ABSTRACT
Numerous risk factors have been linked to invasive candidiasis; however, they are nonspecific and often trigger empiric antifungal therapy in a large number of patients. Identification of more precise predictors could promote judicious use of empiric echinocandins. In this retrospective review, 127 patients with blood cultures positive for Candida spp. were compared to a randomly selected cohort of 134 patients on empiric micafungin for ≥3 days and with blood cultures negative for Candida spp. Factors associated with candidemia included total parenteral nutrition (TPN; 26.0% vs 15.7%, P = 0.040), multifocal Candida colonization (23.6% vs 3.0%, P < 0.001), and positive 1,3-β-d-glucan assay (95.0% vs 35.0%, P < 0.001). Patients without candidemia on empiric micafungin were more likely to receive antibiotic therapy in the previous 10 days (55.9% vs 79.9%, P < 0.001) and to be taking immunosuppressive medications (11.0% vs 30.6%, P < 0.001). Receipt of TPN (odds ratio [OR] = 2.07, 95% confidence interval [CI], 1.02–4.21), severe sepsis (OR = 2.20, 95% CI, 1.00–4.83), and multifocal Candida colonization (OR = 13.87, 95% CI, 4.43–43.37) were independently associated with candidemia in the multivariable logistic regression model. Therefore, the absence of these risk factors, especially in conjunction with a negative 1,3-β-d-glucan assay, may be used to recommend de-escalation of empiric micafungin therapy.
KEYWORDS: 1,3-β-d-glucan assay; antifungal stewardship; Candida score; empiric antifungal therapy; invasive candidiasis; micafungin
Candidemia carries a mortality rate of up to 47%, and appropriate empiric antifungal therapy in patients with suspected candidiasis is paramount.1 However, risk factors for invasive candidiasis are difficult to define because they are numerous and in many cases nonspecific, which would qualify many patients for empiric antifungal therapy. To improve selection, the Candida score and 1,3-β-d-glucan assay are proposed tools to further aid in identifying patients at the highest risk for invasive Candida infection.2–4 Despite current guidelines that recommend using scoring tools and available biomarkers, criteria for the appropriate use and duration of empiric antifungal therapy remain inadequate.5 Identification of more precise risk factors could promote more judicious use of empiric antifungals. We designed a single-center, retrospective case-control study aimed at investigating more specific risk factors for candidemia in both critically ill and noncritically ill patients. The primary objective was to identify factors associated with candidemia in patients at Baylor University Medical Center by comparing patients with candidemia to patients without candidemia prescribed empiric micafungin for ≥3 days.
METHODS
This was a retrospective chart review of patients admitted to Baylor University Medical Center from October 1, 2014, to October 25, 2016. Patients with blood cultures positive for Candida spp. were compared with a randomly selected cohort of patients on empiric micafungin for ≥3 days and with blood cultures negative for Candida spp. Patients less than 18 years old, pregnant patients, prisoners, and patients on prophylactic antifungals were excluded. Patients who had evidence of invasive candidiasis on culture (i.e., abscess culture positive for Candida spp.) but had negative blood cultures were also excluded. Patients were identified using clinical surveillance software (MedMined, BD, Franklin Lakes, NJ). All patients prescribed empiric micafungin during the study period were assigned a random number between 0 and 1 using the “RandNum” function within Microsoft Excel. Numbers were fixed and then ordered from smallest to largest in order to randomize the subjects. This randomization strategy follows the method of simple randomization as described by Altman and Bland.6 Data were collected until the cohort of 134 patients was achieved for the empiric micafungin group. Patient characteristics and culture data were obtained via review of the electronic medical record (Allscripts, Chicago, IL). Cultures and 1,3-β-d-glucan testing were performed at Med Fusion (Lewisville, TX). This study was approved by the institutional review board at Baylor Scott & White Research Institute.
