ABSTRACT
Ventricular dysfunction is common among patients with repaired cyanotic congenital heart disease. To date, no pharmacologic intervention has been demonstrated to be beneficial in this setting. To begin addressing this knowledge gap, we conducted a single-center prospective, randomized, open-label pilot study to investigate the effects of eplerenone on serologic markers of collagen turnover and inflammation, 6-minute walk distance, and quality of life in patients with tetralogy of Fallot (TOF) or transposition of the great arteries with a systemic right ventricle (transposition of the great arteries [TGA]). Patients were randomized to a 3-month drug-free period at the beginning of the treatment period or at the end. All patients received 12 months of eplerenone therapy during the treatment period. Twenty-six patients were enrolled in the trial; 17 completed the study protocol: 8 with TOF and 9 with TGV. Eplerenone had no effect on serum levels of procollagen 1 N-terminal peptide (PINP), procollagen 3 N-terminal peptide (PIIINP), or galectin-3 (G3). Similarly, eplerenone had no effect on 6-minute walk distance or quality of life. In conclusion, PINP and PIIINP levels are as high as or higher in patients with TOF and TGA than in patients with normal cardiac anatomy and heart failure, whereas G3 levels are lower. Eplerenone is well tolerated by adults born with congenital heart disease.
KEYWORDS: Adult congenital heart disease, eplerenone, tetralogy of Fallot, transposition of the great vessels
Among adults born with congenital heart disease (ACHD), heart failure (HF) is a growing problem. In addition to being the most common cause of mortality, HF is an increasingly frequent cause of hospitalization.1,2 Individuals with cyanotic heart disease including tetralogy of Fallot (TOF) and those with a systemic right ventricle with congenital or surgical correction of transposition of the great arteries (TGA) are at particular risk for the development of HF.3 At present, however, there are no pharmacotherapies of proven benefit in this setting. Among patients with HF with structurally normal hearts, cardiac fibrosis is thought to be a common final pathway underlying progressive myocardial dysfunction regardless of HF etiology.3–5 Similarly, in the ACHD population, diffuse myocardial fibrosis has been demonstrated by delayed gadolinium uptake on cardiac magnetic resonance imaging and on postmortem histologic examination in a variety of different lesions.6,7 Of the pharmacologic interventions currently used in HF management, aldosterone blockade has the largest effect size and has been demonstrated to directly prevent myocardial fibrosis.8,9 In the present trial, we thus investigated the effects of eplerenone on serum markers of collagen turnover and inflammation in a population of patients with complex congenital heart disease.
METHODS
We conducted a single-center, prospective, randomized, open-label pilot study of eplerenone versus placebo. The study was approved by the institutional review boards at Washington University School of Medicine and Baylor University Medical Center and was in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines.
All participants were recruited from a clinical database maintained at Washington University School of Medicine. Patients were required to be over 18 years of age; have a diagnosis of TOF or TGA with a systemic right ventricle; be clinically stable; have had no recent surgery and no surgery planned at the time of enrollment; and, if female, not be pregnant or planning to become pregnant during the study period. A complete list of inclusion and exclusion criteria is available in the Supplementary Material.
The primary efficacy endpoints were changes in serologic levels of procollagen 1 N-terminal peptide (PINP), procollagen 3 N-terminal peptide (PIIINP), and galectin-3 (G3). Secondary endpoints included 6-minute walk distance (6MWD) and Short Form 36 (SF-36)–assessed quality of life (QOL). Safety and tolerability endpoints included adverse event rates and individual changes in creatinine and serum potassium (K). Adverse event rates were assessed with monthly telephone calls to patients. Serum creatinine and potassium were measured at study start, at the start of eplerenone therapy, 1 week after initiation of eplerenone therapy, 1 week after increase in eplerenone dose, and at 6 and 12 months.
The principal investigator (AC) randomized participants at the time of their first study visit to have a drug-free period (period without eplerenone) either at the beginning or at the end of the study period (Figure 1). All patients had four study visits. During the study period, patients received oral eplerenone 25 mg daily for 4 weeks; if tolerated, the dose was increased to 50 mg daily for a further 11 months, for a total of 12 months of therapy.
Figure 1.
