TRPV channels, functional TRPV4 channels are formed by homotetramers. Although heterotetrameric TRPV channels involving TRPV4 monomeric subunits have not been reported yet, heterologous expression of functional TRPV4–TRPC1 and TRPV4–TRPC1–TRPP2 heterotetrameric channels has been shown.135 With regard to modulation of functional properties of TRPV4, both PGE2 and PAR-2 signaling have been shown to sensitize channel activity, presumably via PKA and Src phosphorylation of the channel protein.136–139 Although several speculations have been made suggesting upregulation of TRPV4 channel activity by PKC, convincing experimental evidence in support of these have not yet been presented.84 Functional expression of TRPV4 in DRG and TG neurons led to the suggestion that TRPV4 could be involved in the transduction of thermal and mechanical stimuli at both somatic and visceral tissue levels that lead to pain. TRPV4 has been suggested to be involved in nociceptive responses to osmotic stimuli, both hypotonic and hypertonic, leading to mechanical hyperalgesia, as evidenced from reduced mechanical hyperalgesia upon in vivo silencing of this channel in mouse DRG neurons.137,140 Mice lacking functional TRPV4 (Trpv4−/−) exhibit impaired sensitivity to high-threshold mechanical stimuli, and some degree of alterations in selective nociceptive responses to temperature and acid; however, no change has been observed in their responses to noxious temperatures and high-threshold mechanical stimuli.141–143 Interestingly, following tissue injury and inflammation Trpv4−/− mice exhibit increased latency to escape from hot plate, suggesting some contribution of TRPV4 in the development of thermal hyperalgesia.144 Furthermore, attenuated nociceptive responses in experimental rodent models of pancreatitis and irritable bowel syndrome (IBS) in Trpv4−/− mice have also been suggested.145,146 Taken together, TRPV4 presumably serves as a molecular transducer of nociceptive/pain hypersensitivity associated with a number of inflammatory and neuropathic conditions, although more in-depth studies are required to determine the critical and specific role(s) of this channel in specific pain conditions.
3. ION CHANNELS IN THE TRPM SUBFAMILY
3.1 Transient receptor potential melastatin 3
TRPM3 was initially identified and cloned as a nonselective cation channel activated by pregnenolone sulfate; however, more recent reports on activation of TRPM3 at warm/noxious temperatures have brought this channel into the nociceptive TRP channel category.46,147,148 In the nervous system, TRPM3 expression has been reported in both CNS and PNS, including DRG neurons, as well as in a variety of nonneuronal tissues.147,148 Structurally, TRPM3 is a homotetrameric channel with a distinct ~700 amino acid long TRPM-specific domain in the cytoplasmic N-terminus of the protein. Like TRPV1, TRPM3 is a nonselective cation channel with relatively high Ca2+ permeability and has a strong outwardly rectifying current–voltage relationship.147,148 The nociceptive role of TRPM3 came to light more recently with the finding that mice lacking functional TRPM3 (Trpm3−/−) showed significant attenuation of inflammatory thermal hyperalgesia.147 Studies at the cellular level further identified that TRPM3 could be activated by noxious temperatures (>30 °C). Furthermore, in experimental models of CFA-induced inflammatory pain, Trpm3−/− mice exhibited significant attenuation of thermal nociceptive behaviors, as compared to their wild-type littermates,147 suggesting that TRPM3 constitutes yet another noxious heat sensor.
3.2 Transient receptor potential melastatin 8
It had been previously established that neurons in the DRG and TG exhibited cold- and menthol-activated currents, and it was also postulated that both cold and menthol presumably activate the same receptor.149 After the discovery of TRPV1 and TRPV2 as heat-activated ion channels, the continued search for cold-sensing ion channels on sensory neurons by expression cloning led to the identification of TRPM8.150,151 TRPM8 was first identified in prostate epithelial cells as a prostate-specific transcriptional marker; however, its role in sensory neurons was not realized until 2002, when it was identified/cloned from rodent DRG neurons. TRPM8 can be activated by both innocuous or cooling (26−15 °C) and noxious cold (15−8 °C) temperatures, as well as by a number of “cooling agents,” such as menthol and the more potent icilin (reviewed in Refs. 20,152).
3.2.1 Expression and distribution in nervous system
TRPM8 is predominantly expressed in PNS neurons. Antibody-based immunocytochemical analysis in DRGs showed that TRPM8 is expressed in sensory neurons giving rise to mostly C and Aδ fibers.20,84,150,151 Further studies in transgenic mice with EGFP expressed in TRPM8-positive cells have shown that TRPM8-positive neurons project to skin, oral cavity epithelium, regions in the tooth pulp, dentine, and the tongue as well as visceral organs innervated by the pelvic nerve (including the colon) and vagal nerve, such as bronchopulmonary tissue.153–156 TRPM8 is expressed in approximately 5–10% of DRGs and 10–15% of TGs.150,151,157 At the subcellular level, TRPM8 has been shown localized to flotillin-1-containing lipid rafts on the plasma membrane.158 Based on immunocytochemical analysis of brain sections from TRPM8/EGFP reporter mice, no expression of TRPM8 has been detected in brain neurons. Other than sensory neurons, TRPM8 is also expressed in prostate, bladder, lungs, and the urogenital tract.152 However, the functional role of TRPM8 channels in tissues/cells other than sensory neurons is not as well understood as its function in neurons.
3.2.2 Structure
Like other TRP channels, functional TRPM8 channels are homotetramers, with a characteristic long cytoplasmic N-terminus, without any ankyrin repeat, rather comprising of a ~700 amino acid residue TRPM domain (Fig. 3), the function of which still remains unclear. While it has been well established that cold temperature sensitivity of TRPM8 is intrinsic to the channel protein, the exact structural determinants in the channel critical for cooling/cold temperature activation are not well established. Heterologous expression studies on recombinant chimeric TRPV1, TRPV2, and TRPM8 channels have suggested that the cytoplasmic C-terminus is important for temperature activation of TRPM8 (reviewed in Ref. 20). On the other hand, it has also been postulated that the TRPM8 interacts with membrane lipids and those interactions may change under cold temperatures, thereby activating the channel. More is known about the interaction and activation of TRPM8 with its chemical agonists, menthol, and icilin. Mutational analysis and functional Ca2+ imaging in heterologous expression systems have shown that S2 and the TRP domain are critical to activation by icilin and menthol but not cold.159
3.2.3 Functional properties of the channel
As mentioned previously, TRPM8 can be activated by innocuous cooling to noxious cold temperatures (8–26 °C), as well as by cooling agents such as methanol and icilin. Purified TRPM8 proteins incorporated into artificial membranes as well as in excised patches of mammalian cells expressing recombinant TRPM8 retain its activation by cooling/cold temperatures, suggesting that TRPM8 is inherently temperature sensitive.160–162 Menthol and icilin activate TRPM8 by shifting the temperature activation threshold of the channel to higher temperatures, increase the open probability, and cause a hyperpolarizing shift in the voltage dependence of channel activation.56 These combined actions cause channel activation under physiological conditions, which like other TRP channels is a nonselective cation channel that allows Ca2+ and Na+ (at ~3:1 ratio) into the cell, leading to membrane depolarization and AP firing.150,151 One caveat to several studies using menthol is that it not only activates TRPM8 but also activates GABAA,163 TRPA1,164,165 and TRPV3166 and also has inhibitory actions on Nav and certain Cav channels.167,168 All these could contribute to the known effects of menthol-induced reduction in experimental thermal and mechanical pain hypersensitivities. Icilin is also not a specific agonist for TRPM8; it activates TRPA1 with a similar affinity. A number of specific agonists for TRPM8 have been developed for research use (WS-12 and WS-3), which aid in distinguishing the specific role of TRPM8 in sensory neurons, thereby eliminating the off-target effects of using menthol or icilin. Repeated activation of TRPM8 by cooling and/or menthol does not lead to channel desensitization; however, icilin activation leads to channel desensitization, suggesting distinct structural mechanisms of channel activation and desensitization. Overall, the precise mechanisms underlying channel activation still remain unclear.
3.2.4 Modulation of channel expression and function
TRPM8 channel exhibits different threshold of temperature activation in native neurons versus heterologous expression in cells, as well as in purified channels incorporated into liposomes, suggesting that there may be different modulators of the channel under different conditions and in different cell types.152 Like most TRP channels, TRPM8 can be regulated by its interaction with PIP2. In general, an increase in PLC activity by increased Ca2+ influx through the channel or by activation of other cellular signaling cascade (e.g., Gαq signaling) enhances hydrolysis of PIP2, leading to a decrease in channel activity. In other words, PLC-modulated channels exhibit greater desensitization upon activation. This mode of TRPM8 regulation is in contrast to PIP2 action on TRPV1 activity where decreasing TRPV1/PIP2 association leads to increased current and channel sensitization.79,152,160,161 In addition to PLC activity, some reports suggest that increased PKC activity could downregulate TRPM8 activity.169–171 However, a recent study suggests that PKC inhibitors cannot decrease TRPM8 desensitization.172 Studies on various inflammatory mediators such as bradykinin, histamine, serotonin, and ATP, which signal through Gαq/11-coupled receptors, show that these mediators inhibit TRPM8 channel activity via direct interaction of Gαq subunit to the channel protein. Such a mechanism is thought to constitute a mechanism underlying reduced cold sensation under injury/inflammatory conditions. Unlike TRPV1, cAMP and PKA have no effect on TRPM8.173–176 However, regarding channel desensitization, similar to observations on TRPV1 CaM has been suggested to be involved in acute desensitization of TRPM8.177 Endovanniloids and endocannabinoids have also been shown to positively regulate TRPM8.178 Phospholipase A2 (PLA2) activity can increase TRPM8 channel activity.179,180 Arachidonic acid and lysophospholipids are products of PLA2 activity and the latter has been shown to increase the threshold of temperature activation of TRPM8 closer to body temperature.179 When PLA2 activity is inhibited, it decreases icilin but not menthol hypersensitivity. In contrast, arachidonic acid can decrease TRPM8 activity but it is thought that the effects of lysophospholipids are stronger and thus the end result is increased channel activity.152 Overall, posttranslational modifications in TRPM8 channel protein and their impact on channel activity are not well understood.
