Abstract
Background
Airway hyperresponsiveness (AHR) is associated with airway inflammation and a rapid decline in lung function and is a predictor of future risk of COPD among smokers. Alveolar macrophages (AMs) from patients with COPD release a greater amount of matrix metalloproteinase (MMP)-9. We hypothesized that the imbalance between MMP-9 and tissue inhibitor of metalloproteinase-1 (TIMP-1) is related to AHR in smokers.
Patients and methods
Healthy smokers with AHR (AHR + S) or smokers without AHR (AHR − S; divided according to a methacholine challenge test) and nonsmokers without AHR (AHR − NS) were enrolled. Spirometry was performed during enrollment and repeated after 5 years. Initially, AMs recovered from bronchoalveolar lavage (BAL) fluid were cultured in the presence of p38 mitogen-activated protein kinase (MAPK) inhibitor (SB203580), MAPK kinase (MEK) 1/2 (the MEK of extracellular signal-regulated kinase [ERK] inhibitor, PD98059), or medium alone for 24 h. The release of MMP-9 and TIMP-1 in culture supernatants was measured by enzyme-linked immunosorbent assay.
Results
A greater reduction in forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC), FEV1 (as a percentage of the predicted value [%pred]), and maximal mid-expiratory flow (MMEF) was observed among AHR + S in the 5-year period. There was a higher proportion of neutrophils and a lower proportion of AMs in BAL fluid recovered from AHR + S. Compared to AMs from AHR − NS and AHR − S, AMs from nonsmokers with AHR (AHR + NS) released more MMP-9 and less TIMP-1, with an increase in MMP-9/TIMP-1 ratios. The MMP-9/TIMP-1 ratio in smokers was positively correlated with the annual decline in FEV1%pred, FVC%pred, and MMEF%pred. Both SB203580 and PD98059 significantly reduced MMP-9, but not TIMP-1, from AMs of smokers.
Conclusion
AMs of AHR + NS produce excessive MMP-9 over TIMP-1, which may be a predictor of the development of airway obstruction. Inhibition of p38 MAPK and ERK suppresses the generation of MMP-9 by AMs from smokers.
Keywords: smoking, airway hyperresponsiveness, alveolar macrophage, matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1, p38 mitogen-activated protein kinase, extracellular signal-regulated kinase
Take home message
Alveolar macrophages from smokers with airway hyperresponsiveness release a greater amount of matrix metalloproteinase (MMP-9), which can be suppressed by inhibitors of p38 mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase (ERK), and a lesser amount of tissue inhibitor of metalloproteinase-1 (TIMP-1), thus increasing the annual decline in lung function.
Introduction
COPD is the fourth leading cause of death, affecting >300 million people globally.1 The diagnosis of COPD is made in patients with respiratory symptoms, persistent airflow limitation confirmed by a postbronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) of <70%, often associated with cigarette smoking.2 Tobacco smoke inhalation activates epithelial cells and alveolar macrophages (AMs) to release chemoattractants for circulating neutrophils, monocytes, and lymphocytes into the lungs.3 These cells in COPD patients release a greater amount of proteinases and growth factors upon smoking, resulting in the breakdown of lung parenchyma, mucus hypersecretion, and small airway fibrosis, namely emphysema and chronic bronchitis.4–7
Only a subset of cigarette smokers develop COPD, and the host factors leading to airflow limitation are under active investigation. It has been postulated that airway hyperresponsiveness (AHR) predisposes the smokers’ airways to obstruction.8,9 AHR is defined as an increased sensitivity of the airways to direct stimuli (eg, histamine and methacholine) and/or indirect stimuli (eg, mannitol and hypertonic saline). The Lung Health Study demonstrated that AHR was strongly associated with the degree of airway obstruction in current smokers.8 Current smokers with airway obstruction displayed a greater AHR than smokers with normal lung function.10 Thus, smokers with AHR (AHR + S) could be prone to the development of COPD due to chronic airway inflammation and structural alternation.
The excess of protease over antiprotease expression, such as neutrophil elastase versus α1-anti-trypsin and matrix metalloproteinases (MMPs) versus tissue inhibitors of metalloproteinases (TIMPs), has been proposed for cigarette smoke-induced chronic lung disease.11 MMPs are a family of >20 zinc-dependent endopeptidases with enzymatic activities in turnover and degradation of extracellular matrices. MMP-9, also known as gelatinase B or 92 kDa gelatinase, is one of the major elastolytic enzymes produced by AMs from COPD patients but are also secreted by neutrophils, epithelial cells, mast cells, and fibroblasts.3 MMP-9 proteolytically digests extracellular matrix (ECM) proteins including collagens IV, V, VII, X, and XIV, gelatin, and elastin, and activates latent pro-MMP-9 and pro-MMP-13.12 Epigenetically, MMPs could be modulated by methylation of the CpG sites in promoters and chromatin remodeling with histone acetylation.12 Hypomethylation of MMP-9 promotor had been observed in chondrocytes in osteoarthritis, which could be associated with the increased synthesis of the cartilage-degrading enzyme.13 Sirtuin1, a histone deacetylase acting on the activator protein-1 (AP-1) response element in the promotor of MMP-9, inhibits histone 3 acetylation and reduces MMP-9 in COPD.14,15 The activity of MMPs is also modulated transcriptionally by phosphatase and tensin homolog (PTEN) and mitogen-activated protein kinases (MAPKs), posttranscriptionally by microRNAs, and posttranslationally by reversion-inducing cysteine-rich protein with kazal motifs and a four-member family of TIMPs.11 Both active form and precursor form of MMP-9 are antagonized by TIMP-1. The amount of MMP-9 released from AMs of COPD patients and smokers was greater than that of nonsmokers, but the expression of TIMP-1 was higher in sputum in chronic bronchitis and lower in emphysematous lungs.16–19 It is likely that the balance between MMP-9 and TIMP-1 determines the clinical phenotypes of COPD.
MMP-9 inhibition may seem an appealing strategy to treat respiratory diseases including COPD but can hardly be achieved by inhaled corticosteroids, and the effects of MMP inhibitors, blocking antibodies, and antisense technologies have not been widely recognized in clinical practice.20,21 MAPKs, including p38 MAPKs, c-Jun NH2-terminal kinases (JNKs), and extracellular signal-regulated kinases (ERKs), are a family of serine/threonine protein kinases activated in response to external signals (cigarette smoke, wood smoke, colonizing bacteria, oxidative stress, cytokines, and growth factors), resulting in the secretion of many mediators, which then activate further inflammatory cascades, tissue remodeling, and aging process of the lungs.22 MAPK signaling is regulated by successive phosphorylation starting with mitogen-activated protein (MAP) kinase kinase kinases; then MAP kinase kinases such as MAP2K3/6, MAP2K4, and MAPK kinase (MEK) 1/2 (MAP2K1/2); and finally p38 MAPK and ERK, modulating transcription factors such as nuclear factor-κB (NF-κB) and AP-1. Little is known about the effect of MAPK inhibitors on the release of MMP-9 and TIMP-1 from AMs.
