Abstract
Hepatocellular carcinoma (HCC) is the third most common cause of death from cancer, with increasing prevalence worldwide. The mortality rate of HCC is similar to its incidence rate, which reflects its poor prognosis. At present, the diagnosis of HCC is still mostly dependent on invasive biopsy, imaging methods, and serum α-fetoprotein (AFP) testing. Because of the asymptomatic nature of early HCC, biopsy and imaging methods usually detect HCC at the middle–late stages. AFP has limited sensitivity and specificity, as many other nonmalignant liver diseases can also result in a very high serum level of AFP. Therefore, better biomarkers with higher sensitivity and specificity at earlier stages are greatly needed. Since metabolic reprogramming is an essential hallmark of cancer and the liver is the metabolic hub of living systems, it is useful to investigate HCC from a metabolic perspective. As a noninvasive and nondestructive approach, metabolomics provides holistic information on dynamically metabolic responses of living systems to both endogenous and exogenous factors. Therefore, it would be conducive to apply metabolomics in investigating HCC. In this review, we summarize recent metabolomic studies on HCC cellular, animal, and clinicopathologic models with attention to metabolomics as a biomarker in cancer diagnosis. Recent applications of metabolomics with respect to therapeutic and prognostic evaluation of HCC are also covered, with emphasis on the potential of treatment by drugs from natural products. In the last section, the current challenges and trends of future development of metabolomics on HCC are discussed. Overall, metabolomics provides us with novel insight into the diagnosis, prognosis, and therapeutic evaluation of HCC.
Keywords: hepatocellular carcinoma, metabolomics, biomarker, diagnosis and therapy
Introduction
Hepatocellular carcinoma (HCC) is the third most common cause of death from cancer, with increasing prevalence worldwide. The mortality rate of HCC is similar to its incidence rate, which reflects its poor prognosis.1 HCC can be initiated by various factors, including genetic and environmental factors. The majority of HCC comes with a background of liver cirrhosis, primarily induced by two prominent risk factors: hepatitis B and C viruses (HBV and HCV) infection.2 Chronic HBV infection consists of 52.3% and chronic HCV infection 20% of all HCC cases all over the world.3 HBV is the leading etiologic factor for HCC in developing regions, such as western Africa and Southeast Asia, whereas HCV contributes to the most risk in developed countries. These two potent risk factors shape the geographical variation in HCC incidence, which in the developing world is higher than that in the developed world. Also, hereditary conditions, including genetic tyrosinemia, α1-antitrypsin deficiency, and hemochromatosis, can lead to HCC. An escalating amount of lifestyle-related risk factors, such as chronic alcohol abuse, tobacco smoke, betel-quid chewing, high aflatoxin intake, obesity, and diabetes, have also been linked with HCC.4–7 Invalid diagnosis and treatment of HCC often result in its high mortality rate, which poses a big threat to public health. Currently, the diagnosis of HCC is still mostly dependent on invasive biopsy, imaging methods, and serum α-fetoprotein (AFP) testing.8 Because of early HCC asymptomatic nature, biopsy and imaging methods usually detect HCC at middle or late stages, for which there are no effectively therapeutic options. For the clinical diagnosis of HCC, serum AFP, a fetal serum glycoprotein, has been considered a golden biomarker. However, its sensitivity and specificity is very limited, because many other nonmalignant liver diseases, such as acute and chronic hepatitis and liver cirrhosis, can also result in a very high serum level of AFP that is similar to that detected in HCC.9 Therefore, better biomarkers are greatly needed, which would improve clinical diagnosis and therapeutic treatment of HCC at earlier disease stages and ultimately result in lower mortality rates. An ideal biomarker should satisfy the following conditions: target molecules with high sensitivity and specificity; markers to be detectable via a noninvasive way, such as blood and urine; and measurement techniques should be cheap, reliable, and robust across an extensive range of populations.
As the newest “-omics” science, metabolomics is the systematic study of small-molecule metabolites in living systems, which is defined as the “metabolome” (metabolites with an atomic mass <1.5 kDa).10 This approach offers holistic information on dynamically metabolic responses of living systems to both endogenous and exogenous factors. The target of metabolomics is to detect and identify global small-molecule metabolic profiles of complex biological matrices. Compared with other -omics technologies (namely genomics, transcriptomics, and proteomics), where modification of substrates commonly occurs, metabolomics can provide information on metabolites that are directly produced in response to endogenous and exogenous factors.11 It provides potential biomarkers for the diagnosis and monitoring of complex diseases and response to therapeutic intervention.12,13 It has been widely applied in the domains of disease diagnosis, therapeutic monitoring, and pharmacodynamic evaluation.14–16 Cell metabolism plays an important role in cancer, and metabolic reprogramming is an essential hallmark of cancer.17 Cancer cells have been reported to own a significantly unique metabolic phenotype to support their high proliferation rates, which is highlighted by high glycolytic rates, enriched phospholipid turnover, low mitochondrial activity, and decreased bioenergetic expenditure.18 Cancer metabolism has become a “hot spot” and is gaining momentum for better mechanistic research of tumorigenesis. Therefore, it is useful to investigate cancer from a metabolic perspective.
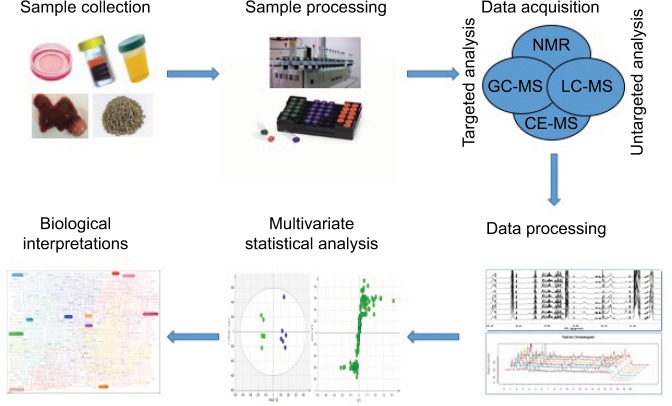
As the metabolic hub of the human body with various metabolic functions, the liver is able to mediate the expression levels of numerous metabolites. It is well accepted that metabolic-profile variations occur prior to imaging diagnosis in HCC patients. Owing to this, HCC has become a disease model of great interest from a metabolic perspective.19,20 More specifically, metabolomics is a noninvasive and nondestructive analysis. Therefore, it would be conducive to apply metabolomics to understand the complex pathophysiology, discover new biomarkers, and evaluate new therapeutic drug targets of HCC. There has been an increasing number of metabolomic studies on HCC over the last few decades.21,22 The typical processing flow of metabolomics studies on HCC is shown in Figure 1. Firstly, samples for HCC are collected. There are different kinds of samples used for HCC, including in vitro HCC cells, in vivo plasma, serum, urine, feces, and liver tumor and nontumor tissue. Collected samples are preprocessed and tested by different approaches. There are two main analytical platforms used in metabolomic studies on HCC: nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS). The latter is usually equipped with different separation instruments, including liquid chromatography (LC), gas chromatography (GC), and capillary electrophoresis (CE). Each platform has its own strengths and weaknesses. After acquisition, the data obtained are processed and analyzed to find metabolites changed majorly by multivariate statistical analysis, such as principal-component analysis and orthogonal projection to latent structure-discriminant analysis. Lastly, according to databases and related literature, the metabolites involved and corresponding pathways are elucidated and the specific biomarkers identified.
Figure 1.
Typical processing flow of metabolomics in HCC.
Notes: Proposed standards for metabolomics on HCC are presented in this schematic view. The first step is to collect the samples. Then, the collected samples are preprocessed and tested by different approaches. After acquisition, obtained data are processed and analyzed by multivariate statistical analysis. Lastly, the underlying biological interpretations are elucidated.
Abbreviations: CE, capillary electrophoresis; GC, gas chromatography; HCC, hepatocellular carcinoma; LC, liquid chromatography; MS, mass spectrometry; NMR, nuclear magnetic resonance.
For this review, we retrieved data from metabolomic studies on HCC in the prior 7 years from the databases PubMed and Google Scholar using the five keywords “hepatocellular carcinoma”, “metabolomics”, “biomarker”, “diagnosis”, and “therapy”. First, we summarized the recent metabolomic studies on HCC cellular, animal, and clinicopathologic models with attention to its use as a biomarker in cancer diagnosis. Subsequently, recent applications of metabolomics with respect to therapeutic and prognostic evaluation of HCC were also focused upon, with emphasis on the potential of treatment by drugs from natural products. In the last section, current challenges of and trends in future development of metabolomics with regard to HCC are discussed. Overall, metabolomics provides us a novel insight into diagnosis, prognosis, and therapeutic evaluation of HCC.
Metabolomics in HCC-model research
In this section, we summarize recent metabolomic studies on HCC cellular, animal, and clinicopathologic models with attention to their use as biomarkers in cancer diagnosis. Table 1 depicts detailed information on four metabolomic studies on HCC cells and nine HCC animal models published recently, and Table 2 includes detailed information on 37 metabolomic studies of human HCC samples. Overall, these studies illustrated the majorly altered metabolites and corresponding metabolic pathways involved in stepwise hepatocarcinogenesis, suggesting that metabolomics could be a promising strategy of biomarker discovery for the early diagnosis of HCC.
Table 1.