3.2.5 Involvement in pain conditions
The exact role of TRPM8 in cold hypersensitivity associated with chronic inflammatory pain conditions like neuropathic pain has not been well established. Most of the work has been done in animal models and extrapolated to human conditions. There is some evidence that chemotherapy drug oxaliplatin-induced neuropathic cold allodynia may be dependent on TRPM8; however, recent evidence now suggests that the noxious cold-sensing channel TRPA1 might be a critical factor in such painful conditions.181 TRPM8 has been suggested to also play a role in visceral pain as peppermint oil (containing menthol) can decrease visceral pain in IBS patients, in some cases but as mentioned before this could be due to effects on other channels mediating excitability (GABAA) that could decrease pain.182 Additionally, TRPM8 has been proposed to play a role in dry eye, airway irritation, and cold-induced urticaria (hives), but the evidence is very preliminary and primarily in rodent models.181
It seems logical that orofacial/dental pain resulting in cold hypersensitivity could involve TRPM8 since TRPM8 is functionally expressed in TG innervating the orofacial region. However, the only evidence supporting this claim is that cold-activated currents in neurons innervating important orofacial structures appear to be dependent on both TRPM8 and TRPA1 activity.183
Interestingly, there is a TRPM8 gene variant associated with increases in migraine susceptibility only in women.184 The exact role of this variant is unknown, as is the association of TRPM8 and migraine but yet this provides intriguing evidence of another pathophysiological role for TRPM8.
TRPM8 knockout (Trpm8−/−) mice exhibit no apparent deficiencies besides a decrease in avoidance behavior to moderately cold temperatures.185–187 Trpm8−/− mice do not completely lack sensation to noxious cold, they still avoid temperatures lower than 0 °C. This residual cold sensation could be due to other cold sensitive channels. Two of the channels implicated are K+ leak channels (TRAAK and TREK1), which close at very low temperatures to increase excitability, and TRPA1, which some groups have shown to be cold sensitive.188,189 As a side note, while these animals have no change in core body temperature compared to wild-type littermate controls, antagonists of the channel can cause transient changes in body temperature, suggesting that TRPM8 is in fact involved in regulation of body temperature and Trpm8−/− undergoes adaption to control body temperature without functional TRPM8.190 It is also important to point out that all menthol sensitivity is not lost in multiple Trpm8−/− mice. This could be due to high concentrations and ability of menthol to activate TRPA1 and TRPV3 as mentioned before (reviewed in Ref. 181).
In addition to the normal physiologic changes in temperature sensation, these Trpm8−/− mice also have attenuated cold hypersensitivity after injury. In two different pain models, chronic constriction injury186 and the second phase of CFA injection186 cold hypersensitivity is attenuated in Trpm8−/− mice compared to wild-type controls. Additionally, there is some evidence that TRPM8 antagonists can reduce visceral pain.191 It is clear that TRPM8 does not only mediate noxious cold but also is important for cold hypersensitivity under injury conditions.
In contrast to TRPM8’s role in cold hypersensitivity, it is also critical to cold and menthol-induced analgesia. In wild-type mice, mild cooling and menthol can reduce acute and inflammatory pain in the formalin injection pain model. In mice that lack functional TRPM8, the analgesic effects of mild cooling are absent for the inflammatory pain phase after formalin injection but still present for the acute phase.187
3.2.6 Drug development targeting TRPM8
Menthol and other cooling agents, as well as cold temperature, are commonly used for their analgesic properties. TRPM8 is expressed on sensory afferents that appear to be nociceptive (some TRPM8 coexpresses with TRPV1153). However, it may also be expressed in different populations of neurons that trigger inhibition of pain signals or increase central inhibition models.192 No specific antagonists for TRPM8 are currently on the market or in the process of clinical trials for the treatment of pain conditions. TRPM8 agonists and antagonists still may be useful in treating an array of diseases from dry eye to cold hyperalgesia but more basic research using more specific pharmacological tools is needed, as well as consideration of TRPM8’s role in thermoregulation.
4. ION CHANNELS IN THE TRPA SUBFAMILY
4.1 Transient receptor potential ankyrin 1
The transient receptor potential subfamily A member 1 (TRPA1) was originally cloned from human lung fibroblasts in 1999.193 While certain key features of the channel were immediately apparent (an abundance of ankyrin repeats in the N-terminus; the 6-TM region and the resemblance to other TRP-related proteins), it was not until 2003 that “ANKTM1” (as TRPA1 was then known) was cloned and became the sixth thermosensitive TRP channel to be identified (after TRPV1–4 and TRPM8).189
4.1.1 Expression and distribution in the nervous system
TRPA1 expression in the nervous system is detectable in DRG, TG, and NG.189,194,198 In the periphery, TRPA1 (much like TRPV1) is predominantly expressed in small-diameter, unmyelinated and partially myelinated C- and Aδ-fibers. Approximately 25% of neurons found to express TRPA1 are also peptidergic, and almost half are isolectin-B4 (IB4)-positive (i.e., nonpeptidergic) in the PNS.194 In terms of coexpression with TRPV1, it appears that sensory afferents do coexpress with TRPV1, but the amount of “overlap” varies considerably between target tissues; colonic afferents have been shown to be predominantly responsive to both mustard oil (a TRPA1 agonist) and capsaicin (a TRPV1 agonist). However, in the skin, only 10% of neurons expressed both channels, a similar proportion expressed TRPA1 alone, and approximately 20% expressed TRPV1 alone.189,194,198 It is important to bear in mind, however, that these figures do not take into account the density of innervation a particular organ receives. In addition to colonic afferents, enterochromaffin cells in the gastrointestinal tract express TRPA1, where it may be involved in manifestation of inflammatory disorders such as Crohn’s disease and colitis.195,196 TRPA1 is also expressed in skin, where keratinocytes showed changes in inflammatory mediator secretion in response to TRPA1 activation.197 Sensory afferents expressing TRPA1 also innervate the bladder and prostate, raising the possibility that TRPA1 is involved in cystitis-associated pain.196
The central projections of TRPA1-expressing sensory neurons extend into the superficial laminae of the SC, where expression is detectable in presynaptic dendrites.198 Consistent with this observation, TRPA1 activation in lamina II of the SC was found to increase glutamate release and evokes excitatory postsynaptic currents.199 In the CNS, TRPA1 expression has been detected in the cerebellum, hippocampus, and forebrain.200 Although the function of TRPA1 in the brain is poorly understood, one potential explanation was put forward when it was reported that TRPA1 regulates resting calcium levels in astrocytes, thereby enhancing extracellular GABA levels and influencing the efficacy of inhibitory synapses.201 TRPA1 expression is found in the autonomic nervous system, where the vasodilation and consequent drop in blood pressure it causes could play a role in autonomic reflexes and disorders associated with neurovascular responses.202 Other internal organs where there is evidence of TRPA1 function include the pancreas,201 the inner ear (where it is known to be involved in mechanotransduction202), dental pulp (where a role in mechanotransduction and cold allodynia has been proposed203), and vascular endothelia and airway epithelial cells202,206, where it modulates chemokine secretion and thereby inflammation.
4.1.2 Structure
The lengthy N-terminal portion of TRPA1 (some 700 of the 1100 total residues) contains as many as 18 ARDs, a number far in excess of the 4–6 repeats typically seen in TRPC and TRPV channels.207,208 Our understanding of the contribution of these repeats to TRPA1 function is far from complete. However, there are suggestions that TRPA1 trafficking to the plasma membrane or insertion therein is compromised by the deletion of ARDs,209 or that (in nonmammalian systems at least) they are vital mediators of mechanotransduction. Until recently, it was assumed that functional TRPA1 channels exist in a homotetrameric configuration. However, TRPV1–TRPA1 concatemers appear to produce functional heterotetrameric channels in heterologous expression systems.16 Whether this occurs endogenously is yet to be determined.
Four potential disulfide bonds have been detected between five cysteine residues in the N-terminus of TRPA1.207 Covalent modification at these sites by electrophilic compounds causes conformational changes, offering a glimpse into the mechanisms by which such compounds modify channel function.13,208,209 Also contained within the N-terminus are three Ca2+-binding EF hand domains, one of which has been shown to be responsible for activating the channel in response to elevated intracellular Ca2+.210 While still categorized as a non-selective cation channel, TRPA1 becomes more Ca2+-permeable upon agonist stimulation.211
4.1.3 Functional properties of the channel
The list of TRPA1 gating modulators, regulators of function, and agonists is extensive. These compounds can be broadly separated into electrophilic (positively charged) and nonelectrophilic modulators. As previously mentioned, the N-terminus of TRPA1 is rich in cysteine residues. It is the negatively charged thiol group in the cysteine side chain with which electrophilic modulators react, resulting in TRPA1 activation.212 The precise structural mechanism by which this activation is elicited remains unclear. One major group of electrophilic modulators is typified by mustard oil (the active component of which is allyl isothiocyanate) and other isothiocyanates, such as those found in wasabi and horseradish.212,215 ROS such as hydrogen peroxide are elevated during injury and inflammation and also cause cysteine oxidation or formation of disulfide bonds between cysteine residues. For example, it has been reported that UVA irradiation prompts production of ROS which results in TRPA1 activation.215 RNS modify the channel by a process known as S-nitrosylation. One such species, nitric oxide, appears to be particularly important for pain sensitization, not only because of its ability to directly modify TRPA1 but also through its actions on cyclic GMP production, as well as generation of the fatty acid product nitro-oleic acid, another known modifier of TRPA1.212,215 In addition to nitric oxide, another “gasotransmitter,” hydrogen sulfide, increases CGRP release in a TRPA1-dependent fashion.216 Certain prostaglandins, such as 15d-PGJ2 are metabolized into electrophilic products, which contain a reactive carbonyl group and are therefore capable of directly activating TRPA1.212 Lipid peroxidation products, such as 4-hydroxynonenal, are produced downstream of ROS and elicit neuropeptide release and neurogenic inflammation. They act through the same covalent cysteine modification mechanism as other electrophilic modulators.217
Human TRPA1 has been shown to be sensitive to acidic pH(interestingly, the same is not true of rodent TRPA1218). Gating of the channel was found to increase in a dose-dependent fashion with increasing proton concentration associated with CO2 or other weak organic acids.219,220 Other compounds broadly characterized as “irritants” are electrophilic in nature, particularly formalin221 and acrolein an unsaturated aldehyde found in vehicle exhaust fumes and tobacco smoke.221,222 Similarly, TRPA1 activation by noxious cold temperatures is also dependent on species. While primate TRPA1 (macaque and human) could be activated at noxious cold temperatures, rodent TRPA1 channels are insensitive to cold temperatures, which is conferred by amino acid residues in the S5–S6 TM domains and the pore loop.223
Anesthetics often have paradoxical pronociceptive effects by acting through TRPA1.224 Propofol and isoflurane excite sensory neurons through acting on TRPA1 and TRPV1.225 It has been suggested that similar effects observed with other general anesthetics could be a substantial contributor to post-operative pain.226 Local anesthetics, such as lidocaine, not only block Nav channels but also activate TRPA1, leading to enhanced spinal release of glutamate.227 Various alcohols can, at millimolar concentrations, activate TRPA1 in a carbon chain length-dependent manner.228 Menthol, classically defined as an agonist of TRPM8, has also been shown to cause activation of TRPA1 at micromolar concentrations, while blocking the channel at higher concentrations.164 The irritant effect of topical nicotine has been attributed to activation of TRPA1.229
An intriguing class of TRPA1 modulators are the PUFAs230; however, the physiological relevance of these noncovalent interactions is unknown. Interestingly, given their structural resemblance to prostaglandins, the fenamate class of nonsteroidal anti-inflammatory drugs were discovered to be TRPA1 agonists and potentiated the effects of other TRPA1 activators, such as AITC.231
TRPA1 was originally described as a cold-sensing ion channel,232 but cinnamaldehyde (which provides cold sensation upon ingestion), a TRPA1 agonist, did not sensitize the response to noxious cold. Furthermore, blockade of TRPA1 in rats had no effect on cold responses.233 However, other groups have reported cold-mediated TRPA1 activation consistent with the original finding.234 As mentioned above, cold temperature activation of TRPA1 was subsequently found to the species-dependent, and is localized to the S5–S6 TM domains and the pore-loop of the channel protein.223 Furthermore, the ankyrin repeat 6 has been suggested to be critical for the thermal sensitivity of TRPA1; specifically three point mutations in this region can individually confer heat sensitivity upon the channel, while leaving chemical sensitivity unaffected.235
TRPA1 is known to be required for responses to noxious chemical stimuli, but there is also a requirement for TRPA1 activity in mechanotransduction.236 The precise ion channel repertoire contributing to mechanosensation and the modulation involved in pain perception are not fully understood. It is clear that although mechanical hypersensitivity is not completely blocked by inhibiting TRPA1 activity, it plays a significant role, since afferents shown to become hypersensitive to mechanical stimuli are TRPA1 positive.236 This is true in skin as well as in the GI tract, where TRPA1 is implicated in normal nociception as well as inflammatory pain.237
4.1.4 Modulation of channel expression and function
There is Ca2+ involvement in both potentiation and desensitization of TRPA1 activity. Initial potentiation relies upon entry of Ca2+ through the pore, whereupon elevation of intracellular Ca2+ concentration produces channel inactivation.238 Also, depletion of the phosphoinositide PIP2 renders TRP channels inactive, and applying PIP2 in the presence of agonists prolongs desensitization. Conversely, scavenging PIP2 accelerates desensitization. This led to the proposal that PIP2 reduces TRPA1’s sensitivity to agonists.239 It has been reported that the gating properties and sensitivities ofTRPA1 are altered with coexpression of TRPV1,240 although whether this relates to Ca2+-induced potentiation and desensitization remains to be established.
