Abstract
Background
It has been repetitively shown that the transcription factors DLX5 and DLX6 are drastically downregulated in endometriotic lesions when compared with eutopic endometrium. These findings suggest that regulatory cascades involving DLX5/6 might be at the origin of endometriosis symptoms such as chronic pelvic pain (CPP). We have shown that inactivation of Dlx5 and Dlx5/6 in the mouse uterus results in an endometrial phenotype reminiscent of endometriosis.
Methods
We focused on genes that present a similar deregulation in endometriosis and in Dlx5/6-null mice in search of new endometriosis targets.
Results
We confirmed a strong reduction of DLX5 expression in endometriosis implants. We identified a signature of 30 genes similarly deregulated in human endometriosis implants and in Dlx5/6-null mouse uteri, reinforcing the notion that the downregulation of Dlx5/6 is an early event in the progress of endometriosis. CACNA2D3, a component of the α2δ family of voltage-dependent calcium channel complex, was strongly overexpressed both in mutant mouse uteri and in endometriosis implants, were also CACNA2D1 and CACNA2D2, other members of the α2δ family involved in nociception, are upregulated.
Conclusion
Comparative analysis of gene expression signatures from endometriosis and mouse models showed that calcium channel subunits α2δ involved in nociception can be targets for the treatment of endometriosis-associated pain. CACNA2D3 has been associated with pain sensitization and heat nociception in animal models. In patients, CACNA2D3 variants were associated with reduced sensitivity to acute noxious stimuli. As α2δs were targets of gabapentinoid analgesics, the results suggested the use of these drugs for the treatment of endometriosis-associated pain. Indeed, recent small-scale clinical studies have shown that gabapentin could be effective in women with CPP. The findings of this study reinforce the need for a large definitive trial.
Keywords: endometriosis, gabapentin, CACNA2D3, Dlx5, pain
Introduction
Endometriosis is characterized by the growth of endometrial-like tissue consisting of glandular tissue and stroma outside the uterine cavity in localized and innervated implants. Remarkably, endometriosis lesions present histopathological and physiological responses that are similar to those of the endometrium. Endometriosis patients frequently experience various pain symptoms including chronic pelvic pain (CPP), dysmenorrhea, dyspareunia, and dyschezia,1 which often result in severe personal, social, and economic difficulties. Treatment of endometriosis-associated pain is difficult due to its poorly understood pathophysiology, complex clinical presentation, and natural history. Endometriosis is generally treated by surgical intervention, analgesic treatment, or hormonal treatment suppressing cyclic ovarian hormone production and reducing or eliminating menses.2 Current therapies present, however, limited efficacy on endometriosis pain, due to high rates of symptom recurrence, and significant side-effects.3,4
Dlx genes comprise a highly conserved family of homeobox genes. Dlx5 and Dlx6 (Dlx5/6) are expressed in GABAergic neurons of the brain including delta opioid receptor-positive GABAergic neurons that are involved in the regulation of chronic pain.5 Besides early roles in neuronogenesis and limb and craniofacial patterning during embryogenesis,6 Dlx5/6 are also involved in the differentiation of steroidogenic tissues including Leydig cells in the testis7 and theca and granulosa cells in the ovary.8 DLX5 and DLX6 are expressed in glandular and lining epithelial cells of mouse and human endometrium9 where their expression is upregulated during the secretory phase10 of the cycle. By targeted inactivation of Dlx5/6 in the mouse uterus, we have demonstrated their central role for uterine adenogenesis and fertility.9
It has been repeatedly shown that the expression of DLX5 and DLX6 is strongly downregulated in endometriotic lesions compared to eutopic endometrium.11–14 Endometriosis implants and Dlx5/6 mutant mouse uteri have, therefore, in common a very low level of Dlx5 expression. Furthermore, Dlx5/6-null endometrial epithelia present a histological appearance reminiscent of that found in endometriosis implants with very few glands and a cuboidal lining epithelium that locally tends to desquamate.9 The histological and molecular similarities between endometriosis implants and Dlx5/6-null mouse uteri have prompted our suggestion that the downregulation of DLX5/6 could be an early event in the progress of endometriosis and that inactivation of these genes in the mouse uterus could constitute a model of the human pathology.9
Here, we show that Dlx5/6-null mouse uteri and ectopic human endometrium share 30 deregulated genes confirming the these mice can be considered a model of the disease; we suggest that these 30 shared genes are a new, more focused, molecular signature of endometriosis. We focus our attention on CACNA2D3, an overexpressed gene that has an evolutionary-conserved role in nociception.15 CACNA2D1, CACNA2D2, and CACNA2D3 constitute the α2δ gene family of glycosylphosphatidyl inositol (GPI)-anchored transmembrane voltage-gated calcium channel (VGCC)-associated subunits. During membrane depolarization, VGCCs permit Ca2 influx, contributing to cell depolarization and functioning as secondary messengers regulating key neuronal mechanisms including gene expression and neurotransmitter release. VGCCs are involved in generating neuropathic pain and are targets of gabapentinoids.16 Remarkably, CACNA2D1 to 3 are significantly increased in endometriosis implants12,13 and should therefore be considered as targets for the treatment of endometriotic pain.
Methods
Endometrial biopsies
A total of 37 ovarian endometriosis and 12 normal endometrium samples were selected from the formalin-fixed, paraffin- embedded tissue collection of the Division of Anatomic Pathology, Department of Surgical Science and Integrated Diagnostics, University of Genoa, Italy. All patients signed an informed consent under local institutional review board-approved protocols for research purposes dealing with the archived endometriosis tissues in the pathology department. Informed consents are available on request (from LM). The Regional Liguria Ethical Committee for medical research approved the use of archive human samples.
Animals
Procedures involving animals were conducted in accordance with the directives of the European Community (council directive 86/609) and the French Agriculture Ministry (council directive 87-848, October 19, 1987, permissions 00782 to GL). The project was reviewed and approved by the “Cuvier” ethical committee of the Muséum National d’Histoire Naturelle and transmitted to the French Ministry of Agriculture. Mice were housed in specific pathogen-free and light-, temperature- (21°C), and humidity (50–60% relative humidity)-controlled conditions. Food and water were available ad libitum. Conditional Dlx5/6flox/flox mutants were generated as described.9 To obtain Pgrcre/+;Dlx5/6flox/flox mice, we crossed Pgrcre/+;Dlx5/6flox/+ males with Dlx5/6flox/flox females.
