Abstract
Obesity is a major global epidemic that sets the stage for diverse multiple pathologies, including cardiovascular disease. The obesity-related low grade chronic inflamed milieu is more pronounced in aging and responsive to cardiac dysfunction in heart failure pathology. Metabolic dysregulation of obesity integrates with immune reservoir in spleen and kidney network. Therefore, an integrative systems biology approach is necessary to delay progressive cardiac alternations. The purpose of this comprehensive review is to largely discuss the impact of obesity on the cardiovascular pathobiology in the context of problems and challenges, with major emphasis on the diversified models, and to study cardiac remodeling in obesity. The information in this article is immensely helpful in teaching advanced undergraduate, graduate, and medical students about the advancement and impact of obesity on cardiovascular health.
Keywords: Cardiac remodeling, obesity, fats, inflammation
Introduction
In recent years, it is evident that a sedentary lifestyle an unhealthy diet enriched with processed and preserved foods, and lack of sleep and exercise have led to an obesity epidemic. The individuals who possess a body mass index (BMI) of 30 or greater are considered obese and overweight. The trend for the obesity population in the United States is tracked by national survey data, which includes the measured heights and weights of participants to calculate BMI. Data from the National Health and Nutrition Examination Survey revealed that the prevalence of obesity among adults in the United States is > 35.5% for men and women(46). It is widely accepted that obesity increases the risk of heart disease, although there is evidence that some obese patients have survival benefits once diagnosed with cardiovascular disease, which begs the question of whether there is a “healthy” obesity. A recent meta-analysis on this subject appears to have demystified this idea. It identified 61,836 individuals from 8 different studies to investigate the associations of BMI and metabolic status with total mortality and cardiovascular events (61). The study highlights that metabolically healthy non-obese individuals have lower risk of total mortality and cardiovascular events than the metabolically healthy obese group. This increased risk in obese but metabolically healthy people was only observed in studies with 10 or more years of follow-up, suggesting a possible explanation for the façade of "healthy" obesity (35, 92). Primarily, obesity is marked by chronic low-grade inflammation that is responsible for the genesis of many diseases, including insulin resistance, type 2 diabetes, metabolic syndrome, atherosclerosis and hyperlipidemia (68) (70). The cluster of these metabolic abnormalities makes individuals prone to the incidence of myocardial infarction (MI) events and subsequent heart failure pathology.
Nutrition, inflammation and cardiovascular disease
The fatty acid composition of leukocytes, other immune cells, and non-immune cells changed according to the intake of fatty acid(s) in the diet. The essential and conditional fatty acids play an immunomodulatory role, which is useful for the management of chronic inflammation processes, such as autoimmune diseases (lupus) (32). Traditionally, specific immune cell-derived cytokines or percentage population of a specific cell population is prominent markers of inflammation in obesity and cardiovascular disease. Undernutrition or over-nutrition is the driving force that alters the immune system network in heart failure pathology, particularly in aging (52). At this point, due to obesity-related changes in microenvironment, it is unclear whether these cytokines are causes or end stage consequences of the disease process (Figure 1). It was well-established in the 18–19th century that acute inflammation is host-protective, self-healing, and characterized by five cardinal signs: rubor (redness), calor (increased heat), tumor (swelling), dolor (pain), and functio laesa (loss of function) (96). The first four were described by Celsus in the eighteenth century, and the fifth was added by Virchow in the nineteenth century (115). Edema or swollen limbs are the main signs of chronic inflammation in advanced heart failure patients and are often accompanied by fatigue and pulmonary congestion (94). Of note, the edematous milieu in response to acute inflammatory response is essential, but the triggers that develop chronic heart failure setting are unknown.
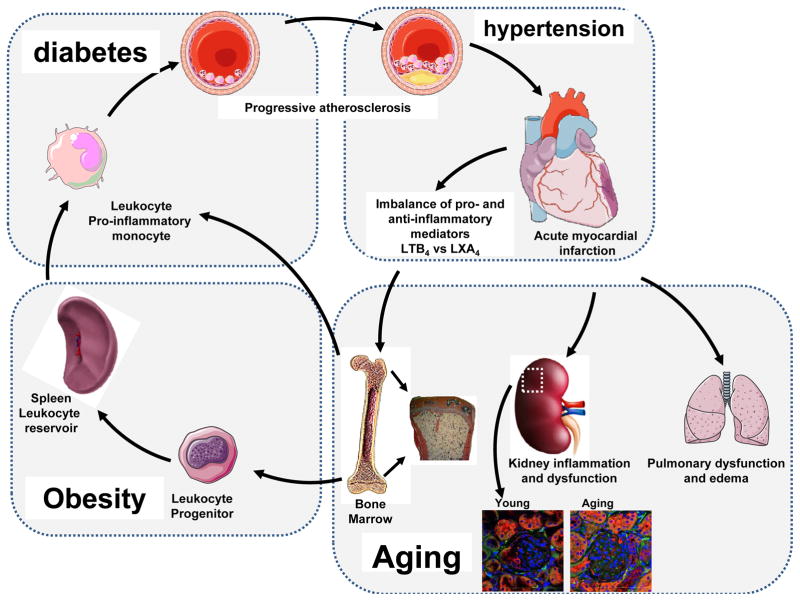
Figure 1.
Obesity and related metabolic dysregulation set the platform for a vicious pro-inflammatory environment for post-MI pathology with marked dysregulation of the cardiosplenic and cardiorenal network in heart failure pathology. Bone marrow adiposity, particularly in obese, diabetes and aging populations, supplies pro-inflammatory monocytes and enhances the atherosclerotic conditions that simulate an MI event, as well as subsequent renal and pulmonary edema and chronic inflammation heart failure pathology. Increased macrophage (DAPI/F480/CD169-red) density in aging mice kidney (18 months) compared with young mice (2 months old) shows chronic glomeruli inflammation.
Failure and success of pharmacological versus nutritional approaches
Cyclooxygenase (COX)-2 is a lipid/fatty acid metabolizing enzyme also known as prostaglandin-endoperoxide synthase, and its inhibition is attempted to develop safe non-steroidal inflammatory drugs. However, use of COX-2 inhibition is associated with a series of cardiovascular adverse effects, including the development of stroke, atrial fibrillation, and MI events (64) (95). COX-2 uses arachidonic acid as the fatty acid substrate and is responsible for the generation of prostaglandins, prostanoids, prostacyclins, and thromboxane. Failure of COX-2 inhibitors (e.g. rofecoxib and valdecoxib) after FDA approval indicates the complexity of nutrition and lipid metabolism machinery in cardiovascular disease pathology. Likewise, single and specific cytokine hypothesis resulted in disappointing results in acute and chronic heart failure trials (36). Pre-clinical studies showed that treatment or overexpression of TNF-α leads to aggravation of HF, and inhibition of TNF-α recovers left ventricle function in heart failure pathology (18, 37). However, the overall results in clinical trials were harmful to heart failure patients. A comprehensive summary of RENWAL, RENIASSANCE and RECOVER trials suggest that use of TNF-α inhibitors is a deceptive strategy to control inflammation in chronic heart failure patients. Thus, it is necessary to delineate the physiological, adaptive and pathological role of TNF-α in response to MI. In relation to the failure of pharmacological strategies, it is also important to consider the nutritional and dietary approach to prevent and treat cardiovascular diseases such as heart failure. In particular, omega-3 fatty acids intake is cardioprotective, though the mechanism of action is unclear. The number of reports from preclinical and clinical studies, as well as the epidemiological studies, suggested that the supplementation of dietary omega-3 fatty acids could be beneficial for heart failure patients (87). The double blind and randomized GISSI-HF trial showed that long-term supplementation of fish oil (1g) to heart failure patients reduced endpoints of all-causes mortality and readmission for cardiovascular complications (108). The Recent PREDIMED study supports the GISSI-HF outcome that reveals prior cardiovascular disease, relatively higher fish intake, and dietary alpha linoleic acid (one of the omega-3 fatty acid) sourced from walnuts and olive oil limit all-causes mortality, but the protection from cardiac death comes only from fish-derived long-chain omega-3 fatty acids (101). Nineteen countries comprehensive studies of free-living populations suggest that concentrations of seafood and plant-derived ω-3 fatty acids biomarkers are associated with a modestly lower incidence of fatal CHD (33). With examples of omega-3 fatty acids in the prevention of cardiovascular disease, dietary approaches outweigh pharmacological approaches in effectiveness.
Distinct challenges in cardiovascular world
In addition to the advancement of technology, mortality-related cardiovascular disease is a prominent challenge to developed and developing nations. Sedentary lifestyle and prevalence of processed food intake alter the body’s metabolic parameters, including increased number of acute coronary syndrome events (4) (90). In response to acute coronary syndrome or MI, the left ventricle experiences dilative changes in size, shape, and function. Though post-MI, overall patient’s survival increased the total mortality also increased within five years due to heart failure progression and pathological remodeling. Traditionally, the number of drugs and dietary intervention are tested with primary focus on inflammation relevant to prevention strategy, but with limited focus on integrative approach. For simplification, cardiovascular disease challenges are divided into the following four categories (94).
MI-prone subjects – obesity, diabetes, hypertension and aging
Post-MI morbid patients – 2–17% die within year (non-resolving patients)
Less morbid patients but prone to death - >45% patients die with-in five years post- MI
Advanced chronic heart failure patients alive after five years but with limited mobility and high discomfort
Integrative approach for heart failure management
Aging is an inevitable and inherent factor, and coupled with obesity, diabetes, hypertension and other metabolic disorders, it leads to the progressive development of cardiac dysfunction and failure (80, 112). Heart-failure free survival is feasible for a period of 3 to 15 years in the absence of metabolic syndromes (2). MI-induced pathological remodeling not only damages myocardium, but also impacts kidney and spleen network to control physiological homeostatic mechanism (52). Therefore, comprehensive and systemic biology integrative approaches are necessary for the prevention and treatment of cardiovascular disease. Application of diversified ‘omics’ approaches such as proteomics, metabolomics and lipidomics allow for understanding of the chemical milieu that facilitates intra-intercellular chemical communication (28, 29, 53, 71). Specifically, circulating leukocytes and immune cells are sensitive to surrounding microenvironment in order to promote physiological or pathological function. Immune cells express a broad range of ligands that are vulnerable to disease pathology acts in coordination with the nervous system as sixth sense in response to stimuli like infection or myocardial damage (15). One hypothesis is that after MI, overactive inflammation is one possible cause of heart failure; likewise a variety of chronic inflammatory disease such autoimmune disease lupus, asthmas, and allergies share frustrated resolution (36, 49). Recent microbiome studies emphasized the importance of nutrient bacterial metabolites to sensitize or desensitize immune cells (111). Nutrition is one of the prime critical factors for atherogenesis, atheroprogression, and atherosclerosis, and atherosclerosis forms fatty streaks in the coronary vessel due to unresolved or un-cleared inflammation (111, 117). Immune cells (e.g. monocytes and macrophages) are exclusive defensive cells that respond to action (exercise) and inaction, the sleep and awake cycle, and overnutrition and undernutrition to maintain homeostatic cardiac physiology (67, 88). Imbalance of these three pairs leads to progressive changes in immune system response to cardiac damage repair. Traditionally, in obesity research, the low-grade inflammation is much more focused rather than overnutrition or undernutrition; however, the global and systems biology integrative approaches are essential for a possible method for the prevention of heart failure and long-term, heart-failure-free survival.