Arachidonic acid metabolites are an important class of TRP channel modulators. Epoxyeicosatrienoic acid, for example, is known to sensitize TRPA1 in inflammation. It is released by activated sensory neurons and activates second-order neurons in the superficial laminae of the dorsal horn of the SC.241 Lipoxygenases are another proinflammatory lipid species that have been shown to activate TRPA1 as well as TRPV1.242 TRPA1 is known to be sensitized by bradykinin,232 which presumably causes activation of PKC, but the exact mechanism and TRPA1 residues involved are unknown.
4.1.5 Involvement in pain conditions
It is abundantly clear that TRPA1 is modulated by known regulators of sensory neuron sensitivity, such as NGF, glial cell-derived neurotrophic factor, and artemin. These mediators can enhance the expression of TRPV1 and TRPA1 and thereby noxious cold sensitivity.243 The TRPA1 knockout (Trpa1−/−) mice display behavioral deficits in response to mechanical stimulation, AITC, and cold temperatures.244 While it seems reasonable to assume these observations also apply to human TRPA1 (for example, AITC also causes mechanical hyperalgesia in humans245), caution must be exercised. Primate and rodent TRPA1 share approximately 80% amino acid identity and are reported to differ in their responses to menthol and caffeine.246 They also differ in which cysteine residues are modified by electrophiles such as AITC.212
TRPA1 activity is involved in a number of inflammatory pain states, typified by allergic contact dermatitis,247 where neurogenic inflammation is a key pathophysiological driver. Neurogenic inflammation is also a symptom of pathological TRPA1 activation in bladder, whereupon inflammatory pain ensues.248 The prototypical pruritogen chloroquine is known to exert its effects at least in part by acting on TRPA1, an observation consistent with TRPA1’s proposed role in chronic itch.249 In chronic itch models, TRPA1 is proposed to be involved not only in the transduction of the itching sensation but also in the pathophysiological changes in skin associated with chronic itch.250
Familial episodic pain syndrome (FEPS) is associated with a mutation in TRPA1 at the amino acid residue asparagine at 855. This residue in the TM domain S4 is mutated to a serine, resulting in a fivefold increase in inward current.251 While somewhat less clear than the FEPS mutation, there does also appear to be an epigenetic phenomenon related to TRPA1. People with elevated pain sensitivity show differential DNA methylation in the vicinity of the TRPA1 gene. It is possible that such differences are a contributor to individual differences in pain sensitivity.252
Intriguingly, changes in expression of TRPA1 splice variants have been observed in mouse neuropathic pain models, but it has yet to be determined if similar process occurs in humans.253 Many conditions that result in oxidative stress and enhanced production of ROS lead to pain through activation of TRPA1. Diabetes is one such example.254,255 The cancer chemotherapeutics paclitaxel and oxaliplatin also lead to ROS production and TRPA1 activation, culminating in mechanical allodynia.255,256 Furthermore, it has been suggested that ROS may originate from macrophages infiltrating nerves and activating TRPA1 in vincristine-induced allodynia.253,257
TRPA1 is involved in both irritant-induced and migraine headache.258 This appears to be based on activation of TRPA1 in dural afferents.259 TRPA1 expression in trigeminal ganglion neurons is consistent with its importance in orofacial and dental pain.205
In osteoarthritis models, activation of TRPA1, along with TRPV1, leads to elevated levels of TNFα, a proinflammatory cytokine crucial to the development of osteoarthritis.260 TRPA1-expressing visceral afferents are involved in visceral hypersensitivity, i.e., IBS and colitis, where channel activation leads to neuropeptide-mediated neurogenic inflammation.261 TRPA1, in combination with TRPV1, contributes to pain downstream of PAR-2-stimulated pancreatitis.262
Curiously, the antinociceptive effects of acetaminophen and tetrahydrocannabinol are lost in the TRPA1 knockout mouse, offering an insight into the mechanisms through which these compounds exert analgesia.263
A remarkable finding was reported recently where LPS, the bacterial cell wall component, was characterized as a novel “irritant” molecule acting directly on TRPA1, independent of any canonical Toll-like receptor signaling.264
Although not necessarily painful, TRPA1 is a key part of chemosensory airway reflexes in response to irritants. Trigeminal afferents expressing TRPA1 are important in mediating sneezing and coughing.265
4.1.6 Drug development targeting TRPA1
Given the diverse pain states in which TRPA1 has been implicated, and the relative absence of serious adverse effects of TRPA1 blockade/deletion, particularly an absence of thermoregulatory side effects, it stands to reason that it represents an attractive target for analgesic drug development. There are nonelectrophilic antagonists, such as HC-030031,266 and electrophilic antagonists, such as AP-18 and A967079,267,268 all of which have demonstrated efficacy in rodent models of inflammatory and neuropathic pain. Indeed, spinally delivered blockade of TRPA1 reduced low-intensity mechanical pain and inflammation269; the same approach also proved effective in a rat osteoarthritis model.119 GRC17536, a TRPA1 antagonist developed by Glenmark pharmaceuticals, has recently passed phase I clinical trials as an antitussive therapeutic.270
Acknowledgments
Research work on TRP channels in nociception and in our laboratory is supported by research grants from the US National Institutes of Health (NIH/NINDS-NS069869 to D.P.M.; NIH/NCI-CA171927 and NIH/NINDS-NS045549 to A.D.M.), US Department of Defense (DoD/PCRP-PC101096 to D.P.M.), American Pain Society (APS-FLP1483 to D.P.M.), and the International Association for the Study of Pain (IASP-1881650 to D.P.M.). We would like to apologize to the authors of several research and review papers, as well as book chapters in the TRP channel sensory biology area, whose work could not be cited in this chapter due to space limitations.