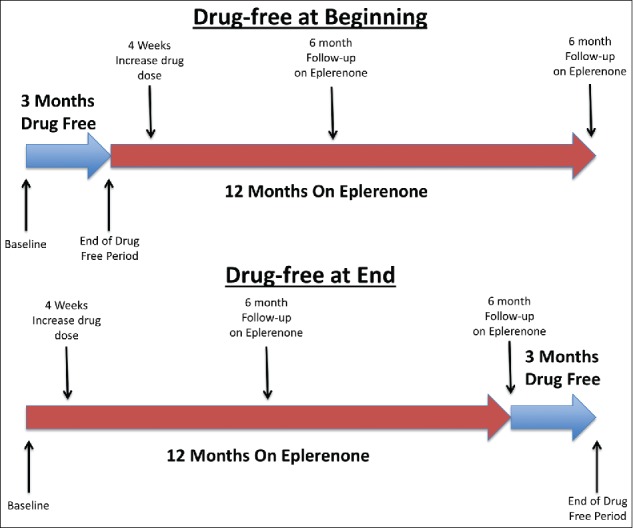
Graphic depiction of trial events and randomization scheme.
Serum for assessment of the primary outcomes was collected from each patient at each study visit in conventional evacuated serum tubes with a clot activator. Samples were centrifuged using a hematologic centrifuge for 15 minutes within 30 minutes of collection. Serum was then drawn from the tubes using plastic transfer pipettes into numerically coded plastic 2-mL freezer tubes and immediately frozen at −80°C. Serum levels of PINP, PIIINP, and G3 were evaluated for all samples simultaneously at the conclusion of the study in the Core Laboratory for Clinical Studies at Washington University School of Medicine by staff blinded to the study protocol and sample identity. Specifically, serum PINP and PIIINP levels were assessed using the radioimmunoassay kit from Orion Diagnostica (Espoo, Finland), and G3 levels were assessed using enzyme-linked immunosorbent assay kits from BG Medicine (Waltham, MA, USA). All samples were run in duplicate and the average variation between duplicates was 4.16%, 5.51%, and 3.01%, respectively.
6MWD testing was performed by various members of the research team using a standardized protocol. Specifically, blood pressure, pulse, and oxygen saturation were assessed at baseline and at the conclusion of the walk. Patients walked through a 100-foot-long straight hallway with low traffic and distance was recorded. Patients were advised to walk as briskly as possible and were advised to try to avoid needing to stop during the 6-minute period.
Patients completed SF-36 questionnaires in a quiet outpatient clinic room without interruption at each visit prior to 6MWD testing. Scoring followed the usual protocol recommended by the developers of the questionnaire.10 In addition to the physical functioning domain score, a nonstandard “total score” is reported here, which was calculated as the average score of each of the eight domains equally weighted. This is not a standardized or validated use of the SF-36 and is included here to demonstrate potential overall effects of the intervention across all aspects of health-related QOL.
Sample size was estimated based on data published in the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival biomarker substudy.11 In this study, there was no change in PINP levels; therefore, power was centered on PIIINP levels. A pretherapy mean PIIINP level of 4.5 ± 1.87 was obtained from the placebo arm at month 3. The posttherapy mean of 3.7 ± 1.66 was obtained from the month 9 eplerenone group. Powering the study at 80% with type I error set to 5%, the number of patients needed to identify a difference was 56. The sample size assumed a conservative mild-to-moderate correlation of 0.30 between pre- and posttherapy values and was based on a paired t test. Target enrollment was 70 subjects to allow for an anticipated 25% to 30% dropout based on previous prospective trials in the ACHD population.12,13
There was insufficient data to permit power analysis for significance of changes in G3 levels, 6MWD, or QOL. The present study served as a pilot for these endpoints.
Normality was assessed via the Shapiro-Wilk test and equality of variances was assessed via the folded F-test. Sphericity was assessed via Mauchly's test. Due to the small sample size, we elected to employ transformations to achieve normality in skewed endpoints as opposed to nonparametric testing. However, for tests based on pre and post differences, because negative values were present, the Wilcoxon rank sum test was used instead of transformation. To determine whether eplerenone had an effect on the primary or secondary outcomes, we performed repeated measures analysis of variance to examine complete cases only. To determine whether a pattern existed among endpoints during the drug-free period based on timing of the period, we performed a two-sample t test on the paired differences in patients before and after the drug-free period. Continuous variables are reported as mean ± standard deviations or medians (quartile 1, quartile 3) if skewed. Categorical variables are reported as frequencies and percentages.