We hypothesized that the imbalance between MMP-9 and TIMP-1 contributes to AHR, which appears to be an early feature of COPD in susceptible smokers. We compared the evolution of lung function over a 5-year period, as well as the generation of MMP-9 and TIMP-1 from AMs in nonsmokers without AHR (AHR − NS) and smokers without AHR (AHR − S) and AHR + S. We also examined whether the inhibition of p38 MAPK and ERK modulates the release of MMP-9 or TIMP-1 from AMs in smokers.
Patients and methods
Studied population
Subjects aged between 20 and 75 years were recruited from our outpatient department, Chang Gung Memorial Hospital. All enrolled subjects had no abnormal radiographic findings on their chest radiograph and had a normal pulmonary function tests and negative bronchodilator response at the initial visits. Current tobacco smokers with a smoking history of at least 20 pack-years were divided into AHR − S (n=13, smokers with a dose of methacholine causing a 20% fall in FEV1 [PC20] ≥16 mg/mL in a methacholine challenge test, described later in the “Lung function and methacholine challenge testing” section) and AHR + S (n=20, smokers with a PC20 of <16 mg/mL), and only nonsmokers who had a negative methacholine challenge test were enrolled (AHR − NS, n=24). None of the recruited subjects had upper airway infection within 6 months before enrollment, tuberculosis, asthma, bronchiectasis, and systemic lupus erythematosus or took antibiotics, inhaled or systemic corticosteroids, immunosuppressants, or regular medications for extrapulmonary diseases before entering in to the study. Thirty-one subjects received fiberoptic bronchoscopy, including 10 AHR − NS, 13 AHR − S, and 8 AHR + S. All patients provided written informed consent, and the study was approved by the Ethics Committee of Chang Gung Hospital (92-099).
Lung function and methacholine challenge testing
Measurement of lung function at the beginning and a follow-up visit of ~6 years later for each enrolled participant was performed by a Spiroanalyzer ST-350R (Fukuda Sangyo Co., Ltd, Tokyo, Japan). The best of three reproducible values was chosen. A normal lung function was defined by an FEV1/FVC of ≥75%, an FEV1 of ≥80% of the predicted value (%pred), an FVC of ≥80%pred, and a maximal mid-expiratory flow (MMEF; also known as forced expiratory flow at 25%–75%) of ≥60%pred in the absence of a significant rise in FEV1 (12% and 200 mL) after bronchodilators. The rate of decline in FEV1, FVC, and MMEF was computed by dividing the total change in the 5-year follow-up period by 5, which represented an average annual rate of change.23 Methacholine challenge test was performed 1 week before bronchoscopy.24 The FEV1 was measured 5 min after the inhalation of phosphate-buffered saline or methacholine solution at incremental concentrations via nebulizer until the FEV1 had fallen by >20% of the starting FEV1. The log dose–response curve for methacholine was constructed as the percentage changes in FEV1 from the baseline (postbuffer) value, and the PC20 was measured by linear interpolation. AHR was defined as a PC20 of <16 mg/mL.
Bronchoalveolar lavage (BAL) and preparation of BAL cells
Lower respiratory cells were collected by BAL using fiberoptic bronchosocopy.25 After premedication with intravenous atropine (0.6 mg) and midazolam (5–10 mg) and topically applied laryngeal lidocaine, a fiberoptic bronchoscope was passed through the nasal passages into the trachea under oxygen supplementation. BAL was performed using six aliquots (50 mL each) of sterile and warm 0.9% saline solution into the right fourth or fifth subsegmental bronchus. Retrieved fluid was pooled, filtered through two layers of sterile gauze, and centrifuged at 600× g for 20 min at 4°C. The cell pellet was washed sequentially and resuspended in RPMI-1640 (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 5% heat-inactivated fetal calf serum (FCS; Flow Laboratories, Paisley, Scotland, UK) at 106 cells/mL. The cell viability was determined by trypan blue exclusion. The differential cell counts were performed by counting 500 cells on cytocentrifuge preparations using the modified Wright–Giemsa stain.
Cells in BAL fluid were plated in six-well Petri dishes at a concentration of 106 cells/mL for 24 h at 37°C, 5% CO2. The medium was replaced by 1 mL of fresh complete medium after culture for 24 h, and the adherent AM was incubated for a further 24 h in the presence of medium alone, medium + SB20358 (10 μM, a p38 MAPK inhibitor), or medium + PD98059 (30 μM, an MEK inhibitor). Supernatants were stored at −20°C until experimental assay.
Enzyme-linked immunosorbent assays for measurement of MMP-9 and TIMP-1 released by AMs in the culture supernatants
MMP-9 and TIMP-1 secreted by AMs in supernatants were assayed using commercially available quantitative sandwich-type enzyme-linked immunoassay kits (Amersham Life Sciences, Arlington Heights, IL, USA). Briefly, monoclonal primary antibodies were coated onto a microtiter plate, washed with PBS/Tween and blocked with PBS/10% FCS (200 μL). Standards and culture supernatant samples were then added, followed by horseradish peroxide-conjugated secondary antibodies, 5,5′-tetramethyl benzidine and hydrogen peroxide. Termination of the reaction was done by sulfuric acid (1.0 M). The color change was read spectrophotometrically at a wavelength of 450 mM, and the quantification of MMP-9 and TIMP-1 was performed by comparing the optical density of the sample with the standard curve.
Statistical analysis
Results are reported as mean ± standard error of mean. The Chi-square test was used to determine whether there was a significant difference between the expected frequencies and the observed frequencies in more categories. Differences among AHR − NS, AHR − S, and AHR + S were estimated by Kruskal–Wallis one-way ANOVA test, followed by Dunn’s post hoc multiple comparisons. The difference in smoking history between AHR − S and AHR + S was determined by Mann–Whitney test. The difference between categorical variables (sex and smoking habit) was determined by Chi-square test. To evaluate the effect of p38 MAPK and ERK inhibition, results were analyzed using the Friedman one-way ANOVA test, followed by Dunn’s post hoc multiple comparisons to the determine the differences between the control group and each treatment group. Correlations were determined by Spearman’s rank correlation. A P-value of <0.05 was accepted as statistically significant.