Summary of recent metabolomics studies on HCC cellular and animal models
| Reference | PubMed ID | Study | Method | Significantly changed metabolites or pathways | Main findings | Validation |
|---|---|---|---|---|---|---|
| 25 | 25672227 | HepG2.2.15 and HepG2 cells | 1H-NMR | HBV infection upregulated the biosynthesis of hexosamine and phosphatidylcholine, induces oxidative stress, and stimulates central carbon metabolism and nucleotide synthesis | HBV-associated hepatocellular carcinoma could be attributed to GFAT1-activated hexosamine biosynthesis and CHKA-activated phosphatidylcholine biosynthesis | Yes |
| 27 | 27075403 | HepG2 cells infected by Ad-HBx | 1H-NMR and gene-expression profiles | HBx disrupted metabolism of glucose, lipids, and amino acids, especially nucleic acids | HBx initially induces DNA damage and then disrupts nucleic acid metabolism, which in turn blocks DNA repair and induces HCC | Yes |
| 28 | 24163401 | HepG2.2.15 and HepG2 cells | 1H-NMR | siRNA to E4F1 included reduction in glutamate, glutathione, acetate, and leucine, as well as increases in phosphocholine, lipid glycerol, and several lipid species | E4F1 may neutralize the capacity of HBx to activate a p53-dependent, metabolic, and growth-arrest phenotype in liver cells | No |
| 29 | 28112229 | HepG2 cells transfected with HBc | 1H-NMR and proteomics | Glycolysis and amino acid metabolism were significantly upregulated by HBc | MLX might be recruited and enriched by HBc in the nucleus to regulate glycolysis pathways | No |
| 30 | 24763554 | PNPLA3 silencing and overexpression of Huh7 cells | GC-MS and LC-MS | Silencing of PNPLA3 declined amino acid metabolism and elevated the levels of myoinositol, cysteine sulfinic acid, polyunsaturated fatty acids, lysolipids, and sphingolipids, whereas overexpression of PNPLA3 mirrored metabolic changes in the opposite direction | These results suggest a critical role of PNPLA3 in the modulation of liver metabolism beyond its classical participation in triacylglycerol remodeling | No |
| 32 | 24127579 | Primary hepatocytes from DEN-induced HCC C57BL/6J | LC-MS/MS | Loss of ERRα promotes hepatocyte necrosis over apoptosis in response to DEN, due to a deficiency in energy production | Loss of ERRα activity promotes HCC by independent but synergistic mechanisms in hepatocytes and Kupffer cells | No |
| 33 | 22084000 | Serum from DEN-induced rat HCC model | LC-MS | Three metabolites – taurocholic acid, lysophosphoethanolamine 16:0, and lysophosphatidylcholine 22:5 – were defined as “marker metabolites” | Three marker metabolites were effective for discrimination of HCC patients, better than AFP | Yes |
| 34 | 26526930 | Serum from DEN-induced rat HCC model | CE-TOF-MS | A novel biomarker pattern of creatine:betaine ratio that reflected the balance of methylation was identified | This ratio biomarker can also improve the diagnostic performance of AFP | Yes |
| 35 | 27578360 | Serum from DEN-induced rat HCC model | LC-MS | A ratio of LPC 18:1/FFA 20:5 was identified as the potential biomarker for HCC | The better performance of ATSD-DN suggested its potential to present time-series changes well and effectively extract early-warning information | Yes |
| 36 | 20814984 | Serum and urine from HLM rat model | GC/TOFMS | Glutamate metabolism and glycolysis were increased, while the TCA cycle was decreased in both HCC and HLM | Metabolism of glucuronic acid, amino acids, and nucleic acids increased only in HLM | No |
| 37 | 20890798 | Tumor tissue from HLM rat model | 1H-NMR | Tumor tissue from HLM showed changes in glucose, lactate, choline, lipids, and some amino acids, such as glycine | Alterations in glycolysis and the metabolism of glycine and choline occur during HCC invasion and metastasis | No |
| 38 | 25594851 | Serum and liver from HBx transgenic mouse model | GC-MS | Lipid (fatty acids, triglycerides, and cholesterol) profiles changed significantly during development of HBx tumorigenesis | Metabolic syndrome plays an important role in HBV tumorigenesis, and dysregulation of lipid metabolism may predict disease progression to HCC in chronic HBV patients | No |
| 39 | 28941178 | Liver tissues from Ras-Tg mice model | Transcriptomics and GC-TOF-MS-based metabolomics | Hras12V oncogene induced perturbations of glycolysis, pentose phosphate pathway, TCA cycle, lipid metabolism, bile-acid synthesis, and redox homeostasis | These altered metabolic processes may play crucial roles in the carcinogenesis, development, and pathological characteristics of HCC | No |
| 40 | 21763219 | Serum from nude mice bearing HepG2 cells | UHPLC/QTOF-MS | Metabolic alterations of LPCs were observed in liver injury and HCC | LPC profile in serum may be biomarker for liver injury and HCC | No |
Abbreviations: Ad, adenovirus; AFP, α-fetoprotein; ATSD-DN, analysis of time-series data based on dynamic networks; CE, capillary electrophoresis; CHKA, choline kinase alpha; DEN, diethylnitrosamine; ERRα, estrogen-related receptor alpha; FFA, free fatty acid; GC, gas chromatography; GFAT1, glutamine-fructose-6-phosphate amidotransferase 1; HBc, hepatitis B virus core protein; HBx, hepatitis B virus X protein; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HLM, HCC with lung metastasis; LC, liquid chromatography; LPC, lysophosphatidylcholine; MLX, Max-like protein X; MS, mass spectrometry; NMR, nuclear magnetic resonance; QTOF, quadrupole time of flight; TCA, tricarboxylic acid; TOF, time of flight; UHPLC, ultrahigh-performance LC.
Table 2.
Summary of recent metabolomic studies on HCC clinicopathologic models
| Reference | PubMed ID | Study | Method | Significantly changed metabolites or pathways | Main findings | Validation | ||
|---|---|---|---|---|---|---|---|---|
| 41 | 26130468 | Serum from 114 HCC cases and 222 matched controls | 1H-NMR | HCC metabolic counterpart displayed positive loading on ethanol, glutamate, and phenylalanine | This study devised a way to bridge lifestyle variables to HCC risk through NMR metabolomic data | No | ||
| 42 | 26399231 | Serum from 114 primary HCC cases and 222 matched controls | 1H-NMR | A metabolic pattern associated with HCC risk comprising perturbations in fatty-acid oxidation and amino acid, lipid, and carbohydrate metabolism was observed | Results showed clear metabolic alterations from early stages of HCC development, with application for better etiologic understanding, prevention, and early detection of HCC | No | ||
| 43 | 21833635 | Serum from 41 HCC patients and 38 healthy controls | LC-MS | The serum metabolite 1-methyladenosine was identified as the characteristic metabolite for HCC | A combination of 1-methyladenosine and AFP exhibited significantly improved sensitivity | No | ||
| 44 | 23889541 | Serum from 29 HCC patients and 30 age-matched healthy controls | UHPLC/QqQ-MS | Lower lysophosphatidylcholine, medium-chain acylcarnitines and branched-chain amino acid, as well as enriched long-chain acylcarnitines and aromatic amino acid were observed in HCC patients | This work provides an approach to acquire multiple-reaction-monitoring ion pairs from real samples and a foundation to achieve pseudotargeted metabolomic analysis | No | ||
| 45 | 24611595 | Serum from 30 HCC and 30 healthy volunteers | LC-MS | In total, 609 and 1,084 ion pairs were found meeting one or more criteria for fusion | The developed method can be an effective tool to process high-resolution mass spectrometry data in “omics” studies | No | ||
| 46 | 26805550 | Urine from 25 HCC patients and 12 matched healthy controls | LC-QTOF-MS | Citric acid cycle, bile-acid biosynthesis, urea-cycle metabolism, and tryptophan metabolism were significantly changed in HCC group | Five marker metabolites were effective for diagnosis of human HCC | Yes | ||
| 47 | 23313056 | Urine from 25 HCC patients and 12 control subjects | UHPLC-QTOF-HD-MS | Glycocholic acid expression was upregulated in urine samples associated with HCC | The network generation clearly enhances the interpretation and understanding of mechanisms for glycocholic acid | No | ||
| 48 | 21518826 | Serum and urine from 82 HCC patients, 24 benign-liver-tumor patients, and 71 healthy controls | GC-TOF-MS and UHPLC-QTOF-MS | 43 serum metabolites and 31 urine metabolites were identified in HCC patients involving the metabolisms of glycolysis, free fatty acids, bile acids, methionine, and urea cycle | The identified biomarkers differentiated HCC patients with AFP levels lower than 20 ng/mL from healthy controls with 100% accuracy | No | ||
| 49 | 23136190 | Serum from 93 lung carcinomata, 28 small HCC, and 33 large HCC | 1H-NMR | Compared with cirrhosis, levels of glutamate, acetate, and N-acetyl glycoproteins increased, while levels of lipids and glutamine decreased in large HCC | Serum NMR-based metabolomics identified metabolic fingerprints that could be specific to large HCC in cirrhotic livers | No | ||
| 50 | 22882828 | Serum from 78 HCC cases and 184 cirrhotic controls | UHPLC-QTOF-MS | Metabolites involved in sphingolipid metabolism and phospholipid catabolism upregulated in sera of HCC vs those with liver cirrhosis; downregulated metabolites included those involved in bile-acid biosynthesis (specifically cholesterol metabolism) | Metabolic profiling is a promising tool to identify candidate metabolic biomarkers for early detection of HCC cases in high-risk population of cirrhotic patients | Yes | ||
| 51 | 23078175 | Serum from 40 HCC patients and 49 cirrhosis patients from Egypt | UHPLC-QTOF-MS | Significant differences between HCC and cirrhotic controls in levels of bile-acid metabolites, long-chain carnitines, and small peptides were observed | This was the first MS-based metabolic biomarker-discovery study on Egyptian subjects | No | ||
| 52 | 24382646 | Serum from 43 HCC patients, 42 lung cirrhosis patients, and 18 healthy volunteers | NMR and LC-MS | HCC induced disturbances of citrate cycle, amino acid catabolism, fatty-acid oxidation, and phospholipid metabolism and synthesis of ketone bodies, bile-acid metabolism, and sphingolipid metabolism | These potential biomarkers appeared to have diagnostic and/or prognostic values for HCC, which deserve to be further investigated | Yes | ||
| 53 | 24853826 | Serum from 30 healthy controls, 25 cirrhosis subjects, and 22 HCC patients | CE-TOF-MS | Tryptophan, glutamine, and 2-hydroxybutyric acid were finally established | Serum-biomarker model enabled the discrimination of small HCC from precancer cirrhosis with an AUC of 0.976 | Yes | ||
| 54 | 24661807 | Serum from 30 HCC patients, 27 hepatitis C cirrhosis disease controls and 30 healthy volunteers | GC-MS and UPLC-MS | Elevated 12-hydroxyeicosatetraenoic acid (12-HETE), 15-HETE, sphingosine, xanthine, serine, glycine, aspartate, and acylcarnitines were strongly associated with HCC | Aberrant amino acid biosynthesis, cell-turnover regulation, reactive oxygen-species neutralization, and eicosanoid pathways may be hallmarks of HCC | No | ||
| 55 | 26030804 | Plasma from 40 HCC cases and 49 patients with liver cirrhosis | GC-QMS and GC-TOF-MS | Significant changes in levels of glutamic acid, citric acid, lactic acid, valine, isoleucine, leucine, α-tocopherol, cholesterol, and sorbose were observed | Findings indicated upregulation of metabolites involved in branched-chain amino acid metabolism | Yes | ||
| 56 | 27913395 | Plasma from 63 HCC cases and 65 cirrhotic controls | GC-MS | Eleven metabolites and three clinical covariates that differentiated HCC cases from cirrhotic controls were identified | This study demonstrated the combination of metabolites and clinical covariates as an effective approach for early detection of HCC in patients with liver cirrhosis | Yes | ||
| 57 | 28340971 | Plasma from healthy subjects (n=20), patients with ESLD (n=99), and patients after LTx (n=7) | GC- or LC-MS | There was a significant difference in glutathione/metabolic profiles from patients with ESLD vs healthy subjects and patients after LTx | Glutathione species and metabolic prints defined liver-disease severity and may serve as surrogates for the detection of HCC in patients with established cirrhosis | No | ||
| 58 | 22200553 | Serum from 82 HCC, 48 lung cancer, and 90 healthy subjects | UHPLC-MS | There were significant disturbances of key metabolic pathways in HCC patients, such as organic acids, phospholipids, fatty acids, bile acids, and gut-flora metabolism | glycochenodeoxycholic acid was suggested to be an important indicator for HCC diagnosis and disease prognosis | Yes | ||
| 59 | 25483141 | Urine from 27 lung cirrhosis subjects, 33 HCC subjects, and 26 healthy individuals | QTrap LC-MS | Hydantoin-5-propionic acid and butyrylcarnitine (carnitine C 4:0) were identified as combinational markers to distinguish HCC from lung cirrhosis | The established pseudotargeted method is a complementary one of targeted and nontargeted methods for metabolomic study | Yes | ||