All analyses were conducted in SAS (Version 9.4, SAS Institute Inc., Cary, NC).
RESULTS
The study was terminated early due to poor enrollment and patient interest. Twenty-six patients initially enrolled in the trial, of whom only 17 completed the prescribed number of study visits (Figure 2). The primary reason for noncompletion was unwillingness to complete study visits due to time, travel distance, financial concerns, or loss of interest in participation. One patient discontinued due to side effects (chest pain and hypotension). One patient randomized to the drug-free period at the end of the study completed all three study visits while on the study drug and was therefore included in the primary analysis. Complete demographic data for all participants can be found in Table 1.
Figure 2.
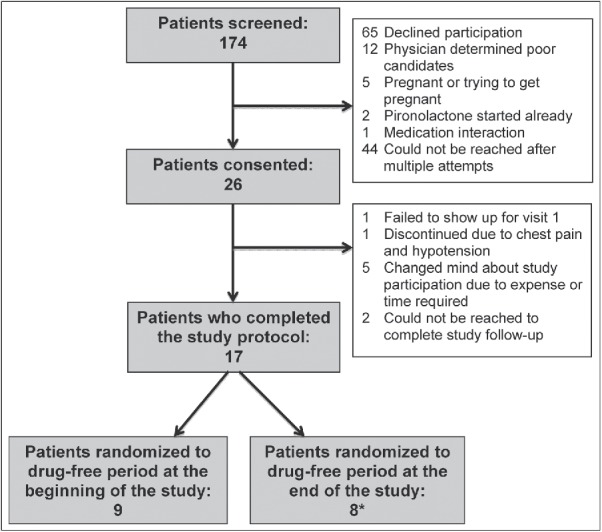
Flowsheet depicting trial dropout rate and reasons. *One patient was randomized to a drug-free period at the end of the trial who completed all on-drug study visits but failed to show up for the 3-month visit off drug. This individual was included in the final analysis of drug effects compared to baseline.
Table 1.
Study population characteristics (n = 26).
| Variable | Value |
|---|---|
| Age (years) | 37.2 ± 10.5 |
| Gender (female) | 9 (34.6%) |
| Smoker | 2 (7.7%) |
| Diagnosis | |
| TOF | 13 (50%) |
| TGA | 13 (50%) |
| Cyanotic | 0 (0.0%) |
| Arrhythmia | 9 (35%) |
| Atrial | 8 (89%) |
| Ventricular | 1 (11%) |
| Drug free at beginning | 14 (54%) |
| Medications | |
| Beta-blocker | 13 (50%) |
| ACEi/ARB | 17 (65%) |
| Antiarrhythmic | 4 (15%) |
| Digoxin | 1 (4%) |
| Loop diuretic | 5 (19%) |
TOF indicates tetralogy of Fallot; TGA, transposition of the great arteries; ACEi/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker.
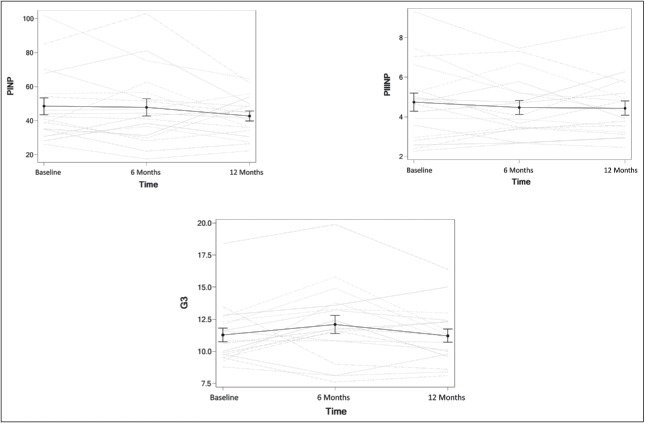
Eplerenone had no effect on serum levels of PINP, PIIINP, or G3 between baseline and 12 months (P = 0.39, 0.49, and 0.21, respectively; Figure 3). Similarly, when separated by lesion, there was no lesion-specific difference in the effect of eplerenone on serum levels of PINP, PIIINP, or G3 (P = 0.12, 0.15, and 0.73, respectively; Table 2). There was no time dependence for the primary outcome.
Figure 3.
