ABSTRACT
We describe a patient with history of dextro-transposition of the great vessels, ventricular septal defect, and pulmonary valve replacement who presented with fatigue, prolonged fever, and leg edema. He was found to have kidney injury, pancytopenia, and liver congestion. Echocardiogram revealed thickened leaflets with prolapsing vegetation on the pulmonary valve. Given the negative blood cultures, high Bartonella henselae immunogobulin G titer (≥1:1024) and positive immunoglobulin M titer (≥1:20), he was diagnosed with Bartonella endocarditis complicated with glomerulonephritis.
KEYWORDS: Bartonella henselae, culture-negative endocarditis, glomerulonephritis, prosthetic valve
B artonella species are rare causes of infective endocarditis (IE).1,2 Patients with Bartonella endocarditis usually present with signs and symptoms of IE, but blood cultures are usually negative.2 Bartonella henselae usually affects patients with previous valvular disease, prosthetic or bioprosthetic valves, or congenital heart defects.3,4 In rare instances, patients with Bartonella endocarditis may develop glomerulonephritis.5,6
CASE DESCRIPTION
A 29-year-old man had complete transposition of the great arteries and ventricular septal defect, for which he received Rastelli repair at age 4 and right ventricle–to–pulmonary artery conduit replacement with a porcine valve at age 18 for conduit stenosis. He initially presented with fatigue, prolonged fever, and leg edema. His temperature was 101°F. A 5/6 harsh systolic murmur was heard over the left sternal border, his jugular veins were distended, his liver was enlarged, and his ankles were edematous. His erythrocyte sedimentation rate was 58 (normal range 0–15 mm/hr), C-reactive protein was 5.1 (normal range 0.0–0.3 mg/dL), creatinine was 3.3 mg/dL, red blood cells were 2.83 M/μL, white blood cells were 3.2 K/μL, and platelets were 105 K/μL. Echocardiogram showed a left ventricular ejection fraction of 35% to 40%. The pulmonic valve had thickened cusps and a prolapsing mass. He was treated with vancomycin, doxycycline, and piperacillin/tazobactam.
The blood cultures, drawn at the time of presentation, showed no growth. The urine protein-creatinine ratio was 4.4 and his 24-hour urine protein was 6.4 g. C3 was 61.4 mg/dL (normal range 90–180 mg/dL), complement C4 was 9.4 mg/dL (normal range 10–40 mg/dL), and serum albumin was 2.3 g/dL. Antineutrophil cytoplasmic antibody in the serum was positive. A kidney biopsy revealed focal segmental proliferative glomerulonephritis with incomplete crescent formation. Electron microscopy showed small mesangial electron-dense deposits and widespread foot process effacement. The mesangial regions were positive for the following immunofluorescence markers: immunoglobulin G (IgG), immunoglobulin M (IgM), C3, C1q, and kappa and lambda light chains. Though not specific, IgM immunofluorescence demonstrated the brightest signal, suggestive of a recent or ongoing infection (Figure 1).
Figure 1.
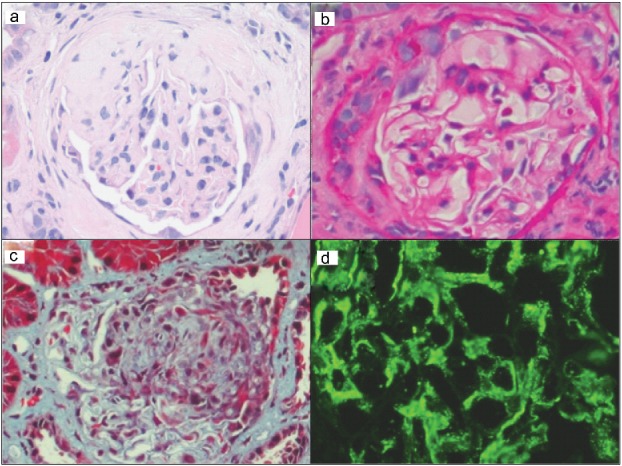
Photomicrographs of the patient's kidney biopsy demonstrating focal segmental proliferative glomerulonephritis with incomplete crescent formation. Representative areas showing segmental sclerosis with crescent formation in a glomerulus on (a) hematoxylin and eosin–stained tissue section, (b) periodic acid–Schiff-stained tissue section, and (c) trichrome-stained tissue section (200× magnification). (d) Bright granular immunoglobulin M immunofluorescence positivity in a mesangial pattern.
Brucella antibody, Coxiella burnetii IgG and IgM, and Ehrlichia IgG were negative. Bartonella henselae serology was positive (IgG titer ≥ 1:1024 and IgM titer ≥ 1:20). The patient took care of cats in his neighborhood. His antibiotic regimen was changed to ceftriaxone, doxycycline, and vancomycin during his hospital stay. He also received prednisone for glomerulonephritis and his creatinine gradually decreased to 2 mg/dL. The fever and lower extremity edema resolved, and he was discharged home on prednisone and 6 weeks of intravenous doxycycline after a stay of 13 days in the hospital. Four weeks later, his creatinine was still elevated at 2.0 mg/dL, the B. henselae IgG was >1:1024, and B. henselae IgM was negative.
