ABSTRACT
Optimal mechanical ventilation management in patients with the acute respiratory distress syndrome (ARDS) involves the use of low tidal volumes and limited plateau pressure. Refractory hypoxemia may not respond to this strategy, requiring other interventions. The use of prone positioning in severe ARDS resulted in improvement in 28-day survival. To determine whether mechanical ventilation strategies or other parameters affected survival in patients undergoing prone positioning, a retrospective analysis was conducted of a consecutive series of patients with severe ARDS treated with prone positioning. Demographic and clinical information involving mechanical ventilation strategies, as well as other variables associated with prone positioning, was collected. The rate of in-hospital mortality was obtained, and previously described parameters were compared between survivors and nonsurvivors. Forty-three patients with severe ARDS were treated with prone positioning, and 27 (63%) died in the intensive care unit. Only three parameters were significant predictors of survival: APACHE II score (P = 0.03), plateau pressure (P = 0.02), and driving pressure (P = 0.04). The ability of each of these parameters to predict mortality was assessed with receiver operating characteristic curves. The area under the curve values for APACHE II, plateau pressure, and driving pressure were 0.74, 0.69, and 0.67, respectively. In conclusion, in a group of patients with severe ARDS treated with prone positioning, only APACHE II, plateau pressure, and driving pressure were associated with mortality in the intensive care unit.
KEYWORDS: Acute respiratory distress syndrome, mechanical ventilation, prone positioning
Therapeutic strategies for the management of the acute respiratory distress syndrome (ARDS) have evolved over time. A landmark study demonstrated survival benefits with the utilization of low tidal volumes and limited plateau pressures.1 On occasion, the aforementioned strategy may not improve the presence of severe hypoxemia. Hence, “rescue treatments” have been investigated. Mechanical ventilation strategies, such as airway pressure release ventilation2,3 and high-frequency oscillatory ventilation,4,5 have presented inconsistent results. Inhaled vasodilators, such as nitric oxide6,7 or epoprostenol,8 led to an improvement in oxygenation for a limited period of time. Perhaps the two most important discoveries since the publication of the ARMA trial (“Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome”)1 have been the utilization of neuromuscular blocking agents9 and prone positioning.10 Prone positioning has been advocated for >40 years as a method to improve aeration in dorsal areas of the lung.11 After some negative outcome trials,12–15 the PROSEVA study (“Prone Positioning in Severe Acute Respiratory Distress Syndrome”)10 showed a 16% reduction in 28-day mortality. The aforementioned trial prompted new interest in this old therapeutic strategy. The present study investigated whether mechanical ventilation parameters were associated with survival in ARDS patients treated with prone positioning.
METHODS
This study involved a retrospective analysis of a consecutive series of adult patients with severe ARDS treated with prone positioning between November 2013 and December 2016. All included patients were treated with a kinetic bed (Rotoprone). Data collection included demographic information, such as gender, age, Acute Physiology and Chronic Health Evaluation (APACHE) II score, body mass index, and height. Clinical information included reason for ARDS, the ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2) upon prone positioning, time from ARDS diagnosis to prone positioning, total time on prone positioning, and mechanical ventilation data such as mode of ventilation, tidal volumes (expressed as tidal volume per ideal body weight), plateau pressures, positive-end expiratory pressures (PEEP), and driving pressures (ΔP), calculated as tidal volume divided by the static compliance of the respiratory system (ΔP = TV/Crs). Information on the utilization of neuromuscular blocking agents, vasopressors for at least 12 hours, high-dose corticosteroids (defined as 0.5 mg/kg every 6 hours), and inhaled vasodilators (i.e., inhaled nitric oxide or epoprostenol) during prone positioning was also collected. Of note, in order to collect mechanical ventilation data, documentation from ventilator checks routinely performed by respiratory therapists was examined. Average daily values of tidal volumes, plateau pressures, and PEEP were obtained throughout the entire stay on prone positioning. The timing of ARDS diagnosis was considered the time of endotracheal intubation or the time of arrival in the intensive care unit (ICU) if the patient arrived in the hospital already intubated. The primary outcome of the study was ICU mortality.
Data were summarized by the patient's survival status and overall using frequencies and percentages for categorical variables. Means, standard deviations, medians, and ranges were used to describe continuous variables. Pearson chi-square and Fisher exact test were used to evaluate the association between a patient's survival status and the categorical measurements. Logistic regression was utilized to evaluate the association between a patient's survival status and continuous variables. Receiver operating characteristic curve (ROC) analysis was used to estimate the optimal cut point for the predictors.