Immunohistochemistry
Fixed tissue samples were embedded in paraffin, and 5 mm thick sections were cut. Slides were dewaxed in xylol and re-hydrated in ethanol solutions. Antigen exposure was achieved by submerging slides in sodium citrate 0.01 M, pH 6.0 (Sigma-Aldrich Co., St Louis, MO, USA) and heating at 90°C with pressure in a 2100-Retriever device (Prestige Medical, Northridge CA, USA). Endogenous peroxidase activity was quenched by incubation with 3% (vol/vol) H2O2 in methanol (Merck Millipore, Billerica, MA, USA) or with peroxidase-blocking solution from Dako EnVision + System HRP kit (K4011; Agilent Technologies, Santa Clara, CA, USA) for 30 minutes. Tissue sections were then blocked with 5% (wt/vol) bovine serum albumin (BSA) (Sigma-Aldrich Co.) or 10% of fetal calf serum (FCS) in PBS at room temperature for 2 hours and then incubated with anti-DLX5 (NBP1-19547; Novus Biologicals, Littleton, CO, USA) polyclonal rabbit primary antibody at 1/200 dilution, or anti-CACNA2D3 at 1/250 dilution (NBP1-30557; Novus Biologicals), or anti-CBS at 1/400 dilution (1478-1-AP; Proteintech, Manchester, UK), or anti-LTBP2 at 1/250 dilution (17708-1-AP; Proteintech, UK), or anti-GJB3 at 1/50 dilution (12880-1-AP; Proteintech, UK), or anti-NOXA1 at 1/100 dilution (CUSABIO, Huston, TX, USA), or anti-KIAA1324 at 1/200 dilution (Thermo Fisher Scientific, Waltham, MA, USA) in PBS 5% FCS overnight at 4°C in a humidified chamber. Normal rabbit IgGs were used as negative control. Next, slides were washed with PBS and detection of the primary antibody was achieved by using the Histostain-SP Broad Spectrum kit (Thermo Fisher Scientific) or goat antirabbit antibody linked to horseradish peroxidase (Dako EnVision + System HRP kit; K4011). Counterstain was done using the hematoxylin-stabilized solution 1/5 (RAL Diagnostics, Martillac, France) according to standard procedures. Images were visualized under an Olympus BX-51 microscope and captured using the Image-Pro Plus v.6.2 software (Media Cybernetics, Rockville, MD, USA).
RNAseq
RNA library preparation was carried out according to the Illumina TruSeq Stranded Total RNA Sample Prep protocol, following Ribo-Zero Gold Deplete procedure (Illumina, San Diego, CA, USA). From a total amount of 1.5 µg of total RNA, both cytoplasmic RNA and mitochondrial ribosomal RNA (rRNA) were removed using specific biotinylated oligos and Ribo-Zero rRNA removal beads. RNA, purified from uteri of three PgrCre/+;Dlx5/6flox/flox and three Dlx5/6flox/flox mice at PN25, was fragmented by the addition of divalent cations and the incubation at 94°C for 4 minutes. The first cDNA strand was synthesized using random primers and a reverse transcriptase. DNA polymerase I synthesized the second cDNA strand and generated blunt-ended ds cDNA, while RNase H removed the RNA template. An A nucleotide was added to the 3′ ends, followed by the ligation of multiple indexing adapters that are required for the pooling and the hybridization with the flow cell. These DNA products were purified, and only those having adapter molecules on both ends were enriched with PCR by using a Cocktail Primer that anneals specifically to the adapters. The obtained libraries were validated by Bioanalyzer (Agilent DNA 1000; Agilent Technologies) and pooled and sequenced using the Illumina HiSeq 2000 sequencing system. The global quality of each library was checked using the FastQC software (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). To reduce any PCR amplification bias, duplicated reads were removed before the duplicated reads before the mapping based on a 100% sequence identity between reads. Then, five nucleotides were trimmed at both 5′ and 3′ ends of all remnant reads using FASTX-Toolkit v0.0.14 (http://hannonlab.cshl.edu/fastx_toolkit/index.html). Reads were mapped against the mouse mm10 genome (Version GRCm38.p3) using the TopHat2 software v2.0.1017 with default parameters. Only uniquely mapped reads were kept and were counted on genes’ exons using the HTSeq-count software v0.6.1p118 with “union” mode using the Mus musculus Ensembl annotation (release 79). Only coding and expressed genes were considered. “R” package DESeq2 v1.8.019 was used for statistical analyses to determine differential gene expression levels (cutoff: adjusted P-value ≤0.1). Samples’ homogeneity was checked realizing a principal component analysis with “R” package FactoMineR, following which, one mutant mouse was not considered for further analysis.
To identify mouse and human orthologs, we used the BioMart tool, provided by Ensembl, Heidelberg, Germany.
From 424 genes differentially expressed in Pgrcre/+; Dlx5/6flox/flox mice, we found 391 human orthologs, which have been used for comparison.
Quantitative real-time PCR (qPCR)
Total RNA was isolated from five mouse uterine samples per group using an RNeasy Mini Kit (Qiagen NV, Venlo, the Netherlands) according the manufacturer’s instructions. On-Column Deoxyribonuclease I (Hoffman-La Roche Ltd., Basel, Switzerland) digestion was incorporated into an RNA isolation procedure to remove potential genomic DNA contamination. RNA concentration and the ratio of the absorbance at 260 and 280 nm were measured using a Nano-Drop 2000 spectrophotometer (Thermo Fisher Scientific). Reverse transcription was carried out using 600 or 200 ng total RNA and Primscript (Ozyme) reverse transcriptase to obtain cDNA. qPCR was performed using the QuantStudio 6 Flex Real-Time PCR System (Thermo Fisher Scientific). The PCR program consisted of 95°C for 10 minutes, 40 cycles 95°C for 15 seconds, and 60°C for 10 minutes. Relative gene expression was measured using SYBR Green mix gene expression assays (Thermo Fisher Scientific). Primers utilized are shown in Table 1. To measure the relative amount of PCR products, the Ct geometric mean of Sdha and Hprt1, was subtracted from the Ct of genes of interest to derive ΔCt. The ΔCt of mutant animals was compared with ΔCt of control animals, and the difference was assigned as ΔΔCt. The fold change between two samples was then calculated as 2−ΔΔCt.
Table 1.