One year later, he was admitted with supraventricular tachycardia and syncope. He admitted that he did not finish the course of treatment with doxycycline. Echocardiogram showed severe right ventricular–to–pulmonary artery conduit stenosis with prolapse of pulmonic valve cusps and peak and mean gradients of 58 and 32 mm Hg, right ventricular hypertrophy, and moderate right ventricular systolic dysfunction. Echocardiogram did not detect vegetation. His creatinine was 3.5 mg/dL, and repeat kidney biopsy revealed chronic end-stage glomerulonephritis. Bartonella IgG was persistently elevated. He underwent successful Melody transcatheter pulmonary valve placement of his severely stenotic pulmonary valve conduit, because he was high surgical risk. He was discharged on doxycycline.
DISCUSSION
Intracellular bacteria (i.e., Bartonella spp. and Coxiella spp.) are the most common organisms associated with blood culture–negative endocarditis.2 Bartonella spp., in particular, are small, intracellular gram-negative bacteria associated with cats and fleas as vectors for their subspecies—B. henselae and B. quintana, respectively.1,2,5 Congenital heart disease and prosthetic valve repair have been identified as risk factors for the development of B. henselae endocarditis, particularly in the pediatric population.4,7 Serology, polymerase chain reaction, or histology is needed to identify Bartonella spp., when suspected.2
IE may present with renal comorbidities such as perinephric abscesses, infarction from emboli, or glomerulonephritis.8 Though IE is associated with hypocomplementic glomerulonephritis, as was seen in this case, glomerulonephritis as a complication of B. henselae endocarditis is rare, with few reported cases.6–8 Multiple studies report that nearly 60% of patients with Bartonella spp. endocarditis have positive antineutrophil cytoplasmic antibody, as was seen in our patient, and some cases may imitate other systemic diseases such as granulomatosis with polyangiitis.5,6 Renal biopsies in these patients may reveal necrotic crescentic disease or diffuse proliferative glomerular disease.6 Most cases of Bartonella spp. IE with glomerulonephritis have loop and mesangial deposits of IgM and IgG complexes.6
Although the exact antibiotic regimens for treatment of Bartonella endocarditis have not been universally agreed upon, the antibiotic regimen consisting of ceftriaxone (6 weeks) plus gentamicin (2 weeks) with/without doxycycline (6 weeks) has been recommended for treatment of suspected Bartonella endocarditis in patients with a prosthetic valve (late, >1 year). The suggested treatment of documented Bartonella endocarditis (culture positive) in patients with a prosthetic valve (late, >1 year) includes doxycycline (6 weeks) and gentamicin (2 weeks).9 In the vast majority of cases (90% in adults and 85% in children), management of B. henselae endocarditis involves valvular surgery.6,8 Follow-up appointments for patients with IE without signs of systemic toxicity should be within 1 month of completion of antibiotic therapy.9 Though some studies have associated Melody valve placement with increased incidence of IE, there is no increased risk of repeat IE in patients with a history of IE who receive a Melody valve.10
References
- 1.Adelson K, Paris J, Horton JR, Hernandez-Tellez L, Ricks D, Morrison RS, Smith CB. Standardized criteria for palliative care consultation on a solid tumor oncology service reduces downstream health care use. J Oncol Pract. 2017;13(5):e431–e440. [DOI] [PubMed] [Google Scholar]
- 2.Subedi S, Jennings Z, Chen SC. Laboratory approach to the diagnosis of culture-negative infective endocarditis. Heart Lung Circ. 2017;26(8):763–771. [DOI] [PubMed] [Google Scholar]
- 3.Papineni P, Carroll A, Radvan J, et al.. Management of Bartonella prosthetic valve endocarditis without cardiac surgery. Emerg Infect Dis. 2017;23(5):861–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edouard S, Nabet C, Lepidi H, Fournier PE, Raoult D. Bartonella, a common cause of endocarditis: a report on 106 cases and review. J Clin Microbiol. 2015;53(3):824–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sada R, Uno S, Hosokawa N, Komiya T. Prosthetic valve endocarditis caused by Bartonella henselae presenting as recurrent fever imitating granulomatosis with polyangiitis. J Formos Med Assoc. 2017;116(11):907–909. doi: 10.1016/j.jfma.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Raybould JE, Raybould AL, Morales MK, et al.. Bartonella endocarditis and pauci-immune glomerulonephritis: a case report and review of the literature. Infect Dis Clin Pract (Baltim Md). 2016;24(5):254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Georgievskaya Z, Nowalk AJ, Randhawa P, Picarsic J. Bartonella henselae endocarditis and glomerulonephritis with dominant C3 deposition in a 21-year-old male with a Melody transcatheter pulmonary valve: case report and review of the literature. Pediatr Dev Pathol. 2014;17(4):312–320. [DOI] [PubMed] [Google Scholar]
- 8.Ouellette CP, Joshi S, Texter K, Jaggi P. Multiorgan involvement confounding the diagnosis of Bartonella henselae infective endocarditis in children with congenital heart disease. Pediatr Infect Dis J. 2017;36(5):516–520. [DOI] [PubMed] [Google Scholar]
- 9.Baddour LM, Wilson WR, Bayer AS, et al.. Infective endocarditis diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation. 2005;111(23):e394–e434. [DOI] [PubMed] [Google Scholar]
- 10.Patel M, Malekzadeh-Milani S, Ladouceur M, Iserin L, Boudjemline Y. Percutaneous pulmonary valve endocarditis: incidence, prevention and management. Arch Cardiovasc Dis. 2014;107(11):615–624. doi: 10.1016/j.acvd.2014.07.052. [DOI] [PubMed] [Google Scholar]