Effects of eplerenone on serologic markers procollagen 1 N-terminal peptide (PINP), procollagen 3 N-terminal peptide (PIIINP), and galectin-3 (G3). Gray lines represent individual patients; black lines represent means.
Table 2.
Outcomes by diagnosis.
| Endpoint | Diagnosis | Start of drug | 6 Months eplerenone | 12 Months eplerenone | P for time | P for diagnosis |
|---|---|---|---|---|---|---|
| PINP (μg/mL) | TGA | 52.2 ± 24.9 | 55.7 ± 24.2 | 47.6 ± 10.8 | 0.21 | 0.12 |
| TOF | 43.7 ± 14.2 | 38.0 ± 14.4 | 36.5 ± 11.6 | |||
| PIIINP (μg/mL) | TGA | 4.8 ± 1.5 | 4.8 ± 1.5 | 4.5 ± 1.1 | 0.46 | 0.15 |
| TOF | 4.7 ± 2.5 | 4.1 ± 1.6 | 4.4 ± 2.0 | |||
| G3 (ng/mL) | TGA | 11.1 ± 1.5 | 11.7 ± 2.3 | 11.1 ± 2.1 | 0.21 | 0.73 |
| TOF | 10.7 (9.5, 12.2) | 12.6 ± 3.9 | 11.4 ± 2.4 | |||
| 6MWD (feet) | TGA | 1688.2 ± 254.8 | 1650.4 ± 219.7 | 1711.2 ± 253.5 | 0.23 | 0.35 |
| TOF | 1575.1 ± 181.3 | 1542.3 ± 242.7 | 1641.5 ± 268.6 | |||
| SF-36 total | TGA | 78.5 ± 13.7 | 82.8 (77.1, 85.3) | 79.2 ± 8.8 | 0.44 | 0.68 |
| TOF | 88.2 (79.2, 91.2) | 87.8 (70.2, 89.0) | 85.6 (75.0, 88.6) | |||
| SF-36 physical | TGA | 82.5 ± 14.8 | 83.0 ± 11.1 | 83.5 ± 8.5 | 0.37 | 0.61 |
| TOF | 95.0 (65.0, 97.5) | 96.3 (67.5, 100.0) | 95.0 (72.5, 95.0) | |||
| Serum creatinine (mg/dL) | TGA | 1.0 ± 0.2 | 0.9 (0.9, 1.1) | 1.0 ± 0.2 | 0.52 | 0.49 |
| TOF | 0.9 ± 0.3 | 0.9 (0.7, 1.2) | 0.9 ± 0.1 | |||
| Serum potassium (mEq/L) | TGA | 4.5 ± 0.5 | 4.4 ± 0.5 | 4.5 ± 0.4 | 0.48 | 0.29 |
| TOF | 4.4 ± 0.5 | 4.5 (4.2, 5.2) | 4.3 ± 0.4 |
PINP indicates procollagen 1 N-terminal peptide; PIIINP, procollagen 3 N-terminal peptide; G3, galectin-3; 6MWD, 6-minute walk distance; SF-36, Short Form 36; TGA, transposition of the great arteries; TOF, tetralogy of Fallot.
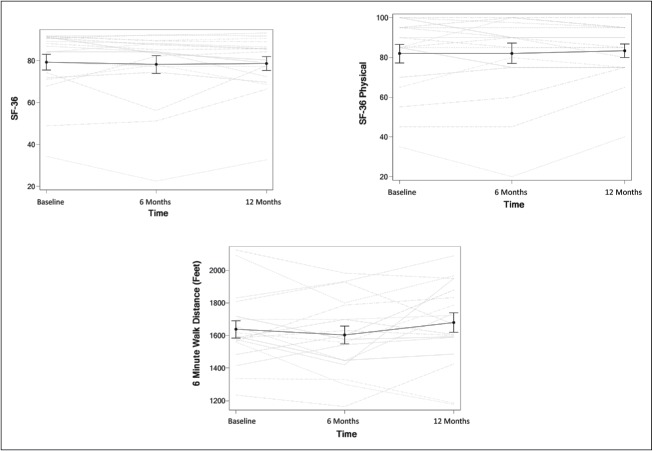
Eplerenone therapy had no effect on 6MWD, SF-36 total score, or physical functioning score (P = 0.22, 0.57, and 0.42, respectively; Figure 4). Similarly, when separated by lesion, there was no lesion-specific difference in the effect of eplerenone on 6MWD, SF-36 total score, or physical functioning score (P = 0.35, 0.68, and 0.61, respectively; Table 2). There was no time dependence for any of the secondary outcomes.