References
- 1.Loeser JD, Treede RD. The Kyoto protocol of IASP basic pain terminology. Pain. 2008;137:473–477. doi: 10.1016/j.pain.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 2.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. http://dx.doi.org/10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu LJ, Sweet TB, Clapham DE. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 5.Montell C. Physiology, phylogeny, and functions of the TRP superfamily of cation channels. Sci STKE. 2001;2001:re1. doi: 10.1126/stke.2001.90.re1. [DOI] [PubMed] [Google Scholar]
- 6.Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224:285–287. doi: 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- 7.Minke B. The history of the Drosophila TRP channel: the birth of a new channel superfamily. J Neurogenet. 2010;24:216–233. doi: 10.3109/01677063.2010.514369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao E, Liao M, Cheng Y, Julius D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature. 2013;504:113–118. doi: 10.1038/nature12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504:107–112. doi: 10.1038/nature12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annu Rev Physiol. 2006;68:685–717. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- 11.Schaefer M. Homo- and heteromeric assembly of TRP channel subunits. Pflugers Arch. 2005;451:35–42. doi: 10.1007/s00424-005-1467-6. [DOI] [PubMed] [Google Scholar]
- 12.Valente P, García-Sanz N, Gomis A, et al. Identification of molecular determinants of channel gating in the transient receptor potential box of vanilloid receptor I. FASEB J. 2008;22:3298–3309. doi: 10.1096/fj.08-107425. [DOI] [PubMed] [Google Scholar]
- 13.Matta JA, Ahern GP. Voltage is a partial activator of rat thermosensitive TRP channels. J Physiol. 2007;585:469–482. doi: 10.1113/jphysiol.2007.144287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akopian AN. Regulation of nociceptive transmission at the periphery via TRPA1–TRPV1 interactions. Curr Pharm Biotechnol. 2011;12:89–94. doi: 10.2174/138920111793937952. [DOI] [PubMed] [Google Scholar]
- 15.Cheng W, Yang F, Takanishi CL, Zheng J. Thermosensitive TRPV channel subunits coassemble into heteromeric channels with intermediate conductance and gating properties. J Gen Physiol. 2007;129:191–207. doi: 10.1085/jgp.200709731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer MJ, Balasuriya D, Jeggle P, et al. Direct evidence for functional TRPV1/TRPA1 heteromers. Pflugers Arch. 2014;466:2229–2241. doi: 10.1007/s00424-014-1497-z. [DOI] [PubMed] [Google Scholar]
- 17.Hellwig N, Albrecht N, Harteneck C, Schultz G, Schaefer M. Homo- and heteromeric assembly of TRPV channel subunits. J Cell Sci. 2005;118:917–928. doi: 10.1242/jcs.01675. [DOI] [PubMed] [Google Scholar]
- 18.Kobori T, Smith GD, Sandford R, Edwardson JM. The transient receptor potential (TRP) channels TRPP2 and TRPC1 form a heterotetramer with a 2:2 stoichiometry and an alternating subunit arrangement. J Biol Chem. 2009;284:35507–35513. doi: 10.1074/jbc.M109.060228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 20.Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 21.Cavanaugh DJ, Lee H, Lo L, et al. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci USA. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang SJ, Oh JM, Valtschanoff JG. Expression of the vanilloid receptor TRPV1 in rat dorsal root ganglion neurons supports different roles of the receptor in visceral and cutaneous afferents. Brain Res. 2005;1047:261–266. doi: 10.1016/j.brainres.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi K, Fukuoka T, Obata K, et al. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 24.Tominaga M, Caterina MJ, Malmberg AB, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 25.Yu L, Yang F, Luo H, et al. The role of TRPV1 in different subtypes of dorsal root ganglion neurons in rat chronic inflammatory nociception induced by complete Freund’s adjuvant. Mol Pain. 2008;4:61. doi: 10.1186/1744-8069-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zacharova G, Palecek J. Parvalbumin and TRPV1 receptor expression in dorsal root ganglion neurons after acute peripheral inflammation. Physiol Res. 2009;58:305–309. doi: 10.33549/physiolres.931738. [DOI] [PubMed] [Google Scholar]
- 27.Brito R, Sheth S, Mukherjea D, Rybak LP, Ramkumar V. TRPV1: a potential drug target for treating various diseases. Cells. 2014;3:517–545. doi: 10.3390/cells3020517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeffry JA, Yu SQ, Sikand P, Parihar A, Evans MS, Premkumar LS. Selective targeting of TRPV1 expressing sensory nerve terminals in the spinal cord for long lasting analgesia. PLoS One. 2009;4:e7021. doi: 10.1371/journal.pone.0007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spicarova D, Palecek J. The role of the TRPV1 endogenous agonist N-Oleoyldopamine in modulation of nociceptive signaling at the spinal cord level. J Neurophysiol. 2009;102:234–243. doi: 10.1152/jn.00024.2009. [DOI] [PubMed] [Google Scholar]
- 30.Spicarova D, Palecek J. The role of spinal cord vanilloid (TRPV1) receptors in pain modulation. Physiol Res. 2008;57(Suppl 3):S69–S77. doi: 10.33549/physiolres.931601. [DOI] [PubMed] [Google Scholar]
- 31.Spicarova D, Nerandzic V, Palecek J. Update on the role of spinal cord TRPV1 receptors in pain modulation. Physiol Res. 2014;63(Suppl 1):S225–S236. doi: 10.33549/physiolres.932713. [DOI] [PubMed] [Google Scholar]
- 32.Cavanaugh DJ, Chesler AT, Braz JM, Shah NM, Julius D, Basbaum AI. Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J Neurosci. 2011;31:10119–10127. doi: 10.1523/JNEUROSCI.1299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA. TRPV1-lineage neurons are required for thermal sensation. EMBO J. 2011;30:582–593. doi: 10.1038/emboj.2010.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vodo S, Arcelli D, Fiorenzani P, et al. Gonadal ERalpha/beta, AR and TRPV1 gene expression: modulation by pain and morphine treatment in male and female rats. Physiol Behav. 2013;110–111:80–86. doi: 10.1016/j.physbeh.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Kedei N, Szabo T, Lile JD, et al. Analysis of the native quaternary structure of vanilloid receptor 1. J Biol Chem. 2001;276:28613–28619. doi: 10.1074/jbc.M103272200. [DOI] [PubMed] [Google Scholar]
- 36.Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron. 2007;54:905–918. doi: 10.1016/j.neuron.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 37.Moiseenkova-Bell VY, Stanciu LA, Serysheva II, Tobe BJ, Wensel TG. Structure of TRPV1 channel revealed by electron cryomicroscopy. Proc Natl Acad Sci USA. 2008;105:7451–7455. doi: 10.1073/pnas.0711835105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henderson R. Structural biology: ion channel seen by electron microscopy. Nature. 2013;504:93–94. doi: 10.1038/504093a. [DOI] [PubMed] [Google Scholar]
- 39.Cao E, Cordero-Morales Julio F, Liu B, Qin F, Julius D. TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron. 2013;77:667–679. doi: 10.1016/j.neuron.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jordt SE, Tominaga M, Julius D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci USA. 2000;97:8134–8139. doi: 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhaka A, Uzzell V, Dubin AE, et al. TRPV1 is activated by both acidic and basic pH. J Neurosci. 2009;29:153–158. doi: 10.1523/JNEUROSCI.4901-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang SM, Bisogno T, Trevisani M, et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci USA. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hwang SW, Cho H, Kwak J, et al. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zygmunt PM, Petersson J, Andersson DA, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 45.Nieto-Posadas A, Picazo-Juarez G, Llorente I, et al. Lysophosphatidic acid directly activates TRPV1 through a C-terminal binding site. Nat Chem Biol. 2012;8:78–85. doi: 10.1038/nchembio.712. [DOI] [PubMed] [Google Scholar]
- 46.Vriens J, Appendino G, Nilius B. Pharmacology of vanilloid transient receptor potential cation channels. Mol Pharmacol. 2009;75:1262–1279. doi: 10.1124/mol.109.055624. [DOI] [PubMed] [Google Scholar]
- 47.Kozai D, Ogawa N, Mori Y. Redox regulation of transient receptor potential channels. Antioxid Redox Signal. 2014;21:971–986. doi: 10.1089/ars.2013.5616. [DOI] [PubMed] [Google Scholar]
- 48.Szallasi A, Szabo T, Biro T, et al. Resiniferatoxin-type phorboid vanilloids display capsaicin-like selectivity at native vanilloid receptors on rat DRG neurons and at the cloned vanilloid receptor VR1. Br J Pharmacol. 1999;128:428–434. doi: 10.1038/sj.bjp.0702810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bohlen CJ, Priel A, Zhou S, King D, Siemens J, Julius D. A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain. Cell. 2010;141:834–845. doi: 10.1016/j.cell.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siemens J, Zhou S, Piskorowski R, et al. Spider toxins activate the capsaicin receptor to produce inflammatory pain. Nature. 2006;444:208–212. doi: 10.1038/nature05285. [DOI] [PubMed] [Google Scholar]
- 51.Trevisani M, Patacchini R, Nicoletti P, et al. Hydrogen sulfide causes vanilloid receptor 1-mediated neurogenic inflammation in the airways. Br J Pharmacol. 2005;145:1123–1131. doi: 10.1038/sj.bjp.0706277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gavva NR, Klionsky L, Qu Y, et al. Molecular determinants of vanilloid sensitivity in TRPV1. J Biol Chem. 2004;279:20283–20295. doi: 10.1074/jbc.M312577200. [DOI] [PubMed] [Google Scholar]
- 53.Jordt SE, Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- 54.Long SB, Campbell EB, Mackinnon R. Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science. 2005;309:903–908. doi: 10.1126/science.1116270. [DOI] [PubMed] [Google Scholar]
- 55.Pathak MM, Yarov-Yarovoy V, Agarwal G, et al. Closing in on the resting state of the Shaker K(+) channel. Neuron. 2007;56:124–140. doi: 10.1016/j.neuron.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 56.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- 57.Chung M-K, Guler AD, Caterina MJ. TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat Neurosci. 2008;11:555–564. doi: 10.1038/nn.2102. [DOI] [PubMed] [Google Scholar]
- 58.Mohapatra DP, Wang SY, Wang GK, Nau C. A tyrosine residue in TM6 of the vanilloid receptor TRPV1 involved in desensitization and calcium permeability of capsaicin-activated currents. Mol Cell Neurosci. 2003;23:314–324. doi: 10.1016/s1044-7431(03)00054-x. [DOI] [PubMed] [Google Scholar]