Patient data collected included presence of diabetes mellitus, cirrhosis, acute gastrointestinal perforation, intermittent hemodialysis, history of candidemia, immunosuppressant use, neutropenia, uncontrolled HIV/AIDS defined as CD4 < 200 cells/μL, receipt of total parenteral nutrition (TPN), multifocal Candida colonization, 1,3-β-d-glucan assay result, duration of hospitalization, length of stay, intensive care unit length of stay, and 30-day mortality. Data on patient location, severe sepsis or septic shock within 48 hours, presence of central venous catheter for >48 hours, hospitalization within 90 days, antibiotic use within 10 days, antifungal exposure within 30 days, and abdominal surgery within 6 weeks were all collected with respect to culture collection time or initiation of empiric micafungin. Information on severe sepsis or septic shock was collected based on diagnosis as documented in the progress notes. For patients with multiple results for the 1,3-β-d-glucan assay, the test result utilized was the one collected nearest to the culture collection time or initiation of empiric micafungin therapy. Candida scores were calculated as described by León et al.6 Culture data collected included organism isolated and patient location at time of culture collection. Medication-induced immunosuppression was defined as ongoing systemic immunosuppressant therapy with tacrolimus, sirolimus, mycophenolate, monoclonal antibodies, or corticosteroids at doses equivalent to ≥2 mg/kg/day of prednisone. Multifocal Candida colonization was defined as isolation of Candida spp. from two or more sterile body sites, even if Candida species were different. In the absence of culture evidence for multifocal Candida colonization, it was assumed that the patient was not colonized.
Patient characteristics were compared between patients with and without candidemia using chi-square tests for categorical variables and t tests for continuous variables. In examining factors associated with the development of candidemia, a multivariable logistic regression model was used, including history of solid organ transplant, presence of central venous catheter, antifungal therapy within 30 days, receipt of TPN, abdominal surgery within previous 6 weeks, severe sepsis or septic shock, multifocal Candida colonization, and Candida score ≥ 3. These factors were chosen based on the findings from prior published studies and clinical relevance. All analyses were performed using STATA (Version 14.0, StataCorp, College Station, TX).
RESULTS
We identified 146 unique patients with blood cultures positive for Candida spp. and 1425 unique patients on empiric micafungin during the study period. Exclusion criteria for both groups are detailed in Figure 1. This study included 127 patients with candidemia and a randomly selected cohort of 134 patients without candidemia prescribed empiric micafungin for ≥3 days. Table 1 describes baseline characteristics for length of stay prior to culture collection or start of empiric micafungin as well as location of culture collection or start of empiric micafungin, respectively. There were no significant differences for gender and age between the groups.
Figure 1.
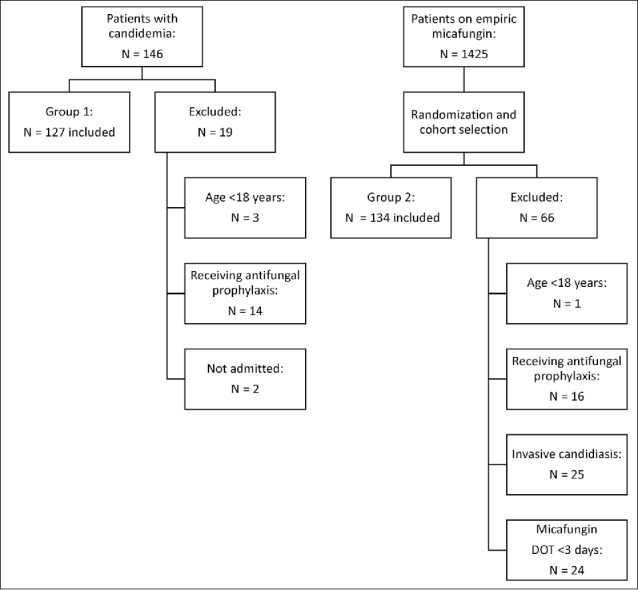
Patient selection.
Table 1.
Baseline characteristics
| Candidemia |
|||
|---|---|---|---|
| Characteristic | Yes (n = 127) | No (n = 134) | P value |
| Male | 73 (58%) | 76 (57%) | 0.901 |
| Age in years, mean ± SD | 55.9 ± 16.0 | 53.2 ± 15.7 | 0.170 |
| Length of stay prior to culture collection in days, mean ± SD | 9.9 ± 15.7 | — | — |
| Intensive care unit length of stay prior to culture in days, mean ± SD | 7.7 ± 9.7 | — | — |
| Culture collection location | |||
| Emergency department | 25 (20%) | — | — |
| Intensive care unit | 58 (46%) | — | — |
| Medical/surgical units | 32 (25%) | — | — |
| Oncology, nonintensive care unit | 12 (9%) | — | — |
| Length of stay prior to micafungin in days, mean ± SD | — | 7.8 ± 9.5 | — |
| Intensive care unit length of stay prior to micafungin in days, mean ± SD | — | 5.7 ± 6.6 | — |
| Micafungin duration of therapy in days, mean ± SD | — | 7.9 ± 7.9 | — |
| Micafungin start location | |||
| Emergency department | — | 0 | — |
| Intensive care unit | — | 86 (64%) | — |
| Medical/surgical units | — | 32 (24%) | — |
| Oncology, nonintensive care unit | — | 16 (12%) | — |
Factors associated with candidemia included receipt of TPN, multifocal Candida colonization, and positive 1,3-β-d-glucan assay (Table 2). Patients without candidemia who were prescribed empiric micafungin were more likely to have received antibiotic therapy in the previous 10 days (55.9% vs 79.9%, P < 0.001) and more likely to be taking immunosuppressive medications (11.0% vs 30.6%, P < 0.001). There were no significant associations with regard to any of the other risk factors considered (Table 2).
