ABSTRACT
Chronic neutrophilic leukemia is a rare myeloproliferative disorder characterized by a sustained peripheral blood neutrophilia, absence of the BCR/ABL oncoprotein, bone marrow hypercellularity with less than 5% myeloblasts and normal neutrophil maturation, and no dysplasia. This leukemia has been associated with mutations in the colony-stimulating factor 3 receptor (CSF3R) that may activate this receptor, leading to the proliferation of neutrophils that are the hallmark of chronic neutrophilic leukemia. We present a case of chronic neutrophilic leukemia and discuss the criteria for diagnosis and the significance of mutations found in this leukemia.
KEYWORDS: Chronic neutrophilic leukemia, CSF3R, myeloproliferative disorder, RUNX1, SETBP1, SRSF2
CASE PRESENTATION
A 53-year-old man presented to the emergency room with abdominal pain. He was a previous smoker (50 pack-years) and stated that he had recently been experiencing night sweats. The past history was otherwise unremarkable. Blood work at the time revealed a leukocytosis of 40 × 109/L (Figure 1a) and hyperuricemia (10.1 mg/dL). Mild splenomegaly was present. Flow cytometry detected a very small (0.1) population of kappa clonal plasma cells. A bone marrow examination showed a hypercellular (100%) marrow with granulocytic hyperplasia and numerous polymorphonuclear cells (Figures 1b and 1c). A plasma cell infiltrate was not appreciated. Subsequent lab work revealed the absence of the BCR/ABL oncoprotein but the presence of colony-stimulating factor 3 receptor (CSF3R) and set binding protein 1 (SETBP1) mutations. These findings were consistent with a diagnosis of chronic neutrophilic leukemia (CNL). The patient was started on ruxolitinib and scheduled for follow-up.
Figure 1.
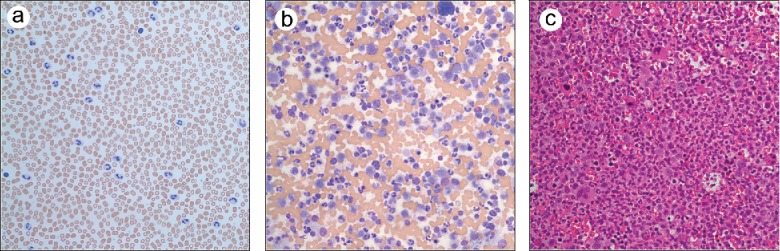
(a) Peripheral blood (Wrights, ×500). (b) Bone marrow aspirate (Wrights, ×500). (c) Bone marrow core biopsy (hemotoxylin and eosin, ×400).
Follow-up at 1 month showed a slight drop in the white blood cell count and uric acid as well as an improvement in appetite and decrease in night sweats. A second bone marrow aspirate was still hypercellular with granulocytic hyperplasia with less than 10% immature granulocytes and no increase in blasts. A repeat mutation panel now revealed a serine/arginine-rich splicing factor 2 (SRSF2) mutation and a variant RUNX1 mutation of undetermined significance. The mutations for CSF3R and SETBP1 were not detected on this analysis. The patient is currently stable with a sustained moderate leukocytosis and remains on ruxolitinib. He is being considered for a hematopoietic stem cell transplant.