Results
More rapid development in airflow limitation in AHR + S
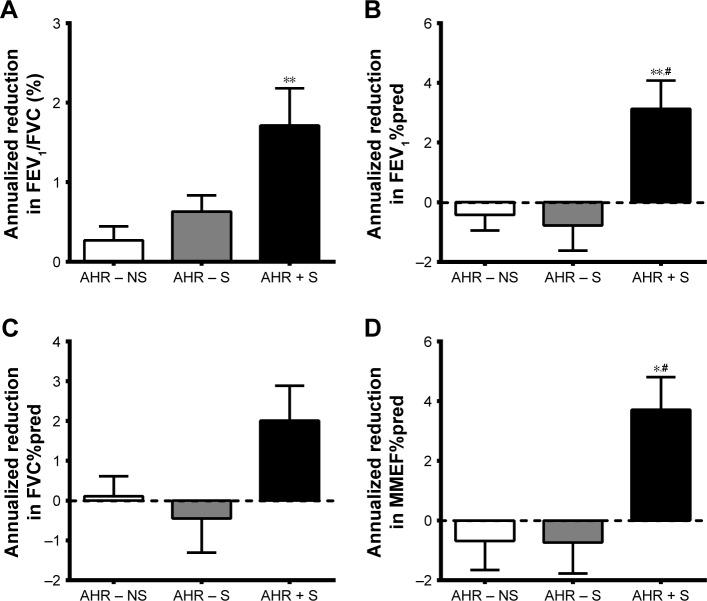
As shown in Table 1, the history of tobacco smoking during initial enrollment and the numbers of participants remaining smoking during the 5-year follow-up visit between AHR − S and AHR + S were not significantly different. The baseline pulmonary function test results were within normal ranges, but AHR + S had lower FEV1/FVC and FEV1%pred compared to AHR − NS and lower MMEF%pred compared to AHR − NS and AHR − S. AHR + S had a greater annual decline in a 5-year period from baseline in FEV1/FVC, FEV1%pred, and MMEF%pred (Figure 1). The FEV1%pred and MMEF%pred of AHR + S dropped below normal, while there was no significant change of lung function in AHR − NS and AHR − S, indicating a correlation between AHR and the development of airflow limitation. The smoking history (pack-years) was not correlated with the rate of annual decline in FEV1/FVC (r=0.031, P=0.866), FEV1%pred (r=0.131, P=0.514), FVC%pred (r=0.213, P=0.235), and MMEF%pred (r=−0.045, P=0.863).
Table 1.
Patient demographics and lung function
| Characteristics | AHR − NS (n=24) | AHR − S (n=13) | AHR + S (n=20) | P-value |
|---|---|---|---|---|
| Age (years) | 42.0±3.2 | 49.8±4.5 | 36.0±3.4 | 0.107 |
| Male/female | 6/18 | 7/6 | 11/9 | 0.083a |
| Body mass index (kg/m2) | 23.2±0.9 | 26.1±1.5 | 24.2±1.2 | 0.324 |
| Blood eosinophil (%) | 2.5±0.8 | 1.5±0.5 | 5.5±2.2 | 0.374 |
| Total IgE (IU/mL) | 104.1±25.1 | 255.3±78.4 | 356.7±124.8 | 0.136 |
| Smoking history (pack-years) | – | 31.1±2.7 | 27.6±2.3 | 0.215b |
| Remain smoking at year 5 | – | 10 | 16 | 0.833a |
| Spirometry | ||||
| FEV1/FVC (%) | ||||
| Year 0 | 87.8±1.1 | 86.1±0.8 | 83.7±1.4* | 0.034 |
| Year 5 | 86.5±1.0 | 83.0±1.3$ | 75.1±2.2***,$$$ | 0.0002 |
| FEV1%pred | ||||
| Year 0 | 98.9±2.3 | 96.8±3.4 | 87.7±2.5* | 0.013 |
| Year 5 | 98.6±2.7 | 98.1±3.8 | 72.7±4.8****,##,$$ | ,0.0001 |
| FVC%pred | ||||
| Year 0 | 98.5±2.1 | 94.9±2.9 | 93.9±2.0 | 0.356 |
| Year 5 | 98.0±2.4 | 97.1±4.0 | 83.9±4.8*,$ | 0.0111 |
| MMEF%pred | ||||
| Year 0 | 92.1±5.9 | 90.5±5.1 | 66.8±7.5#,* | 0.004 |
| Year 5 | 97.9±5.5 | 91.9±4.1 | 59.8±7.8**,#,$ | 0.0011 |
Notes: Data are presented as mean ± standard error of the mean. AHR−NS, nonsmokers without AHR; AHR−S, smokers without AHR; AHR+S, smokers with AHR.
P<0.05 versus AHR−NS.
P<0.01 versus AHR−NS.
P<0.001 versus AHR−NS.
P<0.0001 versus AHR−NS.
P<0.05 versus AHR−S.
P<0.01 versus AHR−S.
P<0.05 versus lung function at Year 0.
P<0.01 versus lung function at Year 0.
P<0.0001 versus lung function at Year 0;
Determined by Mann-Whitney test.
Determined by Chi-Square test.
Abbreviations: AHR, airway hyperresponsiveness; IgE, immunoglobulin E; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; MMEF, maximal mid-expiratory flow; %pred, percentage of the predicted value.
Figure 1.
More rapid decline in airflow in AHR + S.
Notes: Spirometry was performed during the recruitment of the AHR − NS (n=24), AHR − S (n=13) and AHR + S (n=20) and was repeated 5 years later. Annualized reduction from baseline in FEV1/FVC (A), FEV1%pred (B), FVC%pred (C) and MMEF%pred (D) is presented as individual data point, mean and standard error of mean. *P<0.05, **P<0.01 versus AHR−NS; #P<0.05 versus AHR−S. AHR−NS, nonsmokers without AHR; AHR−S, smokes without AHR; AHR + S, smokers with AHR.
Abbreviations: AHR, airway hyperresponsiveness; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; MMEF, maximal mid-expiratory flow; %pred, as a percentage of the predicted value.
Higher percentage of neutrophils in cells in BAL fluid recovered from AHR + S
Table 2 summarizes total and differential cell counts in BAL fluid, with a significant increase in cellularity recovered from BAL fluid of AHR − S compared to that of AHR − NS and AHR + S. The concentration of neutrophils was higher in AHR + S compared to those of AHR − S and AHR − NS. The number of AMs in 1 mL of BAL fluid was higher from AHR − S than from nonsmoker and AHR + S.
Table 2.