Obesity prevalence and the cardiovascular outcome
The World Health Organization defines overweight and obese as having an abnormal or excessive fat accumulation that poses a risk to cardiovascular disease and all-causes mortality (90). The body mass index (BMI), a person’s weight (in kilograms) divided by the square of his or her height (in meters), is well-accepted as the traditional measure of obesity. A person with a BMI of 20–25 kg/m2 is considered healthy, and above 25 kg/m2 is considered overweight. Furthermore, obesity is subdivided into three classes according to BMI: of Class I (30–35 kg/m2), Class II (35–40 kg/m2) and Class III (<40 kg/m2). Recently, a new method was identified to quantify the risk specifically associated with abdominal obesity, known as A Body Shape Index (ABSI). ABSI is based on waist circumference and is adjusted for height and weight: ABSI ≡ WC / (BMI (2/3) x height(1/2)). ABSI is claimed to be a more effective predictor of mortality than BMI, the most common measure used to define obesity (74). Obesity facilitates low-grade chronic inflammation that leads to the development of metabolic syndrome. Being overweight or obesity increases in the general population the risk of atherogenesis, MI and subsequent chronic heart failure with or without metabolic syndrome (110).
Diet-induced obesity, nutrition and left ventricle function
A classical study compared milk and lard fat to determine if a high fat diet was sufficient to cause cardiac dysfunction in mice (19). There were 3 distinct approaches used to evaluate the effect of obesity on left ventricle. The first aspect was subjecting mice to two different high fat diets, lard or milk, and following them over a period of 6 months. The second point involved high-fat feeding during or before and during heart failure. The final part of the study included a commonly used mouse model of overt diabetes, hyperglycemia, and obesity (db/db mice) being subjected to pressure overload and ischemia-reperfusion. In all aspects of the study, cardiac dysfunction was determined by echocardiographic measurements. In the first part, researchers showed that mice on the lard diet exhibited higher fractional shortening compared to low-fat diet and milk, but this difference was attributed to a reduction in systolic diameter in the lard diet group compared to the low-fat group. This study concludes that chronic fat feeding (6 months) is inadequate to develop left ventricle dilation or cardiac dysfunction. In the second part, there was no significance difference observed in the mortality in the mice that were fed a high-fat diet before ligation induced-infarction and mice that were not on a high-fat diet. Both groups had significant changes post-MI, but no difference was observed in LV end systolic and diastolic pressures between the high fat and standard diet groups. These findings led the researchers to make the conclusion that exposure to a high-fat diet does not alter the progression of heart failure in mice. This claim was further supported by the observation that there are no differences in mitochondrial function between the groups. The results of the second aspect of the study led the researchers into their final part of the study, investigating the effects of hyperglycemia and obesity on heart failure progression. Using the db/db mice model and their heterozygous littermates as two cohorts, the mice were subjected to permanent coronary ligation, myocardial ischemia/reperfusion or transverse aortic constriction (TAC). Only the TAC portion was continued after significant mortalities were encountered using other methods. The results found a baseline difference in ejection fractions and end systolic volumes between groups, but not ECG differences between the groups following TAC. These results led researchers to test if cardiac dysfunction during TAC can be attributed to hypoinsulinemia. This was tested by injecting mice with streptozotocin and subjecting them to TAC with cardiac function being assessed via ECG. The results showed no changes in the ejection fractions and fractional shortening between the groups throughout 8 weeks of TAC. The first conclusion drawn from these results was that increased fat intake, either before or after MI, does not exacerbate cardiac dysfunction. The second was that severe hyperglycemia or hypoinsulinemia does not cause any significant adverse effects during pressure overload-induced heart failure. The results also suggest the possibility that a high fat diet confers no greater risk to cardiac function once heart failure has been induced by surgical infarct. The researchers are poised in their conclusion that high-fat diet alone may not be sufficient to produce systolic dysfunction in mice, since mice exposed to the high-fat diet before or after infarct-induced heart failure show no exacerbation of cardiac dysfunction. Mice with hyperglycemia or hypoinsulinemia also exhibited no change in cardiac dysfunction. Presented findings and their conclusions raise the notion that certain mouse models may fail to recapture complex physiology and lipid metabolism of humans because feeding rodents on a high-fat diet could be merely copying the phenotype in humans consuming a Western diet. Future studies could involve including a high-fat diet combined with a high sucrose diet to accurately mimic a human diet, and also could explore the contribution of potential environmental factors to high-fat-diet-induced cardiac dysfunction that had been previously reported (19).
In recent years, obesity has become a reason for concern and reported to be associated with type II diabetes mellitus, metabolic disorders, hypertension and cardiovascular disease (106, 107, 113). The studies have revealed obesity as one of the relevant comorbidities of patients with ischemic heart disease leading to increase in mortality and development of congestive heart failure after MI. Using the murine model of diet-induced obesity, Thakkar et al have investigated the impact of diet-induced obesity after myocardial ischemia-reperfusion injury in mice (109). In mice, diet-induced obesity (DIO) develops hyperinsulinemia and insulin resistance and hepatic steatosis, with significant ectopic lipid deposition in the heart with cardiac hypertrophy and non-significant changes in blood pressure. The DIO mice showed the altered cytokines profile with increase in proinflammatory markers. The obese mice showed hypertrophic remodeling to MI with reperfusion and increase in LV chamber size in infarcted hearts compared with lean mice. The study is supported by Lopez et al., where excess n-6 fatty acid-induced obesity developed early post-MI LV dysfunction, impaired resolution of inflammation, and delayed LV healing in aging, along with dysregulation of proinflammatory analytes such as a 12-hydroxyeicosatetraenoic acid (12-S-HETE) (80). In a recent study, Halade and colleagues showed that the excess intake of fatty acid is deleterious in aging, leading to adverse MI-remodeling. Quantitative analysis of fat metabolites using mass spectrometry in young vs. aging mice displayed higher levels of arachidonic acid (AA) and minimal formation of pro-resolving, D- and E- series resolvins in aging mice fed with excess n-6 diet. The excess n-6 feeding in aging mice also displayed higher pro-inflammatory (CD11b+F4/80+Ly6Chi) immune population (Figure 2). The study highlighted the systems biology approach and focused on the cardiorenal axis. The study showed dysregulation of metabolites and cytokines not only in heart, but the n-6 fatty acid feeding also induced renal inflammation impacting kidney, with increase in NGAL, TNF-α and IL-1β (52). In contrast, the study by Poncelas et al., where B6D2F1 mice of both genders when fed on the high-fat diet (HFD) or standard diet for six months and then subjected to 45 min of coronary occlusion and 28 days of reperfusion, mice developed obesity with hypercholesterolemia and hyperinsulinemia in the absence of hyperglycemia or hypertension (93), though no change was observed in ventricular mass, volume or function, or in vascular reactivity during feeding. HFD showed the reduction in infarct size and cardiac dilation, as well as improved left ventricular function as compared to control diet animals attenuating the consequences of transient coronary occlusion as shown by a marker. A study by Heaberlin et al. in KKAy mouse model of obesity and type 2 diabetes showed improved cardiac function through reduced inflammation, extracellular matrix accumulation, and neovascularization in the infarct region with decreasing survival post-MI. KKAy mice showed increased post-MI mortality, while survivors had improved cardiac function accompanied by reduced macrophages, collagen I and III, and neovascularization (60). Considering the human, in a meta-analysis study with a sample size of 36,803 participants, there were 14,883 incident cases of MI event. This showed that both overweight and obesity increased the risk of AMI (126). Obesity is considered to be an independent risk factor for MI analyzed, using data from a case study (104). Human studies have shown that the obesity is considered to be a metabolic syndrome which doubles the cardiovascular outcomes and mortality (86). Being overweight and obese independently increased the risk of heart disease in patients with type 2 diabetes. However, the paradoxical studies are also published with mice and human data. The long-term mortality was lower in the patients that were obese and older after non-ST-segment-elevation MI compared to those of with healthy weight (89). Though the obesity paradox is found in both mice and human studies, has been observed that age and gender both are the crucial factors in determining the outcomes of obesity-induced cardiovascular risk factors.
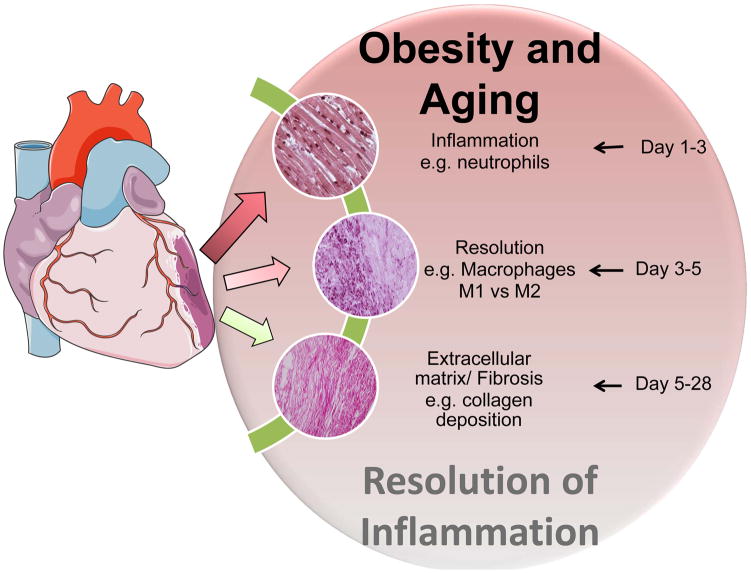
Figure 2.
Obesity superimposed on aging magnify non-resolving inflammation. Post-MI acute inflammation triggers changes in the kinetics and leukocytes quantity from acute phase (d1–d3), resolution phase (d3–d7) and fibrotic/chronic phase (d5–d28). This results in imbalance in generation of resolving and non-resolving lipid in the acute and resolving phases of myocardium healing thereby impaired resolution of inflammation. Levels of resolving and pro-inflammatory lipid mediators determine the resolving capacity of myocardium healing in heart failure pathology.