- 59.Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci. 1997;17:3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loo L, Shepherd AJ, Mickle AD, et al. The C-type natriuretic peptide induces thermal hyperalgesia through a noncanonical Gbetagamma-dependent modulation of TRPV1 channel. J Neurosci. 2012;32:11942–11955. doi: 10.1523/JNEUROSCI.1330-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Docherty RJ, Yeats JC, Bevan S, Boddeke HW. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflugers Arch. 1996;431:828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- 62.Mohapatra DP, Nau C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem. 2005;280:13424–13432. doi: 10.1074/jbc.M410917200. [DOI] [PubMed] [Google Scholar]
- 63.Numazaki M, Tominaga T, Takeuchi K, Murayama N, Toyooka H, Tominaga M. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc Natl Acad Sci USA. 2003;100:8002–8006. doi: 10.1073/pnas.1337252100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Melemedjian OK, Asiedu MN, Tillu DV, et al. IL-6- and NGF-induced rapid control of protein synthesis and nociceptive plasticity via convergent signaling to the eIF4F complex. J Neurosci. 2010;30:15113–15123. doi: 10.1523/JNEUROSCI.3947-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Melemedjian OK, Tillu DV, Moy JK, et al. Local translation and retrograde axonal transport of CREB regulates IL-6-induced nociceptive plasticity. Mol Pain. 2014;10:45. doi: 10.1186/1744-8069-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhave G, Hu H-J, Glauner KS, et al. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci USA. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau Iv RW. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- 68.Jin X, Morsy N, Winston J, Pasricha PJ, Garrett K, Akbarali HI. Modulation of TRPV1 by nonreceptor tyrosine kinase, c-Src kinase. Am J Physiol Cell Physiol. 2004;287:C558–C563. doi: 10.1152/ajpcell.00113.2004. [DOI] [PubMed] [Google Scholar]
- 69.Jung J, Shin JS, Lee SY, et al. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J Biol Chem. 2004;279:7048–7054. doi: 10.1074/jbc.M311448200. [DOI] [PubMed] [Google Scholar]
- 70.Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- 71.Pareek TK, Keller J, Kesavapany S, et al. Cyclin-dependent kinase 5 modulates nociceptive signaling through direct phosphorylation of transient receptor potential vanilloid 1. Proc Natl Acad Sci USA. 2007;104:660–665. doi: 10.1073/pnas.0609916104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol. 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mohapatra DP, Nau C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem. 2003;278:50080–50090. doi: 10.1074/jbc.M306619200. [DOI] [PubMed] [Google Scholar]
- 75.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 76.Zhang X, McNaughton PA. Why pain gets worse: the mechanism of heat hyperalgesia. J Gen Physiol. 2006;128:491–493. doi: 10.1085/jgp.200609676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chuang HH, Prescott ED, Kong H, et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 78.Lukacs V, Yudin Y, Hammond GR, Sharma E, Fukami K, Rohacs T. Distinctive changes in plasma membrane phosphoinositides underlie differential regulation of TRPV1 in nociceptive neurons. J Neurosci. 2013;33:11451–11463. doi: 10.1523/JNEUROSCI.5637-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rohacs T. Regulation of transient receptor potential channels by the phospholipase C pathway. Adv Biol Regul. 2013;53:341–355. doi: 10.1016/j.jbior.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tominaga M, Tominaga T. Structure and function of TRPV1. Pflugers Arch. 2005;451:143–150. doi: 10.1007/s00424-005-1457-8. [DOI] [PubMed] [Google Scholar]
- 81.Sanz-Salvador L, Andres-Borderia A, Ferrer-Montiel A, Planells-Cases R. Agonistand Ca2+-dependent desensitization of TRPV1 channel targets the receptor to lysosomes for degradation. J Biol Chem. 2012;287:19462–19471. doi: 10.1074/jbc.M111.289751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caterina MJ, Leffler A, Malmberg AB, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 83.Davis JB, Gray J, Gunthorpe MJ, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 84.Bourinet E, Altier C, Hildebrand ME, Trang T, Salter MW, Zamponi GW. Calcium-permeable ion channels in pain signaling. Physiol Rev. 2014;94:81–140. doi: 10.1152/physrev.00023.2013. [DOI] [PubMed] [Google Scholar]
- 85.Barton NJ, McQueen DS, Thomson D, et al. Attenuation of experimental arthritis in TRPV1R knockout mice. Exp Mol Pathol. 2006;81:166–170. doi: 10.1016/j.yexmp.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 86.Kelly S, Chapman RJ, Woodhams S, et al. Increased function of pronociceptive TRPV1 at the level of the joint in a rat model of osteoarthritis pain. Ann Rheum Dis. 2015;74:252–259. doi: 10.1136/annrheumdis-2013-203413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Valdes AM, De Wilde G, Doherty SA, et al. The Ile585Val TRPV1 variant is involved in risk of painful knee osteoarthritis. Ann Rheum Dis. 2011;70:1556–1561. doi: 10.1136/ard.2010.148122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ghilardi JR, Rohrich H, Lindsay TH, et al. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci. 2005;25:3126–3131. doi: 10.1523/JNEUROSCI.3815-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mantyh P. Bone cancer pain: causes, consequences, and therapeutic opportunities. Pain. 2013;154(Suppl 1):S54–S62. doi: 10.1016/j.pain.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 90.Akerman S, Kaube H, Goadsby PJ. Anandamide acts as a vasodilator of dural blood vessels in vivo by activating TRPV1 receptors. Br J Pharmacol. 2004;142:1354–1360. doi: 10.1038/sj.bjp.0705896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Geppetti P, Capone JG, Trevisani M, Nicoletti P, Zagli G, Tola MR. CGRP and migraine: neurogenic inflammation revisited. J Headache Pain. 2005;6:61–70. doi: 10.1007/s10194-005-0153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trevisani M, Gazzieri D, Benvenuti F, et al. Ethanol causes inflammation in the airways by a neurogenic and TRPV1-dependent mechanism. J Pharmacol Exp Ther. 2004;309:1167–1173. doi: 10.1124/jpet.103.064162. [DOI] [PubMed] [Google Scholar]
- 93.Trevisani M, Smart D, Gunthorpe MJ, et al. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci. 2002;5:546–551. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- 94.Kim HY, Chung G, Jo HJ, et al. Characterization of dental nociceptive neurons. J Dent Res. 2011;90:771–776. doi: 10.1177/0022034511399906. [DOI] [PubMed] [Google Scholar]
- 95.Park CK, Kim MS, Fang Z, et al. Functional expression of thermo-transient receptor potential channels in dental primary afferent neurons: implication for tooth pain. J Biol Chem. 2006;281:17304–17311. doi: 10.1074/jbc.M511072200. [DOI] [PubMed] [Google Scholar]
- 96.Chung MK, Lee J, Duraes G, Ro JY. Lipopolysaccharide-induced pulpitis up-regulates TRPV1 in trigeminal ganglia. J Dent Res. 2011;90:1103–1107. doi: 10.1177/0022034511413284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chung G, Oh SB. TRP channels in dental pain. Open Pain J. 2013;6:31–36. [Google Scholar]
- 98.Rong W, Hillsley K, Davis JB, Hicks G, Winchester WJ, Grundy D. Jejunal afferent nerve sensitivity in wild-type and TRPV1 knockout mice. J Physiol. 2004;560:867–881. doi: 10.1113/jphysiol.2004.071746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kimball ES, Wallace NH, Schneider CR, D’Andrea MR, Hornby PJ. Vanilloid receptor 1 antagonists attenuate disease severity in dextran sulphate sodium-induced colitis in mice. Neurogastroenterol Motil. 2004;16:811–818. doi: 10.1111/j.1365-2982.2004.00549.x. [DOI] [PubMed] [Google Scholar]
- 100.Chan CL, Facer P, Davis JB, et al. Sensory fibres expressing capsaicin receptor TRPV1 in patients with rectal hypersensitivity and faecal urgency. Lancet. 2003;361:385–391. doi: 10.1016/s0140-6736(03)12392-6. [DOI] [PubMed] [Google Scholar]
- 101.Wang ZY, Wang P, Merriam FV, Bjorling DE. Lack of TRPV1 inhibits cystitis-induced increased mechanical sensitivity in mice. Pain. 2008;139:158–167. doi: 10.1016/j.pain.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 102.Jones RC, 3rd, Otsuka E, Wagstrom E, Jensen CS, Price MP, Gebhart GF. Short-term sensitization of colon mechanoreceptors is associated with long-term hypersensitivity to colon distention in the mouse. Gastroenterology. 2007;133:184–194. doi: 10.1053/j.gastro.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 103.Jones RC, 3rd, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25:10981–10989. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Massa F, Sibaev A, Marsicano G, Blaudzun H, Storr M, Lutz B. Vanilloid receptor (TRPV1)-deficient mice show increased susceptibility to dinitrobenzene sulfonic acid induced colitis. J Mol Med. 2006;84:142–146. doi: 10.1007/s00109-005-0016-2. [DOI] [PubMed] [Google Scholar]
- 105.Storr M. TRPV1 in colitis: is it a good or a bad receptor? A viewpoint. Neurogastroenterol Motil. 2007;19:625–629. doi: 10.1111/j.1365-2982.2007.00946.x. [DOI] [PubMed] [Google Scholar]
- 106.Cruz F, Guimaraes M, Silva C, Reis M. Suppression of bladder hyperreflexia by intravesical resiniferatoxin. Lancet. 1997;350:640–641. doi: 10.1016/S0140-6736(05)63330-2. [DOI] [PubMed] [Google Scholar]
- 107.Dang K, Bielefeldt K, Gebhart GF. Differential responses of bladder lumbosacral and thoracolumbar dorsal root ganglion neurons to purinergic agonists, protons, and capsaicin. J Neurosci. 2005;25:3973–3984. doi: 10.1523/JNEUROSCI.5239-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Premkumar LS, Sikand P. TRPV1: a target for next generation analgesics. Curr Neuropharmacol. 2008;6:151–163. doi: 10.2174/157015908784533888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dinis P, Charrua A, Avelino A, Cruz F. Intravesical resiniferatoxin decreases spinal c-fos expression and increases bladder volume to reflex micturition in rats with chronic inflamed urinary bladders. BJU Int. 2004;94:153–157. doi: 10.1111/j.1464-4096.2004.04855.x. [DOI] [PubMed] [Google Scholar]
- 110.Gavva NR, Treanor JJ, Garami A, et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain. 2008;136:202–210. doi: 10.1016/j.pain.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 111.Liu MG, Zhuo M. No requirement of TRPV1 in long-term potentiation or long-term depression in the anterior cingulate cortex. Mol Pain. 2014;7:27. doi: 10.1186/1756-6606-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bhattacharya A, Scott BP, Nasser N, et al. Pharmacology and antitussive efficacy of 4-(3-trifluoromethyl-pyridin-2-yl)-piperazine-1-carboxylic acid (5-trifluoromethylpyridin-2-yl)-amide (JNJ17203212), a transient receptor potential vanilloid 1 antagonist in guinea pigs. J Pharmacol Exp Ther. 2007;323:665–674. doi: 10.1124/jpet.107.127258. [DOI] [PubMed] [Google Scholar]
- 113.Geppetti P, Materazzi S, Nicoletti P. The transient receptor potential vanilloid 1: role in airway inflammation and disease. Eur J Pharmacol. 2006;533:207–214. doi: 10.1016/j.ejphar.2005.12.063. [DOI] [PubMed] [Google Scholar]
- 114.Gunthorpe MJ, Szallasi A. Peripheral TRPV1 receptors as targets for drug development: new molecules and mechanisms. Curr Pharm Des. 2008;14:32–41. doi: 10.2174/138161208783330754. [DOI] [PubMed] [Google Scholar]