Bronchoalveolar lavage fluid findings from nonsmokers and smokers
| AHR − NS (n=10) | AHR − S (n=13) | AHR + S (n=8) | |
|---|---|---|---|
| Total number of cells, 104 | 508.0±43.6 | 2,125.4±104.6*** | 1,179.8±74.3# |
| Recovered volume (mL) | 62.4±3.1 | 57.4±2.4 | 56.3±3.6 |
| Cellularity, 104 cells/mL | 8.2±0.7 | 37.0±0.7*** | 21.0±0.3# |
| Cell viability (%) | 92.7±0.5 | 92.5±0.9 | 92.3±2.4 |
| AM (%) | 96.1±1.0 | 98.5±2.5 | 82.8±1.5#### |
| 104 cells/mL | 7.9±0.62 | 36.4±0.9*** | 17.4±0.4# |
| Lymphocytes (%) | 2.6±1.0 | 0.7±0.1 | 0.6±0.1 |
| 104 cells/mL | 0.2±0.1 | 0.3±0.1 | 0.11±0.1 |
| Neutrophils (%) | 1.0±0.3 | 0.7±0.1 | 15.5±5.6**,### |
| 104 cells/mL | 0.1±0.0 | 0.3±0.1 | 3.3±1.2***,## |
Notes: Data are presented as mean ± standard error. AHR − NS, nonsmokers without AHR; AHR − S, smokers without AHR; AHR + S, smokers with AHR.
P<0.01 versus AHR − NS.
P<0.0001 versus AHR − NS.
P<0.05 versus AHR − NS.
P<0.01 versus AHR − NS.
P<0.001 versus AHR − NS.
P<0.0001 versus AHR − NS.
Abbreviations: AHR, airway hyperresponsiveness; AM, alveolar macrophage.
Higher MMP-9 and lower TIMP-1 productions by AMs from AHR + S
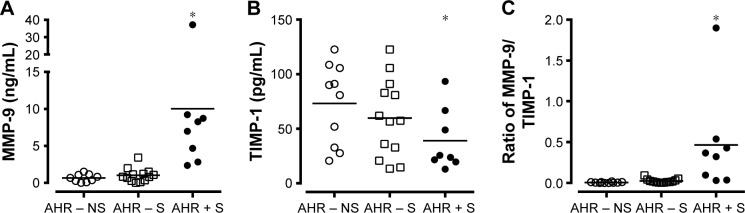
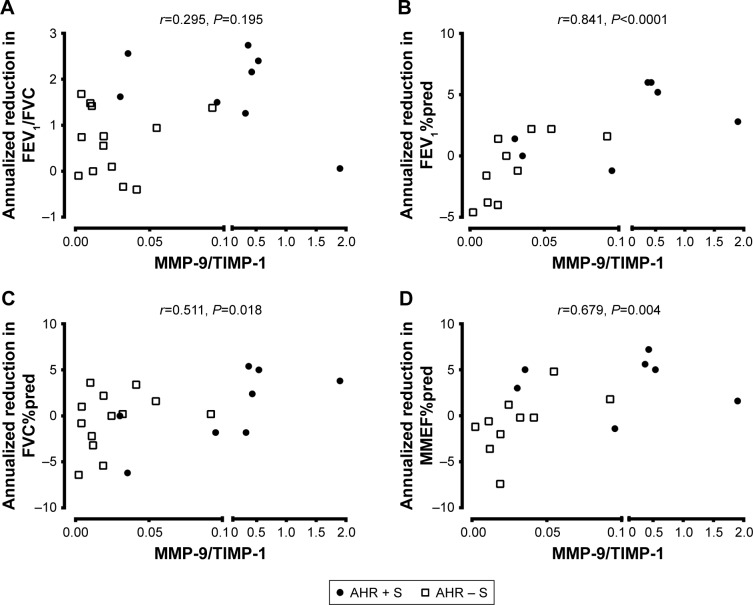
The level of MMP-9 in culture supernatant released from AMs was higher in AHR + S (10.0±4.0 ng/mL, n=8, P<0.05) than in AHR − S (1.0±0.3 ng/mL, n=13) and AHR – NS (0.7±0.2 ng/mL, n=10) (Figure 2A). There was a lower level of TIMP-1 released by AMs in AHR + S (39.0±10.0 ng/mL, P<0.05) than in AHR − NS (73.1±11.7 ng/mL) (Figure 2B). AHR + S had a significant increase in the molar ratio of MMP-9 to TIMP-1 (0.46±0.22, P<0.05) released from AM compared to AHR − S (0.025±0.007) and AHR – NS (0.006±0.002) (Figure 2C). Moreover, the MMP-9/TIMP-1 ratio in smokers was positively correlated with the annual decline in FEV1%pred, FVC%pred, and MMEF%pred, suggesting that the imbalance between MMP-9 and TIMP-1 is related to airflow obstruction (Figure 3).
Figure 2.
Greater release of MMP-9 (A) but lesser release of TIMP-1 (B) by cultured AMs from AHR + S.
Notes: AMs from AHR−NS (n=10), AHR−S (n=13) or AHR+S (n=8) were incubated for 24 hr. The level of MMP-9 (A) and TIMP-1 (B) in the culture supernatants was determined by enzyme-linked immunosorbent assays. The molar ratio between MMP-9 and TIMP-1 was also calculated (C). Values are shown as individual data point, mean, and standard error of mean. *P<0.05 compared to AHR − NS. AHR − NS, nonsmokers without AHR; AHR − S, smokers without AHR; AHR + S, smokers with AHR.
Abbreviations: AHR, airway hyperresponsiveness; AMs, alveolar macrophages; MMP-9, matrix metalloproteinase-9; TIMP-1, tissue inhibitor of metalloproteinase-1.
Figure 3.
Association between MMP-9/TIMP-1 ratio and the development of airflow limitation.
Notes: Spirometry and bronchoscopy were performed during the recruitment, and spirometry was repeated 5 years later. AMs from 21 smokers, including 13 AHR − S and 8 AHR + S, were incubated for 24 h. The level of MMP-9 and TIMP-1 in the culture supernatants was determined by enzyme-linked immunosorbent assays. The MMP-9/TIMP-1 ratio was less related to the reduction in FEV1/FVC (A) but was positively associated with the annualized reduction in FEV1%pred (B), FVC%pred (C), and MMEF%pred (D). AHR − S, smokers without AHR; AHR + S, smokers with AHR.
Abbreviations: AHR, airway hyperresponsiveness; AMs, alveolar macrophages; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; MMEF, maximal mid-expiratory flow; MMP-9, matrix metalloproteinase-9; %pred, percentage of the predicted value; TIMP-1, tissue inhibitor of metalloproteinase-1.