Obesity is associated with the shift in structural and functional parameters of the heart, such as LV hypertrophy, left atrial enlargement and impairment of LV systolic/diastolic function. It is thought that long-term obesity leads to an exacerbation of heart failure, but there are no firm conclusions on the particular role obesity plays in the development of heart failure (1). Previous studies have shown that obesity is a major contributor to cardiac complications and all-causes mortality, independently of association with other cardiovascular risk factors. Obesity is characterized by bone and liver adiposity, and excessive chronic inflammation of adipose tissue with marked molecular and cellular dysregulation in various organs (Figure 3) (51, 55, 56). The high metabolic requirements of the excess adipose tissue cause larger amount of cardiac work, resulting in an increase in LV mass. Obesity-mediated cardiac remodeling is characterized by a shift to a concentric geometry. Obese individual’s heart exhibits many differences from the normal heart; epicardial fat is thicker and the left atrium increases in size (8). The left ventricle of obese individuals shows normal ejection fractions, increased stroke volume, and reduced strain, mid wall shortening, and filling rate. The LV structure also shows an increase in relative wall thickness (8). Thus, obesity increases the risks due to the structural and functional cardiac changes coupled with a prevalence to develop coexisting conditions that also contribute to cardiac dysfunction. Obesity impacts on the structure and function of the heart and the obvious connection between nutrition and obesity. The role of nutrition in LV function and CVD is of fundamental interest. Recent studies were performed in rodents to assess the role of diet in the progression of heart failure. Dietary lipids were presumed as the membrane phospholipids; now it is clear that these lipidic molecules coordinate signaling event and acts as ligands for nuclear receptors (105). Recent studies performed in rodents suggested that high-fat diet and low in carbohydrate prevents development and progression of heart failure when compared to low fat and high carbohydrate diets (105). There is need of two sets of dietary recommendations, one for individuals who are at risk for heart failure and another one for those who have established heart failure.
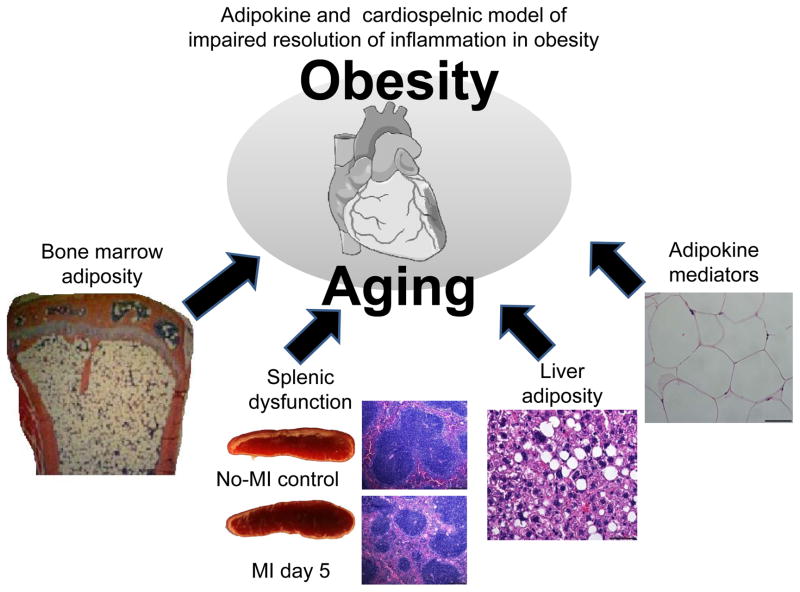
Figure 3.
Multiple mediators generated in the spleen, adipose tissue, liver and bone marrow contributes to myocardial healing. Adipokines (liver adiposity), bone marrow, and spleen collectively coordinate low-grade inflammation in heart failure. The low-grade inflammation is sustained during myocardial healing leading to impaired resolution of inflammation in obesity.
Fatty acids signaling in myocardium metabolic remodeling
In a study of the Burmese python as a model of acute cardiac metabolic regulation, there was an indication of an increase in heart mass after consuming a meal. Most of the mammalian models typically show modest hypertrophy (~10 to 20%) after weeks of stimulation; however, the python heart shows a massive hypertrophy as it grows by 40% with 48 to 72 hours after the consumption of a large meal. The increase in heart mass is regulated by the increase in AMPK, AKT, GSK3 β and mTOR phosphorylation in the postprandial python heart. At the same time, the plasma non-esterified fatty acids and triglycerides are significantly increased after an enormous meal without an accumulation of neutral lipids. Treatment of neonatal rat ventricular myocytes with postprandial plasma from fed python significantly increased their cell size, indicating the role of circulating fatty acids in hypertrophy. The complex mixture of the fatty acids is myristic acid (C14:0), palmitic acid (16:0), and (C16:1). Palmitoleic acid feeding to the python also results in an increase of heart mass. Even with the well-established pro-apoptotic action of the palmitic acid in cardiomyocytes, there is no evidence of this in the python plasma treated myocytes. Interestingly, the infusion of the complex fatty acid mixture into the python for seven days causes the development of cardiac hypertrophy with no activation of fetal genes or signs of fibrosis. This overall data suggests the beneficial role of fatty acids in heart growth characterized by cellular hypertrophy in the absence of hyperplasia.(99)
Effects of obesity on ECGs (electrocardiogram) in humans and mice
Obesity in humans is associated with long QT in electrocardiogram (ECG) measurements, an increase in the frequency of premature ventricular complex and sudden cardiac death. A study was conducted to test the hypothesis that the obese heart possesses a decreased expression of voltage-gated potassium channels, leading to the resulting long QT in ECG measurements (65). Obese humans display impaired repolarization in cardiomyocytes due to the prolonging of the QT interval. There is also a known significant association between obesity and atrial fibrillation and arrhythmias. Sudden cardiac death increase in obese patients is usually due to arrhythmias (65). Similar observations regarding the electrophysiology of the obese heart have been made using a diet-induced obesity model in mice. Diet-induced obesity is the superior model in transgenic mice to induce obesity because overexpression of a single gene in a mouse may not result in the same pathophysiology provided by a diet-induced obesity model. The study used the diet-induced obesity model to investigate the electrophysiological conditions present in obese mice (65). This study concluded that protein kinase D is activated in obese hearts, leading to a reduction of cyclic AMP response element binding protein (CREB). This leads to a decreased expression of potassium channels, thus resulting in long QT and pro-arrhythmic electrophysiological remodeling in the obese heart. Diet-induced obesity mice showed similar ECG characteristics to humans, experiencing a significant increase in QT interval and ventricular ectopy when compared to non-obese controls (65). However, obese people show more atrial fibrillation than diet-induced obesity mice that exhibit signs of ventricular arrhythmias.
Obesity in humans is also associated with an increased risk of developing atrial fibrillation (AF) (48). Several co-morbidities, such as hypertension, coronary artery disease, congestive heart failure, and obstructive sleep apnea are shared between obesity and AF (48). The increase of AF risk in obese patients is associated with a transformation of the atrial electrophysiology that can be observed in these individuals’ ECG. The study showed P wave indices (PWI) that were analyzed in obese patients and found that the amount of pericardial fat was correlated to P-wave duration and P-wave terminal force, and that also PR interval and P-wave amplitude was significantly associated with increasing BMI (48). The effects of obesity on cardiovascular health in mice and humans are evident in the accompanying changes reflected in ECG.
Translational, genetic obesity and surgical animal models to test heart function
To understand the mechanisms of cardiac remodeling in the context of obesity several animal models have been studied. These models are closely associated with the overweight or obesity in humans and with coincident morbidities such as impaired glucose tolerance, insulin resistance, diabetes, and hypertension. To evaluate tissue pathology in a time-dependent manner, rodents have been the choice of model to perform studies. Rodent models are classified into two main categories: transgenic mice with targeted mutations or diet-induced obesity mice achieved by feeding the animals on the high-fat diet. Furthermore, these two rodent models are used with minimum invasive surgery to study heart failure following MI. Most laboratory models of obesity are small inbreed rodents (rats or mice) and are maintained in small enclosures with a particular diet meant to develop obesity. The choice of model for a particular experiment depends upon the goal of the study, time and cost; therefore, young animals are preferred. To evaluate exact mechanism, transgenic models or models with spontaneous mutations may be used for specific mechanism or for validation of therapeutic target to determine whether it engages a specific target or pathway in vivo. In 1959, a spontaneous mutation leading to the markedly obese phenotype in the Lepob/Lepob mouse was first recognized (84). The characterization and discovery of the “ob” gene product, leptin in 1994 intensified the motivation to perform research on obesity genetics (125). To this day, the “ob” gene is one of the most-studied genes of obesity research. The production of bioactive leptin is prevented by a single-base, spontaneous mutation of the “ob” gene. Leptin is synthesized predominantly in white adipocytes, and its secretion is directly proportional to the amount of stored triglyceride. Phenotypically, hyperphagia, reduced energy expenditure, and hypothermia are distinct features of early obesity due to lack of leptin (42). Hypercorticosteronemia, insulin resistance associated with hyperglycemia and hyperinsulinemia, hypothyroidism and growth hormone deficiency are further defects which lead to the decrease in linear growth. A drawback of this model is that Lepob/Lepob mice are infertile. Lepob/Lepob mice develop obesity that can be treated effectively by the administration of exogenous leptin since leptin levels are found to be highly elevated in MI (82). This model of overweight and obesity is extensively used to study obesity-induced MI. The leptin receptor-deficient “db/db” mouse, also called the Leprdb/Lepdb mouse, is phenotypically similar to the Lepob/Lepob mouse (118). These mice are so-named because there is more marked hyperglycemia on some background strains. Lepdb/Lepdb mice are also characterized by hyperphagia and reduced energy expenditure, leading to marked early-onset of obesity. Also, these mice are hypothermic, have decreased linear growth due to growth hormone deficiency, and are infertile (24) (25). The significant difference from the Lep ob/Lepob mouse are resistance to leptin due to spontaneous mutation in leptin receptor. These mice also suffer from morbid obesity, but their leptin levels are markedly elevated. The db/db mouse has an increased susceptibility to myocardial ischemia-reperfusion injury (69) (76). The increased severity of MI in the db/db mouse makes it an excellent model to investigate heart failure in the setting of obesity-induced diabetes.