Table 2.
Results of univariate analysis
| Candidemia |
|||
|---|---|---|---|
| Variable | Yes (n = 127) | No (n = 134) | P value |
| Diabetes mellitus | 36 (28%) | 37 (28%) | 0.895 |
| Cirrhosis | 13 (10%) | 16 (12%) | 0.661 |
| Hemodialysis, intermittent | 8 (6%) | 12 (9%) | 0.420 |
| History of candidemia | 5 (4%) | 3 (2%) | 0.426 |
| Acute gastrointestinal perforation | 13 (10%) | 14 (10%) | 0.955 |
| Medication-induced immunosuppression | 14 (11%) | 41 (31%) | <0.001 |
| Absolute neutrophil count <100 cells/mm3 | 4 (3%) | 10 (8%) | 0.122 |
| HIV/AIDS (CD4 < 200) cells/μL | 2 (2%) | 5 (4%) | 0.281 |
| Central venous catheter | 90 (71%) | 80 (60%) | 0.059 |
| Solid organ transplant | 15 (12%) | 25 (19%) | 0.125 |
| Hospitalization in previous 90 days | 75 (59%) | 80 (60%) | 0.915 |
| Antibiotic therapy in previous 10 days | 71 (56%) | 107 (80%) | <0.001 |
| Antifungal therapy in previous 30 days | 19 (15%) | 16 (12%) | 0.474 |
| Total parenteral nutrition | 33 (26%) | 21 (16%) | 0.040 |
| Multifocal Candida colonization | 30 (24%) | 4 (3%) | <0.001 |
| Abdominal surgery in previous 6 weeks | 27 (21%) | 26 (19%) | 0.709 |
| Severe sepsis or septic shock | 31 (24%) | 23 (17%) | 0.149 |
| Candida score ≥ 3 | 15 (12%) | 9 (7%) | 0.155 |
| Positive 1,3-β-d-glucan assay | 19/20 (95%) | 14/40 (35%) | 0.001 |
Fifteen patients with candidemia had a Candida score ≥ 3 and 9 patients without candidemia had a Candida score < 3. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for Candida score ≥ 3 were 11.8% (95% confidence interval [CI], 6.76%–18.7%), 93.3% (95% CI, 87.6%–96.9%), 62.5% (95% CI, 40.6%–81.2%), and 52.7% (95% CI, 46.2%–59.2%), respectively. Results for the 1,3-β-d-glucan assay were obtained for 64 patients. Among patients with candidemia and 1,3-β-d-glucan assay results, 19 (95%) of 20 had a positive 1,3-β-d-glucan assay. For patients without candidemia, 26 (65%) of 40 had a negative 1,3-β-d-glucan assay result. There were four patients with indeterminate results for the 1,3-β-d-glucan assay. The 1,3-β-d-glucan assay had a sensitivity of 95.0% (95% CI, 75.1%–99.9%), specificity of 65.0% (95% CI, 48.3%–79.4%), PPV of 57.6% (95% CI, 39.2%–74.5%), and NPV of 96.3% (95% CI, 81.0%–99.9%).
A multivariable logistic regression model was used, including history of solid organ transplant, presence of central venous catheter, antifungal therapy within 30 days, receipt of TPN, abdominal surgery within previous 6 weeks, severe sepsis or septic shock, multifocal Candida colonization, and Candida score ≥ 3. These factors were chosen based on the findings from prior published studies and clinical relevance. An area under the receiver operating characteristic (AUROC) curve of 0.71 was estimated from this model as displayed in Figure 2. Risk factors independently associated with candidemia in the multivariable logistic regression model included receipt of TPN, severe sepsis, and multifocal Candida colonization (Table 3).