| 60 | 21275434 | Urine from 20 healthy controls, 20 lung cirrhosis and 18 HCC patients | 1H-NMR | Discriminatory metabolites included glycine, trimethylamine-N-oxide, hippurate, citrate, creatinine, creatine, and carnitine | This was the first study to identify similarly altered urine metabolic profiles of HCC in two etiologically and ethnically distinct populations | No | ||
| 61 | 27862090 | Urine from 40 lung cirrhosis, 55 HCC, and 45 healthy male subjects | GC-MS | 13 marker metabolites (glycine, serine, threonine, proline, urea, phosphate, pyrimidine, arabinose, xylitol, hippuric acid, citric acid, xylonic acid, and glycerol) responsible for the separation of HCC group from healthy subjects | Metabolic profile using GC-MS established optimized diagnostic model to discriminate between HCC patients and healthy subjects | No | ||
| 62 | 24817358 | Urine from 21 healthy controls, 21 lung cirrhosis, and 28 HCC patients | LC-MS | Severe disorders of steroid-hormone network and holistically decreased urinary steroid hormone pattern in cirrhotic and early HCC patients were observed | A panel of two urinary steroid hormones (epitestosterone and allotetrahydrocortisol) displayed excellent diagnostic capability distinguishing early HCC from cirrhosis | No | ||
| 63 | 21458633 | Feces from 23 healthy, 22 lung cirrhosis, and 23 HCC individuals | UHPLC/QTOF-MS | Compared with healthy controls, lysophosphatidylcholines significantly increased, while bile acids and bile pigments significantly decreased in lung cirrhosis and HCC | Results demonstrate the potential of UHPLC-MS as an efficient and convenient method for the early diagnosis of cirrhosis and HCC | No | ||
| 64 | 21900402 | Plasma from 20 HCC, 7 lung cirrhosis, 22 acute myeloid leukemia patients, and 6 healthy volunteers | UHPLC-ESI-QTOF-MS, UHPLC-ESI-TQ-MS, and GC-MS | HCC associated with increased plasma levels of glycodeoxycholate, deoxycholate 3-sulfate, and bilirubin | This investigation illustrates the power of new discovery technologies represented by the UHPLC-ESI-QTOF-MS platform combined with the targeted, quantitative platforms of UHPLC-ESI-TQ-MS and GC-MS for conducting metabolomic investigations | No | ||
| 65 | 22946841 | Serum from 28 lung cirrhosis (22 with HBV infection and 6 with HCV infection), 69 HCC (38 with HBV infection and 31 with HCV infection), and 31 healthy volunteers | UHPLC-QTOF-MS | Serum endocannabinoids anandamide (AEA) and palmitoylethanolamide (PEA) significantly elevated in HCC with HCV compared to corresponding chronic liver diseases | AEA, PEA, or their combination are potential biomarkers to distinguish the HCC from cirrhosis infected with HCV | No | ||
| 66 | 24707821 | Serum from 30 lung cirrhosis (22 infected with HBV and 8 with HCV), 70 HCC (39 with HBV infection and 31 with HCV infection), and 31 healthy volunteers | LC-MS | Multi-TGDR global model selected 45 metabolites with no misclassification and multi-TGDR local selected 48 metabolites with no misclassification | Multi-TGDR local is recommended because it has similar predictive performance and requires the same computing time as multi-TGDR global, but may provide class-specific inference | No | ||
| 67 | 24666728 | Serum from 37 HCC patients and 21 HCV patients | HPLC–MS | The levels of cholylglycine, xanthine, uric acid, dioleoylphosphatidylcholine, arachidonyl lysolecithin, 3-hydroxycapric acid, and d-leucic acid were found to alter in HCC | The use of biological significance as a selection process prior to PLS-DA modeling may offer improved probabilities for translation of newly discovered biomarkers to clinical application | No | ||
| 68 | 24957758 | Serum samples from patients with HCC (n=40) and HCV (n=22) | 1H-NMR | Three metabolites (choline, valine, and creatinine) significantly altered in HCC | Metabolite profiling could provide an alternative approach for HCC screening in HCV patients | No | ||
| 69 | 23856972 | Serum from 30 HCC patients with underlying HCV and cirrhosis and 22 HCV patients with cirrhosis but without HCC | LC-MS/MS | A number of perturbed metabolic pathways, including amino acid, purine, and nucleotide metabolism, were identified | These results provide a promising methodology to distinguish cirrhotic HCV patients who are at high risk of developing HCC from those who have already progressed to HCC | Yes | ||
| 70 | 26658617 | Serum from 49 HBV patients, 52 lung cirrhosis patients, 39 HCC patients, and 61 healthy subjects | GC-TOF-MS | β-glutamate and asparagine for HCC vs lung cirrhosis, palmitic acid for lung cirrhosis vs HBV, 5-methoxytryptamine, malic acid, and phenylalanine for HBV vs NC were selected as potential liver-disease-specific biomarkers | All metabolic perturbations in these liver diseases associated with pathways for energy metabolism, macromolecular synthesis, and maintaining redox balance | No | ||
| 71 | 28969038 | Serum from 49 HBV-cirrhosis patients, 51 HCC patients and 39 healthy subjects | GC-MS and LC-MS | Malate, citrate, succinate, lysine, carnitine, proline, ornithine, serine, phenylalanine, tyrosine, arachidonic acid, arabinose, galactose, uric acid, and several prostaglandins and leukotrienes implicated in pathological processes in HBV-cirrhosis and HCC | These identified biomarkers possessed strong potential to distinguish and diagnose HCC from healthy controls and HBV-cirrhosis patients | No | ||
| 72 | 24939728 | Serum from 30 healthy controls, 30 chronic hepatitis B (CHB) patients, 30 lung cirrhosis patients, and 30 HCC patients | UHPLC-MS | Based on the 27 selected feature pairs, HCC and chronic liver diseases were accurately distinguished using principal-component analysis | Certain profound metabolic disturbances related to liver-disease development were revealed by the feature pairs | Yes | ||
| 73 | 22349331 | Serum from 30 healthy volunteers and 90 patients with liver diseases (30 CHB, 30 lung cirrhosis, and 30 HCC) | LC-MS | Long-chain acylcarnitines accumulated, whereas free carnitine and medium- and short-chain acylcarnitines decreased with the severity of nonmalignant liver diseases | General changing extent was smaller in HCC than in lung cirrhosis, possibly due to the special energy-consumption mechanism of tumor cells | No | ||
| 74 | 21334394 | Serum from 248 patients with 9 types of liver disease and healthy controls | CE-MS | Measurement of γ-glutamyl dipeptide levels distinguished among different liver diseases | γ-Glutamyl dipeptides are novel biomarkers for liver diseases | No | ||
| 75 | 23824744 | 50 pairs of liver cancer samples and matched normal tissues | LC-MS | Glycolysis, gluconeogenesis, and β-oxidation increased, while TCA cycle and Δ12 desaturase decreased in HCC tumors | Combination biomarker of betaine and propionylcarnitine useful for diagnosis of HCC. with a supplementary role for AFP | Yes | ||
| 76 | 23801834 | Central tumor tissue, adjacent tissue, and distant tissue from 10 HBV-related HCC patients | UHPLC-MS | 14 metabolites were identified as characteristic metabolites that showed significant differences in levels between central tumor tissue and distant tumor tissue | Characteristic metabolites and metabolic pathways highly related to HCC pathogenesis and progression identified through metabolic-profiling analysis of HCC-tissue homogenates | No | ||
| 77 | 23376425 | Paired tumor and nontumor tissues from 30 patients with HCC | Gene expression, LC-MS, and GC-MS | Monounsaturated palmitic acid, a product of SCD, elevated in aggressive HCC | A lipogenic network that involves SCD and palmitate signaling was identified to be associated with HCC progression and patient outcomes | Yes | ||
| 78 | 23463346 | 31 pairs of HCC tumors and corresponding nontumor liver tissue | Transcriptomics and GC-MS-based metabolomics | Levels of glucose, malate, myoinositol, alanine, linoleic acid, and glycerol 2- and -3-phosphate decreased in HCC | Tissue metabolomics yielded precise biochemical information regarding HCC-tumor metabolic remodeling from mitochondrial oxidation to aerobic glycolysis | No | ||
| 79 | 26538415 | 10 ICC and 6 HCC samples and their respective surrounding nontumor tissues | Transcriptomics and CE-TOF-MS-based metabolomics | There were 14 compounds, 62 mRNAs and 17 miRNAs with two distinct patterns: tumor and nontumor, and ICC and non-ICC | ICC and HCC have different carcinogenic mechanism, so knowing the specific profile of genes and compounds can be useful in diagnosing ICC | No | ||
| 80 | 24497316 | Human HCC, lung cirrhosis, and nontumor liver tissue | Transcriptomics, NMR, and LC-MS/MS-based metabolomics | Aspartate metabolism, branched-chain amino acid metabolism, and TCA metabolism differentiable in HCC compared to nontumor liver | Alanine, succinate, lactate, glycerophosphoethanolamine, and inorganic phosphate were potential biomarkers | No | ||
Abbreviations: AFP, α-fetoprotein; CE, capillary electrophoresis; ESI, electrospray ionization; ESLD, end-stage liver disease; HBV, hepatitis B virus; HCV, hepatitis C virus; GC, gas chromatography; HCC, hepatocellular carcinoma; HD, high-definition; ICC, intrahepatic cholangiocarcinoma; LC, liquid chromatography; LTx, liver transplantation; MS, mass spectrometry; NC, normal controls; NMR, nuclear magnetic resonance; PLS-DA, projection to latent structure-discriminant analysis; QqQ-MS, triple-quadrupole mass spectrometry; QTOF, quadrupole time of flight; SCD, stearoyl-CoA-desaturase; TCA, tricarboxylic acid; TGDR, threshold gradient descent regularization; TOF, time of flight; TQ, triple quadrupole; UHPLC, ultrahigh-performance LC.
Cell pathology model research
HBV infection involves intricate interactions between the virus and host cells that can induce HCC. It has been reported that there was a correlation between HBV infection and metabolic alterations in host cells.23,24 To study the underlying mechanisms of metabolic alterations caused by HBV infection, NMR-based metabolomics was used to investigate the metabolic features of HepG2.2.15 cells derived from HepG2 with stable expression and replication of HBV, which is a commonly used cell model to study HBV infection.25 The results indicated that HBV infection contributed to HCC by upregulation of the glutamine-fructose-6-phosphate amidotransferase 1 (GFAT1)-activated hexosamine biosynthesis and choline kinase alpha (CHKA)-activated phosphatidylcholine biosynthesis. Hepatitis B virus X protein (HBx), a multifunctional oncoprotein, was reported to be associated with HBV replication, DNA repair, cell-cycle progression, transcriptional regulation, and to play an essential role in HBV-related HCC.26 Yue et al used an NMR-based metabolomic approach to systematically investigate the effects of this specific protein on hepatocarcinogenesis.27 They found that HBx disrupted the metabolism of glucose, lipids, and amino acids, especially nucleic acids. Further investigation of the effects of HBx on nucleic acid metabolism in gene-expression profiles showed that 29 genes correlating with DNA damage and repair in HBx-expressing HepG2 cells were differentially expressed. Together, their results revealed that HBx initially caused DNA damage and then perturbed nucleic acid metabolism, which in turn blocked DNA repair and led to HCC. E4F1, a cellular target of the E1A adenoviral oncoprotein, was reported to interact with HBx. Based on combined analyses of 1H-NMR metabolomics and molecular biology technologies, Dai et al confirmed that E4F1 may contribute to the proliferation of HBV-infected HCC cells by neutralizing the capacity of HBx to activate a p53-dependent metabolic and growth arrest phenotype.28 HBc, encoded by the HBV genome, was also reported to play an essential role in HBV-related HCC. Xie et al combined analyses of proteomics and metabolomics to investigate the function of HBc in HBV-related HCC.29 They found that HBc upregulated glycolysis and amino acid metabolism, and the enriched recruitment and enrichment of Mlx by HBc in the nucleus were linked to glycolysis pathways. PNPLA3 is a gene regulating both acylglycerol O-acyltransferase and triacylglycerol lipase activities. To investigate its role in HCC, Min et al performed GC-MS and LC-MS metabolic profiling of Huh7 cells with PNPLA3 siRNA silencing and overexpression.30 Silencing of PNPLA3 was revealed to reduce amino acid metabolism and elevate levels of myoinositol, cysteine sulfinic acid, polyunsaturated fatty acids, lysolipids, and sphingolipids. Overexpression of PNPLA3 mirrored metabolic changes in the opposite direction. Taken together, their results revealed a central role of PNPLA3 in the regulation of liver metabolism, besides its traditional role in the remodeling of triacylglycerol.