DISCUSSION
In 2016, the World Health Organization classification of hematologic malignancies was updated to reflect advances in the understanding of CNL.1 In CNL, the presence of kinase mutations that activated kinase signaling to promote the expansion of neutrophils has been incorporated into the diagnostic criteria.1 A high prevalence of CSF3R is found in about 80% of cases.2,3 Also common are mutations in SETBP1 (14%–50%) and various spliceosome proteins.2,4,5 Both CSF3R and SETBP1 were found in our case, which is very supportive of a diagnosis of CNL. Patients can still be classified as having CNL in the absence of activating mutations if they meet other criteria such as neutrophilic leukocytosis >25 × 109/L for at least 3 months, hypercellular bone marrow with granulocytic hyperplasia and no dysplasia, splenomegaly, absence of the BCR/ABL oncoprotein, and no detectable underlying reason for a reactive neutrophilia.1 Reactive neutrophilia has been associated with underlying plasma cell neoplasms.6 In the case of a plasma cell neoplasm, the diagnosis of CNL can still be made if there is evidence of myeloid cell clonality. Our case had a very minute population of clonal plasma cells (0.1%), which may be coincidental monoclonal gammopathy of undetermined significance given the tiny population of plasma cells. Our case also had CSF3R and SETBP1 mutations, which support the diagnosis of CNL. Because mutant CSF3R has been associated with dysregulated Janus kinase/signal transducers and activators of transcription signaling, it has been suggested that ruxolitinib may be efficacious in treating CNL, and there are some anecdotal cases suggesting some clinical benefit of ruxolitinib.4,5 However, there have been other cases in which a CSF3R mutation was present but there was no response to ruxolitinib which suggests that additional genetic events such as an SETB1 mutation may abrogate the response.7–9 It is also interesting that on the patient's last bone marrow biopsy, an SRSF2 mutation and RUNX1 gene variant were detected. It is known that both SRSF2 and RUNX1 abnormalities have been found in early stages of myelodysplastic syndromes.10,11 The significance of these findings remains to be seen in this patient. Further studies are necessary on the occurrence of these mutations and their interactions in CNL that will provide insight into the biogenics and therapeutic targeting of this rare but serious myeloid malignancy.
References
- 1.Arber DA, Orazi A, Hasserjian R, et al.. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 2.Pardanani A, Lasho TL, Laborde RR, et al.. CSF3R T618I is a highly prevalent and specific mutation in chronic neutrophilic leukemia. Leukemia. 2013;27(9):1870–1873. doi: 10.1038/leu.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maxson JE, Gotlib J, Pollyea DA, et al.. Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. N Engl J Med. 2013;368(19):1781–1790. doi: 10.1056/NEJMoa1214514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gotlib J, Maxson JE, George TI, Tyner JW. The new genetics of chronic neutrophilic leukemia and atypical CML: implications for diagnosis and treatment. Blood. 2013;122(10):1707–1711. doi: 10.1182/blood-2013-05-500959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui Y, Li B, Gale RP, et al.. CSF3R, SETBP1 and CALR mutations in chronic neutrophilic leukemia. J Hematol Oncol. 2014;7(1):77–81. doi: 10.1186/s13045-014-0077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dincol G, Nalcaci M, Dogan O, et al.. Coexistence of chronic neutrophilic leukemia with multiple myeloma. Leuk Lymphoma. 2002;43(3):649–651. doi: 10.1080/10428190290012218. [DOI] [PubMed] [Google Scholar]
- 7.Ammatuna E, Eefting M, van Lom K, Kavelaars FG, Valk PJ, Touw IP. Atypical chronic myeloid leukemia with concomitant CSF3R T616I and SETBP1 mutations unresponsive to the JAK inhibitor ruxolitinib. Ann Hematol. 2015;94(5):879–880. doi: 10.1007/s00277-014-2272-0. [DOI] [PubMed] [Google Scholar]
- 8.Lasho TL, Mims A, Elliott MA, Finke C, Pardanani A, Tefferi A. Chronic neutrophilic leukemia with concurrent CSF3R and SETBP1 mutations: single colony clonality studies, in vitro sensitivity to JAK inhibitors and lack of treatment response to ruxolitinib. Leukemia. 2014;28(6):1363–1365. doi: 10.1038/leu.2014.39. [DOI] [PubMed] [Google Scholar]
- 9.Maxson JE, Tyner JW. Genomics of chronic neutrophilic leukemia. Blood. 2017;129(6):715–722. doi: 10.1182/blood-2016-10-695981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouyang Y, Qiao C, Chen Y, Zhang SJ. Clinical significance of CSF3R, SRSF2 and SETBP1 mutations in chronic neutrophilic leukemia and chronic myelomonocytic leukemia. Oncotarget. 2017;8(13):20834–20841. PMID:28209919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sood R, Kamikubo Y, Liu P. Role of RUNX1 in hematological malignancies. Blood. 2017;129(15):2070–2082. doi: 10.1182/blood-2016-10-687830. [DOI] [PMC free article] [PubMed] [Google Scholar]