RESULTS
Over 38 months, 43 severe ARDS patients were treated with prone positioning. The mean patient age (± SD) was 54 ± 15 years, and 29 (67%) were men. Average body mass index and APACHE II scores were 32 ± 12 and 27 ± 5, respectively. Upon prone positioning, the average PaO2/FiO2 ratio was 98 ± 50. The mean tidal volume for the entire group of patients was 7 ± 2 cc/kg, while mean plateau and PEEP pressures were 32 ± 7 cm H2O and 12 ± 4 cm H2O, respectively. The elapsed time from ARDS diagnosis to prone positioning was 84 ± 97 hours, and the average total time of prone positioning per patient was 50 ± 44 hours.
Among the 43 patients, 41 (95%) were treated with neuromuscular blocking agents, 19 (44%) received high-dose corticosteroids, and 6 (14%) were treated with inhaled vasodilators concomitantly with prone positioning. Patients were ventilated with either of two ventilators (Puritan Bennett 840, Medtronic, Minneapolis, MN; or Carescape R860, General Electric, Boston, MA). Twenty-eight (65%) patients were ventilated with assist/control volume-limited time-cycled mode, and 12 (28%) were ventilated with assist/control, pressure-limited time-cycled mode. Two (5%) were ventilated with airway pressure release ventilation, and 1 (2%) was ventilated with high-frequency oscillatory ventilation.
Twenty-seven (63%) patients died during the course of the ICU admission. Two of these patients transitioned from prone positioning to extracorporeal membrane oxygenation (ECMO) therapy prior to expiration. No ICU survivor from prone positioning required ECMO treatment. Survivors and nonsurvivors are compared in terms of demographic and clinical information (Table 1) and in terms of mechanical ventilation parameters during the first 4 days of prone positioning (Table 2). As APACHE II, plateau, and ΔP were significant predictors of ICU survival in the univariate analysis, ROC curves were constructed to assess their ability to predict ICU mortality (Figure 1). Furthermore, cut-off points in each ROC curve representing values with the higher possible combination of sensitivity and specificity for prediction of ICU mortality were evaluated (Table 3).
Table 1.
Comparison of demographic and clinical data between intensive care unit nonsurvivors versus survivors
| Nonsurvivors (n = 27) | Survivors (n = 16) | P value | |
|---|---|---|---|
| Age (years): median (IQR) | 57 (45–67) | 55 (34–63) | 0.22 |
| Male | 20 (69%) | 9 (31%) | 0.31 |
| Body mass index (kg/m2): Median (IQR) | 27 (24–30) | 31 (26–42) | 0.12 |
| Height (cm): Median (IQR) | 170 (162–175) | 173 (163–182) | 0.71 |
| APACHE II: Median (IQR) | 30 (24–33) | 26 (23–26) | 0.03 |
| Reason for ARDS | 0.46 | ||
| Aspiration | 4 (15%) | 3 (19%) | |
| H1N1 | 2 (7%) | 1 (6%) | |
| Pancreatitis | 1 (4%) | 2 (12%) | |
| Pneumonia | 16 (59%) | 10 (62%) | |
| Sepsis | 4 (15%) | 0 (0%) | |
| Mechanical ventilation mode | |||
| Volume control | 15 (56%) | 13 (81%) | 0.11 |
| Pressure control | 9 (33%) | 3 (19%) | 0.48 |
| Airway pressure release ventilation | 2 (7%) | 0 (0%) | 0.52 |
| High-frequency oscillatory ventilation | 1 (4%) | 0 (0%) | 0.99 |
| Tidal volume (mL/kg) during prone: Median (IQR) | 8 (6–8) | 7 (6–8) | 0.55 |
| PEEP during prone (cm H2O): Median (IQR) | 13 (10–15) | 13 (11–15) | 0.87 |
| Plateau during prone (cm H2O) (median/IQR) | 34 (31–40) | 30 (26–35) | 0.02 |
| Driving pressure during prone (cm H2O): Median (IQR) | 22 (14–29) | 17 (15–20) | 0.04 |
| Neuromuscular blocking agents | 26 (96%) | 15 (94%) | 0.99 |
| Corticosteroids | 13 (48%) | 6 (38%) | 0.54 |
| Vasopressors | 17 (63%) | 10 (63%) | 0.99 |
| Inhaled vasodilators | 5 (19%) | 1 (6%) | 0.38 |
| PaO2/FiO2 upon prone: Median (IQR) | 87 (65–132) | 72 (63–115) | 0.17 |
| Time from ARDS diagnosis to prone (h): Median (IQR) | 48 (27–112) | 84 (6–120) | 0.38 |
| Time of first prone (h): Median (IQR) | 25 (9–44) | 40 (20–51) | 0.15 |
| Total time on prone (h): Median (IQR) | 31 (9–62) | 45 (20–87) | 0.39 |
IQR indicates interquartile range (25%–75%); APACHE, Acute Physiology and Chronic Health Evaluation; ARDS, acute respiratory distress syndrome; PEEP, positive end-expiratory pressure; PaO2/FiO2, ratio of arterial pressure of oxygen divided by fraction of inspired oxygen.