Animal pathologic model research
Different HCC animal models have been established to investigate the pathogenesis of HCC and identify specific biomarkers. Based on these models, a large number of metabolomic studies have been performed. Chemicals can lead to tumorigenesis in the liver of rodents, and diethylnitrosamine (DEN) has been reported to be the most commonly used hepatocarcinogen in rodents. It has been reported that the genetic and histologic signatures of the DEN-induced HCC rodent model are similar to those of human HCC.31 Several metabolomic studies have been conducted to compare the metabolic profiles of control and HCC using DEN-induced rodents. For example, Hong et al used DEN-induced C57BL/6J model to investigate the role of estrogen-related receptor α (ERRα) in HCC.32 LC-MS/MS-based metabolomic analysis revealed that in response to DEN, the loss of ERRα led to hepatocyte necrosis via apoptosis, which induced HCC through independent but synergistic mechanisms of hepatocytes and Kupffer cells. Tan et al used LC-MS-based metabolomics to identify potential biomarkers from the serum of a DEN-induced rat HCC model.33 Three metabolites – lysophosphoethanolamine 16:0, lysophosphatidylcholine (LPC) 22:5, and taurocholic acid – were identified as biomarkers and effective in discriminating HCC patients better than AFP in sensitivity and specificity. Similar samples were also analyzed via CE–time of flight (TOF)–MS-based metabolomics.34 To improve the detection of early risk of HCC, Huang et al proposed a new strategy for analysis of time-series data based on dynamic networks (ATSD-DN) using the DEN-induced rat HCC model.35 LC-MS-based metabolomic analysis of serum identified a ratio of lysophosphatidylcholine (LPC) 18:1/free fatty acid 20:5 as a biomarker of HCC. The better performance of ATSD-DN suggested its potential to represent time-series changes well and effectively extract early-warning information. Also, the DEN-induced rat HCC model can develop lung metastatic nodules and has also been used to investigate the metastasis of HCC, which contributes to the poor prognosis and high mortality rate of HCC. Li et al used a DEN-induced rat HCC with lung metastasis (HLM) model to study serum and urine metabolic profiles via GC/TOF-MS metabolomics.36 They found that glutamate metabolism and glycolysis were increased and the tricarboxylic acid (TCA) cycle decreased in both HCC and HLM. Especially, the metabolism of glucuronic acid, amino acids, and nucleic acids were increased only in HLM, which revealed potential biomarkers for HCC invasion and metastasis. Wang et al also used this model to study tumor-tissue metabolic profiles via 1H-NMR metabolomics.37
The transgenic mouse model is also used to investigate the pathogenesis of HCC. Teng et al used an HBx transgenic mouse model to study HBV-associated HCC pathogenesis.38 GC-MS-based metabolic profiles of serum and liver revealed that lipid (fatty acids, triglycerides, and cholesterol) profiles changed significantly during the development of HBx tumorigenesis, supporting the view that metabolic syndrome is essential in HBV tumorigenesis. Fan et al used a Ras-Tg mice model to study Hras12V oncogene-associated HCC pathogenesis.39 The combined analysis of transcriptomics and GC-TOF-MS-based metabolomics revealed that Hras12V induced perturbations of glycolysis, the pentose phosphate pathway, the TCA cycle, lipid metabolism, bile-acid synthesis, and redox homeostasis. These altered metabolic pathways may be essential to HCC. In addition, an HCC-xenograft model has also been used. Alcohol abuse is one of the main causes of liver injury that can promote HCC. To investigate the underlying pathogenesis of HCC from liver injury, an HCC-xenograft model was built by subcutaneously inoculating HepG2 cells into nude mice.40 Ultrahigh-performance LC (UHPLC)/quadrupole TOF (QTOF)-MS-based metabolic profiles of serum revealed metabolic alterations of LPCs in liver injury and HCC, suggesting that the serum profile of LPC may be a biomarker for liver injury and HCC.
Clinicopathologic model research
Apart from research on HCC cellular and animal pathologic models, there have been a larger number of metabolomic studies on HCC clinicopathologic models. Since blood, urine, and feces can be easily obtained from humans without any invasion, they are commonly used in human metabolomic studies. A number of metabolomic studies of blood and urine to distinguish HCC from healthy controls have been reported. Assi et al performed 1H-NMR-based metabolomics on prediagnostic serum from 114 first-incident, primary HCC cases and 222 matched controls identified from among the participants of a large European prospective cohort.41,42 They found that the changed metabolites in the HCC group were positively related to HCC risk. LC-MS-based metabolomic studies have also been done.43–47 Chen et al performed LC-MS-based metabolomics on serum from 41 HCC patients and 38 healthy controls.43 They identified the serum 1-methyladenosine as a characteristic metabolite for HCC, and a combination of 1-methyladenosine and AFP showed significantly improved sensitivity. Chen et al conducted UHPLC/triple – quadrupole MS-based metabolomics on serum from 29 HCC patients and 30 age-matched healthy controls.44 Lower LPC, medium-chain acylcarnitines, branched-chain amino acids and enriched long-chain acylcarnitines, and aromatic amino acids were observed in HCC patients. Liang et al conducted LC-QTOF-MS-based metabolomics on urine from 25 HCC patients and 12 matched healthy controls.46 The citric acid cycle, bile-acid biosynthesis, urea-cycle metabolism, and tryptophan metabolism were significantly changed in the HCC group. In addition, a study on serum and urine metabolic profiles from 82 HCC patients, 24 benign liver tumor patients, and 71 healthy controls was conducted by a combination of GC-TOF-MS and UHPLC-QTOF-MS metabolomics:48 43 serum metabolites and 31 urine metabolites were identified in HCC patients involving the metabolism of glycolysis, free fatty acids, bile acids, methionine, and the urea cycle. The identified biomarkers differentiated HCC patients with AFP levels lower than 20 ng/mL from healthy controls with 100% accuracy.
The early and accurate discrimination of HCC from other high-risk liver diseases, such as HBV, HCV, and liver cirrhosis, can improve the prognosis of HCC patients. As the majority of HCC comes with a background of liver cirrhosis, many studies have attempted to discriminate the serum, plasma, urine, or feces metabolic profiles of HCC from liver cirrhosis.49–64 For example, Nahon et al performed 1H-NMR-based metabolomics on serum from 93 liver cirrhosis, 28 small HCC, and 33 large HCC samples.49 Compared with cirrhosis, levels of glutamate, acetate, and N-acetyl glycoproteins were increased and levels of lipids and glutamine largely decreased in HCC. A combination of NMR and LC-MS-based metabolomics was applied to study serum metabolic profiles from 43 HCC patients, 42 liver cirrhosis patients, and 18 healthy volunteers.52 The results revealed that HCC induced disturbances of the citrate cycle, amino acid catabolism, fatty-acid oxidation, phospholipid metabolism, synthesis of ketone bodies, bile-acid metabolism, and sphingolipid metabolism. Sanabria et al conducted GC/LC-MS-based metabolomic analyses on plasma from healthy subjects (n=20), patients with end-stage liver disease (n=99), and patients after liver transplantation (n=7). The results showed glutathione species may define liver disease severity and serve as surrogates for the early detection of HCC in patients with established cirrhosis.57 Shao et al conducted QTrap LC-MS-based metabolomics on urine from 27 liver cirrhosis subjects, 33 HCC subjects, and 26 healthy individuals,59 identifying hydantoin-5-propionic acid and butyrylcarnitine (carnitine C4:0) as combinational biomarkers to distinguish HCC from liver cirrhosis. Cao et al performed UHPLC/QTOF-MS-based metabolomics on feces from 23 healthy individuals, 22 with liver cirrhosis, and 23 with HCC.63 Compared with healthy controls, LPC was significantly increased and bile acids and bile pigments significantly decreased in liver cirrhosis and HCC, which were identified as potential biomarkers. Since HBV/HCV infection is the prominent inducer of HCC, there have been several metabolomic investigations on the metabolic differences between hepatitis patients and HCC patients.65–74 Bowers et al performed HPLC-MS-based metabolomics on serum from 37 HCC patients and 21 HCV patients.67 Levels of cholylglycine, xanthine, uric acid, dioleoylphosphatidylcholine, arachidonyl lysolecithin, 3-hydroxycapric acid, and D-leucic acid were found to be altered in HCC, and may be potential biomarkers to distinguish HCC from HCV. Wei et al distinguished HCC from HCV by three metabolites (choline, valine, and creatinine) with 1H-NMR-based serum metabolomics.68 Gao et al performed GC-TOF-MS-based metabolomics on serum from 49 HBV patients, 52 liver cirrhosis patients, 39 HCC patients, and 61 healthy subjects (normal controls [NC]).70 β-Glutamate and asparagine for HCC versus liver cirrhosis, palmitic acid for liver cirrhosis versus HBV, and 5-methoxytryptamine, malic acid, and phenylalanine for HBV versus NC were selected as potential liver disease-specific biomarkers. Gong et al used a combination of untargeted metabolomics and targeted eicosanoid analysis on serum from 49 HBV-cirrhosis patients, 51 HCC patients, and 39 NC. The biomarkers identified in this study showed high potential to differentiate HCC from NC and HBV-cirrhosis patients.71
Besides the commonly used blood and urine samples in clinical metabolomic studies, there have also been metabolomic studies based on human tissue.75–80 The liver is the most essential metabolic organ, and liver extracts can provide complex metabolic information. Additionally, integration of metabolomics with other -omics technologies can be achieved when the sample used is tissue. For example, Budhu et al performed an integration of gene-expression technology, LC-MS, and GC-MS-based metabolomics on paired tumor and nontumor tissues from 30 HCC patients:77 169 genes and 28 metabolites were identified to be related to HCC. Specifically, monounsaturated palmitic acid, a product of stearoyl-CoA-desaturase, was elevated in aggressive HCC and confirmed to increase the invasion and migration of HCC cells in vitro. Beyoğlu et al conducted an integration of transcriptomics and GC-MS-based metabolomics on 31 pairs of HCC tumors and corresponding nontumor liver tissues.78 The metabolomic results showed that levels of glucose, malate, myoinositol, alanine, linoleic acid, and glycerol 2- and 3-phosphate were decreased in HCC. Transcriptomics classified HCC in G1–G6 subgroups, and suggested that the high serum level of AFP in G1 was connected to the overexpression of lipid catabolic enzymes. Altogether, HCC highlighted metabolic remodeling from mitochondrial oxidation to aerobic glycolysis. In addition, the combination of metabolomics with other -omics technologies could also be used to study the differences between HCC and intrahepatic cholangiocarcinoma.79
Metabolomics in treatment evaluation
Besides metabolomic studies on HCC, recent applications of metabolomics with respect to therapeutic and prognostic evaluation of HCC have also focused on the potential of treatment by drugs from natural products (Table 3). Metabolomics provides comprehensive metabolic profiles of living systems in response to drug treatment or surgery, which further helps us to better understand the underlying mechanism of anti-HCC agents and predict the risk of tumor recurrence.
Table 3.