Figure 2.
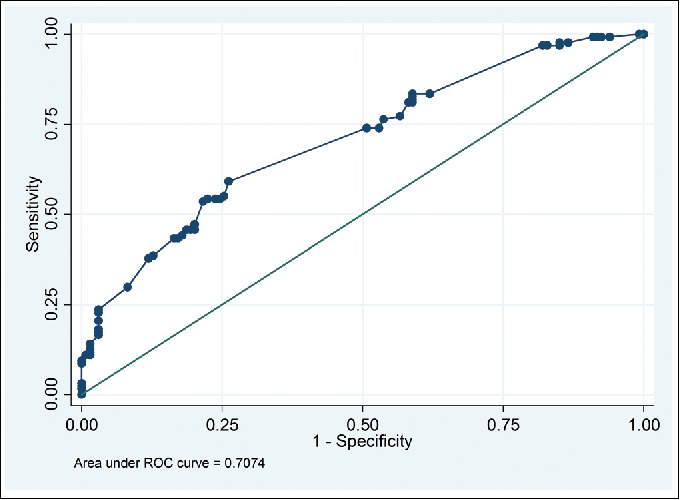
Receiver operating characteristic curve from multivariable logistic regression model.
Table 3.
Factors associated with candidemia: Results of multivariable logistic regression model
| Variable | Adjusted odds ratio (95% confidence interval) | P value |
|---|---|---|
| Central venous catheter | 1.47 (0.82–2.61) | 0.194 |
| Solid organ transplant | 0.44 (0.2–0.98) | 0.044 |
| Antifungal therapy in previous 30 days | 0.95 (0.42–2.13) | 0.905 |
| Total parenteral nutrition | 2.07 (1.02–4.21) | 0.045 |
| Abdominal surgery in previous 6 weeks | 1.04 (0.50–2.14) | 0.924 |
| Severe sepsis or septic shock | 2.20 (1.00–4.83) | 0.05 |
| Multifocal Candida colonization | 13.87 (4.43–43.47) | <0.001 |
| Candida score ≥ 3 | 0.43 (0.12–1.56) | 0.200 |
There was no difference in mean length of stay (25.2 ± 25.1 days vs 27.3 ± 34.3 days, P = 0.625), mean intensive care unit length of stay (9.3 ± 15.8 days vs 11.0 ± 12.4 days, P = 0.338), or 30-day all-cause mortality (32.3% vs 23.9%, P = 0.131) between patients with candidemia and patients on empiric micafungin. The distribution of Candida species in positive blood cultures from patients with candidemia was as follows: C. albicans (37.8%), C. glabrata (30.7%), C. parapsilosis (13.4%), C. tropicalis (11.8%), other Candida spp. (4.7%), and C. krusei (1.6%).
DISCUSSION
In this retrospective case-control study with a total of 261 patients, only TPN, multifocal Candida colonization, and severe sepsis proved to have a significant association with invasive candidiasis as defined by candidemia. Other previously described risk factors, such as gastrointestinal perforation and recent abdominal surgery, were not significant in this study.2 Of the three significant factors in this study, multifocal Candida colonization is the most problematic because patients often do not have enough culture data to make this determination. For the purposes of the study, lack of evidence of culture data from nonsterile body sites was categorized as not colonized. Thus, evaluating Candida colonization in clinical practice may not prove to be beneficial.
In addition to assessing patients based on risk factors, we evaluated tools such as the Candida score and 1,3-β-d-glucan assay. Candida score did not have a statistically significant association in univariate or multivariable logistic regression models. The operating characteristics for Candida score may have differed from the original study by León et al4 (sensitivity 81%, specificity 74%) due to the diversity of this patient population as well as the low number of observations for Candida score ≥ 3. In the current study, the 1,3-β-d-glucan assay had an excellent NPV and poor PPV. This is consistent with a study comparing the blood samples of 36 healthy patients to samples from 15 patients with candidemia and 14 patients with bacteremia in which the sensitivity, specificity, PPV, and NPV of the 1,3-β-d-glucan assay were 93.3%, 77.2%, 51.9%, and 97.8%, respectively.7 With consistently reported high NPV, the 1,3-β-d-glucan assay may play a major role in de-escalation of empiric antifungal therapy.3,7 For the current study, candidiasis was defined as blood cultures positive for Candida spp. To control for localized forms of invasive candidiasis (i.e., abscess), we intentionally excluded patients with negative blood cultures but clinically significant cultures that isolated Candida spp. It is unclear whether the 1,3 β-d-glucan assay has similar utility in this subset of patients.