- 115.Jia Y, McLeod RL, Hey JA. TRPV1 receptor: a target for the treatment of pain, cough, airway disease and urinary incontinence. Drug News Perspect. 2005;18:165–171. doi: 10.1358/dnp.2005.18.3.892761. [DOI] [PubMed] [Google Scholar]
- 116.Amadesi S, Cottrell GS, Divino L, et al. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and mice. J Physiol. 2006;575:555–571. doi: 10.1113/jphysiol.2006.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gatti R, Andre E, Amadesi S, et al. Protease-activated receptor-2 activation exaggerates TRPV1-mediated cough in guinea pigs. J Appl Physiol. 2006;101:506–511. doi: 10.1152/japplphysiol.01558.2005. [DOI] [PubMed] [Google Scholar]
- 118.Razavi R, Chan Y, Afifiyan FN, et al. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006;127:1123–1135. doi: 10.1016/j.cell.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 119.McGaraughty S, Chu K, Perner R, DiDomenico S, Kort M, Kym P. TRPA1 modulation of spontaneous and mechanically evoked firing of spinal neurons in uninjured, osteoarthritic, and inflamed rats. Mol Pain. 2010;6:14. doi: 10.1186/1744-8069-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Reilly RM, McDonald HA, Puttfarcken PS, et al. Pharmacology of modality-specific transient receptor potential vanilloid-1 antagonists that do not alter body temperature. J Pharmacol Exp Ther. 2012;342:416–428. doi: 10.1124/jpet.111.190314. [DOI] [PubMed] [Google Scholar]
- 121.Brown DC, Iadarola MJ, Perkowski SZ, et al. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology. 2005;103:1052–1059. doi: 10.1097/00000542-200511000-00020. [DOI] [PubMed] [Google Scholar]
- 122.Iadarola MJ, Gonnella GL. Resiniferatoxin for pain treatment: an interventional approach to personalized pain medicine. Open Pain J. 2013;6:95–107. doi: 10.2174/1876386301306010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Payne CK, Mosbaugh PG, Forrest JB, et al. Intravesical resiniferatoxin for the treatment of interstitial cystitis: a randomized, double-blind, placebo controlled trial. J Urol. 2005;173:1590–1594. doi: 10.1097/01.ju.0000154631.92150.ef. [DOI] [PubMed] [Google Scholar]
- 124.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 125.Stokes AJ, Shimoda LM, Koblan-Huberson M, Adra CN, Turner H. A TRPV2-PKA signaling module for transduction of physical stimuli in mast cells. J Exp Med. 2004;200:137–147. doi: 10.1084/jem.20032082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Link TM, Park U, Vonakis BM, Raben DM, Soloski MJ, Caterina MJ. TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat Immunol. 2010;11:232–239. doi: 10.1038/ni.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Park U, Vastani N, Guan Y, Raja SN, Koltzenburg M, Caterina MJ. TRP vanilloid 2 knock-out mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. J Neurosci. 2011;31:11425–11436. doi: 10.1523/JNEUROSCI.1384-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Peier AM, Reeve AJ, Andersson DA, et al. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- 129.Xu H, Ramsey IS, Kotecha SA, et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- 130.Yang P, Zhu MX. Trpv3. Handb Exp Pharmacol. 2014;222:273–291. doi: 10.1007/978-3-642-54215-2_11. [DOI] [PubMed] [Google Scholar]
- 131.Ho TC, Horn NA, Huynh T, Kelava L, Lansman JB. Evidence TRPV4 contributes to mechanosensitive ion channels in mouse skeletal muscle fibers. Channels (Austin) 2012;6:246–254. doi: 10.4161/chan.20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nilius B, Voets T. The puzzle of TRPV4 channelopathies. EMBO Rep. 2013;14:152–163. doi: 10.1038/embor.2012.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ye L, Kleiner S, Wu J, et al. TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell. 2012;151:96–110. doi: 10.1016/j.cell.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 135.Du J, Ma X, Shen B, Huang Y, Birnbaumer L, Yao X. TRPV4, TRPC1, and TRPP2 assemble to form a flow-sensitive heteromeric channel. FASEB J. 2014;28:4677–4685. doi: 10.1096/fj.14-251652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alessandri-Haber N, Dina OA, Joseph EK, Reichling DB, Levine JD. Interaction of transient receptor potential vanilloid 4, integrin, and SRC tyrosine kinase in mechanical hyperalgesia. J Neurosci. 2008;28:1046–1057. doi: 10.1523/JNEUROSCI.4497-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Alessandri-Haber N, Yeh JJ, Boyd AE, et al. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. doi: 10.1016/s0896-6273(03)00462-8. [DOI] [PubMed] [Google Scholar]
- 138.Denadai-Souza A, Martin L, de Paula MA, et al. Role of transient receptor potential vanilloid 4 in rat joint inflammation. Arthritis Rheum. 2012;64:1848–1858. doi: 10.1002/art.34345. [DOI] [PubMed] [Google Scholar]
- 139.Wegierski T, Lewandrowski U, Muller B, Sickmann A, Walz G. Tyrosine phosphorylation modulates the activity of TRPV4 in response to defined stimuli. J Biol Chem. 2009;284:2923–2933. doi: 10.1074/jbc.M805357200. [DOI] [PubMed] [Google Scholar]
- 140.Alessandri-Haber N, Joseph E, Dina OA, Liedtke W, Levine JD. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain. 2005;118:70–79. doi: 10.1016/j.pain.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 141.Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. Proc Natl Acad Sci USA. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liedtke W, Tobin DM, Bargmann CI, Friedman JM. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2003;100(Suppl 2):14531–14536. doi: 10.1073/pnas.2235619100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- 144.Todaka H, Taniguchi J, Satoh J, Mizuno A, Suzuki M. Warm temperature-sensitive transient receptor potential vanilloid 4 (TRPV4) plays an essential role in thermal hyperalgesia. J Biol Chem. 2004;279:35133–35138. doi: 10.1074/jbc.M406260200. [DOI] [PubMed] [Google Scholar]
- 145.Ceppa E, Cattaruzza F, Lyo V, et al. Transient receptor potential ion channels V4 and A1 contribute to pancreatitis pain in mice. Am J Physiol Gastrointest Liver Physiol. 2010;299:G556–G571. doi: 10.1152/ajpgi.00433.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.D’Aldebert E, Cenac N, Rousset P, et al. Transient receptor potential vanilloid 4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology. 2011;140:275–285. doi: 10.1053/j.gastro.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 147.Vriens J, Owsianik G, Hofmann T, et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron. 2011;70:482–494. doi: 10.1016/j.neuron.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 148.Wagner TF, Loch S, Lambert S, et al. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat Cell Biol. 2008;10:1421–1430. doi: 10.1038/ncb1801. [DOI] [PubMed] [Google Scholar]
- 149.Reid G, Flonta ML. Physiology. Cold current in thermoreceptive neurons. Nature. 2001;413:480. doi: 10.1038/35097164. [DOI] [PubMed] [Google Scholar]
- 150.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 151.Peier AM, Moqrich A, Hergarden AC, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 152.Yudin Y, Rohacs T. Regulation of TRPM8 channel activity. Mol Cell Endocrinol. 2012;353:68–74. doi: 10.1016/j.mce.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dhaka A, Earley TJ, Watson J, Patapoutian A. Visualizing cold spots: TRPM8- expressing sensory neurons and their projections. J Neurosci. 2008;28:566–575. doi: 10.1523/JNEUROSCI.3976-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Harrington AM, Hughes PA, Martin CM, et al. A novel role for TRPM8 in visceral afferent function. Pain. 2011;152:1459–1468. doi: 10.1016/j.pain.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 155.Takashima Y, Daniels RL, Knowlton W, Teng J, Liman ER, McKemy DD. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. J Neurosci. 2007;27:14147–14157. doi: 10.1523/JNEUROSCI.4578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xing H, Ling JX, Chen M, et al. TRPM8 mechanism of autonomic nerve response to cold in respiratory airway. Mol Pain. 2008;4:22. doi: 10.1186/1744-8069-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Abe J, Hosokawa H, Okazawa M, et al. TRPM8 protein localization in trigeminal ganglion and taste papillae. Brain Res Mol Brain Res. 2005;136:91–98. doi: 10.1016/j.molbrainres.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 158.Morenilla-Palao C, Pertusa M, Meseguer V, Cabedo H, Viana F. Lipid raft segregation modulates TRPM8 channel activity. J Biol Chem. 2009;284:9215–9224. doi: 10.1074/jbc.M807228200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bandell M, Dubin AE, Petrus MJ, et al. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat Neurosci. 2006;9:493–500. doi: 10.1038/nn1665. [DOI] [PubMed] [Google Scholar]
- 160.Liu B, Qin F. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:1674–1681. doi: 10.1523/JNEUROSCI.3632-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- 162.Zakharian E, Cao C, Rohacs T. Gating of transient receptor potential melastatin 8 - (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers. J Neurosci. 2010;30:12526–12534. doi: 10.1523/JNEUROSCI.3189-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tani M, Onimaru H, Ikeda K, Kawakami K, Homma I. Menthol inhibits the respiratory rhythm in brainstem preparations of the newborn rats. Neuroreport. 2010;21:1095–1099. doi: 10.1097/WNR.0b013e3283405bad. [DOI] [PubMed] [Google Scholar]
- 164.Karashima Y, Damann N, Prenen J, et al. Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci. 2007;27:9874–9884. doi: 10.1523/JNEUROSCI.2221-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xiao B, Dubin AE, Bursulaya B, Viswanath V, Jegla TJ, Patapoutian A. Identification of transmembrane domain 5 as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels. J Neurosci. 2008;28:9640–9651. doi: 10.1523/JNEUROSCI.2772-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. More than cool: promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci. 2006;32:335–343. doi: 10.1016/j.mcn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 167.Gaudioso C, Hao J, Martin-Eauclaire MF, Gabriac M, Delmas P. Menthol pain relief through cumulative inactivation of voltage-gated sodium channels. Pain. 2012;153:473–484. doi: 10.1016/j.pain.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 168.Swandulla D, Carbone E, Schafer K, Lux HD. Effect of menthol on two types of Ca currents in cultured sensory neurons of vertebrates. Pflugers Arch. 1987;409:52–59. doi: 10.1007/BF00584749. [DOI] [PubMed] [Google Scholar]