Summary of recent metabolomic applications with respect to efficacy of anti-HCC agents and prognostic evaluation
| Reference | PubMed ID | Treatment | Sample | Method | Significantly changed metabolites/pathways | Main findings | Validation |
|---|---|---|---|---|---|---|---|
| 81 | 21508352 | Belinostat and bortezomib | Hep1 cells | NMR | Cotreatment induced increased amino acids and oxidative stress | Cotreatment displayed synergistic antiproliferative property | No |
| 82 | 26092946 | Sorafenib and everolimus | HepG2 cells | 2-D HRMAS 1H-NMR | Addition of everolimus to sorafenib caused changes in pyruvate, amino acid, methane, glyoxylate, and dicarboxylate glycolysis/gluconeogenesis and glycerophospholipid and purine metabolism | Sorafenib and everolimus have differential effects on HepG2 cells, and this phenomenon may explain (in part) the synergistic effects of sorafenib–everolimus combination therapy | No |
| 84 | 26700591 | Geranylgeranic (GGA) acid | HuH7 cells | UHPLC/TOF-MS | GGA increased fructose 6-phosphate and spermine while decreasing fructose 1,6-diphosphate and spermidine | GGA may shift HuH7 cells from aerobic glycolysis to mitochondrial respiration via upregulating TIGAR and SCO2 protein levels | No |
| 85 | 21843511 | Dehydroepiandrosterone | SK-Hep1 cells | RRLC-TOF-MS | DHEA treatment caused changes in metabolism of glutathione, lipid, SAM. and lysine | The mitochondrial dysfunction caused by decreased SAM production and cardiolipin depletion underlie the antiproliferative effect of DHEA | Yes |
| 86 | 28296595 | R-α-lipoic acid (RLA) | H4IIEC3 cells | GC-MS | RLA treatment inhibited gluconeogenesis via suppressing Thr–Gly–Ser pathways, glycolysis, and lactic acid production | This study may provide mechanistic insight into how RLA induces apoptosis in HCC cells | No |
| 87 | 24376596 | Acyclic retinoid (ACR) | JHH7 cells | NMR and CE-TOF-MS | ACR treatment suppressed ATP | ACR played its selective anti-HCC effect via suppressing the enhanced energy metabolism of HCC but not normal hepatic cells | No |
| 88 | 21327189 | Palmitate (PA) and oleate (OA) | H4IIEC3 cells | GC-MS | Metabolite levels for TCA-cycle intermediates, PPP intermediates, and energy-storage metabolites changed between PA treatment alone and PA-OA cotreatment | Abnormal PPP fluxes and increased adenosine levels might contribute to differences between PA treatment alone and PA-OA cotreatment | No |
| 89 | 28884001 | 6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic acid | Serum from DEN-induced hepatocarcinogenic rats | 1H-NMR | M1 treatment modulated fatty acids, low-density lipoproteins, acetoacetate, choline, lysine, leucine, isoleucine, tyrosine, pyruvate, and creatine to normal levels | Ameliorations of these markers may be linked to repair of inflammation damage, improvement in energy metabolism, and reconstruction of cell-membrane injury | No |
| 90 | 27077962 | Hispidulin | Urine samples from mice bearing H22 cells | UHPLC-QTOF-MS | These changed pathways include pantothenate and CoA biosynthesis, glycine, serine, and threonine metabolism, nicotinate and nicotinamide metabolism, steroid-hormone biosynthesis; pyrimidine metabolism, and glyoxylate and dicarboxylate metabolism | 4-Phosphopantothenoylcysteine, glycine, niacinamide, cortisol, uracil, and 5-thymidylic acid are potential biomarkers that may explain the link between hispidulin and the metabolism of mice bearing neoplasms (H22) | No |
| 91 | 27416811 | Physapubenolide (PB) | Plasma and liver tissue from mice bearing H22 cells | GC-MS | PB disturbed metabolic pattern and significantly decreased lactate production | PB induces apoptosis and decrease in glycolysis through the Akt–p53 pathway | Yes |
| 92 | 26744170 | Acyclic retinoid (ACR) | Liver tissue samples from DEN-induced HCC mouse model | CE-TOF-MS and LC-TOF-MS | ACR significantly counteracted against acceleration of lipogenesis but not glucose metabolism in DEN-treated mouse liver | Inhibition of linoleic acid metabolites, such as arachidonic acid, a proinflammatory precursor, played a crucial role in prevention by ACR | No |
| 93 | 28108381 | Shuihonghuazi (SHHZF) formula | Liver tissue from DEN-induced HCC rat model | HPLC/ESI-TOF-MS | SHHZF inhibited abnormal metabolism of fatty-acid and bile-acid metabolism | Anti-HCC property of SHHZF may be achieved via regulating the activities of lysophospholipase D, MTHFR, and PEMT | No |
| 94 | 24582150 | Surgical resection | Plasma of 18 late-recurrent and 22 early-recurrent HCC patients | LC−MS | Metabolic differences found to be related to amino acid, bile-acid, cholesterol, fatty-acid, phospholipid, and carbohydrate metabolism | Decreased levels of linolenic acid, docosahexaenoic acid, and polyunsaturated eicosapentaenoic acid the specific biomarker for early recurrence | No |
| 95 | 22768978 | Surgical resection | Urinary samples from 19 pairs of matched preoperative and postoperative HCC patients and 20 healthy volunteers | GC-TOF-MS | Metabolism of TCA cycle, amino acids, nucleosides, and gut flora significantly changed after surgical resection | Five metabolites (acotinic acid, phenylalanine, ethanolamine, ribose, and lactic acid) were identified as the biomarkers to predict early recurrence | No |
| 96 | 27015127 | Radiofrequency ablation | Serum samples from 120 HCC patients before and after radiofrequency ablation | 1H-NMR | Impairment of glucose and lipid metabolism in liver cancer recurrence after curative treatment was found | Statistical model showed significant differences depending on whether liver disease had a viral or nonviral etiology before radiofrequency ablation | No |
| 97 | 27322079 | TACE or surgical treatments | Plasma of 57 HCC patients (33 underwent surgical treatment, 18 TACE), and 60 healthy control subjects | 1H-NMR | HCC characterized by enhanced lipid metabolism and high consumption in response to liver injury | TACE or surgical treatments did not immediately improve the metabolic profiles of HCC patients | No |
Abbreviations: CE, capillary electrophoresis; DEN, diethylnitrosamine; DHEA, dehydroepiandrosterone; ESI, electrospray ionization; GC, gas chromatography HCC, hepatocellular carcinoma; HRMAS, high-resolution magic-angle spinning; M1, 6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic acid; MS, mass spectrometry; MTHFR, methylenetetrahydrofolate reductase; NMR, nuclear magnetic resonance; PEMT, Phosphatidylethanolamine N-methyltransferase; PPP, pentose phosphate pathway; QTOF, quadrupole time of flight; RRLC, rapid-resolution LC; SAM, S-adenosylmethionine; TACE, transcatheter arterial chemoembolization; TCA, tricarboxylic acid; TOF, time of flight; UHPLC, ultrahigh-performance liquid chromatography.
Metabolomics in anti-HCC-agent research
Chemotherapy remains one of the main approaches for the treatment of HCC. Specifically, metabolomics can be used to study the effects of cotreatment of currently approved drugs. NMR-based metabolomics was applied to investigate the effects of belinostat and bortezomib cotreatment on HCC.81 The cotreatment increased amino acids and induced oxidative stress, displaying synergistic antiproliferative properties. A similar synergistic anti-HCC effect was also found in sorafenib–everolimus combination therapy using 2-D high-resolution magic-angle spinning 1H-NMR metabolomics.82 So far, most chemotherapeutic drugs have not been effective for HCC, due to drug resistance and serious side effects.83 Therefore, it is urgently necessary to develop new anti-HCC agents. Due to their multiple biological activities and low toxicity, natural products obtained from Chinese medicine have been explored to improve HCC prognosis and survival rate. The in vitro application of metabolomics for efficacy evaluation of anti-HCC agents from natural products has been shown to be predictive of treatment efficacy. Geranylgeranic acid (GGA), an acyclic diterpenoid, is found in medicinal herbs, such as turmeric. It has been reported to induce the death of HCC Huh7 cells. Iwao et al used UHPLC/TOF-MS-based metabolomics to study the underlying mechanism of its anti-HCC effect.84 Their results showed that GGA could increase fructose 6-phosphate and spermine while decreasing fructose 1,6-diphosphate and spermidine, suggesting that GGA may shift Huh7 cells from aerobic glycolysis to mitochondrial respiration via upregulating TIGAR and SCO2 protein levels. Dehydroepiandrosterone (DHEA), a steroid secreted by the brain, gastrointestinal tract, adrenal cortex and gonads, is reported to have antiproliferative properties. A rapid-resolution LC-TOF-MS metabolomic approach revealed that DHEA treatment caused changes in metabolism of glutathione, lipids, S-adenosylmethionine, and lysine, suggesting that the mitochondrial dysfunction caused by decreased S-adenosylmethionine production and cardiolipin depletion underlay the antiproliferative effect of DHEA.85 Ikuta et al used GC-MS metabolomics to evaluate the effect of R-α-lipoic acid (RLA) on rat HCC H4IIEC3 cells.86 The results showed RLA treatment inhibited gluconeogenesis via suppressing Thr–Gly–Ser pathways, glycolysis, and lactic acid production, providing the mechanism on how RLA induces apoptosis in HCC cells. Acyclic retinoid (ACR), a synthetic retinoid, was shown to prevent HCC but not normal hepatic cells. Qin et al applied NMR and CE-TOF-MS-based metabolomics to investigate the molecular basis of its selective anti-HCC effect.87 It was found that ACR treatment suppressed adenosine triphosphate, suggesting that ACR played a selective anti-HCC effect via suppression of the enhanced energy metabolism of HCC but not normal hepatic cells. Noguchi et al used GC-MS-based metabolomics to investigate the effects of cotreatment of palmitate (PA) and oleate (OA) on HCC.88 They found that metabolite levels for TCA-cycle intermediates, pentose phosphate-pathway intermediates, and energy-storage metabolites changed between PA treatment alone and PA-OA cotreatment. This demonstrated that abnormal pentose phosphate-pathway fluxes and increased adenosine levels might contribute to differences between PA treatment alone and PA-OA cotreatment.
Metabolomics has also been applied to evaluate the efficacy of anti-HCC natural products in vivo. For example, 6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic acid is an isoquinoline alkaloid extracted from Mucuna pruriens seeds and reported to have antiproliferative action. Kumar et al used 1H-NMR-based metabolomics of serum from HCC rats to investigate the underlying mechanism.89 The results showed that 6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic acid treatment modulated fatty acids, low-density lipoproteins, acetoacetate, choline, lysine, leucine, isoleucine, tyrosine, pyruvate, and creatine to normal levels, and amelioration of these markers may be linked to repair of inflammation damage, improvement in energy metabolism, and reconstruction of cell-membrane injury. Li et al applied UHPLC-QTOF-MS-based metabolomics of urine samples from mice bearing H22 cells to investigate the anti-HCC effect of hispidulin, which is found widely in Chinese herbs.90 Physapubenolide is a withanolide extracted from Physalis pubescens. Ma et al applied GC-MS-based metabolomics to explore its anti-HCC mechanism.91 They found that physapubenolide significantly decreased lactate production and suppressed glycolysis via the Akt–p53 pathway. Similar anti-HCC effects of ACR were also explored via CE-TOF-MS- and LC-TOF-MS-based metabolomics of liver tissue samples from the DEN-induced HCC mouse model.92 Metabolomics can also be applied to investigate the anti-HCC effect of traditional Chinese medicine formulae. For example, shuihonghuazi formula (SHHZF) is a traditional Chinese medicine made from four herbs, namely Semen Coicis, Fructus Polygoni Orientalis, Imperatae Rhizoma, and Ophicalcitum. SHHZF has been applied to treat HCC clinically, and its underlying mechanism was investigated in liver tissue from a DEN-induced HCC rat model via HPLC/electrospray ionization–TOF-MS-based metabolomics.93 It was observed that SHHZF inhibited abnormal fatty-acid and bile-acid metabolism, and its anti-HCC property was achieved via regulating the activities of lysophospholipase D, MTHFR, and PEMT.