Figure 4.
Effects of eplerenone on total average Short Form 36 (SF-36) score (SF-36), SF-36 physical functioning score (SF-36 physical), and 6-minute walk distance. Gray lines represent individual patients; black lines represent means.
There were no differences in the any outcome between the beginning and the end of the drug-free period for those randomized to the drug-free period at the beginning or end of the study period (Table 3). Participants randomized to a drug-free period at the end of the study period had higher levels of PIIINP during the drug-free period compared to those randomized to the drug-free period at the beginning (P = 0.003); however, their levels of PIIINP did not change during the drug-free period (Figure 5).
Table 3.
Pooled outcomes.
| Endpoint | Drug free | Start of drug-free period | End of drug-free period | P value |
|---|---|---|---|---|
| PINP (μg/mL) | Beginning | 43 ± 16 | 45 ± 18 | 0.95a |
| End | 49 ± 11 | 52 ± 16 | 0.52 | |
| PIIINP (μg/mL) | Beginning | 4.0 ± 1.1 | 3.7 ± 1.1 | 0.21 |
| End | 5.3 ± 1.6 | 5.8 ± 1.5 | 0.83 | |
| G3 (ng/mL) | Beginning | 10.7 ± 2.8 | 11.1 ± 2.6 | 0.36 |
| End | 10.4 ± 1.5 | 10.0 ± 1.5 | 0.82 | |
| 6MWD (feet) | Beginning | 1641.0 ± 257.2 | 1680.4 ± 243.9 | 0.42 |
| End | 1577.1 ± 289.5 | 1591.3 ± 203.2 | 0.2 | |
| SF-36 total | Beginning | 83.8 ± 9.7 | 83.6 ± 10.5 | 0.83 |
| End | 72.4 ± 16.8 | 65.2 ± 18.8 | 0.06 | |
| SF-36 physical | Beginning | 90.8 ± 11.0 | 90.4 ± 9.5 | 1.00a |
| End | 76.1 ± 15.8 | 69.4 ± 19.0 | 0.13a |
PINP indicates procollagen 1 N-terminal peptide; PIIINP, procollagen 3 N-terminal peptide; G3, galectin-3; 6MWD, 6-minute walk distance; SF-36, Short Form 36.
Includes patient randomized to a drug-free period at the end of the study who failed to show up for the final study visit.
Figure 5.
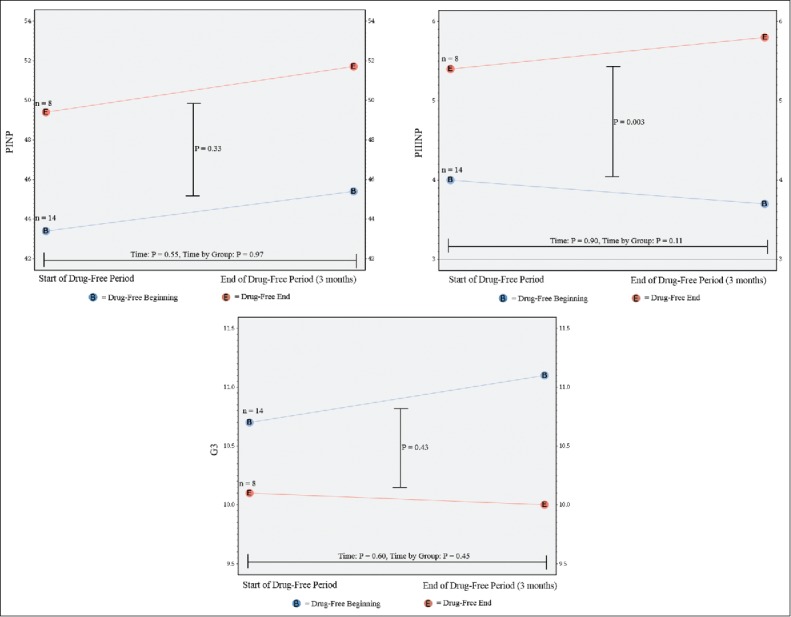
Change in serologic markers procollagen 1 N-terminal peptide (PINP), procollagen 3 N-terminal peptide (PIIINP) and galectin-3 (G3) between the beginning and the end of the drug-free period for those patients randomized to the drug-free period at the beginning (blue) and at the end (red) of the study period.