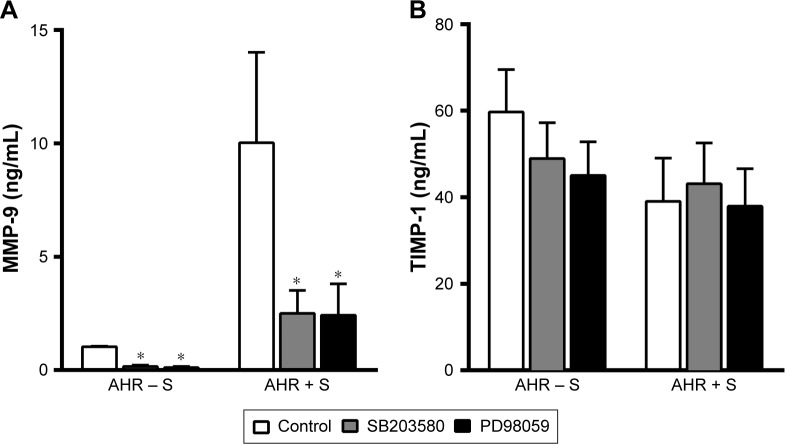
Inhibition of p38 MAPK and ERK reduced the release of MMP-9, but not TIMP-1, by smokers’ AMs
We further investigated whether the modulation of MAP kinase activities alters the release of MMP-9 and TIMP-1 by AMs from smokers. Both p38 MAPK inhibitor SB203580 (10 μM) and MEK inhibitor PD98059 (30 μM) reduced the generation of MMP-9 (2.5±1.0 ng/mL, n=8, P<0.05, and 2.4±1.4 ng/mL, n=8, P<0.05, respectively) compared to untreated controls (10.0±4.0 ng/mL, n=8) by AMs of AHR − S and AHR + S (Figure 4A). In contrast, neither SB203580 nor PD98059 had a significant effect on the production of TIMP-1 by AMs in AHR − S and AHR + S (Figure 4B).
Figure 4.
Inhibition of p38 MAPK and ERK reduced the release of MMP-9, but not TIMP-1, from AMs from smokers.
Notes: AMs from AHR − NS (n=8) or AHR + NS (n=8) were incubated in the presence of SB203580 10 μM (p38 MAPK inhibitor) (A) or PD98059 30 μM (MEK1/2 inhibitor) (B) for 24 h. The release of MMP-9 and TIMP-1 in the culture supernatants was determined by enzyme-linked immunosorbent assays. *P<0.05 compared to untreated controls. AHR − S, smokers without AHR; AHR + S, smokers with AHR.
Abbreviations: AHR, airway hyperresponsiveness; AMs, alveolar macrophages; ERK, extracellular signal-regulated kinase; MAPK, mitogen-activated protein kinase; MEK, MAPK kinase; MMP-9, matrix metalloproteinase-9; TIMP-1, tissue inhibitor of metalloproteinase-1.
Discussion
In this study, the AHR + S had a more significant decline in FEV1/FVC, FEV1, and MMEF over a 5-year period compared to those AHR − S and AHR − NS. AMs from AHR + S released a greater amount of MMP-9 and a lesser amount of TIMP-1 than AMs from AHR − NS and AHR − S, with a higher molar ratio of MMP-9 to TIMP-1. A higher MMP-9/TIMP-1 ratio in AMs was also related to a greater loss of FEV1, FVC, and MMEF in the 5-year period. We also showed that the inhibition of p38 MAPK and ERK signaling pathways was associated with a decrease in MMP-9 production from smokers’ AMs. The results suggested that excessive MMP-9 over TIMP-1 in smokers could be related to AHR, a risk factor of COPD, and might be modulated by MAPK inhibitors.
In agreement with previously reported outcomes, accelerated deterioration of pulmonary function prior to clinical symptoms developed in AHR + S in our study. Tobacco smoking decreases FEV1/FVC, FEV1, and MMEF, indicating airway obstruction and small airway disease in smokers.26 However, the incidence rate of COPD in current smokers is only 19.7/1,000 person-years (95% CI 18.1–21.4), ~2.4 times higher than in former smokers and 4.8 times higher than in never smokers.27 AHR is a fundamental characteristic in asthma and also present in COPD patients and in 12%–22% of healthy subjects.28 Compared to those without AHR, subjects with AHR are more likely to experience respiratory symptoms such as cough, phlegm, dyspnea, persistent wheeze, asthmatic attacks, and progressive airflow obstruction, and COPD patients with AHR suffer from a higher rate of FEV1 decline, more severe air trapping, and a double increased risk of respiratory mortality.29–32 We therefore suggest that AHR may be a risk factor for airway obstruction in smokers. A longitudinal study of AHR − S and AHR + S over a longer period of time will clarify whether AHR leads to a postbronchodilator FEV1/FVC of <70% in smoking population.
Increased numbers of neutrophils and macrophages in respiratory samples in parallel with exaggerated AHR had been observed in both asthmatic smokers and tobacco smoke-treated cats.33,34 Altered behavior of AMs is a key feature of COPD.3 Increased numbers of AMs had been observed in lung parenchyma, BAL fluid, and sputum in COPD.3 AMs produce fibrogenic transforming growth factor (TGF)-β, the most potent inducer of ECM generation, contributing to small airway fibrosis in COPD patients. Cigarette smoke stimulates the generation and release of neutrophils from bone marrow and prolongs the survival of neutrophils in the respiratory organs, possibly mediated by macrophages’ release of mediators, such as granulocyte–macrophage colony-stimulating factors, CXC ligand (CXCL) 8/interleukin (IL)-8, CXCL1/growth-regulated oncogene-α, leukotriene B4, and proteinases including MMP-9.3 We showed an increased absolute number, although not a higher percentage, of AM in BAL fluid from smokers, and AHR + S had more neutrophils than AHR − S and AHR − NS. Recruitment of these inflammatory cells could have potentiated the inflammation and remodeling in the respiratory tracts of these smokers.