Metabolomics in prognostic evaluation
Surgery is the common curative approach for HCC patients, yet predicting the risk of tumor recurrence after surgery is difficult. Prognosis estimation in HCC patients is important, which can offer essential information on diagnosis and thus indicate treatment. Metabolomics is also applied to evaluate the prognosis of HCC. For example, Zhou et al used LC-MS-based metabolomics of plasma to predict early postoperative recurrence among HCC patients.94 They collected plasma samples from 22 early-recurrent and 18 late-recurrent HCC patients. Compared with the late-recurrent HCC group, fatty-acid and bile-acid steroids in the early-recurrent HCC group were found to change greatly. Specifically, decreased levels of linolenic acid, docosahexaenoic acid, and polyunsaturated eicosapentaenoic acid were the specific biomarkers for early recurrence. Ye et al utilized GC-TOF-MS-based metabolomics to investigate the complex physiopathological regulations of HCC after surgical resection.95 They collected urinary samples from 19 pairs of matched preoperative and postoperative HCC patients and 20 healthy volunteers. Their results showed that metabolism of the TCA cycle, amino acids, nucleosides, and gut flora significantly changed after surgical resection, and five metabolites (acotinic acid, phenylalanine, ethanolamine, ribose, and lactic acid) were identified as biomarkers to predict early recurrence. Goossens et al applied 1H-NMR-based metabolomics to investigate the risk of tumor recurrence in HCC patients.96 They collected serum samples from 120 HCC patients before and after radiofrequency ablation. Their results revealed clear differences relying on whether HCC had a viral or nonviral etiology before radiofrequency ablation. Chen et al also applied 1H-NMR-based metabolomics to discriminate plasma metabolic profiles of HCC patients from different therapeutic backgrounds, such as transcatheter arterial chemoembolization or surgical treatment. They found that transcatheter arterial chemoembolization or surgical treatment did not immediately evoke apparent amelioration in the metabolic profiles of HCC patients.97
Current challenges and future trends
Altogether, though great achievements have been made in the field of HCC-biomarker discovery and therapy evaluation by metabolomics, this discipline is still in its infancy, and much exciting work remains to be done.
Current challenges
Although metabolomics has become a hot spot in the scientific community and better understanding of the complex pathophysiology of HCC has been achieved, this discipline still lags behind other -omics technologies to a great extent.98 The current challenges of metabolomics are primarily embodied in the following aspects. First, the most common challenge for metabolomics are its technical limitations. There are about 2,000 metabolites in living systems, and the number of metabolites detected by the current instrumentation is only in the hundreds. Although the development of analytical instrumentation has made much progress, most metabolites cannot be detected due to their dramatic concentration range and great complexity, and these undetectable metabolites may also play an important role in living systems. Second, as a high-throughput pattern-recognition method, metabolomics is based on the analysis of big data. Therefore, studies with small and inadequate samples would cause false-negative results. Third, although most recent studies have offered us detailed information, such as demographic data for human subjects, there is still a lack of consistency on how the samples were selected and classified. For example, liver compensation and the treatment status of human subjects have often been inconsistently reported among different metabolomic studies on HCC. Fourth, a great challenge exists in data analysis of metabolomics, which is the most time-consuming step of metabolomic studies. The limited database and analysis tools may lead to false-negative or false-positive results. Last but not least, a key challenge in metabolomics studies on HCC is bias among the researchers, which is an obstacle to bring metabolomics into clinical application. For example, very different biomarkers can be identified for the same cancer, due to different sample processing, different analytical platforms used, and different data processing.
Future trends
Further trends in metabolomic studies on HCC are as follows (Figure 2). In order to detect as many metabolites as possible, efforts should be made to advance the analysis platforms and computational methods in metabolomic studies. It is anticipated that future metabolomic studies will involve larger epidemiological studies that cover analysis of thousands of samples instead of the presently small sample sizes.99 A recently emerging trend in metabolomics is automation due to the need for quick and accurate quantification in the clinic that not only enlarges throughput but also elevates the reproducibility and reliability among different laboratories.100 Besides, standardization of metabolomic protocols, including sample collecting, handling, storing, techniques, hardware, and data processing, can also improve the reproducibility and reliability of metabolomics.101 A greatly recommended step of metabolomics is to validate metabolomic results. All the key metabolites obtained from metabolomic analysis should be validated to eliminate any unreliable findings by use of an additional set of samples. In the metabolomic studies on HCC we reviewed, only about 20% of publications had their findings validate. Therefore, it is a trend to validate key metabolites obtained by metabolomic analysis for both early HCC diagnosis and therapy evaluation.102 In order to gain a better processing and biological interpretation of metabolomic data, a more comprehensive database will be developed based on global body function, rather than organ-related function. It is widely accepted that a single analytical instrument can only identify a limited number of detectable metabolites. Therefore, an integration of different analytical instruments will be a trend to extend the number of detectable metabolites in biological samples and offer us more information.103 Most of the recent metabolomic studies on HCC have been conducted only at the metabolic level and lacked integrated information on other levels, such as DNA, RNA, and proteins. Since no single technology can provide us the entire spectrum of the HCC phenotype, integration of metabolomic results with markers obtained from the upstream -omics technologies is a new trend to increase our understanding of the pathological progression of HCC, as well as responses to therapy.104 The concept of “precision medicine” (also called personalized medicine) in cancer health care is gradually gaining interest, of which the goal is to use advanced technologies to customize an individual’s medical treatment based on their own biomarker profiles. Given that metabolomics is relatively inexpensive and provides valuable biomarkers in disease diagnosis and therapy monitoring, there can be little doubt that it will be useful in precision medicine to guide clinical strategies from personal disease diagnosis and monitoring to selecting a suitable therapy and finally to following patient prognosis in the future.105 Although the consideration of these challenges and future trends will not be satisfied at present or in the near future, it is essential that they are highly recognized and being discussed on a larger scale.
Figure 2.
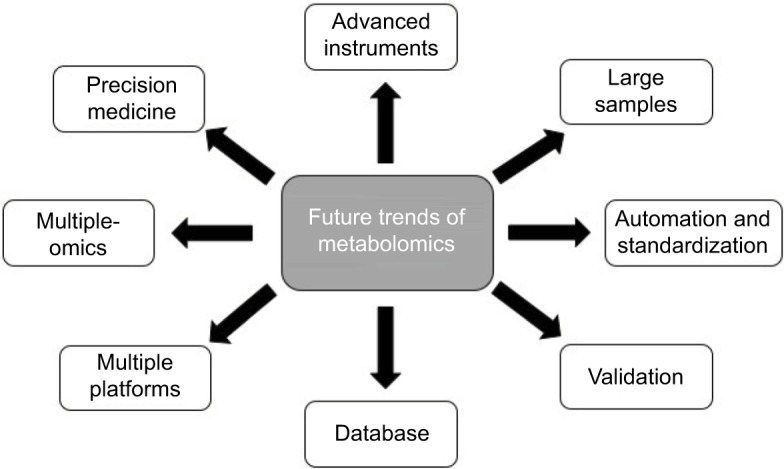
Future trends in metabolomics.
Conclusion
In summary, metabolomics is a burgeoning science with a combination of analytical chemistry, biochemistry, bioinformatics, and medicine, which is well suited to studies of HCC. It can be applied not only to identify the sensitive and specific biomarkers of HCC in a noninvasive and nondestructive way at the early stage but also to evaluate the efficacy of treatment and prognosis. Valuable information regarding HCC diagnosis, prognosis, and therapy can be obtained from these metabolomic studies of HCC. Collectively, metabolomics provides us new insights into the diagnosis, prognosis, and therapeutic evaluation of HCC. With the coming era of precision medicine, metabolomics will undoubtedly play an active role in selecting a suitable therapy tailored to an individual patient in the future. Although technological advances have brought metabolomics into the spotlight, metabolomics is still in its infancy, and much exciting work remains to be done.
Acknowledgments
The study was financially supported by grants from the research council of the University of Hong Kong (project codes 104004092, 104003919, 104004460, 104004745, and 104004746), the Research Grants Committee (RGC) of Hong Kong, HKSAR (project codes 766211, 17152116), Wong’s Donation for Modern Oncology of Chinese Medicine (project code 200006276), and the Shenzhen Basic Research Program (project code JCYJ20140903112959964).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Patel M, Shariff MI, Ladep NG, et al. Hepatocellular carcinoma: diagnostics and screening. J Eval Clin Pract. 2012;18(2):335–342. doi: 10.1111/j.1365-2753.2010.01599.x. [DOI] [PubMed] [Google Scholar]
- 2.Ohata K, Hamasaki K, Toriyama K, Ishikawa H, Nakao K, Eguchi K. High viral load is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis B virus infection. J Gastroenterol Hepatol. 2004;19(6):670–675. doi: 10.1111/j.1440-1746.2004.03360.x. [DOI] [PubMed] [Google Scholar]
- 3.Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127(5 Suppl 1):S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S87–S96. doi: 10.1053/j.gastro.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Tsai JF, Chuang LY, Jeng JE, et al. Betel quid chewing as a risk factor for hepatocellular carcinoma: a case-control study. Br J Cancer. 2001;84(5):709–713. doi: 10.1054/bjoc.1999.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groopman JD, Scholl P, Wang JS. Epidemiology of human aflatoxin exposures and their relationship to liver cancer. Prog Clin Biol Res. 1996;395:211–222. [PubMed] [Google Scholar]
- 7.Regimbeau JM, Colombat M, Mognol P, et al. Obesity and diabetes as a risk factor for hepatocellular carcinoma. Liver Transpl. 2004;10(2 Suppl 1):S69–S73. doi: 10.1002/lt.20033. [DOI] [PubMed] [Google Scholar]
- 8.Justesen P, Fenger C, Høilund-Carlsen PF, et al. Hepatocellulært karcinom og andre levertumorer [Hepatocellular carcinoma and other liver tumors] Ugeskr Laeger. 2006;168(19):1876. Danish. [PubMed] [Google Scholar]
- 9.Taketa K. α-Fetoprotein: reevaluation in hepatology. Hepatology. 1990;12(6):1420–1432. doi: 10.1002/hep.1840120625. [DOI] [PubMed] [Google Scholar]
- 10.Wishart DS, Tzur D, Knox C, et al. HMDB: the Human Metabolome Database. Nucleic Acids Res. 2007;35(Database):D521–D526. doi: 10.1093/nar/gkl923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganti S, Weiss RH. Urine metabolomics for kidney cancer detection and biomarker discovery. Urol Oncol. 2011;29(5):551–557. doi: 10.1016/j.urolonc.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang A, Sun H, Wang P, Han Y, Wang X. Recent and potential developments of biofluid analyses in metabolomics. J Proteomics. 2012;75(4):1079–1088. doi: 10.1016/j.jprot.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Catchpole GS, Beckmann M, Enot DP, et al. Hierarchical metabolomics demonstrates substantial compositional similarity between genetically modified and conventional potato crops. Proc Natl Acad Sci U S A. 2005;102(40):14458–14462. doi: 10.1073/pnas.0503955102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armitage EG, Southam AD. Monitoring cancer prognosis, diagnosis and treatment efficacy using metabolomics and lipidomics. Metabolomics. 2016;12:146. doi: 10.1007/s11306-016-1093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaddurah-Daouk R, Weinshilboum R. Metabolomic signatures for drug response phenotypes: pharmacometabolomics enables precision medicine. Clin Pharmacol Ther. 2015;98(1):71–75. doi: 10.1002/cpt.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu C, Alessandro A, Xia Y. Metabolomic approach in probing drug candidates. Curr Top Med Chem. 2017;17(15):1741–1749. doi: 10.2174/1568026617666161116144146. [DOI] [PubMed] [Google Scholar]