- 169.Abe J, Hosokawa H, Sawada Y, Matsumura K, Kobayashi S. Ca2+-dependent PKC activation mediates menthol-induced desensitization of transient receptor potential M8. Neurosci Lett. 2006;397:140–144. doi: 10.1016/j.neulet.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 170.Daniels RL, Takashima Y, McKemy DD. Activity of the neuronal cold sensor TRPM8 is regulated by phospholipase C via the phospholipid phosphoinositol 4,5-bisphosphate. J Biol Chem. 2009;284:1570–1582. doi: 10.1074/jbc.M807270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Premkumar LS, Raisinghani M, Pingle SC, Long C, Pimentel F. Downregulation of transient receptor potential melastatin 8 by protein kinase C-mediated dephosphorylation. J Neurosci. 2005;25:11322–11329. doi: 10.1523/JNEUROSCI.3006-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yudin Y, Lukacs V, Cao C, Rohacs T. Decrease in phosphatidylinositol 4,5-bisphosphate levels mediates desensitization of the cold sensor TRPM8 channels. J Physiol. 2011;589:6007–6027. doi: 10.1113/jphysiol.2011.220228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bavencoffe A, Gkika D, Kondratskyi A, et al. The transient receptor potential channel TRPM8 is inhibited via the alpha 2A adrenoreceptor signaling pathway. J Biol Chem. 2010;285:9410–9419. doi: 10.1074/jbc.M109.069377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.De Petrocellis L, Starowicz K, Moriello AS, Vivese M, Orlando P, Di Marzo V. Regulation of transient receptor potential channels of melastatin type 8 (TRPM8): effect of cAMP, cannabinoid CB(1) receptors and endovanilloids. Exp Cell Res. 2007;313:1911–1920. doi: 10.1016/j.yexcr.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 175.Linte RM, Ciobanu C, Reid G, Babes A. Desensitization of cold- and menthol-sensitive rat dorsal root ganglion neurones by inflammatory mediators. Exp Brain Res. 2007;178:89–98. doi: 10.1007/s00221-006-0712-3. [DOI] [PubMed] [Google Scholar]
- 176.Sarria I, Gu J. Menthol response and adaptation in nociceptive-like and nonnociceptive-like neurons: role of protein kinases. Mol Pain. 2010;6:47. doi: 10.1186/1744-8069-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sarria I, Ling J, Zhu MX, Gu JG. TRPM8 acute desensitization is mediated by calmodulin and requires PIP(2): distinction from tachyphylaxis. J Neurophysiol. 2011;106:3056–3066. doi: 10.1152/jn.00544.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.De Petrocellis L, Vellani V, Schiano-Moriello A, et al. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J Pharmacol Exp Ther. 2008;325:1007–1015. doi: 10.1124/jpet.107.134809. [DOI] [PubMed] [Google Scholar]
- 179.Andersson DA, Nash M, Bevan S. Modulation of the cold-activated channel TRPM8 by lysophospholipids and polyunsaturated fatty acids. J Neurosci. 2007;27:3347–3355. doi: 10.1523/JNEUROSCI.4846-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vanden Abeele F, Zholos A, Bidaux G, et al. Ca2+-independent phospholipase A2-dependent gating of TRPM8 by lysophospholipids. J Biol Chem. 2006;281:40174–40182. doi: 10.1074/jbc.M605779200. [DOI] [PubMed] [Google Scholar]
- 181.Fernández-Peña C, Viana F. Targeting TRPM8 for pain relief. Open Pain J. 2013;6:154–164. [Google Scholar]
- 182.van Zanten SV. Review: fibre, antispasmodics, and peppermint oil are all effective for irritable bowel syndrome. Evid Based Med. 2009;14:84. doi: 10.1136/ebm.14.3.84. [DOI] [PubMed] [Google Scholar]
- 183.El Karim IA, Linden GJ, Curtis TM, et al. Human odontoblasts express functional thermo-sensitive TRP channels: implications for dentin sensitivity. Pain. 2011;152:2211–2223. doi: 10.1016/j.pain.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 184.Chasman DI, Schurks M, Anttila V, et al. Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nat Genet. 2011;43:695–698. doi: 10.1038/ng.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bautista DM, Siemens J, Glazer JM, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 186.Colburn RW, Lubin ML, Stone DJ, Jr, et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 187.Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 188.Noel J, Zimmermann K, Busserolles J, et al. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 2009;28:1308–1318. doi: 10.1038/emboj.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Story GM, Peier AM, Reeve AJ, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 190.Gavva NR, Davis C, Lehto SG, Rao S, Wang W, Zhu DX. Transient receptor potential melastatin 8 (TRPM8) channels are involved in body temperature regulation. Mol Pain. 2012;8:36. doi: 10.1186/1744-8069-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lashinger ES, Steiginga MS, Hieble JP, et al. AMTB, a TRPM8 channel blocker: evidence in rats for activity in overactive bladder and painful bladder syndrome. Am J Physiol Renal Physiol. 2008;295:F803–F810. doi: 10.1152/ajprenal.90269.2008. [DOI] [PubMed] [Google Scholar]
- 192.McKemy DD. How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol Pain. 2005;1:16. doi: 10.1186/1744-8069-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jaquemar D, Schenker T, Trueb B. An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J Biol Chem. 1999;274:7325–7333. doi: 10.1074/jbc.274.11.7325. [DOI] [PubMed] [Google Scholar]
- 194.Barabas ME, Kossyreva EA, Stucky CL. TRPA1 is functionally expressed primarily by IB4-binding, non-peptidergic mouse and rat sensory neurons. PLoS One. 2012;7:e47988. doi: 10.1371/journal.pone.0047988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Engel MA, Leffler A, Niedermirtl F, et al. TRPA1 and substance P mediate colitis in mice. Gastroenterology. 2011;141:1346–1358. doi: 10.1053/j.gastro.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 196.DeBerry JJ, Schwartz ES, Davis BM. TRPA1 mediates bladder hyperalgesia in a mouse model of cystitis. Pain. 2014;155:1280–1287. doi: 10.1016/j.pain.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Atoyan R, Shander D, Botchkareva NV. Non-neuronal expression of transient receptor potential type A1 (TRPA1) in human skin. J Invest Dermatol. 2009;129:2312–2315. doi: 10.1038/jid.2009.58. [DOI] [PubMed] [Google Scholar]
- 198.Kim YS, Son JY, Kim TH, et al. Expression of transient receptor potential ankyrin 1 (TRPA1) in the rat trigeminal sensory afferents and spinal dorsal horn. J Comp Neurol. 2010;518:687–698. doi: 10.1002/cne.22238. [DOI] [PubMed] [Google Scholar]
- 199.Uta D, Furue H, Pickering AE, et al. TRPA1-expressing primary afferents synapse with a morphologically identified subclass of substantia gelatinosa neurons in the adult rat spinal cord. Eur J Neurosci. 2010;31:1960–1973. doi: 10.1111/j.1460-9568.2010.07255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Fernandes ES, Fernandes MA, Keeble JE. The functions of TRPA1 and TRPV1: moving away from sensory nerves. Br J Pharmacol. 2012;166:510–521. doi: 10.1111/j.1476-5381.2012.01851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shigetomi E, Tong X, Kwan KY, et al. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci. 2011;15:70–80. doi: 10.1038/nn.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pozsgai G, Bodkin JV, Graepel R, et al. Evidence for the pathophysiological relevance of TRPA1 receptors in the cardiovascular system in vivo. Cardiovasc Res. 2010;87:760–768. doi: 10.1093/cvr/cvq118. [DOI] [PubMed] [Google Scholar]
- 203.Cao D-S, Zhong L, Hsieh T-h, et al. Expression of transient receptor potential ankyrin 1 (TRPA1) and its role in insulin release from rat pancreatic beta cells. PLoS One. 2012;7:e38005. doi: 10.1371/journal.pone.0038005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Corey DP, Garcia-Anoveros J, Holt JR, et al. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature. 2004;432:723–730. doi: 10.1038/nature03066. [DOI] [PubMed] [Google Scholar]
- 205.Haas ET, Rowland K, Gautam M. Tooth injury increases expression of the cold sensitive TRP channel TRPA1 in trigeminal neurons. Arch Oral Biol. 2011;56:1604–1609. doi: 10.1016/j.archoralbio.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 206.Nassini R, Pedretti P, Moretto N, et al. Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PLoS One. 2012;7:e42454. doi: 10.1371/journal.pone.0042454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gaudet R. A primer on ankyrin repeat function in TRP channels and beyond. Mol BioSyst. 2008;4:372–379. doi: 10.1039/b801481g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Li J, Mahajan A, Tsai M-D. Ankyrin repeat: a unique motif mediating protein–protein interactions. Biochemistry. 2006;45:15168–15178. doi: 10.1021/bi062188q. [DOI] [PubMed] [Google Scholar]
- 209.Nilius B, Prenen J, Owsianik G. Irritating channels: the case of TRPA1. J Physiol. 2011;589:1543–1549. doi: 10.1113/jphysiol.2010.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang L, Cvetkov TL, Chance MR, Moiseenkova-Bell VY. Identification of in vivo disulfide conformation of TRPA1 ion channel. J Biol Chem. 2012;287:6169–6176. doi: 10.1074/jbc.M111.329748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cvetkov TL, Huynh KW, Cohen MR, Moiseenkova-Bell VY. Molecular architecture and subunit organization of TRPA1 ion channel revealed by electron microscopy. J Biol Chem. 2011;286:38168–38176. doi: 10.1074/jbc.M111.288993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Macpherson LJ, Dubin AE, Evans MJ, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 213.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nat Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]
- 214.Karashima Y, Prenen J, Talavera K, Janssens A, Voets T, Nilius B. Agonist-induced changes in Ca2+ permeation through the nociceptor cation channel TRPA1. Biophys J. 2010;98:773–783. doi: 10.1016/j.bpj.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Takahashi N, Mizuno Y, Kozai D, et al. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels. 2008;2:287–298. doi: 10.4161/chan.2.4.6745. [DOI] [PubMed] [Google Scholar]
- 216.Pozsgai G, Hajna Z, Bagoly T, et al. The role of transient receptor potential ankyrin 1 (TRPA1) receptor activation in hydrogen-sulphide-induced CGRP-release and vasodilation. Eur J Pharmacol. 2012;689:56–64. doi: 10.1016/j.ejphar.2012.05.053. [DOI] [PubMed] [Google Scholar]