The genetically engineered animal model of leptin receptor deficiency is more accurate and is known as s/s (disruption of the LRb/STAT3 signal mouse) (14) (13). This mouse carries a mutation that specifically disrupts the transcription factor STAT3, which is an essential component of the leptin signaling pathway of the long form of the leptin receptor and mediates leptin’s effects on energy homeostasis. The homozygous (s/s) mice are hyperphagic, obese, and fertile. They have normal body length and are less hyperglycemic compared to the Lepdb/Lepdb mouse. These mice develop severe insulin resistance similar to that in Lepdb/Lepdb mice, particularly in the liver. These mouse models are essential in studying STAT3-associated myocardium dysfunctions in obese models (12). The extracellular domain of the leptin receptor is mutated in the obese Zucker (fa/fa or ‘fatty’ rat) and the Koletsky rat. Zucker and Kolesky rats show a similar phenotype of hyperphagia and reduced energy expenditure, leading to morbid obesity (20) with an impaired glucose tolerance- a growth deficit possibly related to a lower activity of the GH/IGF-1 axis and hypothyroidis. Koletsky rats have a nonsense and null mutation that leads to undetectable levels of leptin receptor mRNA expression and lowered fertility (24) (26) (27). The Zucker fatty rats have a fa/fa mutation associated with a processing defect of the leptin receptor, which is generated but retained intracellularly. This leads to reduced numbers of leptin receptors on the cell surface of adipocytes and is associated with decreased leptin binding and signal transduction. When homozygous for a gene (fa/fa), the Zucker rat is obese, moderately insulin resistant, and hypertriglyceridemic, but with no progression in diabetes or cardiovascular complications (6). Thus, these mice are not suitable to study obesity-mediated myocardial dysfunction. Zucker Diabetic Fatty (ZDF) rats are the sub strain of obese Zucker fatty rats displaying early dysregulation of glucose metabolism. ZDF rats fed on high-fat diet develop early diabetes and altered expression of the glucose transporter GLUT4 in skeletal muscle (127). These mice show evident vascular dysfunction in the aorta, coronary arteries, and mesenteric arteries in adult to middle-aged obese ZDF rats with diabetes (91). The ZDF model has proven to be a useful tool in demonstrating the efficacy of pharmaceutical agents directed at myocardial injury and lipidemia (124) (120). This similar rat model is a cross of Zucker (fa/fa) rats with Wistar-Kyoto (WKY) rats. These rats develop obesity and co-morbidities like insulin resistance, hyperinsulinemia, and hyperlipidemia similar to the Zucker (fa/fa) rat. Male Wistar-Kyoto fatty rats develop early hyperglycemia and glucosuria along with insulin resistance (44). These mice have been used to study how the progression of heart failure following coronary artery ligation surgery is accelerated by Type 2 diabetes (21). The PPAR-γ knockout mouse model is tissue-specific (adipose), as the global knockout is embryonically lethal. Ablation or impaired function of PPAR-γ, specifically in adipose tissue, results in an insulin-resistant lipodystrophic phenotype (119). These hypomorphic and the ATKO (adipose tissue-specific knockout mice) displayed average whole body insulin sensitivity when fed a chow diet, and they had hepatic insulin ATKO, where adipose tissue is unable to store lipids and suppress lipolysis appropriately. Thus, when challenged with high-fat feeding, it leads to persistently elevated serum lipid and lipotoxic infiltration of the liver (59). Subsequently, this mouse model develops whole body insulin resistance due to the impaired suppression of gluconeogenesis as a result of the lipotoxic insult (50). The PPAR models reveal greater increases than other models in information regarding the mechanisms of various nuclear transcription factors involved in the cardiovascular system. Since global deletion of PPAR is embryo-lethal, the use of conditional knockout mice (e.g., ECs, VSMCs, macrophages) has been critical to understanding the mechanism of cardiovascular disease. Coronary artery ligation-induced progressive heart failure is the first model applied in dogs that mimic ischemic cardiomyopathy (62). The procedure involves the ligation of the proximal left anterior descending coronary artery (LAD) accompanied by an intubation and left thoracotomy to induce MI. This procedure has a higher mortality rate, nearly 50%, due to malign ventricular tachycardias in the acute phase. TAC in the mouse is an experimental model for pressure overload-induced cardiac hypertrophy and heart failure (100). These mice models initially lead to compensated hypertrophy of the heart, which often is associated with a temporary enhancement of cardiac contractility. With due course of time, however, the response to the chronic hemodynamic overload becomes maladaptive, resulting in cardiac dilatation and heart failure. Rockman et al. validated the first murine TAC model (100). This model has been used as a valuable tool to mimic human hypertensive cardiovascular diseases, and furthermore, to elucidate fundamental signaling processes involved in the cardiac hypertrophic response and heart failure development.
Post-MI remodeling in obesity
Global epidemic obesity is associated with moderate to chronic inflammation. Long standing obesity may result in cardiac changes, such as left ventricular (LV) hypertrophy, left atrial (LA) enlargement, and subclinical impairment of LV systolic and diastolic function. The inflammatory cascade has a critical role in response to cardiac injury, the involvement of inflammatory mediators in repair, and the remodeling of the infarcted left ventricle. The pathogenesis of heart failure post-MI is intricately linked with the development of post-infarction ventricular remodeling (47). Thus, chronic obesity is linked to an increased risk of dysfunction for the heart, and it is assumed that long-term existence of obesity will eventually lead to heart failure (5) (22) (122). In the Framingham Heart Study (73), the population was stratified by BMI and then followed for incident HF that was diagnosed by adjudicated clinical criteria. The study reported that increased BMI was associated with an increased risk of heart failure in both men and women, and the risk was graded across categories of increasing BMI. Though it has been well accepted that obesity increases the risk of developing heart disease, recent reports documented a statistically significant survival benefit in obese patients once they were diagnosed with heart diseases (57) (63) (79) (85) (38). The recent report from our group has shown the improved cardiac function in obese mice models (60). The study shed light on post-MI remodeling in obese and diabetic KKAY mice that showed an attenuated inflammatory response. The decrease in the inflammatory response included reduced macrophage density in the infarcted area with reduced collagen I and III levels and reduced neovascularization in the infarcted area of KKAy mice. The study concluded that diabetes worsened post-MI survival but attenuated LV remodeling in surviving mice, with improved cardiac function in post-MI settings. In another study, obese and hyperphagic mice showed improved post-MI survival with attenuated LV remodeling. This was a result of modifying the leukocyte infiltration kinetics, as well as the angiogenic response in absence brain-derived neurotrophic factor (BDNF) in heterozygous mice (54). Both these studies align with the obesity paradox that obesity may both elicit cardiac disease as well as protect from cardiovascular death. This now requires further mechanistic analyses at the cellular, molecular, systematic and integrative levels.
Saturated fat vs. polyunsaturated fat
The amount and source of dietary fat intake in the human diet can have significant implications on cardiovascular health. In recent years, there has been a shift in Western diets due to the increased use of plant oils over animal fats in cooking and consumption of processed food (87). This change has led to an increased intake of saturated fatty acids (SFA) and polyunsaturated fatty acids (PUFA), in particular the n-6 PUFA, linoleic acid (LA) (16). LA is particularly of cardiovascular interest because it has that elongate to long chain derivative, arachidonic acid (AA) leads to the production of pro-inflammatory compounds. LA is a competitor of the short-chain n-3 PUFA alpha-linoleic acid (ALA) for enzymes required to convert to their long chain derivatives, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), subsequent entry to the plasma membrane. Both SFA and n-3 dietary fatty acids are involved in forming proinflammatory and immunoresolvent mediators (Figure 4; reviewed elsewhere (71)), indicating that diets high in LA may decrease the utilization of the cardioprotective benefits derived from intake of n-3 PUFA. This competition from LA suggests that limiting the intake of LA will result in a reduction of n-6 derived pro-inflammatory mediators, while also enhancing the efficacy of long-chain n-3 PUFA. A recent study was able to show that a diet consisting of low LA taken for four weeks can reduce total LA and total n-6 PUFA content of plasma phospholipids, while also increasing n-3 long-chain PUFA without increasing their consumption due to their improved incorporation (123). It is thought that replacing SFA with PUFA in the diet will result in cardioprotective effects, but it is clear that the amount and type of PUFA in the diet will cause differential effects based on their content (7). A case-control study from Italy examined the FA composition of whole blood in patients with a recent MI and compared with matched controls, finding that PUFA of both n-3 and n-6 series are significantly lower in patients with MI (81). It was also found that MI patients had higher levels of SFA and monounsaturated fatty acids. Another review article on dietary fats in relation to cardiovascular health found evidence that n-6 PUFA promotes inflammation and exacerbates many diseased states while n-3 PUFA works to counter these effects (75). This review refutes earlier evidence that SFA intake increased coronary risk and put forth that replacement of SFA in the diet with carbohydrates, sugar, in particular, has driven an increase in obesity and its corresponding adverse health effects. Many other studies have corroborated the beneficial effects of n-3 PUFA intake on cardiovascular health and outcomes (31) (11) (43) (30) (34) and its ability to reduce atrial fibrillation (58) (72) (121). In summary, current literature shows diversified effects of fat to cardiovascular health. Higher levels of n-3 PUFA in the blood are affected by n-6 PUFA intake. The replacement of SFA with PUFA provides cardioprotective benefits (103) due to the action of n-3 long chain derivatives EPA and DHA. Further research should focus on providing answers on the ideal amounts of n-6 to n-3 PUFA to provide cardiovascular protection and of the extent to which the dietary amounts of EPA and DHA, either individually or synergistically, affect cardiovascular health.
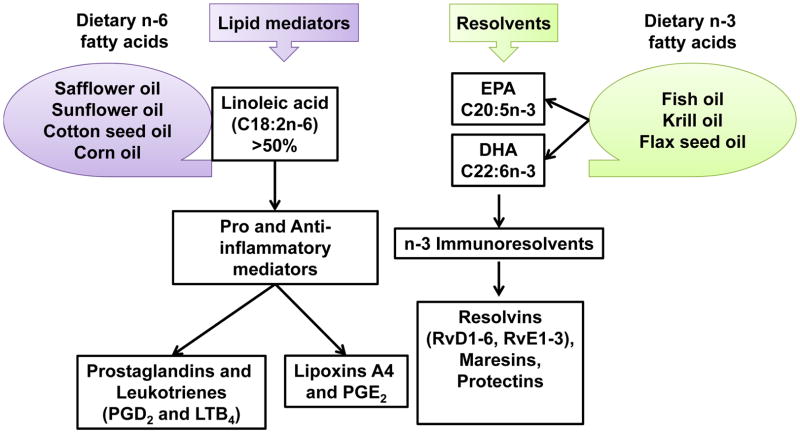
Figure 4.
Diversity of lipid mediators derived from polyunsaturated n-6 fatty acids and immunoresolvents derived from n-3 fatty acid. Dietary sources of n-3 and n-6 fatty acids and their respective lipid metabolites are illustrated to define the differential chemical milieu responsive to dietary factors.
Diet intake and lipotoxicity
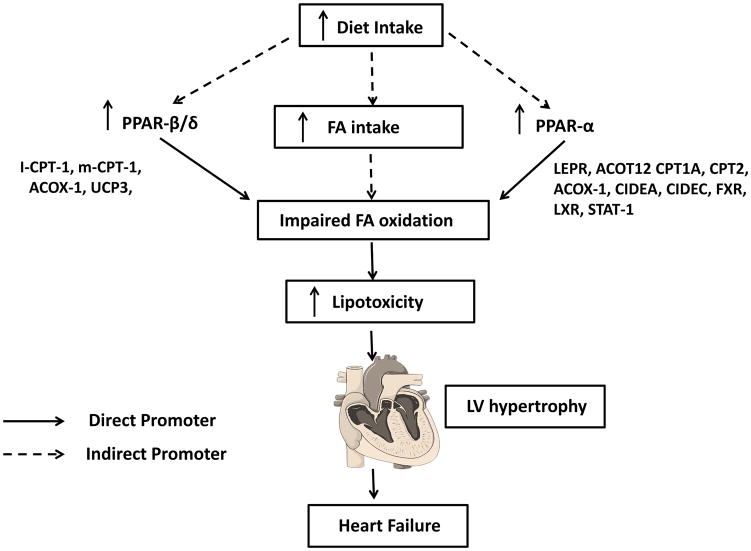
Dietary lipids modulate cardiac function via membrane phospholipids, either as signaling molecules or ligands for nuclear receptors, are predominant substrate for cardiac mitochondria to generate energy through oxidative phosphorylation (105). The peroxisome proliferator–activated receptors (PPARs-α, β, and δ) are one of the first genetic targets for fat. PPARs are members of the nuclear receptors family and can be activated by both dietary fatty acid as well as their metabolic derivatives in the body, and they serve as lipid sensors for myocardial energy metabolism (Figure 5) (41). An abnormality in myocardial energy metabolism, specifically an overall misbalance of mitochondrial oxidative catabolism and anaerobic glycolytic pathways (45) (10) (114), is the primary factor for the myocardium dysfunction and failing heart. These metabolic changes are mediated partially by decreased expression of genes encoding enzymes involved in mitochondrial FAO (fatty acid oxidation) and oxidative phosphorylation pathways secondary to deactivation of the PPAR/PGC-1α axis (45) (9). PPARα deactivation is observed in human heart failure patients (10) (98). PPAR/PGC-1α axis plays an important role in the failing heart indirectly through dietary intake.