- 17.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell. 2012;21(3):297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serkova NJ, Spratlin JL, Eckhardt SG. NMR-based metabolomics: translational application and treatment of cancer. Curr Opin Mol Ther. 2007;9(6):572–585. [PubMed] [Google Scholar]
- 19.Wang X, Zhang A, Sun H. Power of metabolomics in diagnosis and biomarker discovery of hepatocellular carcinoma. Hepatology. 2013;57(5):2072–2077. doi: 10.1002/hep.26130. [DOI] [PubMed] [Google Scholar]
- 20.Chaiteerakij R, Addissie BD, Roberts LR. Update on biomarkers of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2015;13(2):237–245. doi: 10.1016/j.cgh.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimhofer T, Fye H, Taylor-Robinson S, Thursz M, Holmes E. Proteomic and metabonomic biomarkers for hepatocellular carcinoma: a comprehensive review. Br J Cancer. 2015;112(7):1141–1156. doi: 10.1038/bjc.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitian AI, Cabrera R. Disease monitoring of hepatocellular carcinoma through metabolomics. World J Hepatol. 2017;9(1):1–17. doi: 10.4254/wjh.v9.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tong A, Wu L, Lin Q, et al. Proteomic analysis of cellular protein alterations using a hepatitis B virus-producing cellular model. Proteomics. 2008;8(10):2012–2023. doi: 10.1002/pmic.200700849. [DOI] [PubMed] [Google Scholar]
- 24.Yang F, Yan S, He Y, et al. Expression of hepatitis B virus proteins in transgenic mice alters lipid metabolism and induces oxidative stress in the liver. J Hepatol. 2008;48(1):12–19. doi: 10.1016/j.jhep.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Zhu W, Zhang L, et al. The metabolic responses to hepatitis B virus infection shed new light on pathogenesis and targets for treatment. Sci Rep. 2015;5:8421. doi: 10.1038/srep08421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Na TY, Shin YK, Roh KJ, et al. Liver X receptor mediates hepatitis B virus X protein-induced lipogenesis in hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2009;49(4):1122–1131. doi: 10.1002/hep.22740. [DOI] [PubMed] [Google Scholar]
- 27.Yue D, Zhang YW, Cheng LL, et al. Hepatitis B virus X protein (HBx)-induced abnormalities of nucleic acid metabolism revealed by 1H-NMR-based metabonomics. Sci Rep. 2016;6:24430. doi: 10.1038/srep24430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dai Y, Cros MP, Pontoizeau C, Elena-Hermann B, Bonn GK, Hainaut P. Downregulation of transcription factor E4F1 in hepatocarcinoma cells: HBV-dependent effects on autophagy, proliferation and metabolism. Carcinogenesis. 2014;35(3):635–650. doi: 10.1093/carcin/bgt353. [DOI] [PubMed] [Google Scholar]
- 29.Xie Q, Fan F, Wei W, et al. Multi-omics analyses reveal metabolic alterations regulated by hepatitis B virus core protein in hepatocellular carcinoma cells. Sci Rep. 2017;7:41089. doi: 10.1038/srep41089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Min HK, Sookoian S, Pirola CJ, Cheng J, Mirshahi F, Sanyal AJ. Metabolic profiling reveals that PNPLA3 induces widespread effects on metabolism beyond triacylglycerol remodeling in Huh-7 hepatoma cells. Am J Physiol Gastrointest Liver Physiol. 2014;307(1):G66–G76. doi: 10.1152/ajpgi.00335.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang M, Dewaele S, Zhao YP, et al. Serum N-glycome biomarker for monitoring development of DENA-induced hepatocellular carcinoma in rat. Mol Cancer. 2010;9:215. doi: 10.1186/1476-4598-9-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong EJ, Levasseur MP, Dufour CR, Perry MC, Giguere V. Loss of estrogen-related receptor α promotes hepatocarcinogenesis development via metabolic and inflammatory disturbances. Proc Natl Acad Sci U S A. 2013;110(44):17975–17980. doi: 10.1073/pnas.1315319110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan Y, Yin P, Tang L, et al. Metabolomics study of stepwise hepatocarcinogenesis from the model rats to patients: potential biomarkers effective for small hepatocellular carcinoma diagnosis. Mol Cell Proteomics. 2012;11(2):M111.010694. doi: 10.1074/mcp.M111.010694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng J, Huang X, Zhou L, et al. Metabolomics identifies biomarker pattern for early diagnosis of hepatocellular carcinoma: from diethylnitrosamine treated rats to patients. Sci Rep. 2015;5:16101. doi: 10.1038/srep16101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang X, Zeng J, Zhou L, Hu C, Yin P, Lin X. A new strategy for analyzing time-series data using dynamic networks: identifying prospective biomarkers of hepatocellular carcinoma. Sci Rep. 2016;6:32448. doi: 10.1038/srep32448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li ZF, Wang J, Huang C, et al. Gas chromatography/time-of-flight mass spectrometry-based metabonomics of hepatocarcinoma in rats with lung metastasis: elucidation of the metabolic characteristics of hepatocarcinoma at formation and metastasis. Rapid Commun Mass Spectrom. 2010;24(18):2765–2775. doi: 10.1002/rcm.4703. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Zhang S, Li Z, et al. 1H-NMR-based metabolomics of tumor tissue for the metabolic characterization of rat hepatocellular carcinoma formation and metastasis. Tumour Biol. 2011;32(1):223–231. doi: 10.1007/s13277-010-0116-7. [DOI] [PubMed] [Google Scholar]
- 38.Teng CF, Hsieh WC, Yang CW, et al. A biphasic response pattern of lipid metabolomics in the stage progression of hepatitis B virus X tumorigenesis. Mol Carcinog. 2016;55(1):105–114. doi: 10.1002/mc.22266. [DOI] [PubMed] [Google Scholar]
- 39.Fan T, Rong Z, Dong J, et al. Metabolomic and transcriptomic profiling of hepatocellular carcinomas in Hras12V transgenic mice. Cancer Med. 2017;6(10):2370–2384. doi: 10.1002/cam4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li S, Liu H, Jin Y, Lin S, Cai Z, Jiang Y. Metabolomics study of alcohol-induced liver injury and hepatocellular carcinoma xenografts in mice. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(24):2369–2375. doi: 10.1016/j.jchromb.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 41.Assi N, Fages A, Vineis P, et al. A statistical framework to model the meeting-in-the-middle principle using metabolomic data: application to hepatocellular carcinoma in the EPIC study. Mutagenesis. 2015;30(6):743–753. doi: 10.1093/mutage/gev045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fages A, Duarte-Salles T, Stepien M, et al. Metabolomic profiles of hepatocellular carcinoma in a European prospective cohort. BMC Med. 2015;13:242. doi: 10.1186/s12916-015-0462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen F, Xue J, Zhou L, Wu S, Chen Z. Identification of serum biomarkers of hepatocarcinoma through liquid chromatography/mass spectrometry-based metabonomic method. Anal Bioanal Chem. 2011;401(6):1899–1904. doi: 10.1007/s00216-011-5245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen S, Kong H, Lu X, et al. Pseudotargeted metabolomics method and its application in serum biomarker discovery for hepatocellular carcinoma based on ultra high-performance liquid chromatography/triple quadrupole mass spectrometry. Anal Chem. 2013;85(17):8326–8333. doi: 10.1021/ac4016787. [DOI] [PubMed] [Google Scholar]
- 45.Zeng Z, Liu X, Dai W, et al. Ion fusion of high-resolution LC-MS-based metabolomics data to discover more reliable biomarkers. Anal Chem. 2014;86(8):3793–3800. doi: 10.1021/ac500878x. [DOI] [PubMed] [Google Scholar]
- 46.Liang Q, Liu H, Wang C, Li B. Phenotypic characterization analysis of human hepatocarcinoma by urine metabolomics approach. Sci Rep. 2016;6:19763. doi: 10.1038/srep19763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang A, Sun H, Yan G, Han Y, Ye Y, Wang X. Urinary metabolic profiling identifies a key role for glycocholic acid in human liver cancer by ultra-performance liquid-chromatography coupled with high-definition mass spectrometry. Clin Chim Acta. 2013;418:86–90. doi: 10.1016/j.cca.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 48.Chen T, Xie G, Wang X, et al. Serum and urine metabolite profiling reveals potential biomarkers of human hepatocellular carcinoma. Mol Cell Proteomics. 2011;10(7):M110.004945. doi: 10.1074/mcp.M110.004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nahon P, Amathieu R, Triba MN, et al. Identification of serum proton NMR metabolomic fingerprints associated with hepatocellular carcinoma in patients with alcoholic cirrhosis. Clin Cancer Res. 2012;18(24):6714–6722. doi: 10.1158/1078-0432.CCR-12-1099. [DOI] [PubMed] [Google Scholar]
- 50.Ressom HW, Xiao JF, Tuli L, et al. Utilization of metabolomics to identify serum biomarkers for hepatocellular carcinoma in patients with liver cirrhosis. Anal Chim Acta. 2012;743:90–100. doi: 10.1016/j.aca.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao JF, Varghese RS, Zhou B, et al. LC-MS based serum metabolomics for identification of hepatocellular carcinoma biomarkers in Egyptian cohort. J Proteome Res. 2012;11(12):5914–5923. doi: 10.1021/pr300673x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y, Hong Z, Tan G, et al. NMR and LC-MS-based global metabolomics to identify serum biomarkers differentiating hepatocellular carcinoma from liver cirrhosis. Int J Cancer. 2014;135(3):658–668. doi: 10.1002/ijc.28706. [DOI] [PubMed] [Google Scholar]
- 53.Zeng J, Yin P, Tan Y, et al. Metabolomics study of hepatocellular carcinoma: discovery and validation of serum potential biomarkers by using capillary electrophoresis-mass spectrometry. J Proteome Res. 2014;13(7):3420–3431. doi: 10.1021/pr500390y. [DOI] [PubMed] [Google Scholar]
- 54.Fitian AI, Nelson DR, Liu C, Xu Y, Ararat M, Cabrera R. Integrated metabolomic profiling of hepatocellular carcinoma in hepatitis C cirrhosis through GC-MS and UPLC-MS-MS. Liver Int. 2014;34(9):1428–1444. doi: 10.1111/liv.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ranjbar MR, Luo Y, Di Poto C, et al. GC-MS based plasma metabolomics for identification of candidate biomarkers for hepatocellular carcinoma in Egyptian cohort. PLoS One. 2015;10(6):e0127299. doi: 10.1371/journal.pone.0127299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Poto C, Ferrarini A, Zhao Y, et al. Metabolomic characterization of hepatocellular carcinoma in patients with liver cirrhosis for biomarker discovery. Cancer Epidemiol Biomarkers Prev. 2017;26(5):675–683. doi: 10.1158/1055-9965.EPI-16-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanabria JR, Kombu RS, Zhang GF, et al. Glutathione species and metabolomic prints in subjects with liver disease as biological markers for the detection of hepatocellular carcinoma. HPB (Oxford) 2016;18(12):979–990. doi: 10.1016/j.hpb.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang B, Chen D, Chen Y, et al. Metabonomic profiles discriminate hepatocellular carcinoma from liver cirrhosis by ultraperformance liquid chromatography-mass spectrometry. J Proteome Res. 2012;11(2):1217–1227. doi: 10.1021/pr2009252. [DOI] [PubMed] [Google Scholar]
- 59.Shao Y, Zhu B, Zheng R, et al. Development of urinary pseudotargeted LC-MS-based metabolomics method and its application in hepatocellular carcinoma biomarker discovery. J Proteome Res. 2015;14(2):906–916. doi: 10.1021/pr500973d. [DOI] [PubMed] [Google Scholar]
- 60.Shariff MI, Gomaa AI, Cox IJ, et al. Urinary metabolic biomarkers of hepatocellular carcinoma in an Egyptian population: a validation study. J Proteome Res. 2011;10(4):1828–1836. doi: 10.1021/pr101096f. [DOI] [PubMed] [Google Scholar]