Few biomarkers are known to be positively associated with the severity of AHR in COPD apart from adiponectin and eosinophil count.29,35 In the present study, we highlighted the correlation between MMP-9 and AHR in smokers. We uncovered a higher ratio of MMP-9 to TIMP-1 in AMs from AHR + S, which was correlated with a greater reduction in lung function, providing a potential biomarker to predict the development of COPD in smokers. Although it is generally accepted that the excess of MMP-9’s proteolytic activity can lead to lung destruction in emphysema, there is little information linking the pathogenesis of AHR to the impact of MMP-9–TIMP-1 imbalance. AHR could be secondary to increased smooth muscle mass and contractility, infiltration of inflammatory cells, increased airway wall thickening and reduced airway caliber, infiltration of inflammatory cells, and increased production in proinflammatory and fibrogenic mediators.36,37 Increased numbers of AMs, neutrophils, and eosinophils in BAL fluid and elevated mRNA expression of TGF-β and MMP-9 were associated with ozone-induced AHR in a mouse model of emphysema.38 There are at least three potential explanations. First, the mechanical stress elicits an increased production of ECM proteins and MMP-9 relative to TIMP-1.39 Methacholine- or allergen-induced bronchoconstriction increases collagen deposition in sub-epithelial layer in parallel with the upregulation of TGF-β, stimulating the expression of MMP-9 through the activation of ERK1,2, Ras-related C3 botulinum toxin substrate 1–reactive oxygen species–NF-κB pathway, and TGF-β-activated kinase 1–NF-κB pathway, and the mechanical loads applied to the airway structural cells of individuals with AHR might be enhanced.40,41 Second, the disproportionate rise in MMP-9 production worsens airway remodeling and inflammation, which prompts the progression of AHR. It had been reported that asthmatic patients had remarkable expression in MMP-9 (and TIMP-1 to a lesser extent) and more prominent airway remodeling, ie, deposition of collagens and tenascin in the basement membrane, causing airflow obstruction and AHR, although conflicting results showed the other way round in the sputum specimens.42,43 Apart from alveolar wall matrices, MMP-9 has many other substrates such as TGF-β and CXCL8/IL-8. MMP-9 releases latent TGF-β from ECM and then is stimulated by TGF-β in a reciprocal manner, enhancing collagen deposition and small airway fibrosis. A strong correlation has been shown between the reduced airway caliber and AHR in nonasthmatic subjects with COPD, suggesting that AHR may be related to the heightened resistance secondary to geometric change of the airways.36 MMP-9 facilitates chemotaxis of neutrophils by cleaving CXCL8/IL-8 into a more active form, which then amplifies the release of MMP-9 through a positive feedback loop, proteolyzes the basement membrane, and releases the chemotactic fragments from ECM to recruit inflammatory cells, compatible with the higher proportion of neutrophils in the BAL fluid from AHR + S and high MMP-9 in the present study.44 Third, some AHR + S may be asthmatic, although all subjects enrolled in our study had negative bronchodilator test results. Patients with asthma exacerbation have an elevated MMP-9 level in their blood, sputum, and BAL fluid, and the ratio of MMP-9 to TIMP-1 was higher in BAL fluid from children with symptomatic asthma.45 It is conceivable that more MMP-9 and less TIMP-1 released by AMs lead to further inflammatory process and remodeling and is associated with AHR and the subsequent lung function decline in smokers.
We also provided evidence that pharmacological inhibition of p38 MAPK and ERK suppresses the release of MMP-9, but not TIMP-1, from smokers’ AMs in vitro. Cigarette smoking induces marked activation of p38 MAPK and ERK.46 AMs from patients with corticosteroid-insensitive severe asthma and COPD have increased expression of phosphorylated p38 MAPK.47,48 Reduced MMP-9 production has been observed after the inhibition of p38 MAPK, JNK, and ERK using epigallocatechin-3-gallate in phorbol 12-myristate 13-acetate-induced macrophages.49 Cigarette-induced emphysema in Wistar rats is associated with the phosphorylation of p38 MAPK and ERK (c-Jun NH2-terminal kinase) and the activation of NF-κB signaling, leading to an increase in MMP-9 together with a reduction in TIMP-1, while oral erythromycin administration prevented emphysema and inflammation by reducing MMP-9 via the inhibition of p38 MAPK, ERK, and NF-κB.50 Simvastatin attenuates cigarette smoke extract-induced activation of ERK, NF-κB, and AP-1, and upregulation of MMP-9 in rat AMs.51 Inhibition of MAPKs, especially p38 MAPK, appears to be a logical approach to eliminate airway inflammation. Several clinical trials for inhalational p38 MAPK inhibitors, such as RV568 (now known as JNK-49095397), PF-30715455, and AZD-7624, are currently ongoing.52 Clarithromycin and rosiglitazone also block the cigarette smoke extract-induced MMP-9 production in animal models.53,54 Modification of MMP-9 expression by either MAPK inhibitors or approved agents, which modulate MMP-9 expression, may be an additional therapeutic strategy to ameliorate the deterioration of lung function in AHR + S.
Conclusion
Our finding supports the hypothesis that AMs from AHR + S produce an excess of MMP-9 to TIMP-1, accompanied by a more severe deterioration of airway obstruction in these subjects, indicating a new predictor to identify smokers vulnerable to COPD. We also confirm the observation that the generation of MMP-9 can be hampered by the inhibition of p38 MAPK and ERK, which may be a potential therapeutic target to prevent the development of AHR and progressive airflow limitation in smokers.