Figure 5.
Fatty acid metabolism gene network and impaired fatty acid oxidation. Fatty acid intake alters the oxidative metabolism via direct and indirect interaction with the metabolic gene network that generates energy in homeostasis. The imbalance or over and or under activation of metabolic genes leads to lipotoxicity and heart failure.
Impact of Mediterranean and DASH diets on preventing cardiovascular disease
The quality and quantity of the fat intake and other macro or micro nutrients clearly have an effect on cardiovascular health and has led to the development of specific diets aimed at improving cardiovascular health. Two of the most studied diets designed to improve cardiovascular health are the Mediterranean diet and Dietary Approaches to Stop Hypertension (DASH) diet (116).
The Mediterranean diet originated in Crete and Italy; it consists of a high fat intake- 40–50% of daily calories with SFA composing ~8% and monounsaturated fatty acids (MUFA) at 15–25% total calories (39). The diet also consists high fish and plant intake, leading to high n-3 PUFA levels and a much lower ratio of n-6 to n-3 PUFA as compared to other diets. The foods consumed in this diet include fresh fruits/vegetables, whole bread/grains, legumes, nuts, olive oil, moderate dairy products, eggs, fish, and chicken (97). The diet discourages the consumption of red meat and allows reasonable quantities of wine to be taken with meals. The Mediterranean diet (MedDiet) is extensively studied for its effects on CVD incidence and development. One randomized trial, which enrolled 7447 participants at high risk for CVD, tested the effects of two variations of the Mediterranean diet against a control diet on cardiovascular outcomes (40). The results showed that both variations of this diet were associated with a reduction in the incidence of many cardiovascular events. Extensive reports on the subject of the Mediterranean diet found that its intervention is associated with a 38% relative reduction in the risk of CVD clinical events (83). The beneficial cardiovascular effects gained from this diet rise from its ability to cause improved LV function and blood pressure. One group sought to test how LV function changed in response to adherence to the MedDiet by tracking 372 heart failure patients’ LV function and dietary habits (23). The results showed that patients who more closely followed the MedDiet experienced improved LV fill pressure and systolic function of both ventricles. The anti-inflammatory properties inferred from the diet are thought to drive the impact of diastolic filling pressures. Another collection of reports, which included randomized controlled trials where the MedDiet was used as the intervention in adults at high risk of CVD and healthy adults, found that the diet lowered blood pressure in 3 of 5 trials reporting that outcome (97). The utilization of the MedDiet has been positively associated with improved LV function and blood pressures.
Like the MedDiet, the DASH diet has also been shown to have positive effects on cardiovascular outcomes (Figure 6). The DASH diet was a nutritional program begun in the 1990’s that was aimed to reduce hypertension with the hopes that this results in a reduction of CVD events. The main components of the diet are vegetables, fruit, low-fat dairy products, whole grains, chicken, fish and nuts. The diet is low in fat, meat, sweets, and soda. A correctly followed DASH diet will provide more calcium, magnesium, potassium, and dietary fiber, while also providing less fat, SFA, cholesterol, and sodium compared to an average Western diet (39). There have been several studies aimed at evaluating the DASH diet’s ability to reduce blood pressure/hypertension and improved CVD outcome. In one study, obese hypertensives were subjected to DASH diet for three weeks in comparison to a regular diet with added potassium, magnesium, and fiber supplements. The results found that the DASH diet more efficiently lowered blood pressure and improved endothelial function (3). Another study focused on the effects of the DASH diet alone and in conjunction with exercise to see the difference in hypertension relieving capabilities (17). The results showed that exercise along with the DASH diet attenuates the blood pressure lowering more efficiently than the diet alone. Participants who also exercised showed larger blood pressure reductions and also more improvements in vascular and autonomic function along with a reduction in LV mass. Diets that are consistent with the DASH guidelines are also associated with a lower incidence of heart failure (77). The more specific effects of the DASH diet on LV function were elucidated through examining the effect of the DASH diet on patients that had heart failure with preserved ejection fraction (66). These patients that were treated with a sodium-restricted DASH diet showed improvements in ventricular diastolic function, arterial elastance, and ventricular-arterial coupling. A meta-analysis of DASH diet studies found that the diet can significantly protect against CVD, coronary heart disease, stroke and heart failure by 20%, 21%, 19%, and 29%, respectively (102). Similar results of the DASH diet were found in a Chinese population study, showing that adherence to the DASH diet has clear benefits in managing long-term blood pressure and reducing the risk of stroke (78). It is evident that adherence to the DASH diet will be effective in lowering blood pressure and the cardiovascular benefits that go along with improving hypertension (78).
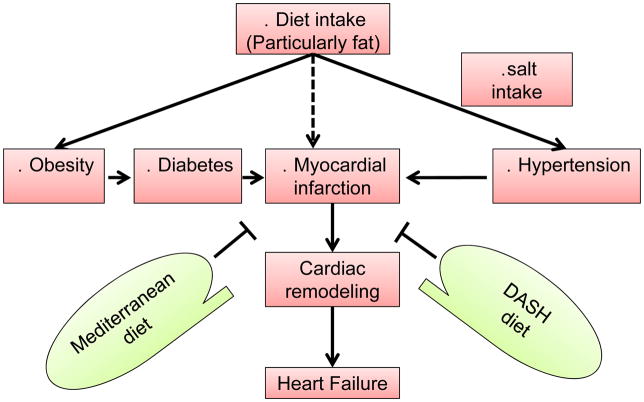
Figure 6.
Impact of salt and fat enriched diet in obesity, diabetes and hypertension leading to MI and heart failure events. Solid lines indicate direct contribution to pathology, and dotted lines indicate indirect effect. Mediterranean and DASH (dietary approaches to stop hypertension) diets show capacity of reducing the overall rate of cardiovascular disease and heart failure events.
The MedDiet and DASH diet both have obvious benefits for cardiovascular outcomes. The MedDiet operates more through its ability to establish healthy n-3 intake and n-6/n-3 PUFA ratios to improve cardiovascular outcome, while the DASH diet aims specifically at lowering blood pressure to improve cardiovascular health (Table 1). Both diets show positive effects on cardiac health development, and their mechanisms of action could provide insight into new ways of improving cardiovascular health.
Table 1.
Summarized features of DASH and Mediterranean diet that showed evidence to reduce cardiovascular events in epidemiological studies. Both diets have many common features.
| Diet features | Mediterranean diet | DASH diet |
|---|---|---|
| Fruit | ↑ Fruit | ↑ Fruit |
| Vegetables | ↑ Vegetables | ↑ Vegetables |
| Dairy products | Moderate amounts of low-fat dairy | moderate amounts of alcohol and dairy products |
| Protein | ↑ Legumes and nuts ↓ Animal protein |
↑ Whole grain and ↓ red or processed meats |
| Fish | ↑ Fish | ↑ Fish |
| Sodium | - | ↓ |
| Sweet | ↓ | ↓ |
DASH: This diet primarily designed to reduce blood pressure.
Differential cardiac health outcome in obesity
By and large, numerous rodent models of diet have been used for studying cardiac pathophysiology and the clinical aspects of diet in heart failure. These studies account for the paradox due to: 1) variation in the fat percentage and its source; 2) age of the animals used in the studies (most likely young rodents are widely used); 3) gender specificity (the selection of only male or female); and 4) dose and duration of diet feeding (short-term vs. long-term). These four factors widely impact the studies and their outcomes; therefore, studies involving the diet or fat intake should be critically designed to translate to the clinical implication of cardiac health.
Future prospective
The manipulation of dietary fat quality and quantity shows promise in the prevention and treatment of heart failure. Additional clinical and animal studies are needed to determine: 1) the optimal diet, i.e. quality and quantity of fatty acids that are the prime substrates for lowering the inflammation with an age-dependent approach; 2) evaluate fat-metabolizing enzyme capacity (genetic machinery) in heart failure pathophysiology; and 3) understanding the role of fatty acid metabolism and fatty acid-derived metabolites in resolving and non-resolving inflammation in heart failure pathology. Thus, studying these three critical points will help scientists and the community in fine-tuning the impact of obesity-related complicated cardiovascular effects on health.
Conclusion
Over nutrition or genetic originated obesity is the multifaceted metabolic disorder that has a long-term dynamic adverse effect on the systemic inflammation and integrative cardiovascular network. Obesity-induced overactive unresolved inflammation and dysregulated interaction of fatty acids with lipid processing enzymes aggravate heart failure pathology. Thus, obesity-mediated cardiac pathology is originated from diverse metabolic factors that coordinate the systemically inflamed milieu to drive cardiovascular dysfunction, particularly in obesity. Cardiac dysfunction-related mechanisms integrate with the splenic and renal network to contribute the progressive non-resolving milieu. Therefore, reduction of risk factors with comprehensive integrative approach will be a suitable strategy to reduce cardiovascular burden. Detailed mechanistic studies of obesity-mediated overactive inflammation in cardiovascular pathobiology are warranted to identify the novel targets and treatment strategy consequently to improve cardiac health.
Acknowledgments
Authors acknowledge the support from National Institutes of Health [AT006704], [HL132989] the award to G.V.H, and the American Heart Association postdoctoral fellowship [POST31000008] to V.K. Authors also acknowledge the use of Servier Medical Art Library for figures.