Table 2.
Comparison of ventilator parameters between survivors and nonsurvivors during prone day 1 to 4
| Day 1 |
Day 2 |
Day 3 |
Day 4 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surv.(n = 16) | NS (n = 27) | P | Surv. (n = 11) | NS (n = 17) | P | Surv. (n = 8) | NS (n = 9) | P | Surv (n = 4) | NS (n = 5) | P | |
| Tidal volume (mL ± SD) | 460 ± 70 | 480 ± 110 | 0.65 | 460 ± 70 | 470 ± 100 | 0.79 | 450 ± 50 | 480 ± 140 | 0.50 | 440 ± 70 | 460 ± 8 | 0.69 |
| PEEP (cm H2O ± SD) | 12 ± 2 | 14 ± 4 | 0.15 | 13 ± 2 | 13 ± 4 | 0.88 | 14 ± 3 | 11 ± 3 | 0.10 | 14 ± 3 | 11 ± 4 | 0.19 |
| Plateau pressure (cm H2O ± SD) | 28 ± 7 | 31 ± 6 | 0.13 | 29 ± 5 | 31 ± 5 | 0.21 | 29 ± 7 | 33 ± 4 | 0.16 | 26 ± 7 | 31 ± 4 | 0.12 |
| Driving pressure (cm H2O ± SD) | 16 ± 5 | 17 ± 7 | 0.76 | 16 ± 6 | 18 ± 6 | 0.31 | 15 ± 7 | 22 ± 6 | 0.06 | 11 ± 5 | 20 ± 5 | 0.02 |
| Hours on prone (h ± SD) | 21 ± 5 | 17 ± 8 | 0.15 | 21 ± 4 | 16 ± 7 | 0.26 | 19 ± 9 | 15 ± 9 | 0.68 | 19 ± 10 | 18 ± 8 | 0.80 |
All values in the table represent the average value in the prone position for each intensive care unit day during a 24-hour period running from 7:00 AM to 6:59 AM. Surv. indicates survivors; NS, nonsurvivors; PEEP, positive-end expiratory pressure; SD, standard deviation.
Figure 1.
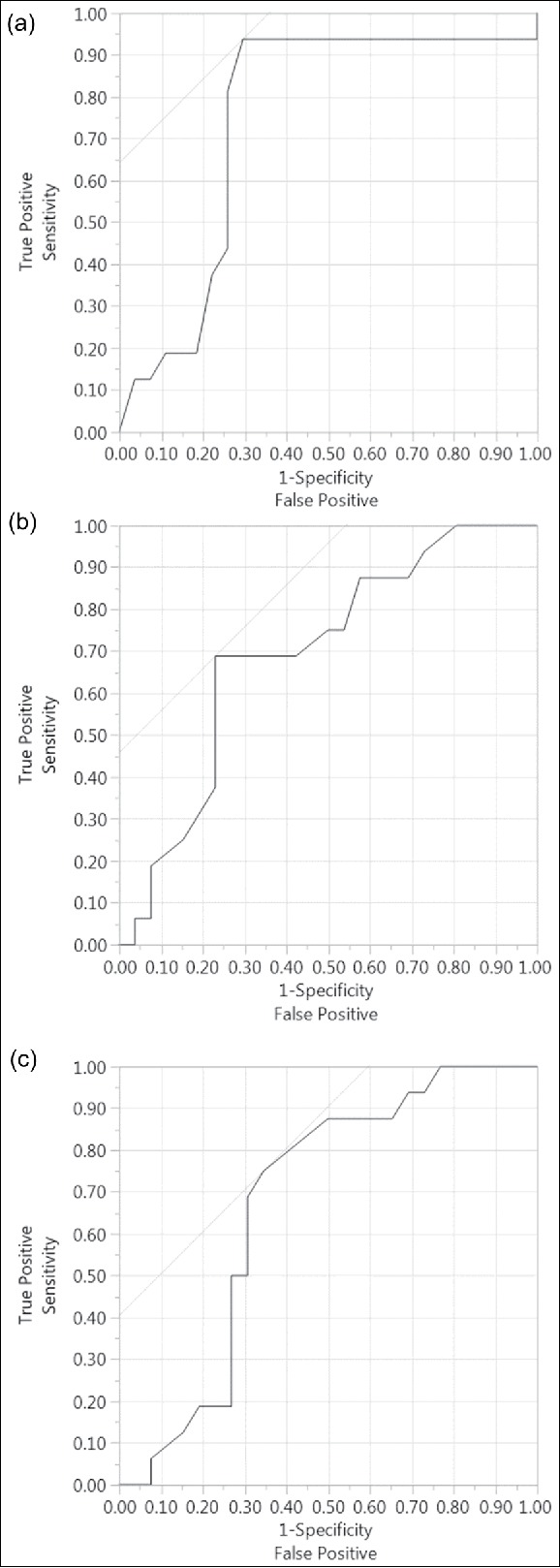
Receiving operating curve in predicting hospital mortality: (a) Acute Physiology and Chronic Health Evaluation (APACHE) II score (area under the curve = 0.74); (b) plateau pressure (area under the curve = 0.69); (c) driving pressure (area under the curve = 0.67).