There was no significant change in serum creatinine or potassium during the study period for any group (Table 2). Six participants reported seven adverse events during the study. Among adverse events, leg cramping, dizziness, and complaints of increased potassium each occurred in one patient, and swelling occurred in two patients. None of these events resulted in eplerenone discontinuation. One patient experienced chest pain and hypotension, which was not thought to be related to the study drug but resulted in drug discontinuation.
DISCUSSION
In the present study, we investigated the levels of serum markers of collagen turnover and inflammation in patients with cyanotic congenital heart disease and the effects of 12 months of eplerenone therapy on those levels. We found that patients with TOF had levels of PINP and PIIINP similar to those seen in patients with HF and normal cardiac anatomy and those with TGA had even higher levels.11 In contrast, we found that G3 levels were significantly lower than those previously seen in association with clinical heart failure among non-ACHD patients.14,15 We found no significant effect of eplerenone therapy on serum markers of collagen turnover, 6MWD, or health-related QOL, although eplerenone was generally well tolerated. The present trial was limited by poor statistical power.
The fact that PINP and PIIINP levels were similar to those found in non-ACHD patients with clinical HF whereas levels of G3 were lower is interesting and potentially informative. PIIINP and G3 have been previously demonstrated to correlate with prognosis in HF with normal cardiac anatomy.14,16 It has been well documented that patients with TOF and TGA have progressive myocardial fibrosis, the ongoing process of which is likely reflected by the relatively elevated PINP and PIIINP levels observed in the present study.3,17–19 Nevertheless, the rate of hospitalization among patients with TOF and TGA, though elevated compared to the general population, is not as high as that in the population of non-ACHD patients with HF.20–22 Elevated serum levels of G3 in HF are associated with incident hospitalization and mortality but much more weakly associated with myocardial fibrosis.14,23 This fact may suggest that the population of patients investigated in the present study was at a relatively lower risk for adverse events than those included in seminal HF trials, demonstrating the efficacy of eplerenone in non–ACHD-related HF, and suggest G3 as a target for further research as a possible predictor of hospitalization risk.24
We elected to investigate aldosterone blockade in the present study for two primary reasons. First, although the etiologic factors underlying HF in ACHD are quite different from those in the majority of patients with normal anatomy and HF, HF in both populations appears to involve progressive fibrous remodeling of the myocardium.3,6 Among the standard pharmacologic interventions for HF, aldosterone receptor blockade has been demonstrated to decrease myocardial fibrosis as well as serum markers of collagen turnover and inflammation, all of which correlate with adverse prognosis.14,16,25 Second, aldosterone blockade has a large effect size compared to beta-blockers and angiotensin–converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs). This is particularly important given difficulties with recruiting and retaining ACHD patients in clinical trials and the relatively lower event rates among ACHD patients compared to those with HF and normal cardiac anatomy.22
The present results are similar to existing data on pharmacologic intervention in the ACHD population in general and highlight a fundamental limitation to nearly all such trials in this patient group. There are no trials of aldosterone blockade in TOF, and the only existing trial of aldosterone blockade in patients with TGV is the eplerenone in systemic right ventricle (EVEDES) trial.26 In the EVEDES trial, 14 patients were randomized to receive eplerenone and 12 were randomized to placebo. The primary outcome was change in right ventricle mass with a secondary outcome of change in serologic markers of collagen turnover and inflammation. Similar to the present study, eplerenone therapy had no significant effect on any endpoint. In the discussion, the authors comment on the difficulty in recruiting patients (only ∼25% of those approached for the EVEDES study agreed to participate). We encountered similar problems, with an even higher nonparticipation rate. Such difficulties with patient recruitment, in combination with a relatively low event rate, make intervention trials in ACHD particularly difficult and have largely led to a near lack of identifiable benefit for any given intervention in the ACHD population.
The results of the present trial in combination with those from the EVEDES trial are nevertheless important for guiding future investigation. Eplerenone was well tolerated both in the present study and in the EVEDES study, with no discernible effect on renal function or serum electrolytes, or adverse impact on patient QOL. Although patients did experience some medication side effects, only one discontinued therapy as a result of events not likely related to the medication, which is comparable to the 13.8% rate of discontinuation seen in the Eplerenone in Mild Patients Hospitalization And Survival Study in Heart Failure trial.24 Despite the negative results in the present trial and EVEDES, the hypothesis that aldosterone blockade might improve outcomes in repaired cyanotic ACHD remains viable and the tolerability of the intervention in ACHD patients should encourage further investigation. Future trials will need to be much larger and multi-institutional.