References
- 1.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiological reviews. 2008;88:389–419. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad FS, Ning H, Rich JD, Yancy CW, Lloyd-Jones DM, Wilkins JT. Hypertension, Obesity, Diabetes, and Heart Failure-Free Survival: The Cardiovascular Disease Lifetime Risk Pooling Project. JACC Heart failure. 2016;4:911–919. doi: 10.1016/j.jchf.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Solaiman Y, Jesri A, Mountford WK, Lackland DT, Zhao Y, Egan BM. DASH lowers blood pressure in obese hypertensives beyond potassium, magnesium and fibre. Journal of human hypertension. 2010;24:237–246. doi: 10.1038/jhh.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, Jr International Diabetes Federation Task Force on E, Prevention, Hational Heart L, Blood I, American Heart A, World Heart F, International Atherosclerosis S, and International Association for the Study of O. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 5.Alpert MA, Lambert CR, Panayiotou H, Terry BE, Cohen MV, Massey CV, Hashimi MW, Mukerji V. Relation of duration of morbid obesity to left ventricular mass, systolic function, and diastolic filling, and effect of weight loss. The American journal of cardiology. 1995;76:1194–1197. doi: 10.1016/s0002-9149(99)80338-5. [DOI] [PubMed] [Google Scholar]
- 6.Amy RM, Dolphin PJ, Pederson RA, Russell JC. Atherogenesis in two strains of obese rats. The fatty Zucker and LA/N-corpulent. Atherosclerosis. 1988;69:199–209. doi: 10.1016/0021-9150(88)90015-9. [DOI] [PubMed] [Google Scholar]
- 7.Ander BP, Dupasquier CM, Prociuk MA, Pierce GN. Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp Clin Cardiol. 2003;8:164–172. [PMC free article] [PubMed] [Google Scholar]
- 8.Aurigemma GP, de Simone G, Fitzgibbons TP. Cardiac remodeling in obesity. Circulation Cardiovascular imaging. 2013;6:142–152. doi: 10.1161/CIRCIMAGING.111.964627. [DOI] [PubMed] [Google Scholar]
- 9.Barger PM, Brandt JM, Leone TC, Weinheimer CJ, Kelly DP. Deactivation of peroxisome proliferator-activated receptor-alpha during cardiac hypertrophic growth. J Clin Invest. 2000;105:1723–1730. doi: 10.1172/JCI9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barger PM, Kelly DP. PPAR signaling in the control of cardiac energy metabolism. Trends Cardiovasc Med. 2000;10:238–245. doi: 10.1016/s1050-1738(00)00077-3. [DOI] [PubMed] [Google Scholar]
- 11.Barrett SJ. The role of omega-3 polyunsaturated fatty acids in cardiovascular health. Alternative therapies in health and medicine. 2013;19(Suppl 1):26–30. [PubMed] [Google Scholar]
- 12.Bates SH, Kulkarni RN, Seifert M, Myers MG., Jr Roles for leptin receptor/STAT3-dependent and -independent signals in the regulation of glucose homeostasis. Cell metabolism. 2005;1:169–178. doi: 10.1016/j.cmet.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Bates SH, Kulkarni RN, Seifert M, Myers MG., Jr Roles for leptin receptor/STAT3-dependent and -independent signals in the regulation of glucose homeostasis. Cell metabolism. 2005;1:169–178. doi: 10.1016/j.cmet.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 15.Blalock JE. The immune system as the sixth sense. Journal of internal medicine. 2005;257:126–138. doi: 10.1111/j.1365-2796.2004.01441.x. [DOI] [PubMed] [Google Scholar]
- 16.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. The American journal of clinical nutrition. 2011;93:950–962. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin PH, Caccia C, Johnson J, Waugh R, Sherwood A. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Archives of internal medicine. 2010;170:126–135. doi: 10.1001/archinternmed.2009.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bozkurt B, Kribbs SB, Clubb FJ, Jr, Michael LH, Didenko VV, Hornsby PJ, Seta Y, Oral H, Spinale FG, Mann DL. Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation. 1998;97:1382–1391. doi: 10.1161/01.cir.97.14.1382. [DOI] [PubMed] [Google Scholar]
- 19.Brainard RE, Watson LJ, Demartino AM, Brittian KR, Readnower RD, Boakye AA, Zhang D, Hoetker JD, Bhatnagar A, Baba SP, Jones SP. High fat feeding in mice is insufficient to induce cardiac dysfunction and does not exacerbate heart failure. PloS one. 2013;8:e83174. doi: 10.1371/journal.pone.0083174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bray GA, York DA. Hypothalamic and genetic obesity in experimental animals: an autonomic and endocrine hypothesis. Physiol Rev. 1979;59:719–809. doi: 10.1152/physrev.1979.59.3.719. [DOI] [PubMed] [Google Scholar]
- 21.Chandler MP, Morgan EE, McElfresh TA, Kung TA, Rennison JH, Hoit BD, Young ME. Heart failure progression is accelerated following myocardial infarction in type 2 diabetic rats. American journal of physiology Heart and circulatory physiology. 2007;293:H1609–1616. doi: 10.1152/ajpheart.01338.2006. [DOI] [PubMed] [Google Scholar]
- 22.Chen YT, Vaccarino V, Williams CS, Butler J, Berkman LF, Krumholz HM. Risk factors for heart failure in the elderly: a prospective community-based study. The American journal of medicine. 1999;106:605–612. doi: 10.1016/s0002-9343(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 23.Chrysohoou C, Pitsavos C, Metallinos G, Antoniou C, Oikonomou E, Kotroyiannis I, Tsantilas A, Tsitsinakis G, Tousoulis D, Panagiotakos DB, Stefanadis C. Cross-sectional relationship of a Mediterranean type diet to diastolic heart function in chronic heart failure patients. Heart and vessels. 2012;27:576–584. doi: 10.1007/s00380-011-0190-9. [DOI] [PubMed] [Google Scholar]
- 24.Chua SC, Jr, Chung WK, Wu-Peng XS, Zhang Y, Liu SM, Tartaglia L, Leibel RL. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science (New York, NY) 1996;271:994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- 25.Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- 26.Crouse JA, Elliott GE, Burgess TL, Chiu L, Bennett L, Moore J, Nicolson M, Pacifici RE. Altered cell surface expression and signaling of leptin receptors containing the fatty mutation. The Journal of biological chemistry. 1998;273:18365–18373. doi: 10.1074/jbc.273.29.18365. [DOI] [PubMed] [Google Scholar]
- 27.da Silva BA, Bjorbaek C, Uotani S, Flier JS. Functional properties of leptin receptor isoforms containing the gln-->pro extracellular domain mutation of the fatty rat. Endocrinology. 1998;139:3681–3690. doi: 10.1210/endo.139.9.6168. [DOI] [PubMed] [Google Scholar]
- 28.de Castro Bras LE, Cates CA, Deleon-Pennell KY, Ma Y, Iyer RP, Halade GV, Yabluchanskiy A, Fields GB, Weintraub ST, Lindsey ML. Citrate Synthase is a Novel In Vivo Matrix Metalloproteinase-9 Substrate that Regulates Mitochondrial Function in the Post-Myocardial Infarction Left Ventricle. Antioxidants & redox signaling. 2014 doi: 10.1089/ars.2013.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Castro Bras LE, Ramirez TA, DeLeon-Pennell KY, Chiao YA, Ma Y, Dai Q, Halade GV, Hakala K, Weintraub ST, Lindsey ML. Texas 3-step decellularization protocol: looking at the cardiac extracellular matrix. Journal of proteomics. 2013;86:43–52. doi: 10.1016/j.jprot.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Caterina R. n-3 fatty acids in cardiovascular disease. The New England journal of medicine. 2011;364:2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- 31.de Oliveira Otto MC, Wu JH, Baylin A, Vaidya D, Rich SS, Tsai MY, Jacobs DR, Jr, Mozaffarian D. Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. Journal of the American Heart Association. 2013;2:e000506. doi: 10.1161/JAHA.113.000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Pablo MA, Alvarez de Cienfuegos G. Modulatory effects of dietary lipids on immune system functions. Immunology and cell biology. 2000;78:31–39. doi: 10.1046/j.1440-1711.2000.00875.x. [DOI] [PubMed] [Google Scholar]
- 33.Del Gobbo LC, Imamura F, Aslibekyan S, Marklund M, Virtanen JK, Wennberg M, Yakoob MY, Chiuve SE, Dela Cruz L, Frazier-Wood AC, Fretts AM, Guallar E, Matsumoto C, Prem K, Tanaka T, Wu JH, Zhou X, Helmer C, Ingelsson E, Yuan JM, Barberger-Gateau P, Campos H, Chaves PH, Djousse L, Giles GG, Gomez-Aracena J, Hodge AM, Hu FB, Jansson JH, Johansson I, Khaw KT, Koh WP, Lemaitre RN, Lind L, Luben RN, Rimm EB, Riserus U, Samieri C, Franks PW, Siscovick DS, Stampfer M, Steffen LM, Steffen BT, Tsai MY, van Dam RM, Voutilainen S, Willett WC, Woodward M, Mozaffarian D. omega-3 Polyunsaturated Fatty Acid Biomarkers and Coronary Heart Disease: Pooling Project of 19 Cohort Studies. JAMA internal medicine. 2016 doi: 10.1001/jamainternmed.2016.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delgado-Lista J, Perez-Martinez P, Lopez-Miranda J, Perez-Jimenez F. Long chain omega-3 fatty acids and cardiovascular disease: a systematic review. The British journal of nutrition. 2012;107(Suppl 2):S201–213. doi: 10.1017/S0007114512001596. [DOI] [PubMed] [Google Scholar]
- 35.Dhana K, Koolhaas CM, van Rossum EFC, Ikram MA, Hofman A, Kavousi M, Franco OH. Metabolically Healthy Obesity and the Risk of Cardiovascular Disease in the Elderly Population. PloS one. 2016;11:e0154273. doi: 10.1371/journal.pone.0154273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dick SA, Epelman S. Chronic Heart Failure and Inflammation: What Do We Really Know? Circulation research. 2016;119:159–176. doi: 10.1161/CIRCRESAHA.116.308030. [DOI] [PubMed] [Google Scholar]
- 37.Diwan A, Dibbs Z, Nemoto S, DeFreitas G, Carabello BA, Sivasubramanian N, Wilson EM, Spinale FG, Mann DL. Targeted overexpression of noncleavable and secreted forms of tumor necrosis factor provokes disparate cardiac phenotypes. Circulation. 2004;109:262–268. doi: 10.1161/01.CIR.0000109642.27985.FA. [DOI] [PubMed] [Google Scholar]
- 38.Doehner W, Clark A, Anker SD. The obesity paradox: weighing the benefit. European heart journal. 2010;31:146–148. doi: 10.1093/eurheartj/ehp339. [DOI] [PubMed] [Google Scholar]
- 39.Eilat-Adar S, Sinai T, Yosefy C, Henkin Y. Nutritional recommendations for cardiovascular disease prevention. Nutrients. 2013;5:3646–3683. doi: 10.3390/nu5093646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pinto X, Basora J, Munoz MA, Sorli JV, Martinez JA, Martinez-Gonzalez MA Investigators PS. Primary prevention of cardiovascular disease with a Mediterranean diet. The New England journal of medicine. 2013;368:1279–1290. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 41.Evans RM, Barish GD, Wang Y-X. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 42.Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, Lechler RI, DePaoli AM, O’Rahilly S. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fezeu LK, Laporte F, Kesse-Guyot E, Andreeva VA, Blacher J, Hercberg S, Galan P. Baseline Plasma Fatty Acids Profile and Incident Cardiovascular Events in the SU.FOL.OM3 Trial: The Evidence Revisited. PloS one. 2014;9:e92548. doi: 10.1371/journal.pone.0092548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Figlewicz DP, Ikeda H, Hunt TR, Stein LJ, Dorsa DM, Woods SC, Porte D., Jr Brain insulin binding is decreased in Wistar Kyoto rats carrying the ‘fa’ gene. Peptides. 1986;7:61–65. doi: 10.1016/0196-9781(86)90062-8. [DOI] [PubMed] [Google Scholar]