PCR primers used in this study
| Genes | Forward primer | Reverse primer | Length (bp) |
|---|---|---|---|
| NOXA1 | CCGCTGGCTAGGATGTACTT | CAGCTGGAAATTGGCCACTC | 150 |
| DMRTA1 | CCCAACTTTCGAGGTTTTCCA | CCCAGAGAATGGTGATGAGTGTT | 126 |
| KIAA1324 | TAGACCTCCACAGTCAGTGGT | GCATTCTCTTCGATGGTGTAGG | 198 |
| SLC27A2 | TCCTCCAAGATGTGCGGTACT | TAGGTGAGCGTCTCGTCTCG | 166 |
| GABRP | ATGAGCTACAGCCTCTATTTGGC | ACCACCGAAATTGGGCCTG | 174 |
| SAPCD2 | GGGCTCTTCTCAGTGTGACA | ACCTTCTTCAGCACTTGTGC | 106 |
| PLCH1 | GGCTCGTTCGCCTCTTTTAC | CTGCCCTCGGTGACTTTGTAG | 118 |
| GJB3 | GCTCCAAGACCTATTGAGTGGC | GCCTGGTGTTACAGTCAAAGTC | 155 |
| DPP6 | CAGAGGCAAAGTGGATAAGCA | TCAACATTCCGCAGTATGACAC | 76 |
| OSCP1 | TGCTTTACGTCCTAGACCAGC | GGTTTGAACAGTTCGTCCATGA | 124 |
| JAZF1 | GCCGAGAACAGGAATCTCTGA | GTAAGGCTGCCACTGCTATGT | 118 |
| CBS | GGGACAAGGATCGAGTCTGGA | AGCACTGTGTGATAATGTGGG | 100 |
| NAIP5 | GCAGTCTCTCGAGGTCTCAG | CACACAGTTCTTTCAGGCCC | 89 |
| EMB | TGAGGGCGATCCCACAGAT | CCGTCACTGAGATATTACAGCTC | 107 |
| CLIP4 | AGAAGGTCGATGTAGCCCAC | TCCGCTACAGTTTTCGGTTC | 124 |
| PIK3AP1 | GTCCCGGATGCCTCTTTCTC | CACAAGTCATTTCCTGCCAGT | 241 |
| LAPTM5 | ACATCGAATTGCCAGCGTAC | CAGCTGCAGTGTCATCAAGG | 80 |
| CTGF | GGGCCTCTTCTGCGATTTC | ATCCAGGCAAGTGCATTGGTA | 151 |
| NAIP2 | TGTGCTCTCAGTCCTTCCTG | AAGCACAGTCATGAGAGGCT | 184 |
| CACNA2D3 | AAGAAATCGACGGTCTCCAAC | GGTCATTGGGGGCTAAGATGAA | 237 |
| LTBP2 | AACAGCACCAACCACTGTATC | CCTGGCATTCTGAGGGTCAAA | 159 |
| TNS3 | ACATGCACTTCACCAACGTC | CTGAGAAACTGCACGTACCG | 130 |
| RAB20 | GCAGTGGCGTTCCTTCAAC | GGAGCCCAGACCATGAAACT | 70 |
| SYTL2 | TCAAAAAGCCGACCAAGAACC | AGGCACTGTGGAAATTCCTGG | 61 |
| LBH | CTGCTCTGACTATCTGAGATCGG | CGGTCAAAGTCTGATGGGTCC | 135 |
| ENC1 | CTGTTTCATAAGTCCTCCTACGC | CACCACTGAACATGGCTTCG | 169 |
| MANBA | GACTGTCCCCGGCTATGTG | AATTCCGTGCTGTAGGTCCA | 126 |
| GALM | CTGCACGATCACTGCTCTG | CACTGCTCCAAAGTAGGGCTG | 115 |
Statistical analysis
The Mann–Whitney unpaired test was conducted using Prism (GraphPad Software, Inc., La Jolla, CA, USA) to calculate the differences between groups of five independent measurements. All values are expressed as mean ± SEM of combined data from replicate experiments. Values P<0.05 were considered statistically significant.
Results
DLX5 distribution in normal endometrium and in endometriosis
First, we analyzed the distribution of DLX5 in normal endometrium from various stages of the menstrual cycle by immunohistochemistry (Figure 1A–B′). DLX5 protein is prevalently present in endometrial glandular and luminal epithelial cells. The intracellular distribution of DLX5 varies at different phases of the cycle: during the proliferative phase, DLX5 is detected both in the nucleus and in the cytoplasm (Figure 1A and A′), while in the secretory phase, DLX5 is predominantly present in the cytoplasm (Figure 1B and B′) where it displays a polarized distribution mostly toward the luminal and basal aspects of the cells.
Figure 1.
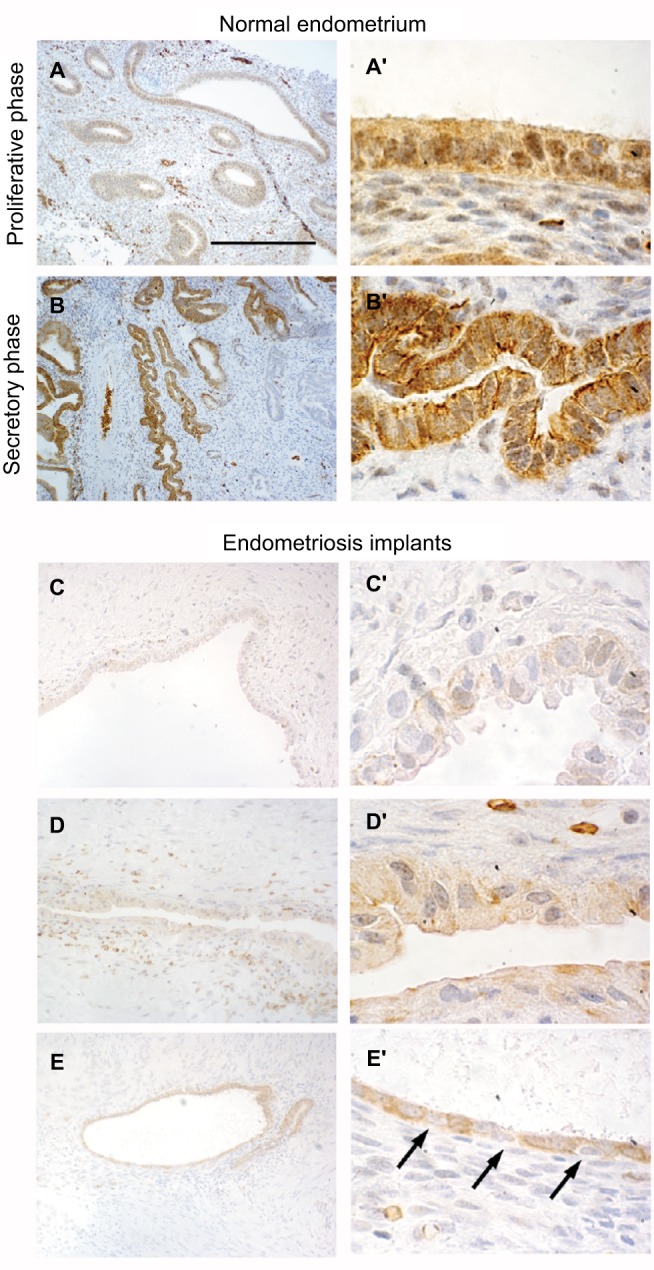
Immunohistolocalization of DLX5 in normal endometrium and ovarian endometriosis.