Patients without candidemia on empiric micafungin were statistically more likely to have received antibiotic therapy in the previous 10 days and to be taking immunosuppressive medications. This is likely a product of the retrospective study design and the prescribing patterns within Baylor University Medical Center. The control group in this study was intentionally chosen to give providers the benefit of the doubt that perhaps these patients had a risk factor that increased their risk for invasive candidiasis. However, these patient characteristics did not prove to be significant for patients with invasive candidiasis as defined by blood cultures positive for candidemia.
Based on findings of the multivariable logistic regression model, the most significant risk factors for candidemia were receipt of TPN, severe sepsis, and multifocal Candida colonization. Although there were significant findings for the 1,3-β-d-glucan assay in univariate analysis, this variable was not included in the model due to a relatively low number of observations overall (64/261). With an AUROC of 0.71, this model did show a significant association with candidemia; however, other scoring tools such as the Candida score6 and the scoring tool developed by Guillamet et al8 reported better performance with AUROC of 0.847 and 0.798, respectively.
Strengths of this study include the diversity of the patient population and consistency with published literature. These findings provide a real-world representation of the patient population and prescribing practices at Baylor University Medical Center. Though this study had a strong sample size with 261 patients overall, it may not have been adequately powered to detect a difference for the risk factors with a low number of observations in this study. There are inherent limitations due to the retrospective and single-center study design; therefore, the findings may not be generalizable to other settings. Results for the 1,3-β-d-glucan assay were only obtained for 64 patients. Intravenous beta-lactam antibiotics and intravenous immunoglobulin exposure have been associated with false positives in the 1,3-β-d-glucan assay; however, these data points were not collected.9
Based on the findings of this study, the absence of significant risk factors (TPN, severe sepsis, and multifocal Candida colonization) in conjunction with a negative 1,3-β-d-glucan assay may be used to recommend de-escalation of empiric micafungin therapy. These findings highlight an opportunity to improve empiric micafungin prescribing patterns, which could potentially decrease antifungal exposure, the development of resistant species, and associated costs. Additional research is warranted to verify the utility of de-escalating empiric antifungals when the 1,3-β-d-glucan assay is negative.
References
- 1.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in U.S. hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 2.Kullberg BJ, Arendrup MC. Invasive candidiasis. N Engl J Med. 2015;373:1445–1456. doi: 10.1056/NEJMra1315399. [DOI] [PubMed] [Google Scholar]
- 3.Levesque E, Rizk F, Noorah Z, et al.. Detection of (1,3)-β-d-glucan for the diagnosis of invasive fungal infection in liver transplant recipients. Int J Mol Sci. 2017;18(4):E862. doi: 10.3390/ijms18040862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.León C, Ruiz-Santana S, Saavedra P, et al; EPCAN Study Group. A bedside scoring system (“Candida score”) for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit Care Med. 2006;34:730–733. doi: 10.1097/01.CCM.0000202208.37364.7D. [DOI] [PubMed] [Google Scholar]
- 5.Pappas PG, Kauffman CA, Andes DR, et al.. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1–e50. doi: 10.1093/cid/civ1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altman DG, Bland JM. How to randomise. BMJ. 1999;319:703–704. doi: 10.1136/bmj.319.7211.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pickering JW, Sant HW, Bowles CA, Roberts WL, Woods GL. Evaluation of a (1→3)-beta-d-glucan assay for diagnosis of invasive fungal infections. J Clin Microbiol. 2005;43:5957–5962. doi: 10.1128/JCM.43.12.5957-5962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guillamet CV, Vazquez R, Micek ST, Ursu O, Kollef M. Development and validation of a clinical prediction rule for candidemia in hospitalized patients with severe sepsis and septic shock. J Crit Care. 2015;30):715–720. doi: 10.1016/j.jcrc.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Marty FM, Lowry CM, Lempitski SJ, Kubiak DW, Finkelman MA, Baden LR. Reactivity of (1–>3)-beta-d-glucan assay with commonly used intravenous antimicrobials. Antimicrob Agents Chemother 2006;50(10):3450–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]