- 217.Trevisani M, Siemens J, Materazzi S, et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.de la Roche J, Eberhardt MJ, Klinger AB, et al. The molecular basis for species-specific activation of human TRPA1 protein by protons involves poorly conserved residues within transmembrane domains 5 and 6. J Biol Chem. 2013;288:20280–20292. doi: 10.1074/jbc.M113.479337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang YY, Chang RB, Allgood SD, Silver WL, Liman ER. A TRPA1-dependent mechanism for the pungent sensation of weak acids. J Gen Physiol. 2011;137:493–505. doi: 10.1085/jgp.201110615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wang YY, Chang RB, Liman ER. TRPA1 is a component of the nociceptive response to CO2. J Neurosci. 2010;30:12958–12963. doi: 10.1523/JNEUROSCI.2715-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.McNamara CR, Mandel-Brehm J, Bautista DM, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bautista DM, Jordt S-E, Nikai T, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 223.Chen J, Kang D, Xu J, et al. Species differences and molecular determinant of TRPA1 cold sensitivity. Nat Commun. 2013;4:2501. doi: 10.1038/ncomms3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Leffler A, Lattrell A, Kronewald S, Niedermirtl F, Nau C. Activation of TRPA1 by membrane permeable local anesthetics. Mol Pain. 2011;7:62. doi: 10.1186/1744-8069-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Fischer MJM, Leffler A, Niedermirtl F, et al. The general anesthetic propofol excites nociceptors by activating TRPV1 and TRPA1 rather than GABAA receptors. J Biol Chem. 2010;285:34781–34792. doi: 10.1074/jbc.M110.143958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Matta JA, Cornett PM, Miyares RL, Abe K, Sahibzada N, Ahern GP. General anesthetics activate a nociceptive ion channel to enhance pain and inflammation. Proc Natl Acad Sci USA. 2008;105:8784–8789. doi: 10.1073/pnas.0711038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Piao L-H, Fujita T, Jiang C-Y, et al. TRPA1 activation by lidocaine in nerve terminals results in glutamate release increase. Biochem Biophys Res Commun. 2009;379:980–984. doi: 10.1016/j.bbrc.2008.12.183. [DOI] [PubMed] [Google Scholar]
- 228.Komatsu T, Uchida K, Fujita F, Zhou Y, Tominaga M. Primary alcohols activate human TRPA1 channel in a carbon chain length-dependent manner. Pflugers Arch. 2012;463:549–559. doi: 10.1007/s00424-011-1069-4. [DOI] [PubMed] [Google Scholar]
- 229.Talavera K, Gees M, Karashima Y, et al. Nicotine activates the chemosensory cation channel TRPA1. Nat Neurosci. 2009;12:1293–1299. doi: 10.1038/nn.2379. [DOI] [PubMed] [Google Scholar]
- 230.Motter AL, Ahern GP. TRPA1 is a polyunsaturated fatty acid sensor in mammals. PLoS One. 2012;7:e38439. doi: 10.1371/journal.pone.0038439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hu H, Tian J, Zhu Y, et al. Activation of TRPA1 channels by fenamate nonsteroidal anti-inflammatory drugs. Pflugers Arch. 2010;459:579–592. doi: 10.1007/s00424-009-0749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bandell M, Story GM, Hwang SW, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 233.Dunham J, Leith J, Lumb B, LF D. Transient receptor potential channel A1 and noxious cold responses in rat cutaneous nociceptors. Neuroscience. 2010;165:1412–1419. doi: 10.1016/j.neuroscience.2009.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Karashima Y, Talavera K, Everaerts W, et al. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci USA. 2009;106:1273–1278. doi: 10.1073/pnas.0808487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jabba S, Goyal R, Sosa-Pagán Jason O, et al. Directionality of temperature activation in mouse TRPA1 ion channel can be inverted by single-point mutations in ankyrin repeat six. Neuron. 2014;82:1017–1031. doi: 10.1016/j.neuron.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Brierley SM, Castro J, Harrington AM, et al. TRPA1 contributes to specific mechanically activated currents and sensory neuron mechanical hypersensitivity. J Physiol. 2011;589:3575–3593. doi: 10.1113/jphysiol.2011.206789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Brierley SM, Hughes PA, Page AJ, et al. The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology. 2009;137:2084–2095. e2083. doi: 10.1053/j.gastro.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wang YY, Chang RB, Waters HN, McKemy DD, Liman ER. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem. 2008;283:32691–32703. doi: 10.1074/jbc.M803568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kim D, Cavanaugh EJ, Simkin D. Inhibition of transient receptor potential A1 channel by phosphatidylinositol-4,5-bisphosphate. Am J Physiol Cell Physiol. 2008;295:C92–C99. doi: 10.1152/ajpcell.00023.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Salas MM, Hargreaves KM, Akopian AN. TRPA1-mediated responses in trigeminal sensory neurons: interaction between TRPA1 and TRPV1. Eur J Neurosci. 2009;29:1568–1578. doi: 10.1111/j.1460-9568.2009.06702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sisignano M, Park C-K, Angioni C, et al. 5,6-EET is released upon neuronal activity and induces mechanical pain hypersensitivity via TRPA1 on central afferent terminals. J Neurosci. 2012;32:6364–6372. doi: 10.1523/JNEUROSCI.5793-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gregus AM, Doolen S, Dumlao DS, et al. Spinal 12-lipoxygenase-derived hepoxilin A3 contributes to inflammatory hyperalgesia via activation of TRPV1 and TRPA1 receptors. Proc Natl Acad Sci USA. 2012;109:6721–6726. doi: 10.1073/pnas.1110460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Elitt CM, McIlwrath SL, Lawson JJ, et al. Artemin overexpression in skin enhances expression of TRPV1 and TRPA1 in cutaneous sensory neurons and leads to behavioral sensitivity to heat and cold. J Neurosci. 2006;26:8578–8587. doi: 10.1523/JNEUROSCI.2185-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kwan KY, Allchorne AJ, Vollrath MA, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 245.Koltzenburg M, Lundberg LER, Torebjörk HE. Dynamic and static components of mechanical hyperalgesia in human hairy skin. Pain. 1992;51:207–219. doi: 10.1016/0304-3959(92)90262-A. [DOI] [PubMed] [Google Scholar]
- 246.Bianchi BR, Zhang X-F, Reilly RM, Kym PR, Yao BB, Chen J. Species comparison and pharmacological characterization of human, monkey, rat, and mouse TRPA1 channels. J Pharmacol Exp Ther. 2012;341:360–368. doi: 10.1124/jpet.111.189902. [DOI] [PubMed] [Google Scholar]
- 247.Liu B, Escalera J, Balakrishna S, et al. TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J. 2013;27:3549–3563. doi: 10.1096/fj.13-229948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Nakamura Y, Une Y, Miyano K, et al. Activation of transient receptor potential ankyrin 1 evokes nociception through substance P release from primary sensory neurons. J Neurochem. 2012;120:1036–1047. doi: 10.1111/j.1471-4159.2011.07628.x. [DOI] [PubMed] [Google Scholar]
- 249.Than JY-XL, Li L, Hasan R, Zhang X. Excitation and modulation of TRPA1, TRPV1, and TRPM8 channel-expressing sensory neurons by the pruritogen chloroquine. J Biol Chem. 2013;288:12818–12827. doi: 10.1074/jbc.M113.450072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wilson SR, Nelson AM, Batia L, et al. The ion channel TRPA1 is required for chronic itch. J Neurosci. 2013;33:9283–9294. doi: 10.1523/JNEUROSCI.5318-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kremeyer B, Lopera F, Cox JJ, et al. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron. 2010;66:671–680. doi: 10.1016/j.neuron.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Bell JT, Loomis AK, Butcher LM, et al. Differential methylation of the TRPA1 promoter in pain sensitivity. Nat Commun. 2014;5:2978. doi: 10.1038/ncomms3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zhou Y, Suzuki Y, Uchida K, Tominaga M. Identification of a splice variant of mouse TRPA1 that regulates TRPA1 activity. Nat Commun. 2013;4:2399. doi: 10.1038/ncomms3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kim S, Hwang S. Emerging roles of TRPA1 in sensation of oxidative stress and its implications in defense and danger. Arch Pharm Res. 2013;36:783–791. doi: 10.1007/s12272-013-0098-2. [DOI] [PubMed] [Google Scholar]
- 255.Barrière DA, Rieusset J, Chanteranne D, et al. Paclitaxel therapy potentiates cold hyperalgesia in streptozotocin-induced diabetic rats through enhanced mitochondrial reactive oxygen species production and TRPA1 sensitization. Pain. 2012;153:553–561. doi: 10.1016/j.pain.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 256.Nassini R, Gees M, Harrison S, et al. Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain. 2011;152:1621–1631. doi: 10.1016/j.pain.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 257.Old EA, Nadkarni S, Grist J, et al. Monocytes expressing CX3CR1 orchestrate the development of vincristine-induced pain. J Clin Invest. 2014;124:2023–2036. doi: 10.1172/JCI71389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Benemei S, De Cesaris F, Fusi C, Rossi E, Lupi C, Geppetti P. TRPA1 and other TRP channels in migraine. J Headache Pain. 2013;14:71. doi: 10.1186/1129-2377-14-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Edelmayer RM, Le LN, Yan J, et al. Activation of TRPA1 on dural afferents: a potential mechanism of headache pain. Pain. 2012;153:1949–1958. doi: 10.1016/j.pain.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Fernandes ES, Russell FA, Spina D, et al. A distinct role for transient receptor potential ankyrin 1, in addition to transient receptor potential vanilloid 1, in tumor necrosis factor α-induced inflammatory hyperalgesia and Freund’s complete adjuvant-induced monarthritis. Arthritis Rheum. 2011;63:819–829. doi: 10.1002/art.30150. [DOI] [PubMed] [Google Scholar]
- 261.Yang J, Li Y, Zuo X, Zhen Y, Yu Y, Gao L. Transient receptor potential ankyrin-1 participates in visceral hyperalgesia following experimental colitis. Neurosci Lett. 2008;440:237–241. doi: 10.1016/j.neulet.2008.05.093. [DOI] [PubMed] [Google Scholar]
- 262.Terada Y, Fujimura M, Nishimura S, et al. Contribution of TRPA1 as a downstream signal of proteinase-activated receptor-2 to pancreatic pain. J Pharmacol Sci. 2013;123:284–287. doi: 10.1254/jphs.13128sc. [DOI] [PubMed] [Google Scholar]
- 263.Andersson DA, Gentry C, Alenmyr L, et al. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Δ9-tetrahydrocannabiorcol. Nat Commun. 2011;2:551. doi: 10.1038/ncomms1559. [DOI] [PubMed] [Google Scholar]
- 264.Meseguer V, Alpizar YA, Luis E, et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat Commun. 2014;5:3125. doi: 10.1038/ncomms4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt S-E. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008;118:1899–1910. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Eid S, Crown E, Moore E, et al. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol Pain. 2008;4:48. doi: 10.1186/1744-8069-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Chen J, Joshi SK, DiDomenico S, et al. Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain. 2011;152:1165–1172. doi: 10.1016/j.pain.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 268.Petrus M, Peier A, Bandell M, et al. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain. 2007;3:40. doi: 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Pertovaara A, Koivisto A. TRPA1 ion channel in the spinal dorsal horn as a therapeutic target in central pain hypersensitivity and cutaneous neurogenic inflammation. Eur J Pharmacol. 2011;666:1–4. doi: 10.1016/j.ejphar.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 270.Mukhopadhyay I, Kulkarni A, Aranake S, et al. Transient receptor potential ankyrin 1 receptor activation in vitro and in vivo by pro-tussive agents: GRC 17536 as a promising anti-tussive therapeutic. PLoS One. 2014;9:e97005. doi: 10.1371/journal.pone.0097005. [DOI] [PMC free article] [PubMed] [Google Scholar]