The present study was inadequately powered and had significant patient heterogeneity, precluding derivation of any real conclusions regarding the efficacy of eplerenone in this patient group. The dropout rate was high but was comparable to that seen in other clinical trials in ACHD.13,27 In the present study, we investigated only serologic markers of collagen turnover, which may or may not correlate with actual histologic myocardial collagen deposition. We selected this outcome because our intention with this small study was to present pilot data to lay the foundation for future larger trials. The addition of cardiac imaging (cardiac magnetic resonance or transthoracic echocardiography) to future protocols would likely add to the strength of data.
Supplementary Material
Funding Statement
This work was supported by an investigator-initiated award from Pfizer Pharmaceuticals trial number WI170964. Clinical Trial Identifier: NCT01971593
Pfizer Pharmaceuticals
ACKNOWLEDGMENTS
Eplerenone for this study was provided by Pfizer Pharmaceuticals as a part of investigator-initiated trial number WI170964.
References
- 1.Verheugt CL, Uiterwaal CS, van der Velde ET, et al.. Mortality in adult congenital heart disease. Eur Heart J. 2010;31(10):1220–1229. doi: 10.1093/eurheartj/ehq032 [DOI] [PubMed] [Google Scholar]
- 2.Opotowsky AR, Siddiqi OK, Webb GD. Trends in hospitalizations for adults with congenital heart disease in the U.S. J Am Coll Cardiol. 2009;54(5):460–467. doi: 10.1016/j.jacc.2009.04.037 [DOI] [PubMed] [Google Scholar]
- 3.Broberg CS, Burchill LJ. Myocardial factor revisited: the importance of myocardial fibrosis in adults with congenital heart disease. Int J Cardiol. 2015;189:204–210. doi: 10.1016/j.ijcard.2015.04.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delcayre C, Swynghedauw B. Molecular mechanisms of myocardial remodeling. The role of aldosterone. J Mol Cell Cardiol. 2002;34(12):1577–1584. doi: 10.1152/physrev.1999.79.1.215 [DOI] [PubMed] [Google Scholar]
- 5.Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83(6):1849–1865. [DOI] [PubMed] [Google Scholar]
- 6.Babu-Narayan SV, Goktekin O, Moon JC, et al.. Late gadolinium enhancement cardiovascular magnetic resonance of the systemic right ventricle in adults with previous atrial redirection surgery for transposition of the great arteries. Circulation. 2005;111(16):2091–2098. doi: 10.1161/01.CIR.0000162463.61626.3B [DOI] [PubMed] [Google Scholar]
- 7.Babu-Narayan SV, Kilner PJ, Li W, et al.. Ventricular fibrosis suggested by cardiovascular magnetic resonance in adults with repaired tetralogy of Fallot and its relationship to adverse markers of clinical outcome. Circulation. 2006;113(3):405–413. doi: 10.1161/CIRCULATIONAHA.105.548727 [DOI] [PubMed] [Google Scholar]
- 8.Pitt B, Zannad F, Remme WJ, et al;. Randomized Aldactone Evaluation Study (RALES) Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341(10):709–717. doi: 10.1056/NEJM199909023411001 [DOI] [PubMed] [Google Scholar]
- 9.Pitt B, Remme W, Zannad F, et al;. Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348(14):1309–1321. doi: 10.1056/NEJMoa03020 [DOI] [PubMed] [Google Scholar]
- 10.RAND Health 36-Item Short Form Survey (SF-36). https://www.rand.org/health/surveys_tools/mos/mos_core_36item.html; accessed November 1, 2017.