Table 3.
Receiver operating characteristic curve analysis: the optimal cut point for the predictors
| Predictors | Optimal cut point | Estimated probability of survival | Specificity | Sensitivity | True Pos. | True Neg. | False Pos. | False Neg. | AUC |
|---|---|---|---|---|---|---|---|---|---|
| APACHE | 27 | 0.368 | 0.704 | 0.938 | 15 | 19 | 8 | 1 | 0.744 |
| Plateau during prone | 30 | 0.445 | 0.769 | 0.688 | 11 | 20 | 6 | 5 | 0.694 |
| Driving pressure | 19 | 0.390 | 0.654 | 0.750 | 12 | 17 | 9 | 4 | 0.673 |
APACHE indicates Acute Physiology and Chronic Health Evaluation score; pos., positive; neg., negative; AUC, area under the curve.
DISCUSSION
The present study reveals that, in patients with severe ARDS treated with prone positioning, only APACHE II score, plateau pressure, and ΔP were associated with ICU mortality. Notably, tidal volumes had no association with this clinical outcome. These findings are consistent with a prior landmark study, which showed that among multiple ventilator variables, ΔP was the most strongly associated with hospital survival at 60 days.16 Interestingly, in the previously described study, individual changes in tidal volume and PEEP were not independently associated with this outcome. As ΔP = TV/Crs = TV/ [TV/(Plateau – PEEP)], it is likely that plateau pressure drives the aforementioned survival benefit. Notably, as shown in Table 3, a plateau pressure of 30 cm H2O was identified as the best cutoff point to predict mortality, with a sensitivity of 77% and specificity of 69%. This cutoff point is concordant with the strategy suggested by the ARMA study.1 Driving pressure may be used as a surrogate of the amount of cyclic parenchymal deformation imposed over preserved lung units, causing ventilator-induced lung injury. Hence, as increased plateau pressure affects compliance of the respiratory system, it may consequently result in more cyclic deformation, more ventilator-induced lung injury, and lower survival.
Interestingly, prior studies12,14 exploring prone positioning in ARDS patients reported ICU mortalities for prone patients ranging from 43% to 51%, whereas the landmark PROSEVA trial reported a 28-day mortality of 16%.10 The ICU mortality in our prone patients was 63%. Several factors may have been involved in this higher mortality. First, as the APACHE II score for the entire ARDS population was 27, the expected ICU mortality ranged from 55% to 65%,17 concordant with the observed outcome. Second, with the exception of the PROSEVA trial, which included ARDS subjects with a PaO2/FiO2 <150 mm Hg, other studies were performed including previously denominated acute lung injury patients, defined as PaO2/FiO2 < 300 mm Hg.13,14 Therefore, the lower level of ARDS severity in prior studies may have been associated with lower mortality rates compared with our cohort. Third, the time elapsed from ARDS diagnosis to prone positioning in our population was 84 hours (3.5 days). The aforementioned elapsed time in the PROSEVA trial10 was <1.5 days, whereas it was <2 days and <3 days in the studies of Mancebo et al12 and Taccone et al,15 respectively. This delay in the implementation of an effective therapy may have also caused higher ICU mortality in our group compared with prior studies.