- 45.Finck BN, Lehman JJ, Barger PM, Kelly DP. Regulatory networks controlling mitochondrial energy production in the developing, hypertrophied, and diabetic heart. Cold Spring Harb Symp Quant Biol. 2002;67:371–382. doi: 10.1101/sqb.2002.67.371. [DOI] [PubMed] [Google Scholar]
- 46.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA : the journal of the American Medical Association. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 47.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nature reviews Cardiology. 2014;11:255–265. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Friedman DJ, Wang N, Meigs JB, Hoffmann U, Massaro JM, Fox CS, Magnani JW. Pericardial Fat is Associated With Atrial Conduction: The Framingham Heart Study. Journal of the American Heart Association. 2014;3:e000477. doi: 10.1161/JAHA.113.000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nature reviews Drug discovery. 2016;15:551–567. doi: 10.1038/nrd.2016.39. [DOI] [PubMed] [Google Scholar]
- 50.Gray SL, Dalla Nora E, Vidal-Puig AJ. Mouse models of PPAR-γ deficiency: dissecting PPAR-γ’s role in metabolic homoeostasis. Biochemical Society Transactions. 2005;33:1053–1058. doi: 10.1042/BST0331053. [DOI] [PubMed] [Google Scholar]
- 51.Halade GV, El Jamali A, Williams PJ, Fajardo RJ, Fernandes G. Obesity-mediated inflammatory microenvironment stimulates osteoclastogenesis and bone loss in mice. Experimental gerontology. 2011;46:43–52. doi: 10.1016/j.exger.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Halade GV, Kain V, Black LM, Prabhu SD, Ingle KA. Aging dysregulates D- and E-series resolvins to modulate cardiosplenic and cardiorenal network following myocardial infarction. Aging. 2016 doi: 10.18632/aging.101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halade GV, Kain V, Black LM, Prabhu SD, Ingle KA. Aging dysregulates D- and E-series resolvins to modulate cardiosplenic and cardiorenal network following myocardial infarction. Aging. 2016;8:2611–2634. doi: 10.18632/aging.101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halade GV, Ma Y, Ramirez TA, Zhang J, Dai Q, Hensler JG, Lopez EF, Ghasemi O, Jin YF, Lindsey ML. Reduced BDNF attenuates inflammation and angiogenesis to improve survival and cardiac function following myocardial infarction in mice. American journal of physiology Heart and circulatory physiology. 2013;305:H1830–1842. doi: 10.1152/ajpheart.00224.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Halade GV, Rahman MM, Williams PJ, Fernandes G. Combination of conjugated linoleic acid with fish oil prevents age-associated bone marrow adiposity in C57Bl/6J mice. The Journal of nutritional biochemistry. 2011;22:459–469. doi: 10.1016/j.jnutbio.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Halade GV, Rahman MM, Williams PJ, Fernandes G. High fat diet-induced animal model of age-associated obesity and osteoporosis. The Journal of nutritional biochemistry. 2010;21:1162–1169. doi: 10.1016/j.jnutbio.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hall JA, French TK, Rasmusson KD, Vesty JC, Roberts CA, Rimmasch HL, Kfoury AG, Renlund DG. The paradox of obesity in patients with heart failure. Journal of the American Academy of Nurse Practitioners. 2005;17:542–546. doi: 10.1111/j.1745-7599.2005.00084.x. [DOI] [PubMed] [Google Scholar]
- 58.Hassan Eftekhari M, Aliasghari F, Babaei-Beigi MA, Hasanzadeh J. Effect of conjugated linoleic acid and omega-3 fatty acid supplementation on inflammatory and oxidative stress markers in atherosclerotic patients. ARYA atherosclerosis. 2013;9:311–318. [PMC free article] [PubMed] [Google Scholar]
- 59.He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heaberlin JR, Ma Y, Zhang J, Ahuja SS, Lindsey ML, Halade GV. Obese and diabetic KKAy mice show increased mortality but improved cardiac function following myocardial infarction. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 2013;22:481–487. doi: 10.1016/j.carpath.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hill JO, Wyatt HR. The myth of healthy obesity. Annals of internal medicine. 2013;159:789–790. doi: 10.7326/0003-4819-159-11-201312030-00016. [DOI] [PubMed] [Google Scholar]
- 62.Hood WB, Jr, McCarthy B, Lown B. Myocardial infarction following coronary ligation in dogs. Hemodynamic effects of isoproterenol and acetylstrophanthidin. Circulation research. 1967;21:191–199. doi: 10.1161/01.res.21.2.191. [DOI] [PubMed] [Google Scholar]
- 63.Horwich TB, Fonarow GC. The impact of obesity on survival in patients with heart failure. Heart failure monitor. 2002;3:8–14. [PubMed] [Google Scholar]
- 64.Howes LG. Selective COX-2 inhibitors, NSAIDs and cardiovascular events - is celecoxib the safest choice? Ther Clin Risk Manag. 2007;3:831–845. [PMC free article] [PubMed] [Google Scholar]
- 65.Huang H, Amin V, Gurin M, Wan E, Thorp E, Homma S, Morrow JP. Diet-induced obesity causes long QT and reduces transcription of voltage-gated potassium channels. Journal of molecular and cellular cardiology. 2013;59:151–158. doi: 10.1016/j.yjmcc.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hummel SL, Seymour EM, Brook RD, Sheth SS, Ghosh E, Zhu S, Weder AB, Kovacs SJ, Kolias TJ. Low-sodium DASH diet improves diastolic function and ventricular-arterial coupling in hypertensive heart failure with preserved ejection fraction. Circulation Heart failure. 2013;6:1165–1171. doi: 10.1161/CIRCHEARTFAILURE.113.000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ingle KA, Kain V, Goel M, Prabhu SD, Young ME, Halade GV. Cardiomyocyte specific Bmal1 deletion in mice triggers diastolic dysfunction, extracellular matrix response and impaired resolution of inflammation. American journal of physiology Heart and circulatory physiology. 2015 doi: 10.1152/ajpheart.00608.2015. ajpheart.00608.02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnson AR, Milner JJ, Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev. 2012;249:218–238. doi: 10.1111/j.1600-065X.2012.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones SP, Girod WG, Granger DN, Palazzo AJ, Lefer DJ. Reperfusion injury is not affected by blockade of P-selectin in the diabetic mouse heart. The American journal of physiology. 1999;277:H763–769. doi: 10.1152/ajpheart.1999.277.2.H763. [DOI] [PubMed] [Google Scholar]
- 70.Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15:6184–6223. doi: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kain V, Prabhu SD, Halade GV. Inflammation revisited: inflammation versus resolution of inflammation following myocardial infarction. Basic research in cardiology. 2014;109:444. doi: 10.1007/s00395-014-0444-7. [DOI] [PubMed] [Google Scholar]
- 72.Kar S. Role of omega-3 fatty acids in the prevention of atrial fibrillation. Reviews in cardiovascular medicine. 2013;14:e82–91. doi: 10.3909/ricm0620. [DOI] [PubMed] [Google Scholar]
- 73.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. The New England journal of medicine. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 74.Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PloS one. 2012;7:e39504. doi: 10.1371/journal.pone.0039504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lawrence GD. Dietary fats and health: dietary recommendations in the context of scientific evidence. Advances in nutrition. 2013;4:294–302. doi: 10.3945/an.113.003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lefer DJ, Scalia R, Jones SP, Sharp BR, Hoffmeyer MR, Farvid AR, Gibson MF, Lefer AM. HMG-CoA reductase inhibition protects the diabetic myocardium from ischemia-reperfusion injury. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2001;15:1454–1456. doi: 10.1096/fj.00-0819fje. [DOI] [PubMed] [Google Scholar]
- 77.Levitan EB, Wolk A, Mittleman MA. Consistency with the DASH diet and incidence of heart failure. Archives of internal medicine. 2009;169:851–857. doi: 10.1001/archinternmed.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin PH, Yeh WT, Svetkey LP, Chuang SY, Chang YC, Wang C, Pan WH. Dietary intakes consistent with the DASH dietary pattern reduce blood pressure increase with age and risk for stroke in a Chinese population. Asia Pacific journal of clinical nutrition. 2013;22:482–491. [PubMed] [Google Scholar]
- 79.Lissin LW, Gauri AJ, Froelicher VF, Ghayoumi A, Myers J, Giacommini J. The prognostic value of body mass index and standard exercise testing in male veterans with congestive heart failure. Journal of cardiac failure. 2002;8:206–215. doi: 10.1054/jcaf.2002.126812. [DOI] [PubMed] [Google Scholar]
- 80.Lopez EF, Kabarowski JH, Ingle KA, Kain V, Barnes S, Crossman DK, Lindsey ML, Halade GV. Obesity superimposed on aging magnifies inflammation and delays the resolving response after myocardial infarction. American journal of physiology Heart and circulatory physiology. 2015;308:H269–280. doi: 10.1152/ajpheart.00604.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marangoni F, Novo G, Perna G, Perrone Filardi P, Pirelli S, Ceroti M, Querci A, Poli A. Omega-6 and omega-3 polyunsaturated fatty acid levels are reduced in whole blood of Italian patients with a recent myocardial infarction: the AGE-IM study. Atherosclerosis. 2014;232:334–338. doi: 10.1016/j.atherosclerosis.2013.11.048. [DOI] [PubMed] [Google Scholar]
- 82.Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. Journal of the American College of Cardiology. 2008;52:1201–1210. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martinez-Gonzalez MA, Bes-Rastrollo M. Dietary patterns, Mediterranean diet, and cardiovascular disease. Current opinion in lipidology. 2014;25:20–26. doi: 10.1097/MOL.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 84.Mayer J, Bates MW, Dickie MM. Hereditary diabetes in genetically obese mice. Science (New York, NY) 1951;113:746–747. doi: 10.1126/science.113.2948.746. [DOI] [PubMed] [Google Scholar]
- 85.Mosterd A, Cost B, Hoes AW, de Bruijne MC, Deckers JW, Hofman A, Grobbee DE. The prognosis of heart failure in the general population: The Rotterdam Study. European heart journal. 2001;22:1318–1327. doi: 10.1053/euhj.2000.2533. [DOI] [PubMed] [Google Scholar]