Acknowledgments
This work was supported by the National Science Research Project (NMRP) grant 92-2314-B-182A-178 – and Chang Gung Memorial Hospital Research Project Grant CMRPG3B1323.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Global Burden of Disease Study C Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 3.Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138(1):16–27. doi: 10.1016/j.jaci.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Chung KF, Adcock IM. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J. 2008;31(6):1334–1356. doi: 10.1183/09031936.00018908. [DOI] [PubMed] [Google Scholar]
- 5.Bchir S, Nasr HB, Bouchet S, et al. Concomitant elevations of MMP-9, NGAL, proMMP-9/NGAL and neutrophil elastase in serum of smokers with chronic obstructive pulmonary disease. J Cell Mol Med. 2017;21(7):1280–1291. doi: 10.1111/jcmm.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Boer WI, Hau CM, van Schadewijk A, Stolk J, van Krieken JH, Hiemstra PS. Expression of epidermal growth factors and their receptors in the bronchial epithelium of subjects with chronic obstructive pulmonary disease. Am J Clin Pathol. 2006;125(2):184–192. doi: 10.1309/W1AX-KGT7-UA37-X257. [DOI] [PubMed] [Google Scholar]
- 7.Lee SH, Lee SH, Kim CH, et al. Increased expression of vascular endothelial growth factor and hypoxia inducible factor-1alpha in lung tissue of patients with chronic bronchitis. Clin Biochem. 2014;47(7–8):552–559. doi: 10.1016/j.clinbiochem.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Tashkin DP, Altose MD, Bleecker ER, et al. The lung health study: airway responsiveness to inhaled methacholine in smokers with mild to moderate airflow limitation. The Lung Health Study Research Group. Am Rev Respir Dis. 1992;145(2 pt 1):301–310. doi: 10.1164/ajrccm/145.2_Pt_1.301. [DOI] [PubMed] [Google Scholar]
- 9.Frew AJ, Kennedy SM, Chan-Yeung M. Methacholine responsiveness, smoking, and atopy as risk factors for accelerated FEV1 decline in male working populations. Am Rev Respir Dis. 1992;146(4):878–883. doi: 10.1164/ajrccm/146.4.878. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Garcia JM, Hernandez JR, Martinez-Muniz MA, et al. Airways reactivity, atopy and bronchoalveolar lavage in male smokers with airflow obstruction. Respiration. 1996;63(4):199–204. doi: 10.1159/000196545. [DOI] [PubMed] [Google Scholar]
- 11.Navratilova Z, Kolek V, Petrek M. Matrix metalloproteinases and their inhibitors in chronic obstructive pulmonary disease. Arch Immunol Ther Exp (Warsz) 2016;64(3):177–193. doi: 10.1007/s00005-015-0375-5. [DOI] [PubMed] [Google Scholar]
- 12.Loffek S, Schilling O, Franzke CW. Series “matrix metalloproteinases in lung health and disease”: biological role of matrix metalloproteinases: a critical balance. Eur Respir J. 2011;38(1):191–208. doi: 10.1183/09031936.00146510. [DOI] [PubMed] [Google Scholar]
- 13.Roach HI, Yamada N, Cheung KS, et al. Association between the abnormal expression of matrix-degrading enzymes by human osteoarthritic chondrocytes and demethylation of specific CpG sites in the promoter regions. Arthritis Rheum. 2005;52(10):3110–3124. doi: 10.1002/art.21300. [DOI] [PubMed] [Google Scholar]
- 14.Nakamaru Y, Vuppusetty C, Wada H, et al. A protein deacetylase SIRT1 is a negative regulator of metalloproteinase-9. FASEB J. 2009;23(9):2810–2819. doi: 10.1096/fj.08-125468. [DOI] [PubMed] [Google Scholar]
- 15.Gao Z, Ye J. Inhibition of transcriptional activity of c-JUN by SIRT1. Biochem Biophys Res Commun. 2008;376(4):793–796. doi: 10.1016/j.bbrc.2008.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohnishi K, Takagi M, Kurokawa Y, Satomi S, Konttinen YT. Matrix metalloproteinase-mediated extracellular matrix protein degradation in human pulmonary emphysema. Lab Invest. 1998;78(9):1077–1087. [PubMed] [Google Scholar]
- 17.Vignola AM, Riccobono L, Mirabella A, et al. Sputum metalloproteinase-9/tissue inhibitor of metalloproteinase-1 ratio correlates with airflow obstruction in asthma and chronic bronchitis. Am J Respir Crit Care Med. 1998;158(6):1945–1950. doi: 10.1164/ajrccm.158.6.9803014. [DOI] [PubMed] [Google Scholar]
- 18.Russell RE, Culpitt SV, DeMatos C, et al. Release and activity of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2002;26(5):602–609. doi: 10.1165/ajrcmb.26.5.4685. [DOI] [PubMed] [Google Scholar]
- 19.Lim S, Roche N, Oliver BG, Mattos W, Barnes PJ, Chung KF. Balance of matrix metalloprotease-9 and tissue inhibitor of metalloprotease-1 from alveolar macrophages in cigarette smokers. Regulation by interleukin-10. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1355–1360. doi: 10.1164/ajrccm.162.4.9910097. [DOI] [PubMed] [Google Scholar]
- 20.Vandenbroucke RE, Dejonckheere E, Libert C. A therapeutic role for matrix metalloproteinase inhibitors in lung diseases? Eur Respir J. 2011;38(5):1200–1214. doi: 10.1183/09031936.00027411. [DOI] [PubMed] [Google Scholar]
- 21.Culpitt SV, Maziak W, Loukidis S, Nightingale JA, Matthews JL, Barnes PJ. Effect of high dose inhaled steroid on cells, cytokines, and proteases in induced sputum in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1635–1639. doi: 10.1164/ajrccm.160.5.9811058. [DOI] [PubMed] [Google Scholar]
- 22.Barnes PJ. Kinases as novel therapeutic targets in asthma and chronic obstructive pulmonary disease. Pharmacol Rev. 2016;68(3):788–815. doi: 10.1124/pr.116.012518. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Zhang HX, Sun BX, et al. Cross-shift airway responses and long-term decline in FEV1 in cotton textile workers. Am J Respir Crit Care Med. 2008;177(3):316–320. doi: 10.1164/rccm.200702-318OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Popa V. ATS guidelines for methacholine and exercise challenge testing. Am J Respir Crit Care Med. 2001;163(1):292–293. doi: 10.1164/ajrccm.163.1.16310b. [DOI] [PubMed] [Google Scholar]
- 25.Wang CH, Liu CY, Lin HC, Yu CT, Chung KF, Kuo HP. Increased exhaled nitric oxide in active pulmonary tuberculosis due to inducible NO synthase upregulation in alveolar macrophages. Eur Respir J. 1998;11(4):809–815. doi: 10.1183/09031936.98.11040809. [DOI] [PubMed] [Google Scholar]
- 26.Kuperman AS, Riker JB. The variable effect of smoking on pulmonary function. Chest. 1973;63(5):655–660. doi: 10.1378/chest.63.5.655. [DOI] [PubMed] [Google Scholar]