Notes: In healthy endometrium, DLX5 protein is detected in glandular epithelia (A, A′, B, and B′). During the proliferative phase (A and A′), the protein is present both in epithelial nuclei and in the cytoplasm. In contrast, during the secretory phase (B and B′) DLX5 presents a polarized distribution in the cytoplasm and is virtually absent from the nuclei. In ovarian endometriosis implants (C–E′), the expression of DLX5 is more variable. Most of the implants present very few glands with a very large lumen and a thin cuboidal epithelia, which is in general constituted by a mixture of DLX5-positive and DLX5-negative (arrows in E′) cells. Scale bar: (A and B) 250 µm; (C–E) 125 µm; and (A′, B′, C′, D′, and E′) 25 µm.
Most endometriotic lesions (Figure 1C–D′) present very few glands with a very large lumen covered by an atypical noncolumnar epithelium, which does not present a uniform phenotype even between different epithelial regions from the same patient (eg, the two facing epithelia in Figure 1D′). In certain regions, epithelial cells are large and noncohesive (Figure 1C′ and D′), while in other territories, they display a flattened phenotype with very little cytoplasm (Figure 1D′ and E′); cells are almost invariably ciliated. The intensity of anti-DLX5 staining is generally low or at the limit of detection (Figure 1C–D′), but occasionally (Figure 1E and E′), a clear signal can be detected in the cytoplasm and in perinuclear regions, while nuclei are mostly negative; frequently, epithelia are constituted by a mixture of DLX5-positive and DLX5-negative (arrows in Figure 1E′) cells.
A shared genetic signature between Dlx5/6-null mouse uteri and endometriosis implants
DLX5 and DLX6 are between the most downregulated genes in endometriosis implants,11–13 and their inactivation in the mouse uterus results in an endometriosis-like phenotype.9 To better investigate the apparent similarity between endometriotic implants and mouse Dlx5/6-null uteri, we have compared the RNAseq profiles of genes deregulated in the mutant mouse uterus with those reported in four independent studies in which the expression profile of endometriosis implants was compared with that of eutopic endometrium.12–14,20 Hawkins et al12 described 2083 deregulated genes in endometriotic lesions; among this set of genes, 997 genes are underexpressed and 1086 genes are overexpressed. Several of these genes were also identified in the three other studies, which we have considered,13,14,20 providing a shared database to identify potential therapeutic targets. Comparison of Dlx5/6-mouse mutant uterus RNAseq dataset with the human datasets permits identifying 30 genes that present a common deregulation in the mouse and in endometriotic lesions: 13 downregulated and 17 upregulated genes (Table 2). If indeed the downregulation of Dlx5/6 is an early event in endometriosis progression, this set of 30 genes could well constitute a more focused molecular signature of endometriosis implants (Figure 2).
Table 2.
Genes commonly deregulated after Dlx5/6 inactivation in the mouse uterus and in human endometriosis implants
| Gene | Comparison of normal and Dlx5/6-null mouse uterus
|
Comparison of normal human endometrium and endometriosis implants
|
|||||
|---|---|---|---|---|---|---|---|
| Log2 fold change | P-value | Log2 fold change Hawkins | P-value Hawkins | Hever | Hull | Eyster | |
| Down | |||||||
| DLX5 | Absent (targeted deletion) | –8,434 | 1.804E-04 | Down | Down | ||
| NOXA1 | –2.25 | 3.51E-07 | –1.816 | 2.012E-03 | Down | ||
| DMRTA1 | –2.11 | 2.65E-06 | –2.158 | 2.628E-04 | |||
| KIAA1324 | –2.07 | 1.96E-07 | –19.062 | 1.180E-05 | Down | Down | Down |
| SLC27A2 | –1.91 | 1.14E-05 | –1.982 | 5.256E-03 | Down | Down | |
| GABRP | –1.81 | 5.56E-06 | –22.036 | 2.671E-05 | Down | ||
| SAPCD2 | –1.55 | 5.04E-04 | –2.155 | 6.115E-03 | Down | ||
| CENPW | –1.36 | 6.92E-04 | –1.905 | 1.224E-04 | Down | ||
| PLCH1 | –1.32 | 4.14E-04 | –3.082 | 5.696E-04 | Down | Down | |
| GJB3 | –1.23 | 2.97E-03 | –1.835 | 5.448E-01 | Down | ||
| DPP6 | –1.21 | 2.80E-03 | –2.917 | 2.170E-04 | Down | ||
| OSCP1 | –0.95 | 3.24E-03 | –1.714 | 1.668E-03 | Down | ||
| JAZF1 | −0.88 | 3.09E-03 | –2.719 | 2.028E-06 | Down | ||
| Up | |||||||
| CBS | 3.09 | 5.77E-27 | 2.491 | 3.844E-03 | Up | Up | |
| NAIP5 | 2.44 | 1.69E-10 | 1.884 | 3.807E-04 | |||
| EMB | 1.76 | 4.38E-08 | 1.532 | 5.658E-03 | |||
| CLIP4 | 1.52 | 1.76E-08 | 2.612 | 2.198E-04 | Up | ||
| PIK3AP1 | 1.48 | 8.44E-06 | 2.566 | 2.857E-03 | Up | ||
| LAPTM5 | 1.45 | 8.85E-05 | 3.092 | 7.976E-03 | Up | Up | |
| CTGF | 1.29 | 3.51E-04 | 1.582 | 7.149E-03 | Up | ||
| NAIP2 | 1.19 | 1.04E-03 | 1.884 | 3.807E-04 | |||
| CACNA2D3 | 1.19 | 2.61E-03 | 2.066 | 2.469E-06 | Up | ||
| LTBP2 | 1.14 | 3.16E-03 | 4.344 | 9.850E-05 | Up | Up | Up |
| TNS3 | 1.12 | 1.22E-05 | 1.583 | 3.773E-03 | Up | Up | |
| RAB20 | 1.08 | 3.26E-04 | 1.827 | 9.691E-03 | |||
| SYTL2 | 0.97 | 1.97E-03 | 1.788 | 8.826E-04 | Up | ||
| LBH | 0.97 | 1.26E-03 | 2.029 | 1.615E-03 | Up | ||
| ENC1 | 0.91 | 2.39E-03 | 2.919 | 3.286E-03 | Up | ||
| MANBA | 0.83 | 1.98E-03 | 2.011 | 2.363E-03 | Up | ||
| GALM | 0.76 | 2.27E-03 | 1.680 | 1.019E-03 | Up | ||
Figure 2.