- 86.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. Journal of the American College of Cardiology. 2010;56:1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 87.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. Journal of the American College of Cardiology. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 88.Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science (New York, NY) 2013;341:1483–1488. doi: 10.1126/science.1240636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O’Brien EC, Fosbol EL, Peng SA, Alexander KP, Roe MT, Peterson ED. Association of body mass index and long-term outcomes in older patients with non-ST-segment-elevation myocardial infarction: results from the CRUSADE Registry. Circ Cardiovasc Qual Outcomes. 2014;7:102–109. doi: 10.1161/CIRCOUTCOMES.113.000421. [DOI] [PubMed] [Google Scholar]
- 90.Olijhoek JK, van der Graaf Y, Banga JD, Algra A, Rabelink TJ, Visseren FL, Group SS. The metabolic syndrome is associated with advanced vascular damage in patients with coronary heart disease, stroke, peripheral arterial disease or abdominal aortic aneurysm. European heart journal. 2004;25:342–348. doi: 10.1016/j.ehj.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 91.Oltman CL, Richou LL, Davidson EP, Coppey LJ, Lund DD, Yorek MA. Progression of coronary and mesenteric vascular dysfunction in Zucker obese and Zucker diabetic fatty rats. American journal of physiology Heart and circulatory physiology. 2006;291:H1780–1787. doi: 10.1152/ajpheart.01297.2005. [DOI] [PubMed] [Google Scholar]
- 92.Phillips CM. Metabolically healthy obesity: definitions, determinants and clinical implications. Rev Endocr Metab Disord. 2013;14:219–227. doi: 10.1007/s11154-013-9252-x. [DOI] [PubMed] [Google Scholar]
- 93.Poncelas M, Inserte J, Vilardosa U, Rodriguez-Sinovas A, Baneras J, Simo R, Garcia-Dorado D. Obesity induced by high fat diet attenuates postinfarct myocardial remodeling and dysfunction in adult B6D2F1 mice. J Mol Cell Cardiol. 2015;84:154–161. doi: 10.1016/j.yjmcc.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 94.Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, Samal UC, Shimokawa H, Budi Siswanto B, Sliwa K, Filippatos G. Heart failure: preventing disease and death worldwide. ESC Heart Failure. 2014;1:4–25. doi: 10.1002/ehf2.12005. [DOI] [PubMed] [Google Scholar]
- 95.Praticò D, Dogné J-M. Selective Cyclooxygenase-2 Inhibitors Development in Cardiovascular Medicine. Circulation. 2005;112:1073–1079. doi: 10.1161/CIRCULATIONAHA.104.524231. [DOI] [PubMed] [Google Scholar]
- 96.Punchard NA, Whelan CJ, Adcock I. The Journal of Inflammation. J Inflamm (Lond) 2004;1:1. doi: 10.1186/1476-9255-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rees K, Hartley L, Flowers N, Clarke A, Hooper L, Thorogood M, Stranges S. ‘Mediterranean’ dietary pattern for the primary prevention of cardiovascular disease. The Cochrane database of systematic reviews. 2013;8:CD009825. doi: 10.1002/14651858.CD009825.pub2. [DOI] [PubMed] [Google Scholar]
- 98.Remondino A, Rosenblatt-Velin N, Montessuit C, Tardy I, Papageorgiou I, Dorsaz PA, Jorge-Costa M, Lerch R. Altered expression of proteins of metabolic regulation during remodeling of the left ventricle after myocardial infarction. J Mol Cell Cardiol. 2000;32:2025–2034. doi: 10.1006/jmcc.2000.1234. [DOI] [PubMed] [Google Scholar]
- 99.Riquelme CA, Magida JA, Harrison BC, Wall CE, Marr TG, Secor SM, Leinwand LA. Fatty acids identified in the Burmese python promote beneficial cardiac growth. Science (New York, NY) 2011;334:528–531. doi: 10.1126/science.1210558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, Ross J, Jr, Chien KR. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sala-Vila A, Guasch-Ferre M, Hu FB, Sanchez-Tainta A, Bullo M, Serra-Mir M, Lopez-Sabater C, Sorli JV, Aros F, Fiol M, Munoz MA, Serra-Majem L, Martinez JA, Corella D, Fito M, Salas-Salvado J, Martinez-Gonzalez MA, Estruch R, Ros E. Dietary alpha-Linolenic Acid, Marine omega-3 Fatty Acids, and Mortality in a Population With High Fish Consumption: Findings From the PREvencion con DIeta MEDiterranea (PREDIMED) Study. Journal of the American Heart Association. 2016;5 doi: 10.1161/JAHA.115.002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Salehi-Abargouei A, Maghsoudi Z, Shirani F, Azadbakht L. Effects of Dietary Approaches to Stop Hypertension (DASH)-style diet on fatal or nonfatal cardiovascular diseases--incidence: a systematic review and meta-analysis on observational prospective studies. Nutrition. 2013;29:611–618. doi: 10.1016/j.nut.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 103.Salter AM. Dietary fatty acids and cardiovascular disease. Animal : an international journal of animal bioscience. 2013;7(Suppl 1):163–171. doi: 10.1017/S1751731111002023. [DOI] [PubMed] [Google Scholar]
- 104.Schargrodsky H, Rozlosnik J, Ciruzzi M, Ruffa R, Paterno C, Ardariz M, Caccavo A, D’Avanzo B, Negri E, La Vecchia C, et al. Body weight and nonfatal myocardial infarction in a case-control study from Argentina. Soz Praventivmed. 1994;39:126–133. doi: 10.1007/BF01299656. [DOI] [PubMed] [Google Scholar]
- 105.Stanley WC, Dabkowski ER, Ribeiro RF, Jr, O’Connell KA. Dietary fat and heart failure: moving from lipotoxicity to lipoprotection. Circulation research. 2012;110:764–776. doi: 10.1161/CIRCRESAHA.111.253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stepien M, Stepien A, Wlazel RN, Paradowski M, Banach M, Rysz J. Obesity indices and inflammatory markers in obese non-diabetic normo- and hypertensive patients: a comparative pilot study. Lipids Health Dis. 2014;13:29. doi: 10.1186/1476-511X-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stepien M, Wlazel RN, Paradowski M, Banach M, Rysz M, Misztal M, Rysz J. Serum concentrations of adiponectin, leptin, resistin, ghrelin and insulin and their association with obesity indices in obese normo- and hypertensive patients - pilot study. Arch Med Sci. 2012;8:431–436. doi: 10.5114/aoms.2012.29397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 109.Thakker GD, Frangogiannis NG, Bujak M, Zymek P, Gaubatz JW, Reddy AK, Taffet G, Michael LH, Entman ML, Ballantyne CM. Effects of diet-induced obesity on inflammation and remodeling after myocardial infarction. American journal of physiology Heart and circulatory physiology. 2006;291:H2504–2514. doi: 10.1152/ajpheart.00322.2006. [DOI] [PubMed] [Google Scholar]
- 110.Thomsen M, Nordestgaard BG. Myocardial Infarction and Ischemic Heart Disease in Overweight and Obesity With and Without Metabolic Syndrome. JAMA internal medicine. 2013 doi: 10.1001/jamainternmed.2013.10522. [DOI] [PubMed] [Google Scholar]
- 111.Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity. 2014;40:833–842. doi: 10.1016/j.immuni.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 112.Thorvaldsen T, Benson L, Stahlberg M, Dahlstrom U, Edner M, Lund LH. Triage of patients with moderate to severe heart failure: who should be referred to a heart failure center? Journal of the American College of Cardiology. 2014;63:661–671. doi: 10.1016/j.jacc.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 113.Tsai AG, Williamson DF, Glick HA. Direct medical cost of overweight and obesity in the USA: a quantitative systematic review. Obes Rev. 2011;12:50–61. doi: 10.1111/j.1467-789X.2009.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.van Bilsen M, Smeets PJ, Gilde AJ, van der Vusse GJ. Metabolic remodelling of the failing heart: the cardiac burn-out syndrome? Cardiovasc Res. 2004;61:218–226. doi: 10.1016/j.cardiores.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 115.van den Tweel JG, Taylor CR. A brief history of pathology: Preface to a forthcoming series that highlights milestones in the evolution of pathology as a discipline. Virchows Arch. 2010;457:3–10. doi: 10.1007/s00428-010-0934-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Van Horn L, Yancy C. Diet prevention and therapy for heart failure? Circulation Heart failure. 2013;6:1109–1111. doi: 10.1161/CIRCHEARTFAILURE.113.000868. [DOI] [PubMed] [Google Scholar]
- 117.Viola J, Soehnlein O. Atherosclerosis - A matter of unresolved inflammation. Seminars in immunology. 2015;27:184–193. doi: 10.1016/j.smim.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 118.Wang B, Chandrasekera PC, Pippin JJ. Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr Diabetes Rev. 2014;10:131–145. doi: 10.2174/1573399810666140508121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang F, Mullican SE, DiSpirito JR, Peed LC, Lazar MA. Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARgamma. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18656–18661. doi: 10.1073/pnas.1314863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wendel AA, Belury MA. Effects of conjugated linoleic acid and troglitazone on lipid accumulation and composition in lean and Zucker diabetic fatty (fa/fa) rats. Lipids. 2006;41:241–247. doi: 10.1007/s11745-006-5093-7. [DOI] [PubMed] [Google Scholar]
- 121.Wilbring M, Ploetze K, Bormann S, Waldow T, Matschke K. Omega-3 Polyunsaturated Fatty Acids Reduce the Incidence of Postoperative Atrial Fibrillation in Patients with History of Prior Myocardial Infarction Undergoing Isolated Coronary Artery Bypass Grafting. The Thoracic and cardiovascular surgeon. 2014 doi: 10.1055/s-0034-1371699. [DOI] [PubMed] [Google Scholar]
- 122.Wilhelmsen L, Rosengren A, Eriksson H, Lappas G. Heart failure in the general population of men--morbidity, risk factors and prognosis. Journal of internal medicine. 2001;249:253–261. doi: 10.1046/j.1365-2796.2001.00801.x. [DOI] [PubMed] [Google Scholar]
- 123.Wood KE, Lau A, Mantzioris E, Gibson RA, Ramsden CE, Muhlhausler BS. A low omega-6 polyunsaturated fatty acid (n-6 PUFA) diet increases omega-3 (n-3) long chain PUFA status in plasma phospholipids in humans. Prostaglandins, leukotrienes, and essential fatty acids. 2014 doi: 10.1016/j.plefa.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yue TL, Bao W, Gu JL, Cui J, Tao L, Ma XL, Ohlstein EH, Jucker BM. Rosiglitazone treatment in Zucker diabetic Fatty rats is associated with ameliorated cardiac insulin resistance and protection from ischemia/reperfusion-induced myocardial injury. Diabetes. 2005;54:554–562. doi: 10.2337/diabetes.54.2.554. [DOI] [PubMed] [Google Scholar]
- 125.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 126.Zhu J, Su X, Li G, Chen J, Tang B, Yang Y. The incidence of acute myocardial infarction in relation to overweight and obesity: a meta-analysis. Arch Med Sci. 2014;10:855–862. doi: 10.5114/aoms.2014.46206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zierath JR, Ryder JW, Doebber T, Woods J, Wu M, Ventre J, Li Z, McCrary C, Berger J, Zhang B, Moller DE. Role of skeletal muscle in thiazolidinedione insulin sensitizer (PPARgamma agonist) action. Endocrinology. 1998;139:5034–5041. doi: 10.1210/endo.139.12.6364. [DOI] [PubMed] [Google Scholar]