Our study presents several strengths. It explores the impact of several ventilator parameters (i.e., TV, PEEP, plateau, and ΔP) in ICU mortality. Per our knowledge, this is the first study in the English literature that addresses that question. Our study included ARDS patients treated with prone positioning based on current standards (PaO2/FiO2 < 150 mm Hg) and had similar rates of use of neuromuscular blocking agents (87% in PROSEVA [10] vs. 95% in this study). Hence, the findings from our study in terms of mechanical ventilation strategies may be applicable to other ICUs that follow similar prone positioning protocols. Despite the previously described strengths, our study presents multiple limitations. First, the retrospective nature of the study creates the possibility of informational bias, as many points of data may have been missing or not accounted for. Secondly, as few patients (N = 43) were treated with prone positioning over the course of 38 months, it is likely that the study was not powered to detect significant differences in other important variables. A larger number of patients may have resulted in different findings. Last, information on important clinical variables, such as heart disease, immunocompromised status, kidney or liver disease, and neurologic impairment was not collected in our database. Hence, it is possible that mortality rates may have been associated with an unbalanced presence of any of these conditions rather than factors exclusively related to prone positioning or mechanical ventilation strategies. In summary, this study reveals that, in severe ARDS patients treated with prone positioning, there is an association between APACHE II score, plateau pressure, and ΔP and ICU mortality.
References
- 1.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A; Acute Respiratory Distress Syndrome Network . Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 2.Habashi NM. Other approaches to open-lung ventilation: airway pressure release ventilation. Crit Care Med. 2005;33(Suppl):S228–S240. doi: 10.1097/01.CCM.0000155920.11893.37. [DOI] [PubMed] [Google Scholar]
- 3.Varpula T, Valta P, Niemi R, Takkunen O, Hynynen M, Pettila VV. Airway pressure release ventilation as a primary ventilatory mode in acute respiratory distress syndrome. Acta Anaesthesiol Scand. 2004;48(6):722–731. doi: 10.1111/j.0001-5172.2004.00411.x. [DOI] [PubMed] [Google Scholar]
- 4.Young D, Lamb SE, Shah S, et al; OSCAR Study Group . High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med. 2013;368(9):806–813. doi: 10.1056/NEJMoa1215716. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson ND, Cook DJ, Guyatt GH, et al; OSCILLATE Trial Investigators, Canadian Critical Care Trials Group . High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013;368(9):795–805. doi: 10.1056/NEJMoa1215554. [DOI] [PubMed] [Google Scholar]
- 6.Dellinger RP, Zimmerman JL, Taylor RW, et al; Inhaled Nitric Oxide in ARDS Study Group . Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Crit Care Med. 1998;26(1):15–23. doi: 10.1097/00003246-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Taylor RW, Zimmerman JL, Dellinger RP, et al; Inhaled Nitric Oxide in ARDS Study Group . Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004;291(13):1603–1609. doi: 10.1001/jama.291.13.1603. [DOI] [PubMed] [Google Scholar]
- 8.Fuller BM, Mohr NM, Skrupky L, Fowler S, Kollef MH, Carpenter CR. The use of inhaled prostaglandins in patients with ARDS: a systematic review and meta-analysis. Chest. 2015;147(6):1510–1522. doi: 10.1378/chest.14-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papazian L, Forel JM, Gacouin A, et al; ACURASYS Study Investigators . Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 10.Guerin C, Reignier J, Richard JC. Prone positioning in the acute respiratory distress syndrome. N Engl J Med. 2013;369(10):980–981. [DOI] [PubMed] [Google Scholar]
- 11.Bryan AC. Conference on the scientific basis of respiratory therapy. Pulmonary physiotherapy in the pediatric age group. Comments of a devil's advocate. Am Rev Respir Dis. 1974;110(6Pt 2):143–144. [DOI] [PubMed] [Google Scholar]
- 12.Mancebo J, Fernandez R, Blanch L, et al;. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;173(11):1233–1239. doi: 10.1164/rccm.200503-353OC. [DOI] [PubMed] [Google Scholar]
- 13.Guerin C, Gaillard S, Lemasson S, et al;. Effects of systematic prone positioning in hypoxemic acute respiratory failure: a randomized controlled trial. JAMA. 2004;292(19):2379–2387. doi: 10.1001/jama.292.19.2379. [DOI] [PubMed] [Google Scholar]
- 14.Gattinoni L, Tognoni G, Pesenti A, et al; Prone-Supine Study Group . Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001;345(8):568–573. doi: 10.1056/NEJMoa010043. [DOI] [PubMed] [Google Scholar]
- 15.Taccone P, Pesenti A, Latini R, et al; Prone-Supine II Study Group . Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2009;302(18):1977–1984. doi: 10.1001/jama.2009.1614. [DOI] [PubMed] [Google Scholar]
- 16.Amato MB, Meade MO, Slutsky AS, et al;. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372(8):747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 17.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]