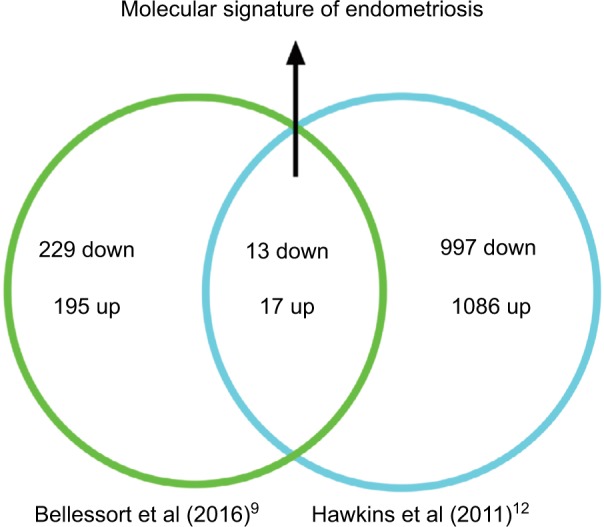
Molecular signature of endometriosis obtained comparing genes deregulated in Dlx5/6 mutant mice uteri and in endometriosis lesions.
Validation by qPCR
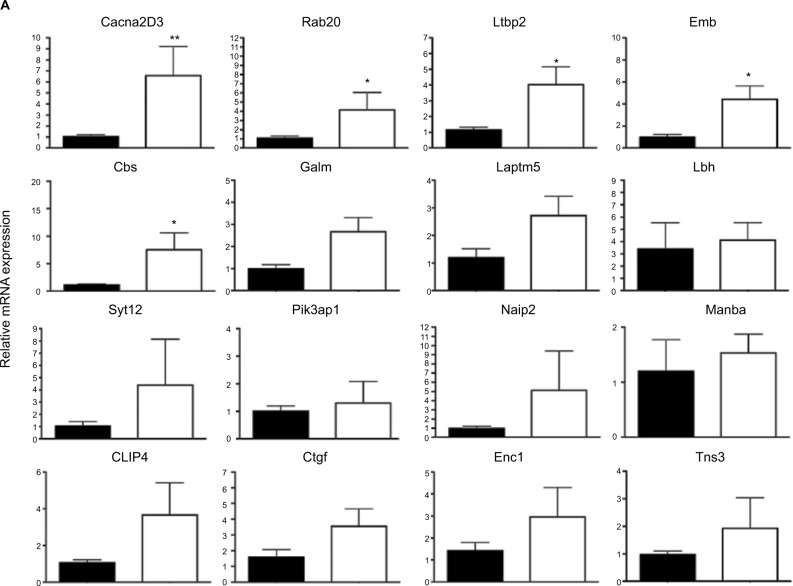
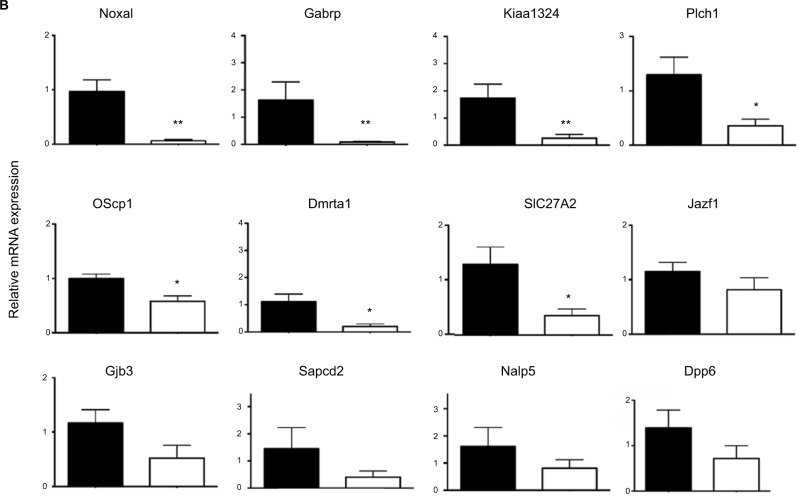
In a first round of validation, we analyzed, by qPCR, the effect of Dlx5/6 inactivation on the expression levels of 28 of the 30 genes identified by RNAseq comparison (Figure 3A and B). In all cases, the trend of variation occurred in the direction predicted by RNAseq either up (Figure 3A) or down (Figure 3B); however, due to high individual variability, the variation reached a level of significance for only 12 genes. Between the upregulated genes, the expression of CACNA2D3 and CBS was, respectively, increased six and seven times. CACNA2D3 together with CACNA2D1 and CACNA2D2 constitutes the family of α2δ VGCC subunits involved in nociception. In Dlx5/6-null mouse uteri, the expression levels of CACNA2D1 and CACNA2D2 were not significantly changed after RNAseq analysis and were not further analyzed.
Figure 3.
qPCR validation of genes upregulated after Dlx5/6 invalidation in the mouse uterus.
Notes: (A) Relative mRNA expression in normal (black) and mutant (white bars) mouse uteri of upregulated genes belonging to our newly identified endometriosis signature. Reference gene: Sdha. *P<0.05, **P<0.01, Mann and Whitney test analysis. Each group was constituted by five independent samples. (B) Relative mRNA expression in normal (black) and mutant (white bars) mouse uteri of downregulated genes identified as commonly deregulated genes belonging to our newly identified endometriosis signature (as shown in Table 2). Reference gene: Sdha. *P<0.05, **P<0.01, Mann and Whitney test analysis. Each group was constituted by five independent samples.
Abbreviation: qPCR, quantitative real-time PCR.
Remarkably, the expression of each of these three genes is significantly increased in endometriosis implants (Data Set Records GDS2835 and GDS3975).12,13 Interestingly, the cystathionine-b-synthase (CBS) gene has been already shown to be hypomethylated and upregulated in endometriosis lesions.21
Altered pattern of gene expression in human endometriosis
To validate the upregulation in endometriosis of the most significantly affected genes, we performed immunohistolocalization on section from normal endometrium and endometriosis implants. CACNA2D3, CBS, and LTBP2 (Figure 4) immunodetection signal was dramatically increased in endometriotic lesions. CACNA2D3 was virtually undetectable on normal endometrium sections (Figure 4A and B) and presented a high level of expression in endometriosis epithelia and smooth muscle cells (Figure 4A′ and B′). In the epithelia, CACNA2D3 immunodetection was predominantly associated to membranes and cytoplasm (arrows in Figure 4B′), while CBS and LTBP2 immunodetection (Figure 4C–D′) was mostly nuclear in endometriosis implants.