- 61.Osman D, Ali O, Obada M, El-Mezayen H, El-Said H. Chromatographic determination of some biomarkers of liver cirrhosis and hepatocellular carcinoma in Egyptian patients. Biomed Chromatogr. 2017;31(6):e3893. doi: 10.1002/bmc.3893. [DOI] [PubMed] [Google Scholar]
- 62.Dai W, Yin P, Chen P, et al. Study of urinary steroid hormone disorders: difference between hepatocellular carcinoma in early stage and cirrhosis. Anal Bioanal Chem. 2014;406(18):4325–4335. doi: 10.1007/s00216-014-7843-3. [DOI] [PubMed] [Google Scholar]
- 63.Cao H, Huang H, Xu W, et al. Fecal metabolome profiling of liver cirrhosis and hepatocellular carcinoma patients by ultra performance liquid chromatography-mass spectrometry. Anal Chim Acta. 2011;691(1–2):68–75. doi: 10.1016/j.aca.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 64.Patterson AD, Maurhofer O, Beyoglu D, et al. Aberrant lipid metabolism in hepatocellular carcinoma revealed by plasma metabolomics and lipid profiling. Cancer Res. 2011;71(21):6590–6600. doi: 10.1158/0008-5472.CAN-11-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou L, Ding L, Yin P, et al. Serum metabolic profiling study of hepatocellular carcinoma infected with hepatitis B or hepatitis C virus by using liquid chromatography-mass spectrometry. J Proteome Res. 2012;11(11):5433–5442. doi: 10.1021/pr300683a. [DOI] [PubMed] [Google Scholar]
- 66.Tian S, Chang HH, Wang C, Jiang J, Wang X, Niu J. Multi-TGDR, a multi-class regularization method, identifies the metabolic profiles of hepatocellular carcinoma and cirrhosis infected with hepatitis B or hepatitis C virus. BMC Bioinformatics. 2014;15:97. doi: 10.1186/1471-2105-15-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bowers J, Hughes E, Skill N, Maluccio M, Raftery D. Detection of hepatocellular carcinoma in hepatitis C patients: biomarker discovery by LC-MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;966:154–162. doi: 10.1016/j.jchromb.2014.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei S, Suryani Y, Gowda GA, Skill N, Maluccio M, Raftery D. Differentiating hepatocellular carcinoma from hepatitis C using metabolite profiling. Metabolites. 2012;2(4):701–716. doi: 10.3390/metabo2040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baniasadi H, Gowda GA, Gu H, et al. Targeted metabolic profiling of hepatocellular carcinoma and hepatitis C using LC-MS/MS. Electrophoresis. 2013;34(19):2910–2917. doi: 10.1002/elps.201300029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao R, Cheng J, Fan C, et al. Serum metabolomics to identify the liver disease-specific biomarkers for the progression of hepatitis to hepatocellular carcinoma. Sci Rep. 2015;5:18175. doi: 10.1038/srep18175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gong ZG, Zhao W, Zhang J, et al. Metabolomics and eicosanoid analysis identified serum biomarkers for distinguishing hepatocellular carcinoma from hepatitis B virus-related cirrhosis. Oncotarget. 2017;8(38):63890–63900. doi: 10.18632/oncotarget.19173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin X, Gao J, Zhou L, Yin P, Xu G. A modified k-TSP algorithm and its application in LC-MS-based metabolomics study of hepatocellular carcinoma and chronic liver diseases. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;966:100–108. doi: 10.1016/j.jchromb.2014.05.044. [DOI] [PubMed] [Google Scholar]
- 73.Zhou L, Wang Q, Yin P, et al. Serum metabolomics reveals the deregulation of fatty acids metabolism in hepatocellular carcinoma and chronic liver diseases. Anal Bioanal Chem. 2012;403(1):203–213. doi: 10.1007/s00216-012-5782-4. [DOI] [PubMed] [Google Scholar]
- 74.Soga T, Sugimoto M, Honma M, et al. Serum metabolomics reveals γ-glutamyl dipeptides as biomarkers for discrimination among different forms of liver disease. J Hepatol. 2011;55(4):896–905. doi: 10.1016/j.jhep.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 75.Huang Q, Tan Y, Yin P, et al. Metabolic characterization of hepatocellular carcinoma using nontargeted tissue metabolomics. Cancer Res. 2013;73(16):4992–5002. doi: 10.1158/0008-5472.CAN-13-0308. [DOI] [PubMed] [Google Scholar]
- 76.Liu SY, Zhang RL, Kang H, Fan ZJ, Du Z. Human liver tissue metabolic profiling research on hepatitis B virus-related hepatocellular carcinoma. World J Gastroenterol. 2013;19(22):3423–3432. doi: 10.3748/wjg.v19.i22.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Budhu A, Roessler S, Zhao X, et al. Integrated metabolite and gene expression profiles identify lipid biomarkers associated with progression of hepatocellular carcinoma and patient outcomes. Gastroenterology. 2013;144(5):1066–1075 e1061. doi: 10.1053/j.gastro.2013.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beyoğlu D, Imbeaud S, Maurhofer O, et al. Tissue metabolomics of hepatocellular carcinoma: tumor energy metabolism and the role of transcriptomic classification. Hepatology. 2013;58(1):229–238. doi: 10.1002/hep.26350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murakami Y, Kubo S, Tamori A, et al. Comprehensive analysis of transcriptome and metabolome analysis in intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Sci Rep. 2015;5:16294. doi: 10.1038/srep16294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Darpolor MM, Basu SS, Worth A, et al. The aspartate metabolism pathway is differentiable in human hepatocellular carcinoma: transcriptomics and 13C-isotope based metabolomics. NMR Biomed. 2014;27(4):381–389. doi: 10.1002/nbm.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spratlin JL, Pitts TM, Kulikowski GN, et al. Synergistic activity of histone deacetylase and proteasome inhibition against pancreatic and hepatocellular cancer cell lines. Anticancer Res. 2011;31(4):1093–1103. [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng JF, Lu J, Wang XZ, Guo WH, Zhang JX. Comparative metabolomic profiling of hepatocellular carcinoma cells treated with sorafenib monotherapy vs. sorafenib-everolimus combination therapy. Med Sci Monit. 2015;21:1781–1791. doi: 10.12659/MSM.894669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Asghar U, Meyer T. Are there opportunities for chemotherapy in the treatment of hepatocellular cancer? J Hepatol. 2012;56(3):686–695. doi: 10.1016/j.jhep.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 84.Iwao C, Shidoji Y. Upregulation of energy metabolism-related, p53-target TIGAR and SCO2 in HuH-7 cells with p53 mutation by geranylgeranoic acid treatment. Biomed Res. 2015;36(6):371–381. doi: 10.2220/biomedres.36.371. [DOI] [PubMed] [Google Scholar]
- 85.Cheng ML, Shiao MS, Chiu DT, Weng SF, Tang HY, Ho HY. Biochemical disorders associated with antiproliferative effect of dehydroepian-drosterone in hepatoma cells as revealed by LC-based metabolomics. Biochem Pharmacol. 2011;82(11):1549–1561. doi: 10.1016/j.bcp.2011.07.104. [DOI] [PubMed] [Google Scholar]
- 86.Ikuta N, Chikamoto K, Asano Y, et al. Time course effect of R-α-lipoic acid on cellular metabolomics in cultured hepatoma cells. J Med Food. 2017;20(3):211–222. doi: 10.1089/jmf.2016.3837. [DOI] [PubMed] [Google Scholar]
- 87.Qin XY, Wei F, Tanokura M, et al. The effect of acyclic retinoid on the metabolomic profiles of hepatocytes and hepatocellular carcinoma cells. PLoS One. 2013;8(12):e82860. doi: 10.1371/journal.pone.0082860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Noguchi Y, Young JD, Aleman JO, Hansen ME, Kelleher JK, Stephanopoulos G. Tracking cellular metabolomics in lipoapoptosis- and steatosis-developing liver cells. Mol Biosyst. 2011;7(5):1409–1419. doi: 10.1039/c0mb00309c. [DOI] [PubMed] [Google Scholar]
- 89.Kumar P, Singh AK, Raj V, et al. 6,7-Dimethoxy-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic acid attenuates hepatocellular carcinoma in rats with NMR-based metabolic perturbations. Future Sci OA. 2017;3(3):FSO202. doi: 10.4155/fsoa-2017-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li F, Li X, Miao Y, et al. UHPLC-MS-based metabolomics analysis on mice bearing neoplasm (H22) for hispidulin. J Pharm Biomed Anal. 2016;125:310–318. doi: 10.1016/j.jpba.2016.03.050. [DOI] [PubMed] [Google Scholar]
- 91.Ma T, Fan BY, Zhang C, et al. Metabonomics applied in exploring the antitumour mechanism of physapubenolide on hepatocellular carcinoma cells by targeting glycolysis through the Akt-p53 pathway. Sci Rep. 2016;6:29926. doi: 10.1038/srep29926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Qin XY, Tatsukawa H, Hitomi K, et al. Metabolome analyses uncovered a novel inhibitory effect of acyclic retinoid on aberrant lipogenesis in a mouse diethylnitrosamine-induced hepatic tumorigenesis model. Cancer Prev Res (Phila) 2016;9(3):205–214. doi: 10.1158/1940-6207.CAPR-15-0326. [DOI] [PubMed] [Google Scholar]
- 93.Bao Y, Wang S, Yang X, Li T, Xia Y, Meng X. Metabolomic study of the intervention effects of shuihonghuazi formula, a traditional Chinese medicinal formulae, on hepatocellular carcinoma (HCC) rats using performance HPLC/ESI-TOF-MS. J Ethnopharmacol. 2017;198:468–478. doi: 10.1016/j.jep.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 94.Zhou L, Liao Y, Yin P, et al. Metabolic profiling study of early and late recurrence of hepatocellular carcinoma based on liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;966:163–170. doi: 10.1016/j.jchromb.2014.01.057. [DOI] [PubMed] [Google Scholar]
- 95.Ye G, Zhu B, Yao Z, et al. Analysis of urinary metabolic signatures of early hepatocellular carcinoma recurrence after surgical removal using gas chromatography-mass spectrometry. J Proteome Res. 2012;11(8):4361–4372. doi: 10.1021/pr300502v. [DOI] [PubMed] [Google Scholar]
- 96.Goossens C, Nahon P, le Moyec L, et al. Sequential serum metabolomic profiling after radiofrequency ablation of hepatocellular carcinoma reveals different response patterns according to etiology. J Proteome Res. 2016;15(5):1446–1454. doi: 10.1021/acs.jproteome.5b01032. [DOI] [PubMed] [Google Scholar]
- 97.Chen Y, Zhou J, Li J, Feng J, Chen Z, Wang X. Plasma metabolomic analysis of human hepatocellular carcinoma: diagnostic and therapeutic study. Oncotarget. 2016;7(30):47332–47342. doi: 10.18632/oncotarget.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Spratlin JL, Serkova NJ, Eckhardt SG. Clinical applications of metabolomics in oncology: a review. Clin Cancer Res. 2009;15(2):431–440. doi: 10.1158/1078-0432.CCR-08-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gika HG, Theodoridis GA, Plumb RS, Wilson ID. Current practice of liquid chromatography-mass spectrometry in metabolomics and metabonomics. J Pharm Biomed Anal. 2014;87:12–25. doi: 10.1016/j.jpba.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 100.Grebe SK, Singh RJ. LC-MS/MS in the clinical laboratory: where to from here? Clin Biochem Rev. 2011;32(1):5–31. [PMC free article] [PubMed] [Google Scholar]
- 101.Vermeersch KA, Styczynski MP. Applications of metabolomics in cancer research. J Carcinog. 2013;12:9. doi: 10.4103/1477-3163.113622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hwang VJ, Weiss RH. Metabolomic profiling for early cancer detection: current status and future prospects. Expert Opin Drug Metab Toxicol. 2016 Sep 13; doi: 10.1080/17425255.2016.1238460. Epub. [DOI] [PubMed] [Google Scholar]
- 103.Goodacre R, Vaidyanathan S, Dunn WB, Harrigan GG, Kell DB. Metabolomics by numbers: acquiring and understanding global metabolite data. Trends Biotechnol. 2004;22(5):245–252. doi: 10.1016/j.tibtech.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 104.Nicholson JK, Lindon JC. Systems biology: metabonomics. Nature. 2008;455(7216):1054–1056. doi: 10.1038/4551054a. [DOI] [PubMed] [Google Scholar]
- 105.Everett JR. Pharmacometabonomics in humans: a new tool for personalized medicine. Pharmacogenomics. 2015;16(7):737–754. doi: 10.2217/pgs.15.20. [DOI] [PubMed] [Google Scholar]