- 11.Iraqi W, Rossignol P, Angioi M, et al.. Extracellular cardiac matrix biomarkers in patients with acute myocardial infarction complicated by left ventricular dysfunction and heart failure: insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) Study. Circulation. 2009;119(18):2471–2479. doi: 10.1161/CIRCULATIONAHA.108.809194 [DOI] [PubMed] [Google Scholar]
- 12.Babu-Narayan SV, Uebing A, Davlouros PA, et al.. Randomised trial of ramipril in repaired tetralogy of Fallot and pulmonary regurgitation: the APPROPRIATE Study (Ace inhibitors for Potential PRevention Of the deleterious effects of Pulmonary Regurgitation In Adults with repaired TEtralogy of Fallot). Int J Cardiol. 2012;154(3):299–305. doi: 10.1016/j.ijcard.2010.09.057 [DOI] [PubMed] [Google Scholar]
- 13.van der Bom T, Winter MM, Bouma BJ, et al.. Effect of valsartan on systemic right ventricular function: a double-blind, randomized, placebo-controlled pilot trial. Circulation. 2013;127(3):322–330. doi: 10.1161/CIRCULATIONAHA.112.135392 [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Andrès N, Rossignol P, Iraqi W, et al.. Association of galectin-3 and fibrosis markers with long-term cardiovascular outcomes in patients with heart failure, left ventricular dysfunction, and dyssynchrony: insights from the CARE-HF (Cardiac Resynchronization in Heart Failure) Trial. Eur J Heart Fail. 2012;14(1):74–81. doi: 10.1093/eurjhf/hfr151 [DOI] [PubMed] [Google Scholar]
- 15.Ho JE, Liu C, Lyass A, et al.. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60(14):1249–1256. doi: 10.1016/j.jacc.2012.04.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zannad F, Alla F, Dousett B, Perez A, Pitt B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the Randomized Aldactone Evaluation Study (RALES). Circulation. 2000;102(22):2700–2706. [DOI] [PubMed] [Google Scholar]
- 17.Bolger AP, Coats AJ, Gatzoulis MA. Congenital heart disease: the original heart failure syndrome. Eur Heart J. 2003;24(10):970–976. [DOI] [PubMed] [Google Scholar]
- 18.Bolger AP, Sharma R, Li W, et al.. Neurohormonal activation and the chronic heart failure syndrome in adults with congenital heart disease. Circulation. 2002;106(1):92–99. [DOI] [PubMed] [Google Scholar]
- 19.Delcayre C, Swynghedauw B. Molecular mechanisms of myocardial remodeling. The role of aldosterone. J Mol Cell Cardiol. 2002;34(12):1577–1584. [DOI] [PubMed] [Google Scholar]
- 20.Negishi J, Ohuchi H, Yasuda K, Miyazaki A, Norifumi N, Yamada O. Unscheduled hospitalization in adults with congenital heart disease. Korean Circ J. 2015;45(1):59–66. doi: 10.4070/kcj.2015.45.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desai AS, Stevenson LW. Rehospitalization for heart failure: predict or prevent? Circulation. 2012;126(4):501–506. doi: 10.1161/CIRCULATIONAHA.112.125435 [DOI] [PubMed] [Google Scholar]
- 22.Cedars A, Benjamin L, Vyhmeister R, et al.. Contemporary hospitalization rate among adults with complex congenital heart disease. World J Pediatr Congenit Heart Surg. 2016;7(3):334–343. doi: 10.1177/2150135116639541 [DOI] [PubMed] [Google Scholar]
- 23.Ho JE, Liu C, Lyass A, et al.. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60(14):1249–1256. doi: 10.1016/j.jacc.2012.04.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zannad F, McMurray JJ, Krum H, et al;. EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21. doi: 10.1056/NEJMoa1009492 [DOI] [PubMed] [Google Scholar]
- 25.Lax A, Sanchez-Mas J, Asensio-Lopez MC, et al.. Mineralocorticoid receptor antagonists modulate galectin-3 and interleukin-33/ST2 signaling in left ventricular systolic dysfunction after acute myocardial infarction. JACC Heart Fail. 2015;3(1):50–58. doi: 10.1016/j.jchf.2014.07.015 [DOI] [PubMed] [Google Scholar]
- 26.Dos L, Pujadas S, Estruch M, et al.. Eplerenone in systemic right ventricle: double-blind randomized clinical trial. The EVEDES Study. Int J Cardiol. 2013;168(6):5167–5173. doi: 10.1016/j.ijcard.2013.07.163 [DOI] [PubMed] [Google Scholar]
- 27.Cedars AM, Saef J, Peterson LR, et al.. Effect of ambrisentan on exercise capacity in adult patients after the Fontan procedure. Am J Cardiol. 2016;117(9):1524–1532. doi: 10.1016/j.amjcard.2016.02.024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.