Figure 4.
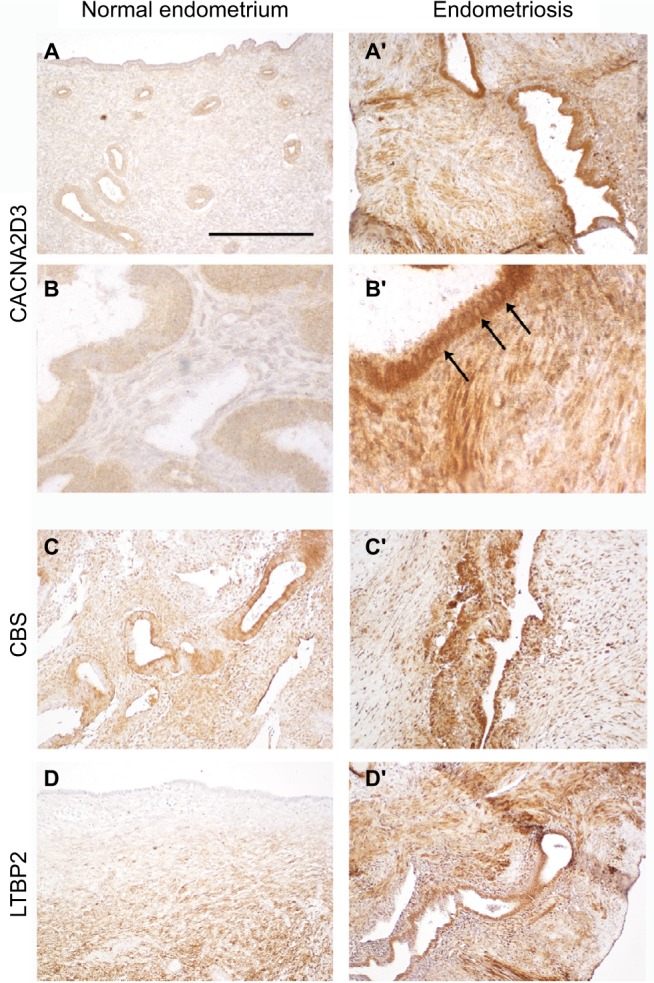
Immunolocalization of CACNA2D3, CBS, and LTBP2 in normal endometrium and in ovarian endometriosis implants.
Notes: Immunolocalization of CACNA2D3, CBS, and LTBP2 on sections from normal endometrium (A–C) and from endometriosis implants (A′, B′, and C′). Arrows indicate CACNA2D3 distribution in epithelial cells of endometriosis implants. Scale bar: (A, A′, C, C′, D, and D′) 250 µm and (B and B′) 62.5 µm.
Discussion
Morphological and genetic observations on mutant mouse uteri and on pathological specimens have prompted the suggestion that low DLX5 and DLX6 expressions could be associated with the progression of endometriosis. We have therefore proposed that Dlx5/6-null mouse uteri could be considered as a model of the disease permitting to get new insight on this pathology.9
Here, we have compared the gene expression signature of Dlx5/6-null mouse uteri with those obtained in four independent studies on endometriosis.12–14,20 Dlx5/6-null uteri and endometriosis implants share a set of 30 commonly deregulated genes supporting the hypothesis that downregulation of Dlx5/6 could be an early event in endometriosis progression. The 30 identified genes constitute a more “focused” genetic signature of the disease that has permitted potential therapeutic targets to be handpicked. We focused, in particular, on genes upregulated in endometriosis as they could be potential targets for therapeutic strategies.
Current endometriosis therapies present limited efficacy, high rates of symptom recurrence, and significant side effects.3,4 For example, the use of GnRH agonists is associated with side effects such as menopausal symptoms and reduced bone mineral density,22 which restrict their use and demand add-back therapy.23 In addition, pain management is one of the major clinical challenges for the treatment of endometriosis.24 Endometriosis implants, although ectopic, are highly innervated. Peritoneal endometriosis lesions present a high density of sensory, cholinergic, and adrenergic nerve fibers that might be involved in the origin of pain.25,26 It has been proposed that a bidirectional relation exists between nerve fibers and endometriotic lesions with a two-way interaction between the implants and the central nervous system.27–29 The interplay between the peripheral and the central nervous system might be at the origin of individual differences in pain perception that can, in some patients, evolve independently from the disease.30 When pathological conditions cause damage to the nervous system, sensory activation thresholds are lowered with exaggerated pain perception even upon mild or absent painful stimulation.
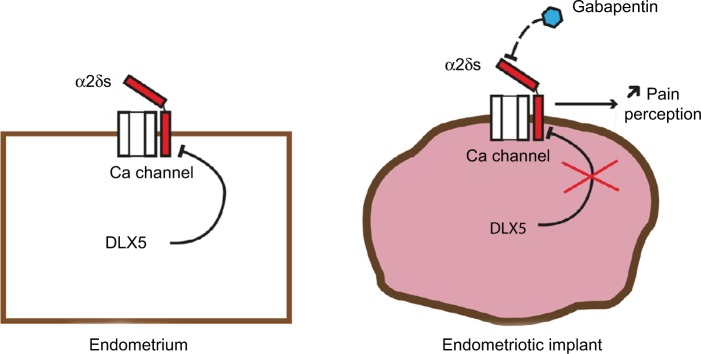
Between the 17 upregulated gene candidates, we focused specifically on CACNA2D3, member of the family of α2δ VGCC subunits involved in nociception (Figure 5). CACNA2D1, CACNA2D2, CACNA2D3, and CACNA2D4 constitute the α2δ family of VGCC subunits.16 VGCC are composed of α1 subunit proteins involved in pore formation31 and many possible splice variants of the α1, β, and α2δ subunits. Such a molecular diversity confers to these channels a variety of specific properties in different cell types and situations. VGCCs play a critical role in neuropathic pain development through the modulation of the release of excitatory neurotransmitters,32 calcium-dependent enzyme activation,33 gene regulation,33–35 and short- and long-term plasticity changes.36–39 Abnormal regulation of VGCC subunits, such as α2δs, may contribute to pain signal transduction through several mechanisms including the induction of abnormal synaptogenesis.40,41 Dysregulation of VGCCs and their subunits has been observed in pathological conditions, including nerve injuries and animal models of endometriosis;42 it is therefore possible that these channels contribute to the origin of endometriosis-associated pain.