- 27.Terzikhan N, Verhamme KM, Hofman A, Stricker BH, Brusselle GG, Lahousse L. Prevalence and incidence of COPD in smokers and non-smokers: the Rotterdam Study. Eur J Epidemiol. 2016;31(8):785–792. doi: 10.1007/s10654-016-0132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lei Y, Gao Y, Chen J, et al. GLCCI1 rs37973: a potential genetic predictor of therapeutic response to inhaled corticosteroids in Chinese chronic obstructive pulmonary disease patients. Sci Rep. 2017;7:42552. doi: 10.1038/srep42552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tkacova R, Dai DL, Vonk JM, et al. Airway hyperresponsiveness in chronic obstructive pulmonary disease: a marker of asthma-chronic obstructive pulmonary disease overlap syndrome? J Allergy Clin Immunol. 2016;138(6):1571–1579.e10. doi: 10.1016/j.jaci.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 30.Xu X, Rijcken B, Schouten JP, Weiss ST. Airways responsiveness and development and remission of chronic respiratory symptoms in adults. Lancet. 1997;350(9089):1431–1434. doi: 10.1016/S0140-6736(97)10041-1. [DOI] [PubMed] [Google Scholar]
- 31.O’Connor GT, Sparrow D, Weiss ST. A prospective longitudinal study of methacholine airway responsiveness as a predictor of pulmonary-function decline: the Normative Aging Study. Am J Respir Crit Care Med. 1995;152(1):87–92. doi: 10.1164/ajrccm.152.1.7599868. [DOI] [PubMed] [Google Scholar]
- 32.Walker PP, Hadcroft J, Costello RW, Calverley PM. Lung function changes following methacholine inhalation in COPD. Respir Med. 2009;103(4):535–541. doi: 10.1016/j.rmed.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Kolahian S, Shahbazfar AA, Tayefi-Nasrabadi H, et al. Tiotropium effects on airway inflammatory events in the cat as an animal model for acute cigarette smoke-induced lung inflammation. Exp Lung Res. 2014;40(6):272–287. doi: 10.3109/01902148.2014.905657. [DOI] [PubMed] [Google Scholar]
- 34.Shimoda T, Obase Y, Kishikawa R, Iwanaga T. Influence of cigarette smoking on airway inflammation and inhaled corticosteroid treatment in patients with asthma. Allergy Asthma Proc. 2016;37(4):50–58. doi: 10.2500/aap.2016.37.3944. [DOI] [PubMed] [Google Scholar]
- 35.Postma DS, Rabe KF. The asthma-COPD overlap syndrome. N Engl J Med. 2015;373(13):1241–1249. doi: 10.1056/NEJMra1411863. [DOI] [PubMed] [Google Scholar]
- 36.Cockcroft DW, Davis BE. Mechanisms of airway hyperresponsiveness. J Allergy Clin Immunol. 2006;118(3):551–559. doi: 10.1016/j.jaci.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Atkinson JJ, Lutey BA, Suzuki Y, et al. The role of matrix metalloproteinase-9 in cigarette smoke-induced emphysema. Am J Respir Crit Care Med. 2011;183(7):876–884. doi: 10.1164/rccm.201005-0718OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li F, Wiegman C, Seiffert JM, et al. Effects of N-acetylcysteine in ozone-induced chronic obstructive pulmonary disease model. PLoS One. 2013;8(11):e80782. doi: 10.1371/journal.pone.0080782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swartz MA, Tschumperlin DJ, Kamm RD, Drazen JM. Mechanical stress is communicated between different cell types to elicit matrix remodeling. Proc Natl Acad Sci U S A. 2001;98(11):6180–6185. doi: 10.1073/pnas.111133298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krstic J, Santibanez JF. Transforming growth factor-beta and matrix metalloproteinases: functional interactions in tumor stroma-infiltrating myeloid cells. Scientific World Journal. 2014;2014:521754. doi: 10.1155/2014/521754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grainge CL, Lau LC, Ward JA, et al. Effect of bronchoconstriction on airway remodeling in asthma. N Engl J Med. 2011;364(21):2006–2015. doi: 10.1056/NEJMoa1014350. [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto H, Niimi A, Takemura M, et al. Relationship of airway wall thickening to an imbalance between matrix metalloproteinase-9 and its inhibitor in asthma. Thorax. 2005;60(4):277–281. doi: 10.1136/thx.2004.028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoshino M, Nakamura Y, Sim J, Shimojo J, Isogai S. Bronchial subepithelial fibrosis and expression of matrix metalloproteinase-9 in asthmatic airway inflammation. J Allergy Clin Immunol. 1998;102(5):783–788. doi: 10.1016/s0091-6749(98)70018-1. [DOI] [PubMed] [Google Scholar]
- 44.Overbeek SA, Braber S, Koelink PJ, et al. Cigarette smoke-induced collagen destruction; key to chronic neutrophilic airway inflammation? PLoS One. 2013;8(1):e55612. doi: 10.1371/journal.pone.0055612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grzela K, Litwiniuk M, Zagorska W, Grzela T. Airway remodeling in chronic obstructive pulmonary disease and asthma: the role of matrix metalloproteinase-9. Arch Immunol Ther Exp (Warsz) 2016;64(1):47–55. doi: 10.1007/s00005-015-0345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koch A, Giembycz M, Stirling RG, et al. Effect of smoking on MAP kinase-induced modulation of IL-8 in human alveolar macrophages. Eur Respir J. 2004;23(6):805–812. doi: 10.1183/09031936.04.00104304. [DOI] [PubMed] [Google Scholar]
- 47.Bhavsar P, Hew M, Khorasani N, et al. Relative corticosteroid insensitivity of alveolar macrophages in severe asthma compared with non-severe asthma. Thorax. 2008;63(9):784–790. doi: 10.1136/thx.2007.090027. [DOI] [PubMed] [Google Scholar]
- 48.Renda T, Baraldo S, Pelaia G, et al. Increased activation of p38 MAPK in COPD. Eur Respir J. 2008;31(1):62–69. doi: 10.1183/09031936.00036707. [DOI] [PubMed] [Google Scholar]
- 49.Wang QM, Wang H, Li YF, et al. Inhibition of EMMPRIN and MMP-9 expression by epigallocatechin-3-gallate through 67-kDa laminin receptor in PMA-induced macrophages. Cell Physiol Biochem. 2016;39(6):2308–2319. doi: 10.1159/000447923. [DOI] [PubMed] [Google Scholar]
- 50.Zhou X, Gu D, Hou G. Erythromycin attenuates metalloprotease/anti-metalloprotease imbalance in cigarette smoke-induced emphysema in rats via the mitogen-activated protein kinase/nuclear factor-kappaB activation pathway. Mol Med Rep. 2017;15(5):2983–2990. doi: 10.3892/mmr.2017.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim SE, Thanh Thuy TT, Lee JH, et al. Simvastatin inhibits induction of matrix metalloproteinase-9 in rat alveolar macrophages exposed to cigarette smoke extract. Exp Mol Med. 2009;41(4):277–287. doi: 10.3858/emm.2009.41.4.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Norman P. Investigational p38 inhibitors for the treatment of chronic obstructive pulmonary disease. Expert Opin Investig Drugs. 2015;24(3):383–392. doi: 10.1517/13543784.2015.1006358. [DOI] [PubMed] [Google Scholar]
- 53.Hou G, Yin Y, Han D, Wang QY, Kang J. Rosiglitazone attenuates the metalloprotease/anti-metalloprotease imbalance in emphysema induced by cigarette smoke: involvement of extracellular signal-regulated kinase and NFkappaB signaling. Int J Chron Obstruct Pulmon Dis. 2015;10:715–724. doi: 10.2147/COPD.S77514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakamura M, Wada H, Honda K, et al. Clarithromycin ameliorates pulmonary inflammation induced by short term cigarette smoke exposure in mice. Pulm Pharmacol Ther. 2015;35:60–66. doi: 10.1016/j.pupt.2015.09.005. [DOI] [PubMed] [Google Scholar]