Figure 5.
Summary diagram.
Note: The reduction of DLX5 expression in endometriosis implants results in an increased expression of α2δ subunits with a potential increase in neuropathic nociception.
Gabapentinoids, including gabapentin (Neurontin; Pfizer, Zürich, CH) and pregabalin (Lyrica; Pfizer), are compounds used for the treatment of neuropathic pain.43,44 As seen by binding of [3H]gabapentin to membranes from COS-7 cells transfected with α2δ cDNA, gabapentinoids bind predominantly to α2δ1, α2δ2 subunits of VGCCs.45–47 However, an evolutionary conserved role in nociception has also been shown for α2δ3, and SNP variants of this subunit are associated with reduced sensitivity to heat and chronic back pain.15
Conclusion
Analgesics, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and gabapentin, are often used for pain relief despite limited evidence of their efficacy in endometriosis.4,48 A recent pilot trial on 47 patients49 and several clinical observations48,50 have suggested that treatment with gaba-pentin alleviates significantly endometriotic pain; however, given the small size of the trials, uncertainty remains.43 Our findings provide evidence supporting a definitive evaluation of the efficacy of gabapentin in the management of endometriosis-associated pain.
Acknowledgments
We thank Dr Elisabeth Da Maia, Department of Pathology, Pitié Salpêtrière Hospital, for her kind help in endometrial and endometriosis section analysis. This research was partially supported by the EU Consortium HUMAN (EU-FP7-HEALTH-602757) to GL and by CNRS and MNHN general support to UMR7221. This study was supported in part by the French Centre National de la Recherche Scientifique, the French National Museum of Natural History, and the EU Consortium HUMAN (EU-FP7-HEALTH-602757).
Footnotes
Author contributions
GL, BB, AB, VG, CDL, and NN-N conceptualized and designed the study. BB, AF, GA, and ED-B acquired the data. PG, CP, and ED-B generated and analyzed the RNAseq data. SA and LM collected the tissue and performed histological analysis. GL, BB, AB, and VG drafted the article. All authors discussed the results and commented on the article. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Morotti M, Vincent K, Becker CM. Mechanisms of pain in endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017;209:8–13. doi: 10.1016/j.ejogrb.2016.07.497. [DOI] [PubMed] [Google Scholar]
- 2.Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker CM, Gattrell WT, Gude K, Singh SS. Reevaluating response and failure of medical treatment of endometriosis: a systematic review. Fertil Steril. 2017;108(1):125–136. doi: 10.1016/j.fertnstert.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunselman GA, Vermeulen N, Becker C, et al. European Society of Human Reproduction and Embryology ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–412. doi: 10.1093/humrep/det457. [DOI] [PubMed] [Google Scholar]
- 5.Chu Sin Chung P, Keyworth HL, Martin-Garcia E, et al. A novel anxiogenic role for the delta opioid receptor expressed in GABAergic forebrain neurons. Biol Psychiatry. 2015;77(4):404–415. doi: 10.1016/j.biopsych.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kraus P, Lufkin T. Dlx homeobox gene control of mammalian limb and craniofacial development. Am J Med Genet A. 2006;140(13):1366–1374. doi: 10.1002/ajmg.a.31252. [DOI] [PubMed] [Google Scholar]
- 7.Nishida H, Miyagawa S, Vieux-Rochas M, et al. Positive regulation of steroidogenic acute regulatory protein gene expression through the interaction between Dlx and GATA-4 for testicular steroidogenesis. Endocrinology. 2008;149(5):2090–2097. doi: 10.1210/en.2007-1265. [DOI] [PubMed] [Google Scholar]
- 8.Bouhali K, Dipietromaria A, Fontaine A, et al. Allelic reduction of Dlx5 and Dlx6 results in early follicular depletion: a new mouse model of primary ovarian insufficiency. Hum Mol Genet. 2011;20(13):2642–2650. doi: 10.1093/hmg/ddr166. [DOI] [PubMed] [Google Scholar]
- 9.Bellessort B, Le Cardinal M, Bachelot A, et al. Dlx5 and Dlx6 control uterine adenogenesis during post-natal maturation: possible consequences for endometriosis. Hum Mol Genet. 2016;25(1):97–108. doi: 10.1093/hmg/ddv452. [DOI] [PubMed] [Google Scholar]
- 10.Talbi S, Hamilton AE, Vo KC, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147(3):1097–1121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- 11.Hu WP, Tay SK, Zhao Y. Endometriosis-specific genes identified by real-time reverse transcription-polymerase chain reaction expression profiling of endometriosis versus autologous uterine endometrium. J Clin Endocrinol Metab. 2006;91(1):228–238. doi: 10.1210/jc.2004-1594. [DOI] [PubMed] [Google Scholar]
- 12.Hawkins SM, Creighton CJ, Han DY, et al. Functional microRNA involved in endometriosis. Mol Endocrinol. 2011;25(5):821–832. doi: 10.1210/me.2010-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hever A, Roth RB, Hevezi P, et al. Human endometriosis is associated with plasma cells and overexpression of B lymphocyte stimulator. Proc Natl Acad Sci U S A. 2007;104(30):12451–12456. doi: 10.1073/pnas.0703451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eyster KM, Boles AL, Brannian JD, Hansen KA. DNA microarray analysis of gene expression markers of endometriosis. Fertil Steril. 2002;77(1):38–42. doi: 10.1016/s0015-0282(01)02955-7. [DOI] [PubMed] [Google Scholar]
- 15.Neely GG, Hess A, Costigan M, et al. A genome-wide Drosophila screen for heat nociception identifies alpha2delta3 as an evolutionarily conserved pain gene. Cell. 2010;143(4):628–638. doi: 10.1016/j.cell.2010.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong N, Park J, Luo ZD. Injury-induced maladaptation and dysregulation of calcium channel alpha2 delta subunit proteins and its contribution to neuropathic pain development. Br J Pharmacol. 2017 Jun 23; doi: 10.1111/bph.13930. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14(4):R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anders S, Pyl PT, Huber W. HTSeq – a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31(2):166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hull ML, Escareno CR, Godsland JM, et al. Endometrial-peritoneal interactions during endometriotic lesion establishment. Am J Pathol. 2008;173(3):700–715. doi: 10.2353/ajpath.2008.071128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borghese B, Barbaux S, Mondon F, et al. Research resource: genome-wide profiling of methylated promoters in endometriosis reveals a subtelomeric location of hypermethylation. Mol Endocrinol. 2010;24(9):1872–1885. doi: 10.1210/me.2010-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DY, Lee JY, Seo JW, Yoon BK, Choi D. Gonadotropin-releasing hormone agonist with add-back treatment is as effective and tolerable as dienogest in preventing pain recurrence after laparoscopic surgery for endometriosis. Arch Gynecol Obstet. 2016;294(6):1257–1263. doi: 10.1007/s00404-016-4184-9. [DOI] [PubMed] [Google Scholar]
- 23.Lee DY, Park HG, Yoon BK, Choi D. Effects of different add-back regimens on hypoestrogenic problems by postoperative gonadotropin-releasing hormone agonist treatment in endometriosis. Obstet Gynecol Sci. 2016;59(1):32–38. doi: 10.5468/ogs.2016.59.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308(5728):1587–1589. doi: 10.1126/science.1111445. [DOI] [PubMed] [Google Scholar]
- 25.Tokushige N, Markham R, Russell P, Fraser IS. Nerve fibres in peritoneal endometriosis. Hum Reprod. 2006;21(11):3001–3007. doi: 10.1093/humrep/del260. [DOI] [PubMed] [Google Scholar]
- 26.Tokushige N, Markham R, Russell P, Fraser IS. High density of small nerve fibres in the functional layer of the endometrium in women with endometriosis. Hum Reprod. 2006;21(3):782–787. doi: 10.1093/humrep/dei368. [DOI] [PubMed] [Google Scholar]
- 27.Morotti M, Vincent K, Brawn J, Zondervan KT, Becker CM. Peripheral changes in endometriosis-associated pain. Hum Reprod Update. 2014;20(5):717–736. doi: 10.1093/humupd/dmu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller EJ, Fraser IS. The importance of pelvic nerve fibers in endometriosis. Womens Health (Lond) 2015;11(5):611–618. doi: 10.2217/whe.15.47. [DOI] [PubMed] [Google Scholar]
- 29.Yan D, Liu X, Guo SW. Nerve fibers and endometriotic lesions: partners in crime in inflicting pains in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017;209:14–24. doi: 10.1016/j.ejogrb.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 30.Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327–346. doi: 10.1093/humupd/dmq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dolphin AC. Voltage-gated calcium channels and their auxiliary subunits: physiology and pathophysiology and pharmacology. J Physiol. 2016;594(19):5369–5390. doi: 10.1113/JP272262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee S. Pharmacological inhibition of voltage-gated Ca(2+) channels for chronic pain relief. Curr Neuropharmacol. 2013;11(6):606–620. doi: 10.2174/1570159X11311060005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park J, Luo ZD. Calcium channel functions in pain processing. Channels (Austin) 2010;4(6):510–517. doi: 10.4161/chan.4.6.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perret D, Luo ZD. Targeting voltage-gated calcium channels for neuropathic pain management. Neurotherapeutics. 2009;6(4):679–692. doi: 10.1016/j.nurt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wheeler DG, Groth RD, Ma H, et al. Ca(V)1 and Ca(V)2 channels engage distinct modes of Ca(2+) signaling to control CREB-dependent gene expression. Cell. 2012;149(5):1112–1124. doi: 10.1016/j.cell.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu XG, Zhou LJ. Long-term potentiation at spinal C-fiber synapses: a target for pathological pain. Curr Pharm Des. 2015;21(7):895–905. doi: 10.2174/1381612820666141027115949. [DOI] [PubMed] [Google Scholar]
- 37.Russo RE, Hounsgaard J. Short-term plasticity in turtle dorsal horn neurons mediated by L-type Ca2+ channels. Neuroscience. 1994;61(2):191–197. doi: 10.1016/0306-4522(94)90222-4. [DOI] [PubMed] [Google Scholar]
- 38.Youn DH, Gerber G, Sather WA. Ionotropic glutamate receptors and voltage-gated Ca(2)(+) channels in long-term potentiation of spinal dorsal horn synapses and pain hypersensitivity. Neural Plast. 2013;2013:654257. doi: 10.1155/2013/654257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naka A, Gruber-Schoffnegger D, Sandkuhler J. Non-Hebbian plasticity at C-fiber synapses in rat spinal cord lamina I neurons. Pain. 2013;154(8):1333–1342. doi: 10.1016/j.pain.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eroglu C, Allen NJ, Susman MW, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139(2):380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park J, Yu YP, Zhou CY, et al. Central mechanisms mediating thrombospondin-4-induced pain states. J Biol Chem. 2016;291(25):13335–13348. doi: 10.1074/jbc.M116.723478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Zhang M, Xie F, et al. Upregulation of alpha(2)delta-1 calcium channel subunit in the spinal cord contributes to pelvic organ cross-sensitization in a rat model of experimentally-induced endometriosis. Neurochem Res. 2015;40(6):1267–1273. doi: 10.1007/s11064-015-1592-3. [DOI] [PubMed] [Google Scholar]
- 43.Moore RA, Wiffen PJ, Derry S, Toelle T, Rice AS. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;4:CD007938. doi: 10.1002/14651858.CD007938.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev. 2015;67(4):821–870. doi: 10.1124/pr.114.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gong HC, Hang J, Kohler W, Li L, Su TZ. Tissue-specific expression and gabapentin-binding properties of calcium channel alpha2delta subunit subtypes. J Membr Biol. 2001;184(1):35–43. doi: 10.1007/s00232-001-0072-7. [DOI] [PubMed] [Google Scholar]
- 46.Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Wood-ruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem. 1996;271(10):5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- 47.Kukkar A, Bali A, Singh N, Jaggi AS. Implications and mechanism of action of gabapentin in neuropathic pain. Arch Pharm Res. 2013;36(3):237–251. doi: 10.1007/s12272-013-0057-y. [DOI] [PubMed] [Google Scholar]
- 48.Speer LM, Mushkbar S, Erbele T. Chronic pelvic pain in women. Am Fam Physician. 2016;93(5):380–387. [PubMed] [Google Scholar]
- 49.Lewis SC, Bhattacharya S, Wu O, et al. Gabapentin for the management of chronic pelvic pain in women (GaPP1): a pilot randomised controlled trial. PLoS One. 2016;11(4):e0153037. doi: 10.1371/journal.pone.0153037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sator-Katzenschlager SM, Scharbert G, Kress HG, et al. Chronic pelvic pain treated with gabapentin and amitriptyline: a randomized controlled pilot study. Wien Klin Wochenschr. 2005;117(21–22):761–768. doi: 10.1007/s00508-005-0464-2. [DOI] [PubMed] [Google Scholar]